Abstract
C-reactive protein (CRP) is an acute-phase reactant frequently used in histochemistry as a marker of ongoing inflammation. Furthermore, CRP is a powerful biomarker for the prediction of coronary artery disease risk. Heat-shock protein 60 (Hsp60) and CRP are complement-activating molecules, and the effect of their interactions on the regulation of complement activation was studied. However, during the first experiments, we learned that polyclonal anti-CRP antibodies cross-react with Hsp60. Therefore, the aim of our present study was to analyze the cross-reactivity of anti-CRP antibodies (Ab) with Hsp60 in solid-phase enzyme immune assays, in epitope studies using a series of overlapping synthetic peptides, and in Ouchterlony analyses. We found that three different commercial rabbit polyclonal antibodies and two monoclonal (9C9 and CRP-8) anti-CRP antibodies specifically recognize recombinant human Hsp60 and recombinant Mycobacterium tuberculosis Hsp65, respectively. Hsp60 was found to inhibit the binding of anti-CRP polyclonal Ab to Hsp60. Six epitope regions of Hsp60 were recognized by the anti-CRP antibodies, and one region (amino acids [AA] 218 to 232) was recognized by monoclonal antibodies CRP-8 and 9C9. This epitope region of Hsp60 displays 26.6% amino acid identity to CRP AA region 77 to 90. These data suggest that the B-cell epitopes shared between CRP and Hsp60 give rise to a true mimicry-based cross-reaction and the induction of cross-reactive antibodies. Our study underlines the importance of thorough study design and careful interpretation of results while using polyclonal anti-CRP antibodies for histochemistry, especially at low dilutions. Furthermore, analytical interference with Hsp60 in CRP assays should also be tested.
C-reactive protein (CRP) is an acute-phase reactant in humans, rabbits, and a number of other mammalian species. It is expressed and secreted primarily by hepatocytes, but recently, local production at sites of inflammation by monocytes has also been reported (20). The serum CRP concentration increases up to 1,000-fold during the acute-phase reaction or inflammation. CRP has been shown to activate the classical complement pathway by C1q binding (7). Furthermore, CRP is active in opsonization and lymphocyte modulation and in natural killer cell, macrophage, neutrophil, and platelet responses (5). It has been reported that human CRP may exist in two antigenically distinct forms, which are known as native CRP (nCRP) and modified CRP (mCRP). Neoepitopes are expressed on mCRP when the native pentameric form of CRP is dissociated into free subunits (10). Commercial anti-CRP antibody preparation may possess a significant proportion of specificities (up to 16% of the total reactivity) directed against CRP neoepitopes (14).
Heat shock proteins (Hsps) are ubiquitous, phylogenetically highly conserved stress proteins, having essential roles in cell survival (3). Hsps are often immunodominant antigens recognized in bacterial, fungal, and parasitic infections and are therefore capable of inducing strong humoral and cellular immune responses in mammals (1). Immunization schedules often involve the administration of the antigen in complete Freund's adjuvant (CFA), followed by booster injections of antigens. CFA is an emulsion of mycobacteria in oil, and Hsp65 is an immunodominant antigen of mycobacteria. It is hardly surprising that Hsp65-reactive T cells and antibodies develop in response to CFA; indeed, this has been demonstrated with rats (13) and rabbits (18).
We have previously shown strong activation of the classical pathway of complement by human Hsp60 (11). Since the expression of Hsp60 and the complement-activating acute-phase reactant CRP are increased at sites of inflammation, we intended to determine whether Hsp60 is able to form complexes with CRP. However, we learned during the first experiment that anti-CRP polyclonal antibodies recognize human Hsp60. Hence, the aim of the present study was to characterize the anti-Hsp60 activity present in anti-CRP antibody preparations. Since anti-CRP antibodies are widely used for immunohistochemistry, our data may be of importance in regard to the interpretation of these studies.
MATERIALS AND METHODS
Proteins and antibodies used.
