Abstract
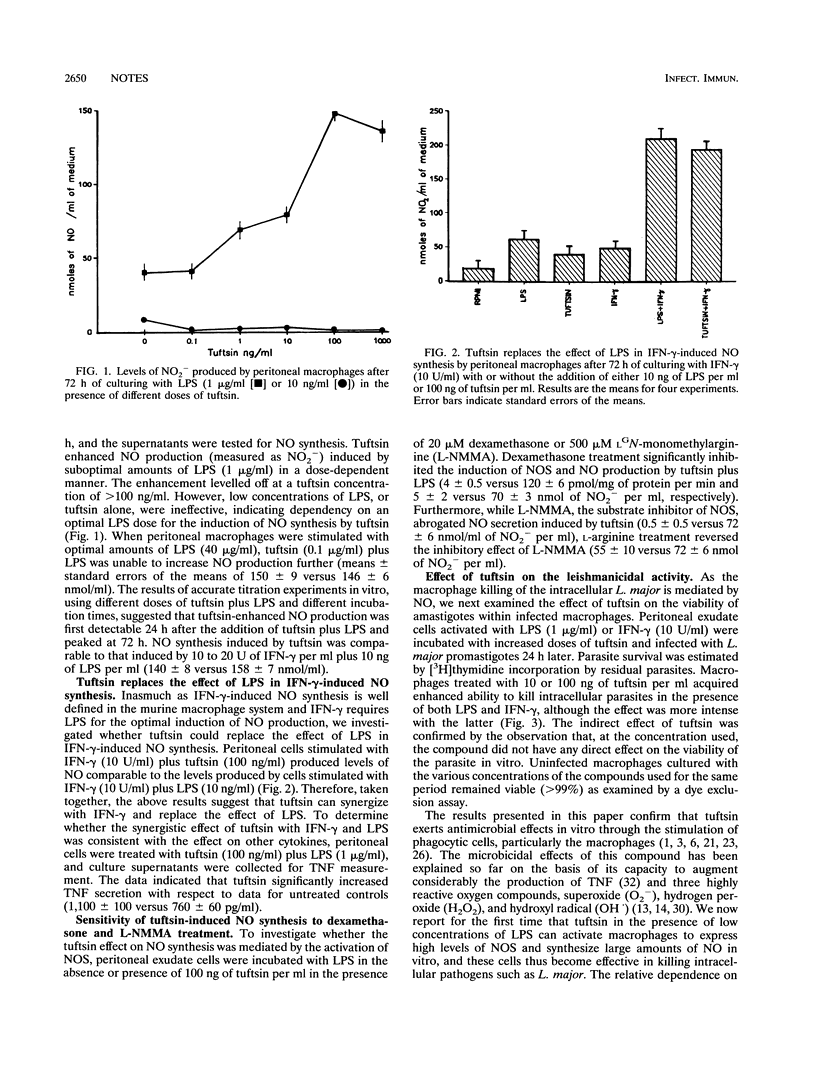
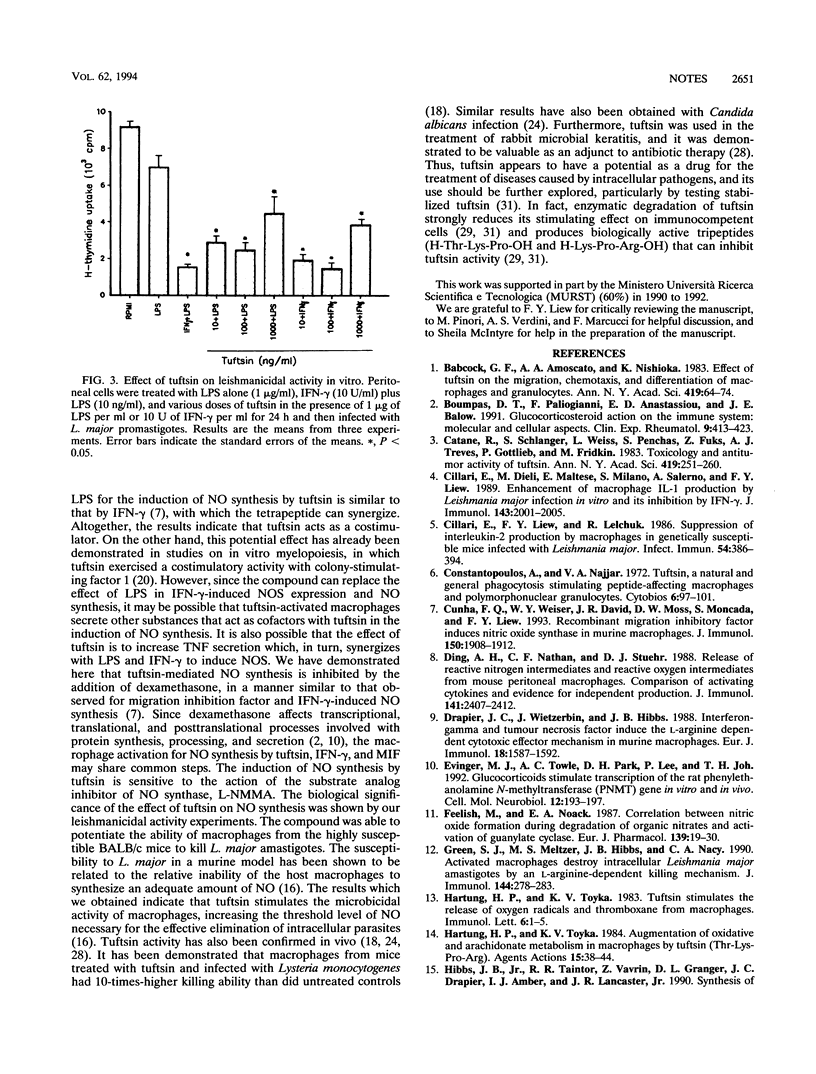
The macrophage-activating tetrapeptide tuftsin was able to activate, in a dose-dependent manner, murine macrophages to express nitric oxide (NO) synthase and to produce NO. Tuftsin required lipopolysaccharides for the optimal induction of NO production and synergized with gamma interferon in the induction of NO synthesis. Tuftsin-dependent NO production was sensitive to inhibition by dexamethasone and the NO synthase specific inhibitor LGN-monomethylarginine (L-NMMA). Murine peritoneal macrophages activated by tuftsin were able to kill the amastigotes of the intracellular protozoan parasite Leishmania major in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. F., Amoscato A. A., Nishioka K. Effect of tuftsin on the migration, chemotaxis, and differentiation of macrophages and granulocytes. Ann N Y Acad Sci. 1983;419:64–74. doi: 10.1111/j.1749-6632.1983.tb37092.x. [DOI] [PubMed] [Google Scholar]
- Boumpas D. T., Paliogianni F., Anastassiou E. D., Balow J. E. Glucocorticosteroid action on the immune system: molecular and cellular aspects. Clin Exp Rheumatol. 1991 Jul-Aug;9(4):413–423. [PubMed] [Google Scholar]
- Catane R., Schlanger S., Weiss L., Penchas S., Fuks Z., Treves A. J., Gottlieb P., Fridkin M. Toxicology and antitumor activity of tuftsin. Ann N Y Acad Sci. 1983;419:251–260. doi: 10.1111/j.1749-6632.1983.tb37111.x. [DOI] [PubMed] [Google Scholar]
- Cillari E., Dieli M., Maltese E., Milano S., Salerno A., Liew F. Y. Enhancement of macrophage IL-1 production by Leishmania major infection in vitro and its inhibition by IFN-gamma. J Immunol. 1989 Sep 15;143(6):2001–2005. [PubMed] [Google Scholar]
