Abstract
Human immunodeficiency virus type 1 (HIV-1)-specific cellular immune responses are elicited in a proportion of infants born to HIV-1-infected mothers and are associated with protection against vertical transmission. To investigate correlates of these HIV-1-specific responses, we examined levels of the immune activation markers neopterin, β2-microglobulin (β2-m), and soluble l-selectin (sl-selectin); the immunomodulatory and hematopoietic factors interleukin-7 (IL-7), stromal-cell-derived factor 1 alpha (CXCL12), and granulocyte-macrophage colony-stimulating factor (GM-CSF); and the immunoregulatory cytokine IL-10 among a group of newborns born to HIV-1-positive mothers who did not receive any antiretroviral drugs for prevention of perinatal HIV-1 transmission. Cellular immune responses to HIV-1 envelope (Env) peptides were also measured. We aimed to determine whether newborns who elicit HIV-1-specific cellular immune responses (Env+) and those who lack these responses (Env−) exhibit unique immune features. Our data confirmed that no Env+ infants acquired HIV-1 infection. Among exposed, uninfected infants, Env+ infants had reduced immune activation (as measured by β2-m and sl-selectin levels in cord blood plasma) compared to Env− infants as well as reduced GM-CSF levels in cord blood plasma. There was also a reduced ability of cord blood mononuclear cells to be induced to produce GM-CSF among Env+ infants. Maternal viral load was lower in Env+ infants, suggesting that exposure to low levels of antigen may be responsible for priming the protective responses. These findings suggest that infants who are able to develop apparently protective HIV-1-specific cellular immune responses have immunological features and viral exposure histories that distinguish them from their nonresponder counterparts, providing new insights into the development of HIV-1 protective immunity.
Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ T-cell responses are considered critical for controlling viral replication once HIV-1 infection is established (22). Surprisingly, these HIV-1-specific cellular immune responses have also been detected among a proportion of infants who remain uninfected despite being born to HIV-1-infected mothers (10, 15, 31), hereafter referred to as “exposed, uninfected” (EU) infants. Since these responses are detectable at birth, it is presumed that they are indicative of events occurring prior to birth, pointing to the likelihood of in utero exposure to HIV-1 or its soluble products in the absence of infection and to the possible immunological ability of some neonates to successfully respond to such exposure and prevent infection. There is strong evidence that these HIV-1-specific cellular immune responses in newborns are markers of protective immunity, and they have been associated with protection against subsequent intrapartum and postpartum infection through breast-feeding (13). To date all that is known of these responses is that they can sometimes be detected, but how and when in utero priming to maternal HIV-1 or its products takes place and how these responses develop remain unexplored.
In animal models, low-dose exposure to virus may selectively activate the cellular arm of the immune system, promoting development of strong adaptive T-cell immunity (26). Therefore, we sought to test the hypothesis that lower maternal viral load is associated with development of HIV-1-specific cell-mediated immune responses among EU newborns. We also aimed to investigate whether there were other immune parameters that distinguish EU infants who develop HIV-1-specific cellular immune responses from those who do not. We examined activation markers (neopterin, β2-microglobulin [β2-m], and soluble l-selectin [sl-selectin]) in cord blood plasma which, like the HIV-1-specific responses, are indicative of past events that occurred prior to birth. These parameters were of interest since there is evidence of increased immune activation at birth (19, 25) among HIV-1-exposed compared to unexposed infants, suggesting that in utero viral exposure may be associated with immune activation. As the activation of T cells results in the coordinate expression of a number of cytokines involved in the differentiation, proliferation, and activation of the hematopoietic system, we also questioned whether elevated levels of cytokines (interleukin [IL-7], IL-10, stromal-cell-derived factor 1 alpha [CXCL12], and granulocyte-macrophage colony-stimulating factor [GM-CSF]) involved in immunomodulation and hematopoiesis would be associated with the development of HIV-1-specific responses. Understanding factors important in the pathway to the development of protective immunity will provide important information for the design and evaluation of HIV-1 vaccines.
MATERIALS AND METHODS
Study population.
The study population included HIV-1-seropositive and -seronegative women delivering live-born infants at Chris Hani Baragwanath Hospital, Soweto, South Africa. Women who had not received any antiretroviral drugs prior to the infant's birth either for prevention of mother-to-child transmission or for HIV-1 treatment (n = 124) were recruited as part of a postexposure prophylaxis trial (11). Women were eligible for the trial if they tested HIV seropositive for the first time after delivery and were drug naive, usually because they received no prenatal care or had attended prenatal clinics where HIV-1 testing was not available at the time. Their infants were randomized to receive either zidovudine or nevirapine to reduce the risk of vertical transmission. This study population was selected because HIV-1-specific T-cell responses are attenuated in neonates whose mothers receive antiretroviral drugs (including zidovudine, lamivudine, and nevirapine) during pregnancy and labor (14, 16). We therefore studied newborns of mothers who did not know their HIV-1 status at delivery and therefore did not receive any antiretroviral drugs prior to delivery. For ethical reasons, postexposure prophylaxis with nevirapine or zidovudine in accordance with random assignment was given to all infants within 72 h of birth. A control group of pregnant HIV-seronegative women (n = 30) was recruited at this site over the same time period.