Recombinant human Hsp60 and recombinant Mycobacterium tuberculosis Hsp65 antigens were obtained from Lionex, Ltd., Braunschweig, Germany. Rabbit polyclonal anti-Hsp65 antibodies were produced by immunizing two New Zealand White rabbits by using a standard protocol. Briefly, following prebleeding to collect preimmune serum, 0.2 mg of antigen was injected intradermally into the rabbits, with CFA (Sigma-Aldrich, St. Louis, MO). Quintuple booster immunizations with 0.2 mg antigen and incomplete Freund's adjuvant were given to the rabbits intradermally every 2 weeks following the initial immunization. Animals were bled 1 week after the second and fourth booster injections to determine titers. One week after the final booster injections, the animals were exsanguinated under deep anesthesia. All experimental procedures were approved by the Animal Care and Ethics Committee of the Faculty of Veterinary Science, Szent István University, and complied with the Hungarian Code of Practice for the Care and Use of Animals for Scientific Purposes. Purified human C-reactive protein (code C-4063) was obtained from Sigma. Goat anti-CRP (Autokit CRP-HS R2; WAKO Chemicals GmbH, Neuss, Germany), rabbit anti-CRP (code C-3527; Sigma-Aldrich, Steinheim, Germany), and rabbit anti-CRP (code A0073; DAKO, Glostrup, Denmark) polyclonal antibodies were purchased. The anti-CRP monoclonal antibody (Ab) panel (clones I-15-1D6, II-15-2C10, III-26-8C10, IV-13-3H12, IV-26-9C9, and IV-13-12D7), the phosphatidylcholine conjugated to keyhole-limpet hemocyanin (PC-KLH), and the recombinant modified CRP were produced as described previously (21) and kindly provided by L. A. Potempa. Anti-CRP monoclonal antibody CRP-8 was obtained from Sigma (code C-1688).
Enzyme-linked immunosorbent assay.
Enzyme-linked immunosorbent assay (ELISA) plates (Greiner, Germany) were coated with 100 μl/well recombinant human Hsp60 (2 μg/ml) or recombinant M. tuberculosis Hsp65 (2 μg/ml) in 0.1 M bicarbonate buffer (pH 9.6) and incubated overnight at 4°C, and uncoated plates were used as controls. Plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20 and blocked with 0.15 M PBS (pH 7.2) containing 0.5% gelatin for 1 h in room temperature. The wells were incubated with 100-μl serial dilutions of goat anti-CRP polyclonal (WAKO) or two different rabbit anti-CRP polyclonal (Sigma and DAKO) antibody preparations and mouse anti-CRP monoclonal antibodies diluted in PBS containing 0.5% gelatin and 0.05% Tween 20 (pH 7.2). Binding was determined using horseradish peroxidase-labeled anti-goat immunoglobulin G (IgG) (Atlantic Antibodies, Stillwater, MN), anti-rabbit IgG (Sigma), or anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL) antibodies and an o-phenylene-diamine (OPD; Sigma) detection system. The optical density (OD) was measured at a λ of 490 nm (reference at a λ of 620 nm), and the mean value of two parallel measurements was calculated.
The same ELISA system was applied to assess the inhibition of binding of rabbit anti-CRP polyclonal antibody and of 9C9 or CRP-8 anti-CRP monoclonal antibodies to human Hsp60 or M. tuberculosis Hsp65 coating the ELISA plates by free inhibitors (with Hsp60, Hsp65, and native CRP and bovine serum albumin [BSA] as controls). In these competitive experiments, the ELISA plates were coated with 1 μg/ml Hsp60 and Hsp65 and indirectly with native CRP through PC-KLH (1:1,000 dilution). The antibodies with the serial dilutions of inhibitors were preincubated for 1 h at room temperature before the addition of the mixture to the Hsp-coated wells. Anti-rabbit or anti-mouse IgG horseradish peroxidase-labeled antibodies were added, and binding was determined by OPD, as described above.
Ouchterlony analysis.
Double-diffusion plates were prepared with 1% agarose (Reanal, Hungary) in 0.2 M K3PO4 buffer (pH 7.8; Merck, Darmstadt, Germany). Wells were filled with 4 μl of samples, and precipitation patterns were read after 24 h of incubation at room temperature by detection with 0.2% amido-black stain after differentiation.
Synthesis of peptides on the tips of polyethylene pins.
Decamer peptides overlapping by five amino acid residues were synthesized using 9-fluorenylmethyloxy carbonyl (Fmoc)-β-alanine-glycine ester-derivatized pins (Chiron Technologies, Australia) as previously described (17). Briefly, the Fmoc-tert-butyl technique was used: the Fmoc protecting group was removed by 20% piperidine-N,N-dimethylformamide (vol/vol). The coupling was performed with diisopropylcarbodiimide-1-hydroxybenzotriazole methodology and monitored with bromophenol blue added to the coupling mixture. The peptides were acetylated at the N terminus, and then the side chain protecting groups were removed with trifluoroacetic acid (TFA)-1,2-ethanedithiol-anisole 38:1:1 (vol/vol/vol), but the unprotected peptides remained covalently attached to the pins.
ELISA for epitope scanning of anti-CRP antibodies.
Binding of anti-CRP polyclonal (Sigma) and monoclonal 9C9 or CRP-8 antibodies to human Hsp60 and M. tuberculosis Hsp65 peptides immobilized on polyethylene pins was detected by modified ELISA. After blocking (PBS, 0.5% gelatin), pins were incubated with 150 μl antibodies diluted 1:500 in PBS containing 0.5% gelatin and 0.05% Tween 20 for 1 h at room temperature. Binding of antibodies was determined using anti-rabbit IgG horseradish peroxidase-labeled antibodies and an OPD detection system. The optical density was measured at 490 nm (reference at 620 nm) and means of duplicates were calculated. Pins were used repeatedly after they were thoroughly cleaned by sonication in disruption buffer (PBS, 1% sodium dodecyl sulfate [SDS], 0.1% 2-mercapto-ethanol).