- Cillari E., Liew F. Y., Lelchuk R. Suppression of interleukin-2 production by macrophages in genetically susceptible mice infected with Leishmania major. Infect Immun. 1986 Nov;54(2):386–394. doi: 10.1128/iai.54.2.386-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha F. Q., Weiser W. Y., David J. R., Moss D. W., Moncada S., Liew F. Y. Recombinant migration inhibitory factor induces nitric oxide synthase in murine macrophages. J Immunol. 1993 Mar 1;150(5):1908–1912. [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Evinger M. J., Towle A. C., Park D. H., Lee P., Joh T. H. Glucocorticoids stimulate transcription of the rat phenylethanolamine N-methyltransferase (PNMT) gene in vivo and in vitro. Cell Mol Neurobiol. 1992 Jun;12(3):193–215. doi: 10.1007/BF00712926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feelisch M., Noack E. A. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol. 1987 Jul 2;139(1):19–30. doi: 10.1016/0014-2999(87)90493-6. [DOI] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hartung H. P., Toyka K. V. Tuftsin stimulates the release of oxygen radicals and thromboxane from macrophages. Immunol Lett. 1983 Jan;6(1):1–6. doi: 10.1016/0165-2478(83)90021-4. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Li Y., Moss D., Parkinson C., Rogers M. V., Moncada S. Resistance to Leishmania major infection correlates with the induction of nitric oxide synthase in murine macrophages. Eur J Immunol. 1991 Dec;21(12):3009–3014. doi: 10.1002/eji.1830211216. [DOI] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Martinez J., Winternitz F. Bactericidal activity of tuftsin. Mol Cell Biochem. 1981 Dec 4;41:123–136. doi: 10.1007/BF00225303. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moore R. N., Osmand A. P., Dunn J. A., Joshi J. G., Rouse B. T. Substance P augmentation of CSF-1-stimulated in vitro myelopoiesis. A two-signal progenitor restricted, tuftsin-like effect. J Immunol. 1988 Oct 15;141(8):2699–2703. [PubMed] [Google Scholar]
- Najjar V. A., Nishioka K. "Tuftsin": a natural phagocytosis stimulating peptide. Nature. 1970 Nov 14;228(5272):672–673. doi: 10.1038/228672a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nishioka K., Babcock G. F., Phillips J. H., Noyes R. D. Antitumor effect of tuftsin. Mol Cell Biochem. 1981 Dec 4;41:13–18. doi: 10.1007/BF00225293. [DOI] [PubMed] [Google Scholar]