Infants of HIV-1-infected mothers were followed prospectively after birth until 3 months of age or until at least 4 weeks after all breast-feeding had stopped to determine their HIV-1 status. HIV-1 DNA PCR was performed on infant peripheral blood samples collected at 6 weeks of age (Roche Diagnostic Systems, Inc., Branchburg, NJ). To establish the timing of infection, samples collected on the day of birth were tested if the 6-week PCR was positive. A positive result on the day of birth was used to infer intrauterine transmission, and a negative result at birth with a positive result at 6 weeks or later was used to infer intrapartum (IP) transmission. No infection could be clearly attributed to postnatal infection. Children testing negative at 6 weeks or older were included as EU. Children born to seronegative women (control group) were not followed after birth.
For this nested case control study, all infants with IP infections (n = 14) and a random selection of 63 EU infants from the cohort of 124 infants born to HIV-1-infected mothers who did not receive any antiretroviral drugs before delivery were selected. Three infants were excluded from the EU group and one from the IP infection group as samples were insufficient for all the tests. Four EU infants with HIV-1-specific T-cell responses, from a related study at the time, were added. Ten control infants were excluded as HIV-1-specific responses were not measured. As quantitating factors in cord blood of infants with intrauterine infections demonstrate the consequences of existing infection, these infants were also excluded from this study (n = 4).
This study was approved by the University of the Witwatersrand Committee for Research on Human Subjects, and informed consent was obtained from the mothers of all infants enrolled in the study.
Blood samples.
Small aliquots (3 to 5 ml) of cord blood were obtained by cordocentesis immediately after delivery of the placenta, and 10 ml peripheral blood was obtained from each mother within 24 h of delivery. Blood samples were drawn into EDTA tubes (Becton Dickinson, Plymouth, United Kingdom). Mononuclear cells (adult peripheral and infant cord blood) and plasma were processed by Ficoll-Paque Plus (Amersham Biosciences, Uppsala, Sweden) gradient centrifugation within 24 h. Plasma (mother and infant) was stored at −70°C, and mononuclear cell samples (mother and infant) were stored in liquid nitrogen until testing.
CBMC isolation.
Mononuclear cells were isolated from cord blood by density centrifugation on Ficoll-Paque Plus at 1,000 × g for 30 min at room temperature. The plasma layer was removed and centrifuged at 850 × g to remove platelets using a Sigma 4K15 centrifuge (Wirsam Scientific, South Africa), aliquoted, and stored at −70°C. The cord blood mononuclear cell (CBMC) layer was removed and washed twice in phosphate-buffered saline (PBS). Contaminating erythrocytes were lysed using 0.15 M NH4Cl, 10 mM KHCO3, and 1 mM sodium EDTA, pH 7.0. The cell suspension containing lysed cells was washed a further two times in PBS, and the viable-cell numbers were determined by trypan blue (Sigma, Steinheim, Germany) exclusion. CBMCs resuspended to a final concentration of 3 × 106 cells/ml in RPMI containing 1% l-glutamine were used to measure HIV-1-specific cellular immune responses.
HIV-1-specific cellular immune responses.
CBMCs (2 × 105 cells/well) were unstimulated or stimulated with a cocktail of five synthetic HIV-1 Env peptides (HIV-1 gp120 peptides T1, T2, and TH4.1 of conserved regions and P18 MN and P18 IIIB of variable regions) at a final concentration of 2.5 μM to measure HIV-1-specific responses and with phytohemagglutinin (PHA; positive control) (Sigma, Steinheim, Germany) used at a final concentration of 12.5 μg/ml. The selection of the HIV-1 stimulus was based on previous studies which have identified these peptides to be broadly immunogenic across major histocompatibility complex haplotypes (2, 4, 12). T-helper-cell responses to these peptides have also been documented in several, independent populations of HIV-1 EU individuals (5, 7, 8, 13, 18).
For each stimulus, cultures were run in duplicate. After 1 h of incubation at 37°C, pooled human serum (10%) was added to each well. In addition, anti-IL-2 receptor antibody (monoclonal anti-TAC) was added to the cultures at a concentration of 2 mg/ml to block IL-2 consumption. After 7 days incubation at 37°C in a moist, 5% CO2 atmosphere, culture supernatants were harvested and stored at −20°C until testing. IL-2 was quantitated in culture supernatants using the Quantiglo human IL-2 enzyme-linked immunosorbent assay (ELISA) system (R&D Systems) with a chemiluminescent detection step to allow maximum sensitivity. Results were expressed as the ratio of IL-2 produced among the stimulated cultures to that of unstimulated cultures. All ratios >3 were considered a positive response to a particular stimulus.