Statistical analysis.
Data are presented as the means of parallel measurements with standard errors of the means. Binding characteristics of different antibodies were compared by the analysis of variance method. A P value of less than 0.05 was considered significant. GraphPad Prism 3.0 was used for data presentation and statistical analysis.
RESULTS
Binding of anti-CRP antibodies to 60-kDa heat shock proteins.
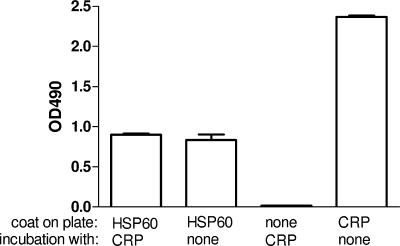
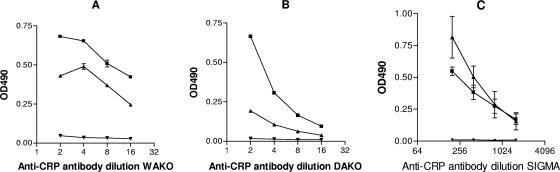
In the first experiment, the formation of potential complexes between microplate-coated Hsp60 and soluble CRP was studied (Fig. 1). However, we learned that the polyclonal rabbit anti-CRP antibodies fixed to the Hsp60-coated plate irrespective of the previous addition of CRP. Thus, we hypothesized that anti-CRP antibody cross-reacts with Hsp60 or contained anti-Hsp60 activity. Therefore, in the upcoming experiments we repeated this observation with different anti-CRP preparations and intended to characterize this reaction thereafter. ELISA plates were coated with recombinant human Hsp60 or recombinant M. tuberculosis Hsp65 and were incubated with polyclonal antibody preparations against CRP. All of the three polyclonal antibody preparations showed dose-dependent binding to heat shock protein (Fig. 2). Binding of the DAKO and WAKO anti-CRP preparations to Hsp60 and Hsp65 was detected at high concentrations, whereas the Sigma antibody preparation showed a marked binding to both Hsps, even at 1:400 dilution.
FIG. 1.
Binding of polyclonal anti-CRP antibody to solid-phase Hsp60 in the presence or absence of CRP. Negative controls were uncoated plates, while positive controls were CRP-coated plates. Each bar represents means ± standard errors of the means of OD values. Results are the sum of three independent experiments, each performed in duplicate.
FIG. 2.
Interaction of polyclonal anti-CRP antibodies with 60-kDa heat shock proteins. Binding of different dilutions of goat (WAKO, panel A) and two different rabbit (DAKO, panel B; SIGMA, panel C) anti-CRP polyclonal antibodies to recombinant human Hsp60 (▪, 2 μg/ml) or recombinant M. tuberculosis Hsp65 (▴, 2 μg/ml) target antigens as detected by ELISA. Control wells were uncoated (▾). Optical densities were measured at 490 nm; means ± standard errors of the means of OD values are shown. Results are the sum of three independent experiments, each performed in duplicate.
In the next experiment, the interaction between the polyclonal anti-CRP antibody (Sigma) and heat shock proteins was studied by an independent, non-surface-coated method, i.e., in a double-diffusion analysis. Polyclonal anti-CRP antibody was added to the central well, and different dilutions of Hsp60, Hsp65, native, and modified CRP were added to the peripheral wells. Recombinant human Hsp60 and M. tuberculosis Hsp65 were precipitated by rabbit polyclonal anti-CRP antibody as well as by native and modified CRP (Fig. 3). Furthermore, it can also be seen that every precipitin band joins at its closest end and fuses, which means that the antigen epitopes are identical and cross-reactive.
FIG. 3.
Precipitation of 60-kDa heat shock proteins with polyclonal anti-CRP antibodies. Ouchterlony analysis of the interactions among Hsp60, Hsp65, native, and modified CRP and polyclonal anti-CRP antibody. Well B, 2 mg/ml native CRP diluted in K3PO4 buffer (pH 7.8); wells C and D, Hsp60 (1 mg/ml and 0.5 mg/ml, respectively); well E, mCRP (2 mg/ml); wells F and A, Hsp65 (1 mg/ml and 2 mg/ml, respectively). Central well, polyclonal anti-CRP antibody (DAKO) diluted 1:10 in K3PO4 buffer (pH 7.8). Experiments were performed with 1% agarose.