- Nishioka K., Hopfer R. L., el-Hagin T., Lopez-Berestein G. Prophylaxis of Candida albicans infection with tuftsin. J Antimicrob Chemother. 1986 Mar;17(3):361–363. doi: 10.1093/jac/17.3.361. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Price R. E., Templeton J. W., Smith R., 3rd, Adams L. G. Modulation of the intracellular survival of Brucella abortus by tuftsin and muramyl dipeptide. Vet Immunol Immunopathol. 1993 Apr;36(3):265–279. doi: 10.1016/0165-2427(93)90024-x. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. C., Zam Z. S., Stern G. A. The effect of tuftsin in the treatment of experimental Pseudomonas keratitis. Cornea. 1986;5(3):181–183. doi: 10.1097/00003226-198605030-00012. [DOI] [PubMed] [Google Scholar]
- Spirer Z., Zakuth V., Golander A., Bogair N., Fridkin M. The effect of Tuftsin on the nitrous blue tetrazolium reduction of normal human polymorphonuclear leukocytes. J Clin Invest. 1975 Jan;55(1):198–200. doi: 10.1172/JCI107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch G. L., Niswander P. W. Adenosine deaminase activity and superoxide formation during phagocytosis at different cell densities. Biochem Med. 1981 Oct;26(2):185–190. doi: 10.1016/0006-2944(81)90045-4. [DOI] [PubMed] [Google Scholar]
- Verdini A. S., Silvestri S., Becherucci C., Longobardi M. G., Parente L., Peppoloni S., Perretti M., Pileri P., Pinori M., Viscomi G. C. Immunostimulation by a partially modified retro-inverso-tuftsin analogue containing Thr1 psi[NHCO](R,S)Lys2 modification. J Med Chem. 1991 Dec;34(12):3372–3379. doi: 10.1021/jm00116a005. [DOI] [PubMed] [Google Scholar]
- Wleklik M. S., Luczak M., Najjar V. A. Tuftsin induced tumor necrosis activity. Mol Cell Biochem. 1987 Jun;75(2):169–174. doi: 10.1007/BF00229905. [DOI] [PubMed] [Google Scholar]