Quantitation of immune activation markers and cytokines in cord blood and mothers' plasma.
In order to quantify levels of T-cell activation, we selected neopterin, β2-m, and sl-selectin as they are early and reliable soluble plasma markers of immune activation events that have already occurred in vivo (neopterin for T cells/monocytes; β2-m for CD8+ T cells, and sl-selectin for T lymphocytes, monocytes, and neutrophils). Maternal and infant (cord blood) plasma levels of the immune activation factors β2-m and sl-selectin were determined using Quantikine ELISA kits (R&D Systems Inc., Minneapolis, MN) by following the manufacturer's instructions. While β2-m levels were determined from undiluted plasma samples, sl-selectin determination required a 100-fold dilution of the samples. Levels of the immune activation factor neopterin were quantitated from undiluted plasma using an Immunotech ELISA system (Beckman Coulter, Marseille, France). The minimum detectable dose of β2-m is <0.2 μg/ml; for sl-selectin it is <0.3 ng/ml, and for neopterin it is <0.2 ng/ml.
Levels of GM-CSF, IL-7, and CXCL12 were determined using the high-sensitivity (h)GM-CSF Biotrak ELISA system (Amersham, Biosciences AB, Uppsala, Sweden), a Quantikine ELISA system (R&D Systems, Minneapolis, MN) for CXCL12, and a Quantikine high-sensitivity ELISA system (R&D Systems, Minneapolis, MN) for IL-7 according to the manufacturers' protocols. Samples were tested undiluted for GM-CSF, IL-7, and CXCL12. The minimum detectable level of GM-CSF was 0.1 pg/ml, and for IL-7 it was <0.1 pg/ml; for CXCL12 the mean minimum detectable dose is 18 pg/ml.
Levels of IL-10 were determined using the high-sensitivity Biotrak ELISA system (Amersham Biosciences). IL-10 levels were determined from plasma samples diluted 10-fold, with the minimum detectable dose being 1 pg/ml.
GM-CSF production assays.
CBMC isolated from infants' blood samples and resuspended at 3 × 106 cells/ml in a total volume of 100 μl RPMI medium containing 1% l-glutamine were unstimulated or stimulated with HIV-1 peptides as described above for T-helper-cell responses and PHA at a final concentration of 12.5 μg/ml. Human serum (10%) was then added to each well. Following a 24-h incubation at 37°C in a moist, 5% CO2 atmosphere, culture supernatants were harvested and stored at −70°C. Supernatants were tested for GM-CSF production using a Biotrak ELISA system (Amersham Biosciences AB, Uppsala, Sweden). GM-CSF was determined after a 5-fold dilution of unstimulated and HIV-1 peptide-stimulated cultures and a 150-fold dilution of PHA-stimulated cultures. The minimum detectable level of GM-CSF was <2 pg/ml.
Quantitation of maternal viral loads.
Plasma HIV-1 RNA levels were measured using the Amplicor RNA monitor assay (Roche Diagnostic Systems, Inc., Branchburg, NJ) with a lower detection limit of 400 RNA copies/ml.
Quantitation of mothers' CD4 T-cell counts.
CD4 T-cell counts were determined using the commercially available FACSCount system from Becton Dickinson (San Jose, CA).
Statistical analysis.
Levels of the different infant and maternal parameters were compared between groups using the nonparametric Mann-Whitney U test. Spearman's rank correlation coefficient was used to describe associations between parameters. Differences between parameters for mothers and infants were tested using the Wilcoxon signed rank test for paired data. Fisher's exact test was used to compare proportions between groups. Statistical analyses were performed using SPSS software (version 11.0; SPSS Inc., Chicago, IL). All statistical tests were two-tailed and considered significant at a P of <0.05.
RESULTS
HIV-1-specific cellular immune responses are associated with lack of transmission.
Clinical characteristics of the mother-infant participants who were included in this nested case control study are presented in Table 1. HIV-1 envelope peptide-stimulated cellular immune responses (Env+) were detected among 13.2% of the EU infants (14/106) whose mothers had not received any antiretroviral drugs. For this study, 42 EU infants without responses (Env−) and 18 EU infants (14/106 plus 4 additional infants from a related study) with responses (Env+) were studied further. No infant with an Env+ response at birth became infected. All infants who later were found to be infected were unresponsive (Env−) at birth. No HIV-1-specific responses were detected among the controls.
TABLE 1.