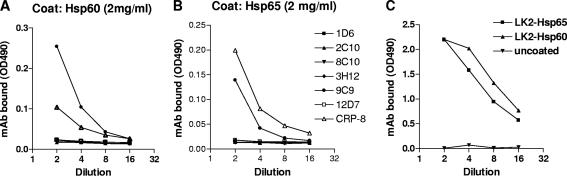
Results of the Ouchterlony analysis prompted us to investigate a panel of mouse anti-CRP monoclonal antibodies for the presence of cross-reactive clones. Seven different anti-CRP monoclonal antibodies (clones 1D6, 2C10, 8C10, 3H12, 9C9, 12D7, and CRP-8) were tested in these experiments together with an anti-Hsp60-specific monoclonal antibody (clone LK2) for positive control. Two out of seven anti-CRP monoclonal antibodies (clones 9C9 and CRP-8) recognized solid-phase heat shock proteins (Fig. 4).
FIG. 4.
Binding of anti-C reactive protein monoclonal antibodies (1D6, 2C10, 8C10,3H12, 9C9, 12D7, and CRP-8) to adsorbed human Hsp60 (A) and mycobacterial Hsp65 (B) target antigens. Supernatant antibodies were used with serial dilutions from 1:2, except CRP-8, which is a purified antibody (serially diluted from 1:200). LK-2 monoclonal anti-Hsp antibody (LK-2-Hsp) was used as a positive control, and uncoated plates were used as negative controls (panel C). Each point represents means ± standard errors of the means of two parallel measurements. The experiment for which results are shown was performed three times in duplicate; the variance between assays was less than 10%.
Inhibition of binding of anti-CRP antibodies to Hsp60 and Hsp65.
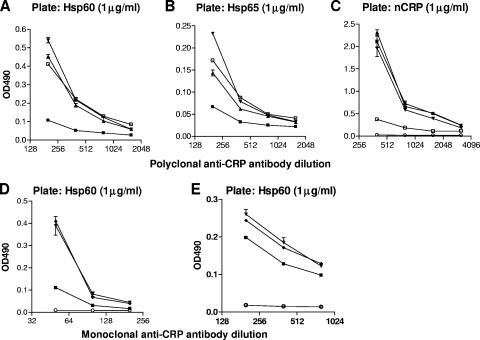
To test whether anti-CRP polyclonal and monoclonal antibodies cross-react with human Hsp60 and M. tuberculosis Hsp65, a competitive inhibition ELISA was performed next. Polyclonal anti-CRP (Sigma) preparation or monoclonal antibodies 9C9 and CRP-8 were preincubated with Hsp60, Hsp65, or CRP before the reaction with solid-phase 60-kDa heat shock proteins in the ELISA plate. The inhibition of anti-CRP antibody binding to CRP was also measured as a control. Hsp60 inhibited the binding of anti-CRP polyclonal Ab to Hsp60 (Fig. 5A). The inhibition level was pronounced at a high concentration of the competitive protein (10 μg/ml). The inhibition of anti-CRP antibody binding to Hsp60 by Hsp65 and native CRP was very weak. A similar reaction was observed on Hsp65-coated plates (Fig. 5B). However, on CRP-coated plates, the binding of anti-CRP antibody was markedly inhibited by soluble CRP, whereas the inhibitory effects of heat shock proteins were very weak, although statistically significant in the case of Hsp65 (Fig. 5C).
FIG. 5.
(A, B, and C) Inhibition of rabbit anti-C reactive protein polyclonal and monoclonal (D) antibody binding to solid-phase adsorbed Hsp60 (A), Hsp65 (B), and native CRP (C) by preincubation with soluble Hsp60 (▪), Hsp65 (▴), and CRP (□) (▾, antibody alone; ○, antibody on uncoated plate) in competitive ELISA. In panels D and E, the interaction of mouse 9C9 or CRP-8 anti-CRP monoclonal antibodies and soluble Hsp60 are shown (▾, antibody alone; ▪, antibody + Hsp60 [10 μg/ml]; ⧫, antibody + BSA [10 μg/ml]; ○, antibody on uncoated plate). The antibodies (polyclonal and CRP-8 monoclonal, 1:200; 9C9 monoclonal, 1:50) and inhibitors (10 μg/ml) were preincubated for 1 h at room temperature and diluted on the plate thereafter. Values represent means ± standard errors of the means of parallel measurements. Results are the sum of three independent experiments, each performed in duplicate. Two-way analysis of variance values are as follows (comparison was to antibody alone): for (A) Hsp60, P < 0.0001; Hsp65, P = 0.0002; CRP, P = 0.0013; for (B) Hsp60, P < 0.0001; Hsp65, P = 0.0004; CRP, P = 0.048; for (C) Hsp60, P = 0.089; Hsp65, P = 0.0157; CRP, P < 0.0001; for (D) Hsp60, P < 0.0001; and for (E) Hsp60, P < 0.0001.