Comparisons of maternal characteristics and levels of the immunomodulatory and immunoregulatory cytokines among infant groups
| Characteristic | Value for infant group
|
|||
|---|---|---|---|---|
| Controla | HIV-1-infected mother
|
|||
| Env−
|
EU Env+ | |||
| EU | IP infection | |||
| No. of infants | 20 | 42 | 13 | 18 |
| PHA responseb | 182.7 (145.9) | 237 (218.9) | 262 (303.6) | 199 (154.4) |
| Env responseb | 1.0 (0.5) | 1.1 (0.5) | 0.9 (0.4) | 4.5 (1.4) |
| Maternal CD4+ T-cell countb,c | 482 (259) | 430 (188.7) | 488 (288) | |
| Maternal HIV-1 RNA (log10)d,e | 4.8 (4.3-5.2) | 4.8 (4.4-5.3) | 4.1 (3.5-4.9) | |
| Neopterin (ng/ml)e | 2.74 (2.2-3.6) | 3.7 (2.8-4.9) | 4.3 (3.3-6.7) | 3.3 (2.9-4.3) |
| β2-m (μg/ml)e,f | 1.6 (1.3-2.0) | 2.2 (1.9-3.4) | 2.2 (1.6-2.8) | 1.9 (1.5-2.4) |
| sl-Selectin (ng/ml)e,g | 554.5 (484-613.5) | 695.4 (568.8-842.8) | 703.1 (581.9-790.4) | 593.6 (509.1-667.1) |
| IL-7 (pg/ml)e | 2.8 (1.8-4.0) | 2.3 (1.2-3.3) | 2.4 (1.0-3.9) | 2.1 (1.4-4.7) |
| CXCL12 (pg/ml)e | 311 (245.2-520) | 325.4 (259-408,8) | 332.7 (277.3-445.9) | 298.2 (214.7-452.2) |
| IL-10 (pg/ml)e | 17.4 (15.6-20.3) | 20.2 (18.1-35.6) | 19.2 (16.8-30.3) | 19 (17.1-21.7) |
| GM-CSF (pg/ml)e,h | 5.2 (4.2-6.9) | 7.7 (3.5-9.90) | 7.0 (3.7-9.1) | 2.0 (0.7-2.2) |
The control group consisted of uninfected Env− infants whose mothers were also uninfected.
Values are means. Values in parentheses are standard deviations.
The mothers of the exposed infants had not received any antiviral drugs.
P was 0.024 in a comparison of EU Env− infants and EU Env+ infants.
Values are medians. Values in parentheses are interquartile ranges, with endpoints being the 25th and 75th percentiles.
P was 0.018 in a comparison of EU Env− infants and EU Env+ infants.
P was 0.013 in a comparison of EU Env− infants and EU Env+ infants.
P was <0.001 in a comparison of EU Env− infants and EU Env+ infants.
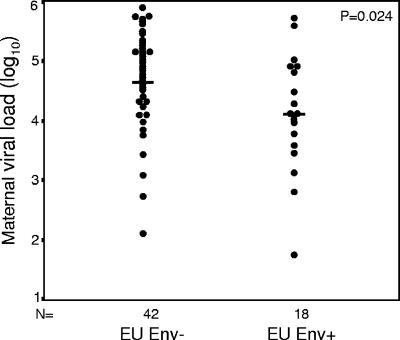
Lower viral loads among mothers of infants with HIV-1-specific responses.
Overall nontransmitting mothers had lower plasma viral loads than transmitting mothers. When results were further stratified by whether or not the infant had HIV-1-specific responses, it was found that nontransmitting mothers of Env+ infants had lower median viral loads (log10 4.1 copies/ml) than nontransmitting mothers of Env− infants and than transmitting mothers (log10 4.8 copies/ml) (Table 1; Fig. 1). There was no significant difference in CD4 T-cell counts between mothers of Env+ and Env− infants.
FIG. 1.
Maternal-viral-load distributions for uninfected infants born to HIV-1-infected mothers (EU) that do not elicit cellular responses to HIV-1 envelope peptides (Env−) and those that do (Env+). Horizontal bars, median values (log10 4.8 and log10 4.1 for Env− and Env+ groups, respectively).
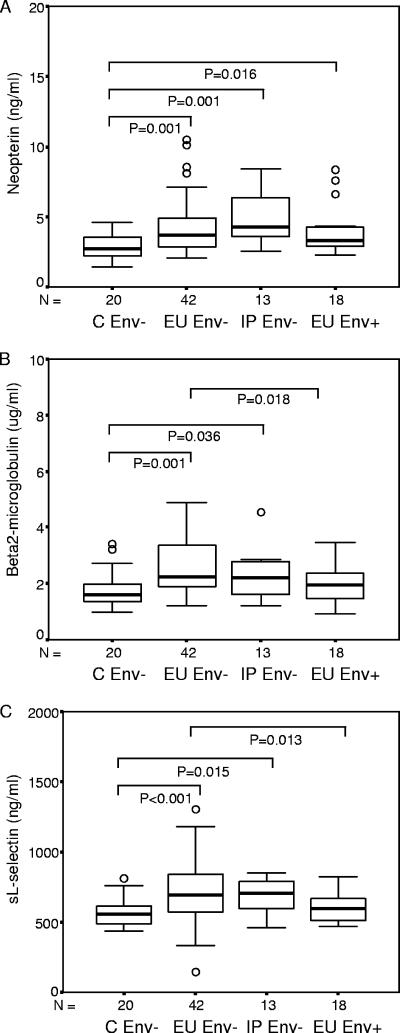
Less immune activation among EU infants with HIV-1-specific responses.
All infants born to HIV-1-seropositive mothers, regardless of their ability to elicit HIV-1-specific responses, exhibited significantly elevated neopterin levels compared to control infants born to HIV-1-negative mothers (Table 1; Fig. 2A). In contrast, β2-m and sl-selectin were elevated relative to unexposed controls only among EU infants lacking HIV-1-specific immune responses (Env−). EU Env+ infants had levels of β2-m and sl-selectin similar to those of control infants, significantly lower than among EU Env− infants (Fig. 2B and C).
FIG. 2.
Levels of neopterin (ng/ml) (A), β2-m (μg/ml) (B), and sl-selectin (ng/ml) (C) for infants born to HIV-seropositive mothers and stratified on the basis of infection outcome (C, uninfected controls) and cellular immune responses to HIV-1 envelope peptides (Env− and Env+ infants). Data are presented as medians (bars inside boxes), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars outside boxes). Significant differences between groups and sample numbers per group are indicated.
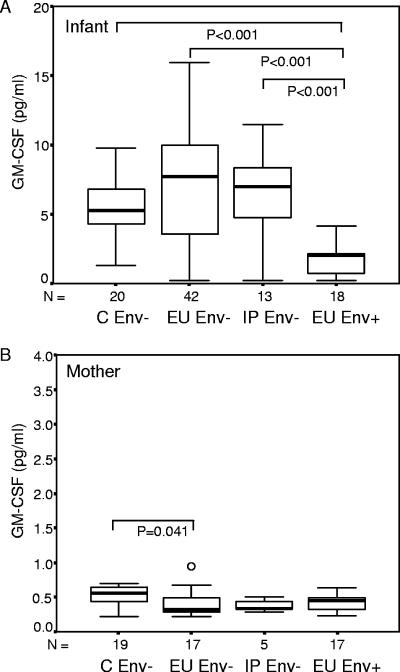
Reduced plasma GM-CSF among EU infants with HIV-1-specific responses.
Env+ infants had significantly reduced levels of GM-CSF in cord blood plasma compared to Env− infants (Fig. 3A). Given these findings we were curious as to whether maternal GM-CSF levels might influence those of their infants. When maternal levels were measured, we found that GM-CSF levels of HIV-1-infected mothers were substantially lower than those of their infants, even among EU Env+ infants and their mothers (P = 0.002). Furthermore, GM-CSF levels of infected mothers tended to be decreased relative to those of uninfected mothers (Fig. 3B). Since infant GM-CSF levels correlated with maternal viral load in the EU group (P = 0.038; r = 0.268), we investigated whether maternal viral load might explain the lower GM-CSF levels observed among infants with HIV-1-specific responses. In a multivariable regression model, GM-CSF levels remained significantly lower among EU Env+ infants (P < 0.001) even after adjusting for maternal viral load. IL-7, CXCL12, and IL-10 (Table 1) levels were similar among Env+ and Env− infants.
FIG. 3.
Infant (A) and maternal (B) plasma GM-CSF levels. Infants and their mothers were stratified on the basis of infection outcome (C, uninfected controls) and HIV-1-specific cellular immune responses to Env peptides (Env− and Env+). Data are presented as medians (bars inside boxes), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars outside boxes). Significant differences between groups and sample numbers per group are indicated. The lowest level of GM-SCF measured in the infants was 0.17 pg/ml.
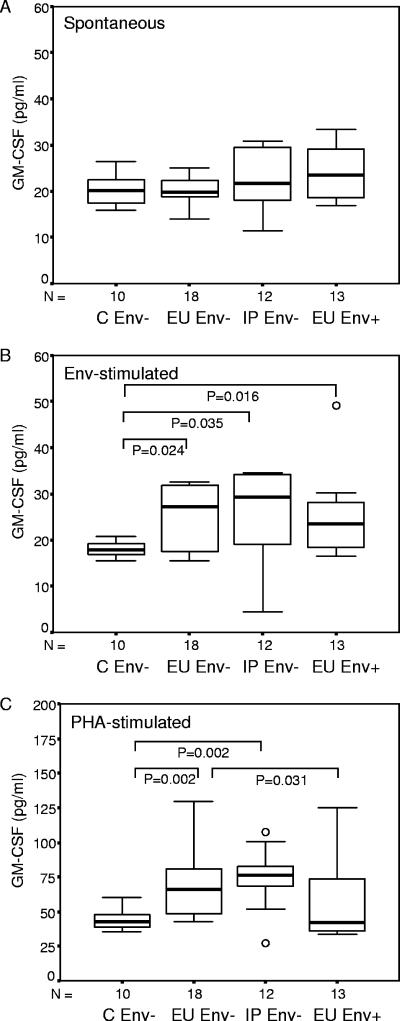
Reduced ability of CBMC to produce GM-CSF among EU infants with HIV-1-specific responses.