Next, the monoclonal antibodies to CRP (clones 9C9 and CRP-8) were preincubated with Hsp60 and added to Hsp60-coated plates. Hsp60 was found to significantly inhibit the binding of both the 9C9 and the CRP-8 monoclonal antibodies to Hsp60 (P < 0.0001) (Fig. 5D and E). BSA applied as a negative control did not have any effect on antibody binding.
Epitope scanning of the binding sites for polyclonal and monoclonal (CRP-8) anti-CRP antibodies on human Hsp60.
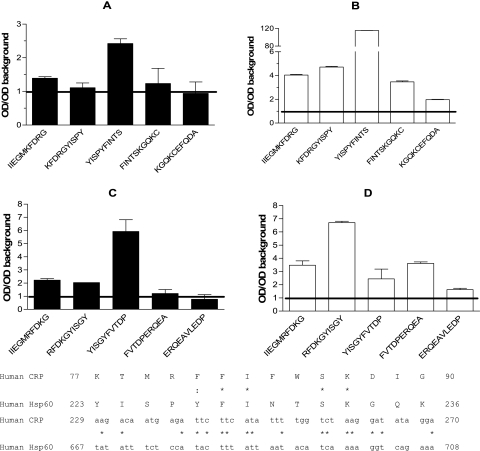
Data of the binding experiments documented that antibodies specific for Hsp60 are present in the anti-CRP antibody preparations. Therefore, the epitope specificities of these antibodies were studied in the next experiment, using a set of overlapping synthetic peptides. Potential antigenic sites were predicted based on hydrophilicity and secondary structure and selected for synthesis as described previously (17). A total of 46 decamer peptides covering 50% of human Hsp60 were synthesized. The binding sites for monoclonal antibodies against CRP (CRP-8 and 9C9) on human Hsp60 were analyzed (Fig. 6). Importantly, the same epitope region located between amino acids 218 and 232 on Hsp60 was found to be recognized by CRP-8 and 9C9 monoclonal antibodies. The corresponding region of Hsp65 was also recognized by both monoclonal antibodies, and 9C9 showed a stronger reaction than CRP-8. Strong and significant binding to different peptides of the epitope, exceeding the level of background values by 2.75- to 116-fold, was found by both monoclonal antibodies. This region showed 26.6% amino acid identity to the CRP AA 77 to 90 region compared with 17.4% identity between the whole molecules analyzed by ClustalW (16). The similarity (nucleotide identity) in the nucleotide sequences of the same regions was 40.48% between the two molecules (Fig. 6).
FIG. 6.
Binding of anti-CRP monoclonal antibodies to 60-kDa heat shock protein peptides. Epitope analysis of human Hsp60 (panels A and B) and M. tuberculosis Hsp65 (panels C and D) protein for the binding sites of monoclonal antibody against C-reactive protein (clone CRP-8, white bars; clone 9C9, black bars). Bars represent means ± errors of the means of different overlapping peptides, corrected with background OD values. Background reactivity is indicated by the horizontal line. The experiment was repeated three times with similar results. Sequence homology between the CRP and the Hsp60 AA region 223 to 236 analyzed by ClustalW program is indicated just below panels C and D. Identical residues are marked with an asterisk, and similar residues are marked with dots.
Results of epitope analysis of the DAKO rabbit anti-CRP antibody are presented in Table 1 (similar results were obtained for the Sigma anti-CRP antibody as well; data not shown). For comparison, a purified IgG preparation from a rabbit immunized with M. tuberculosis Hsp65 was also applied. Six epitope regions of Hsp60 were recognized by the anti-CRP antibodies, whereas anti-Hsp65 IgG cross-reacted with 12 epitope sites of the 10 regions of Hsp60 analyzed. Four out of the six regions recognized by anti-CRP antibodies were exactly the same as the sites recognized by anti-Hsp65 antibodies; the remaining two sites were partly overlapping.
TABLE 1.