As Env+ infants demonstrated dramatically reduced GM-CSF plasma levels, we questioned whether this was possibly associated with an inability of CBMC to produce GM-CSF. Env+ infants demonstrated no altered ability of their CBMC to spontaneously release GM-CSF (Fig. 4A). However, in response to stimulation with HIV-1 Env peptides (Fig. 4B) all groups (Env− and Env+ infants) were able to release significantly larger amounts of GM-CSF than the control group, with a trend to reduced levels among EU Env+ infants compared to the two Env− groups (EU and IP infection). In response to stimulation with PHA, CBMC of Env+ infants produced significantly less GM-CSF than EU Env− infants, suggesting that the capacity to produce GM-CSF mirrors the peripheral blood levels of this factor. A low cellular capacity to produce GM-CSF is therefore associated with the presence of HIV-1-specific cellular immune responses.
FIG. 4.
Ability of CBMC to produce GM-CSF (pg/ml) spontaneously (A) and in response to HIV-1 envelope peptides (B) and PHA (C). Results are stratified on the basis of infection outcome (C, uninfected controls) and HIV-1-specific cellular immune responses to Env peptides (Env− and Env+). Data are presented as medians (bars inside boxes), 25th and 75th percentiles (boxes), and 10th and 90th percentiles (bars outside boxes). Significant differences between GM-CSF levels (pg/ml) of the groups and sample numbers per group are indicated.
DISCUSSION
In our study, no child with HIV-1-specific cellular responses to envelope peptides in cord blood subsequently became infected, supporting the notion that these responses may be markers of protective immunity (9, 10, 13, 31). Furthermore, we demonstrated that infants with these apparently protective Env+ responses exhibited immune capabilities significantly different from those of infants without them. Specifically, we observed (i) reduced activation as determined by measuring plasma levels of β2-m and sl-selectin, (ii) reduced plasma levels of GM-CSF and reduced capacity to produce GM-CSF from CBMC, and (iii) lower viral loads among mothers whose infants had detectable HIV-1-specific responses. These differences are perhaps surprising since our assay of HIV-1-specific cellular immune responses is sure to have missed some true responses below the detection threshold or responses to antigens not used. Nevertheless, there appeared to be distinct features of the EU infants with and without detectable HIV-1-specific responses.
The importance of giving low-dose antigen to induce cell-mediated immune responses was first demonstrated in a study where susceptible BALB/c mice were protected from Leishmania major and increased resistance was achieved in resistant mice (3). In a macaque model, prior exposure to subinfectious doses of simian immunodeficiency virus (SIV) led to SIV-specific T-cell responses that appeared to confer long-term protection against subsequent virus challenge (6). Murine studies have also suggested that the initial antigen dose may be critical to the development of protective immunity in newborns. For example, the inability to develop a cytotoxic T-lymphocyte response to high doses of virus (Cas-Br-M murine leukemia virus) correlated with the induction of a nonprotective type 2 cytokine response and was not the result of immunological immaturity (24). In our data, those infants who elicited an apparently protective HIV-1-specific immune response had mothers with lower plasma cell-free viral loads than infants without these responses. Although maternal plasma viral load is a highly imperfect marker of in utero viral exposure, our data suggest that, for effective immunological priming, exposure to low doses may be necessary if responses that are protective against subsequent virus challenge are to be developed. Our data also suggest that the well-established association between lower maternal viral load and lower risk of transmission may not only be a consequence of reduced viral exposure but may, in addition, mark more effective priming of protective immune responses in the infant.
An important question is what constitutes viral exposure in utero. The placenta plays an important role as a physical barrier to fetal infections such as those with cell-free HIV-1; however, inflammatory reactions, e.g., chorioamnionitis (30), and inflammatory cytokines (20) can cause placental damage facilitating transmission. p24 antigen has also been found to permeate this barrier (1). Defective viral particles or products could potentially prime immune responses in EU infants; for example, it has been demonstrated that HIV-1 mutants with the integrase/endonuclease gene deleted are unable to integrate but can competently produce HIV-1 core and envelope antigens (28).