Reactivity of rabbit polyclonal anti-CRP antibodies with synthetic peptides representing regions of human Hsp60
| Region | Human Hsp60 peptidea | Reactivity (OD/OD control >2)b
|
|
|---|---|---|---|
| Rabbit anti-CRP polyclonal antibody | Rabbit anti-Hsp65 polyclonal antibody | ||
| I | 52AVTMGPKGRT61 | − | + |
| 57PKGRTVIIEQ66 | − | + | |
| 62VIIEQSWGSP71 | + | − | |
| 67SWGSPKVTKD76 | − | − | |
| 72KVTKDGVTVA81 | − | − | |
| II | 117TVLARSIAKE126 | + | + |
| 122SIAKEGFEKI131 | − | − | |
| 127GFEKISKGAN136 | − | − | |
| 132SKGANPVEIR141 | − | − | |
| 137PVEIRRGVML146 | − | + | |
| III | 162VTTPEEIAQV171 | − | − |
| 167EIAQVATISA176 | + | + | |
| 172ATISANGDKE181 | − | − | |
| 177NGDKEIGNII186 | − | − | |
| IV | 203DGKTLNDELE212 | − | − |
| 208DNELEIIEGM217 | − | − | |
| 213IIEGMKFDRG222 | − | − | |
| 218KFDRGYISPY227 | − | + | |
| 223YISPYFINTS232 | + | − | |
| 228FINTSKGQKC237 | − | − | |
| 233KGQKCEFQDA242 | − | − | |
| 238EFQDAYVLLS247 | − | − | |
| 243YVLLSEKKIS252 | − | + | |
| 248EKKISSIQSI257 | − | − | |
| 253SIQSIVPALE262 | − | − | |
| V | 303PGFGDNRKNQ312 | − | + |
| 308NRKNQLKDMA317 | − | + | |
| VI | 368EKRIQEIIEQ377 | − | − |
| 373EIIEQLDVTT382 | + | + | |
| 378LDVTTSEYEK387 | − | − | |
| VII | 394LAKLSDGVAV403 | − | − |
| 399DGVAVLKVGG408 | − | + | |
| 404LKVGGTSDVE413 | − | − | |
| VIII | 436IVLGGGCALL445 | + | + |
| 441GCALLRCIPA450 | − | − | |
| 446RCIPALDSLT455 | − | − | |
| IX | 470RTLKIPAMTI479 | − | + |
| 475PAMTIAKNAG484 | − | − | |
| 480AKNAGVEGSL489 | − | − | |
| 485VEGSLIVEKI494 | − | − | |
| 490IVEKIMQSSS499 | − | − | |
| 495MQSSSEVGYD504 | − | − | |
| 500EVGYDAMAGD509 | − | − | |
| X | 531DAAGVASLLT540 | − | − |
| 536ASLLTTAEVV545 | − | + | |
| 541TAEVVVTEIP550 | − | − | |
Peptides were immobilized on polyethylene pins at their C termini. Numbers represent the position of the sequence in the protein.
Reactivity was defined as follows: ratios of the OD of the given peptide/OD of control peptide (OD background) were calculated for each peptide; ratios >2 were considered positive (+). OD/OD background ratios obtained for each peptide with anti-CRP + anti-rabbit IgG antibody conjugated with peroxidase or anti-Hsp65 + anti-rabbit IgG antibody conjugated with peroxidase were corrected with ratios obtained from the control experiment (anti-rabbit IgG antibody conjugated with peroxidase only).
DISCUSSION
The major novel observation from this study is the description of antigenic cross-reaction between 60-kDa heat shock protein and C-reactive protein. The cross-reacting IgG antibodies characterized in this study are able to recognize human Hsp60 and M. tuberculosis Hsp65 in solid-phase binding experiments and in an Ouchterlony precipitation assay as well. Results of the precipitation assay indicated antigenic cross-reaction between the two proteins. Induction by a mimicry mechanism was supported by the following observations. First, the specificity and dose dependence of the anti-CRP binding to Hsp60 was shown by competitive inhibition of free Hsp60 and Hsp65. Importantly, the binding of the anti-CRP antibodies was significantly inhibited by Hsp60 (Fig. 5). Second, two out of seven monoclonal anti-CRP antibodies were also found to cross-react with 60-kDa heat shock proteins. Furthermore, the two anti-CRP monoclonal antibodies reacting with Hsp60 recognized the same antigenic region on Hsp60 (amino acids [AA] 223 to 236). Finally, according to the ClustalW homology search, there is a homologous region in the CRP molecule (AA 77 to 90) showing 26.6% amino acid identity to Hsp60 (Fig. 6). All of these novel observations indicate a true antigenic cross-reaction between Hsp60 and CRP.