Infants exposed to p24 antigen, defective virus particles, or HIV-1 from aborted infections might be expected as a result of this immune stimulation to display increased antigen-induced lymphokine production and cellular proliferation. Thus we had originally hypothesized that immune activation markers would be higher among those with HIV-1-specific responses as a consequence of this viral exposure. However, our observation that reduced activation was associated with protective immune responses suggests that activation may facilitate transmission and may preclude the development of HIV-1-specific responses. Thus we have a paradoxical situation where priming may be necessary to develop antigen-specific immunity, yet too much antigen stimulation and/or activated response to that stimulation may tip the balance toward infection rather than protection. We have previously described the complex interactions between immune activation and prevention of vertical transmission in the presence (mothers given a single dose of nevirapine at the onset of labor) and absence (infants given only postexposure prophylaxis) of antiretroviral therapy (25). Specifically, the presence of antiretroviral drugs may help drive an activated immune system into an anergic state that may be beneficial in preventing HIV-1 replication (25).
Our most striking result was that Env+ infants had significantly reduced levels of GM-CSF relative to the Env− group, both in cord blood plasma and PHA-stimulated CBMC. It is possible that reduced production may be a consequence of in utero events that have impacted this capacity or that the inherent ability to produce GM-CSF is different in these infants. In support of the first hypothesis, it was interesting that stimulation with Env peptides had the ability to enhance GM-CSF production in these infants above the uninfected control group, yet these levels were not significantly different from those for the EU Env− group. This suggests that GM-CSF production ability was influenced by the nature or potency of the viral stimulus. GM-CSF would be expected to be produced by activated T cells and monocytes in CBMC cultures. It is also possible that cell types responsible for producing GM-CSF are differently represented in the infant groups or are primed differently as a result of differences in in utero exposure to HIV-1.
GM-CSF is known to augment immune responses to antigens in vivo and in vitro (27), and, due to its numerous immunomodulatory properties, it has been administered as a vaccine adjuvant to enhance cellular or humoral responses to specific antigen. It is intriguing that the effects of GM-CSF, as a vaccine adjuvant, are related to type 1 and type 2 dendritic cells (DC), with DC1 priming naive T cells towards a cellular (T-helper-type 1 [Th1]) response and DC2 promoting differentiation to humoral immune responses (Th2). DC ratios shift towards a predominance of DC1 when peripheral blood stem cells are mobilized with GM-CSF and granulocyte colony-stimulating factor (27). This would suggest that hematopoietic growth factors shift T-cell responses. The differences noted between Env+ and Env− infants may well be due to different amounts of GM-CSF produced upon HIV-1 encounter. Consistent with lower maternal viral load as stimulus and supported by reduced levels of immune activation markers, Env+ infants produce less GM-CSF, which in turn could result in a shift towards DC1 cells that drive Th1 responses (Env+ responses). Another explanation lies in the fact that infant levels are approximately 10-fold higher than those in adults, suggesting that the role of GM-CSF in neonates is quite different from that in adults, and may reflect the more naive immune state evident in early life. Levels of GM-CSF in Env+ infants were reduced to almost adult levels, and so this may well be the consequence of a more mature immune status in these infants, possibly as a result of having developed HIV-1-specific immunity through in utero exposure. Immune maturation may have been fast-tracked in these infants as a result of their exposure histories, a process that would be advantageous in the context of reexposure to HIV-1. Whether levels of GM-CSF play a role in the development of T-cell responses or whether altered levels of GM-CSF among Env+ infants are a consequence of these responses remains to be determined.
In conclusion, our data are consistent with an animal study that has demonstrated the development of protective immune responses following exposure to subinfectious doses of SIV (either due to abortive infection or infection with a virus of low replication competency) (6). Our finding that Env+ infants have reduced levels of β2-m (CD8+ T cells) and sl-selectin (T lymphocytes, monocytes, and neutrophils) reflects altered activation of different cell types. Also, the decreased GM-CSF levels and a reduced ability of CBMCs to produce GM-CSF highlights the fact that infants who develop specific immune responses exhibit immune characteristics different from infants without these responses. It is further intriguing that a deficient phenotype of CC chemokine CCL3/CCL3-L1 production has recently been shown, on the same mother-child cohort studied here, to be associated with acquisition of IP infection (Env−) (19). Given the likely role of these molecules in the instruction of adaptive immunity, the deficient production of CCL3/CCL3-L1 in infants with IP infection may compromise the development of primary immune responses against HIV-1 (19). Strategies that optimize the cellular components of the immune system (the activation and expansion of virus-specific CD4+ and CD8+ T-cell responses) (23) are important for HIV-1 vaccine development. Our data have provided new insights as to how protective immune responses might develop. In neonates, induction of the Th1 responses in early life could require a prolonged exposure to low doses of antigen (17), less nonspecific immune activation, and a more mature phenotype in terms of capacity to produce GM-CSF in response to viral antigens, thereby driving CD4+ T cells along the Th1 differentiation pathway (21, 29). Identifying factors involved in the development of protective immune responses and the relationships between such factors will aid in identifying critical components of protective immunity to HIV-1; however, it will be important to determine whether the correlates of protection that develop during natural infection reflect those that are protective during vaccine-induced immunity.