There are, however, two, although not mutually exclusive, explanations for the induction of anti-Hsp60 antibodies in anti-CRP antibody preparations: generation by true antigenic cross-reaction (mimicry)-based mechanism or nonspecific induction during the immunization process by the adjuvant containing Hsp. Detailed analysis of the polyclonal CRP preparations also supports the possibility of induction of anti-Hsp60 antibodies by CFA. All of the rabbit polyclonal anti-CRP antibodies were raised using complete CFA in the animals (DAKO Ltd. and Sigma Chemical Co., personal communication). Some observations of the present study indicate that the anti-Hsp60 reactivity present in the polyclonal anti-CRP preparations may also be raised by the method of immunization, namely, the application of CFA. The anti-Hsp60 antibodies in anti-CRP preparations seem to be specific antibodies with dose-dependent binding activity. Second, the binding of anti-Hsp60-reactive antibodies to Hsp60 can be inhibited by free Hsp60, but the inhibitory activity of free CRP in this reaction is weak but significant (Fig. 5A and B). Some data from the literature also support this possibility. Complete Freund's adjuvant is known to induce anti-Hsp65 antibodies in immunized rabbits, as shown previously in the study by Xu et al. (18) and proposed by Hajeer and Bernstein (6). B-cell epitope analysis was carried out to study potential induction of anti-Hsp60 reactivity present in anti-CRP preparation by CFA immunization. Specificities of antibodies induced by Hsp65 (in CFA) immunization and the anti-Hsp60 reactivity present in anti-CRP preparations were compared. An overlapping set of synthetic peptides representing predicted epitope sites of human Hsp60 was used in these experiments. The immunization of a rabbit with Hsp65 yields a preparation with reactivity to 12 epitope regions on Hsp60, whereas the anti-Hsp60 antibodies present in the anti-CRP preparation recognized 6 epitope regions on Hsp60. Four out of the six regions were the same as the sites recognized by anti-Hsp65 antibodies; the remaining two sites were partly overlapping (Table 1). These data support our assumption that the induction of anti-Hsp60 antibodies in animals immunized with CRP may partially also be a consequence of the usage of CFA. All of the above observations support the possibility that anti-Hsp60, anti-CRP, and cross-reacting antibodies exist in parallel in polyclonal anti-CRP preparations.
There are important practical consequences of our novel results. Serum samples of healthy humans are known to contain soluble Hsp60 (9), and elevated levels of serum-soluble Hsp60 were reported in atherosclerosis (19). These observations raise the possibility of false positivity caused by sHsp60 in CRP assays if Hsp60 cross-reacting monoclonal is applied in the turbidimetric assay. Furthermore, autoantibodies against CRP and Hsp60 in systemic lupus erythematosus patients were reported (2, 4), indicating that cross-reacting antibodies might indeed occur in vivo as well. These autoantibodies could play significant roles in immunomodulation by interfering with biological effects of both Hsp60 and CRP.
The polyclonal antibodies characterized in this study are widely used reagents in immunohistochemistry, used in concentrations comparable to those in our experiments (8, 12, 15, 22). Special attention should be paid to the interpretation of studies using the above reagents. Although some eminent studies apply appropriate controls to rule out undesirable cross-reactions and provide data for the characterization of the antibodies applied, there are several studies reporting the deposition/presence of CRP using antibodies even in low concentrations for detection.
A potential limitation of the present study is the lack of inhibition data for the Hsp60 peptides. This is due to the fact that the overlapping peptides were covalently synthesized onto polyethylene pins and could not be used for inhibition. Thus, the strength of antibody binding to CRP and Hsp60 epitopes could not be compared directly.
In conclusion, the novel observation of antigenic cross-reaction between Hsp60 and CRP reported in this study has two important practical consequences. The cross-reacting antibodies need to be tested in the future for their immunomodulating effects in clinical studies, and ultrasensitive CRP assays must be validated with samples containing amounts of soluble Hsp60 high enough to rule out interference. Furthermore, polyclonal antibody preparations raised against CRP using CFA should be used only with special attention. Appropriate design and interpretation of studies using such antibody reagents are essential for the future.
Acknowledgments
We thank W. van Eden for the anti-Hsp60 monoclonal antibody clone LK2, Larry A. Potempa for the monoclonal anti-CRP antibodies, and Szigeti Antalné for the excellent technical assistance.
Our study was supported by grants from the National Research Fund (OTKA T46837) and the ATHERNET grant of the European Commission (QLG1-CT-2002-90397).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Cohen, I. R., and D. B. Young. 1991. Autoimmunity, microbial immunity and the immunological homunculus. Immunol. Today 12:105-110. [DOI] [PubMed] [Google Scholar]
- 2.Dieude, M., J. L. Senecal, and Y. Raymond. 2004. Induction of endothelial cell apoptosis by heat-shock protein 60-reactive antibodies from anti-endothelial cell autoantibody-positive systemic lupus erythematosus patients. Arthritis Rheum. 50:3221-3231. [DOI] [PubMed] [Google Scholar]
- 3.Dubois, P. 1989. Heat shock proteins and immunity. Res. Immunol. 140:653-659. [DOI] [PubMed] [Google Scholar]
- 4.Figueredo, M. A., A. Rodriguez, M. Ruiz-Yague, M. Romero, A. Fernandez-Cruz, E. Gomez-de la Concha, and R. Patino. 2006. Autoantibodies against C-reactive protein: clinical associations in systemic lupus erythematosus and primary antiphospholipid syndrome. J. Rheumatol. 33:1980-1986. [PubMed] [Google Scholar]
- 5.Gewurz, H., C. Mold, J. Siegel, and B. Fiedel. 1982. C-reactive protein and the acute phase response. Adv. Intern. Med. 27:345-372. [PubMed] [Google Scholar]
- 6.Hajeer, A. H., and R. M. Bernstein. 1993. Antibody to mycobacterial 65-kD heat shock protein in commercial antisera. Clin. Exp. Immunol. 94:544-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mold, C., H. Gewurz, and T. W. Du Clos. 1999. Regulation of complement activation by C-reactive protein. Immunopharmacology 42:23-30. [DOI] [PubMed] [Google Scholar]
- 8.Nozoe, T., D. Korenaga, M. Futatsugi, H. Saeki, Y. Maehara, and K. Sugimachi. 2003. Immunohistochemical expression of C-reactive protein in squamous cell carcinoma of the esophagus—significance as a tumor marker. Cancer Lett. 192:89-95. [DOI] [PubMed] [Google Scholar]
- 9.Pockley, A. G., J. Bulmer, B. M. Hanks, and B. H. Wright. 1999. Identification of human heat shock protein 60 (Hsp60) and anti-Hsp60 antibodies in the peripheral circulation of normal individuals. Cell Stress Chaperones 4:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potempa, L. A., J. N. Siegel, B. A. Fiedel, R. T. Potempa, and H. Gewurz. 1987. Expression, detection and assay of a neoantigen (neo-CRP) associated with a free, human C-reactive protein subunit. Mol. Immunol. 24:531-541. [DOI] [PubMed] [Google Scholar]
- 11.Prohaszka, Z., J. Duba, G. Lakos, E. Kiss, L. Varga, L. Janoskuti, A. Csaszar, I. Karadi, K. Nagy, M. Singh, L. Romics, and G. Fust. 1999. Antibodies against human heat-shock protein (hsp) 60 and mycobacterial hsp65 differ in their antigen specificity and complement-activating ability. Int. Immunol. 11:1363-1370. [DOI] [PubMed] [Google Scholar]
- 12.Quan, L., M. Q. Fujita, B. L. Zhu, K. Ishida, and H. Maeda. 2000. Immunohistochemical distribution of C-reactive protein in the hepatic tissue in forensic autopsy. Forensic Sci. Int. 113:177-182. [DOI] [PubMed] [Google Scholar]
- 13.Ramos-Ruiz, R., J. P. Lopez-Bote, F. Pelayo, V. Larraga, R. van der Zee, and C. Bernabeu. 1991. Cellular and humoral reactivity pattern to the mycobacterial heat shock protein HSP65 in adjuvant arthritis susceptible and resistant Wistar rats. Autoimmunity 9:1-5. [DOI] [PubMed] [Google Scholar]
- 14.Samberg, N. L., R. A. Bray, H. Gewurz, A. L. Landay, and L. A. Potempa. 1988. Preferential expression of neo-CRP epitopes on the surface of human peripheral blood lymphocytes. Cell. Immunol. 116:86-98. [DOI] [PubMed] [Google Scholar]
- 15.Skowasch, D., S. Schrempf, C. J. Preusse, J. A. Likungu, A. Welz, B. Luderitz, and G. Bauriedel. 2006. Tissue resident C reactive protein in degenerative aortic valves: correlation with serum C reactive protein concentrations and modification by statins. Heart 92:495-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uray, K., F. Hudecz, G. Fust, and Z. Prohaszka. 2003. Comparative analysis of linear antibody epitopes on human and mycobacterial 60-kDa heat shock proteins using samples of healthy blood donors. Int. Immunol. 15:1229-1236. [DOI] [PubMed] [Google Scholar]
- 18.Xu, Q., H. Dietrich, H. J. Steiner, A. M. Gown, B. Schoel, G. Mikuz, S. H. Kaufmann, and G. Wick. 1992. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler. Thromb. 12:789-799. [DOI] [PubMed] [Google Scholar]
- 19.Xu, Q., G. Schett, H. Perschinka, M. Mayr, G. Egger, F. Oberhollenzer, J. Willeit, S. Kiechl, and G. Wick. 2000. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation 102:14-20. [DOI] [PubMed] [Google Scholar]
- 20.Yasojima, K., C. Schwab, E. G. McGeer, and P. L. McGeer. 2001. Generation of C-reactive protein and complement components in atherosclerotic plaques. Am. J. Pathol. 158:1039-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ying, S. C., H. Gewurz, C. M. Kinoshita, L. A. Potempa, and J. N. Siegel. 1989. Identification and partial characterization of multiple native and neoantigenic epitopes of human C-reactive protein by using monoclonal antibodies. J. Immunol. 143:221-228. [PubMed] [Google Scholar]
- 22.Zuniga, R., G. S. Markowitz, T. Arkachaisri, E. A. Imperatore, V. D. D'Agati, and J. E. Salmon. 2003. Identification of IgG subclasses and C-reactive protein in lupus nephritis: the relationship between the composition of immune deposits and FCgamma receptor type IIA alleles. Arthritis Rheum. 48:460-470. [DOI] [PubMed] [Google Scholar]