Acknowledgments
This study was supported in part by the Poliomyelitis Research Foundation Major Impact grant of South Africa and by grants from NICHD (HD 42402 and HD 36177). C.T.T. is a Wellcome Trust International Senior Research Fellow (076352/Z/05/Z).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Bawdon, R. E., M. Gravell, R. Hamilton, J. Sever, R. Miller, and C. J. Gibbs. 1994. Studies on the placental transfer of cell-free human immunodeficiency virus and p24 antigen in an ex vivo human placental model. J. Soc. Gynecol. Investig. 1:45-48. [DOI] [PubMed] [Google Scholar]
- 2.Berzofsky, J. A., C. D. Pendleton, M. Clerici, J. Ahlers, D. R. Lucey, S. D. Putney, and G. M. Shearer. 1991. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and humans of multiple MHC types. J. Clin. Investig. 88:876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bretscher, P. A., G. Wei, J. N. Menon, and H. Bielefeldt-Ohmann. 1992. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 257:539-542. [DOI] [PubMed] [Google Scholar]
- 4.Cease, K. B., H. Margalit, J. L. Cornette, S. D. Putney, W. G. Robey, C. Ouyang, H. Z. Streicher, P. J. Fischinger, R. C. Gallo, C. DeLisi, et al. 1987. Helper T-cell antigenic site identification in the acquired immunodeficiency syndrome virus gp120 envelope protein and induction of immunity in mice to the native protein using a 16-residue synthetic peptide. Proc. Natl. Acad. Sci. USA 84:4249-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clerici, M., J. A. Berzofsky, G. M. Shearer, and C. O. Tacket. 1991. Exposure to human immunodeficiency virus (HIV) type I indicated by HIV-specific T helper cell responses before detection of infection by polymerase chain reaction and serum antibodies. J. Infect. Dis. 164:178-182. [DOI] [PubMed] [Google Scholar]
- 6.Clerici, M., E. A. Clark, P. Polacino, I. Axberg, L. Kuller, N. I. Casey, W. R. Morton, G. M. Shearer, and R. E. Benveniste. 1994. T-cell proliferation to subinfectious SIV correlates with lack of infection after challenge of macaques. AIDS 8:1391-1395. [DOI] [PubMed] [Google Scholar]
- 7.Clerici, M., J. V. Giorgi, C. C. Chou, V. K. Gudeman, J. A. Zack, P. Gupta, H. N. Ho, P. G. Nishanian, J. A. Berzofsky, and G. M. Shearer. 1992. Cell-mediated immune response to human immunodeficiency virus (HIV) type 1 in seronegative homosexual men with recent sexual exposure to HIV-1. J. Infect. Dis. 165:1012-1019. [DOI] [PubMed] [Google Scholar]
- 8.Clerici, M., J. M. Levin, H. A. Kessler, A. Harris, J. A. Berzofsky, A. L. Landay, and G. M. Shearer. 1994. HIV-specific T-helper activity in seronegative health care workers exposed to contaminated blood. JAMA 271:42-46. [PubMed] [Google Scholar]
- 9.Clerici, M., M. Saresella, F. Colombo, S. Fossati, N. Sala, D. Bricalli, M. L. Villa, P. Ferrante, L. Dally, and A. Vigano. 2000. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood 96:3866-3871. [PubMed] [Google Scholar]
- 10.Clerici, M., A. V. Sison, J. A. Berzofsky, T. A. Rakusan, C. D. Brandt, M. Ellaurie, M. Villa, C. Colie, D. J. Venzon, J. L. Sever, et al. 1993. Cellular immune factors associated with mother-to-infant transmission of HIV. AIDS 7:1427-1433. [DOI] [PubMed] [Google Scholar]
- 11.Gray, G. E., M. Urban, M. F. Chersich, C. Bolton, R. van Niekerk, A. Violari, W. Stevens, and J. A. McIntyre. 2005. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS 19:1289-1297. [DOI] [PubMed] [Google Scholar]
- 12.Hale, P. M., K. B. Cease, R. A. Houghten, C. Ouyang, S. Putney, K. Javaherian, H. Margalit, J. L. Cornette, J. L. Spouge, C. DeLisi, et al. 1989. T cell multideterminant regions in the human immunodeficiency virus envelope: toward overcoming the problem of major histocompatibility complex restriction. Int. Immunol. 1:409-415. [DOI] [PubMed] [Google Scholar]
- 13.Kuhn, L., A. Coutsoudis, D. Moodley, D. Trabattoni, N. Mngqundaniso, G. M. Shearer, M. Clerici, H. M. Coovadia, and Z. Stein. 2001. T-helper cell responses to HIV envelope peptides in cord blood: protection against intrapartum and breast-feeding transmission. AIDS 15:1-9. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn, L., S. Meddows-Taylor, G. Gray, D. Schramm, and C. T. Tiemessen. 2003. HIV-stimulated IL-2 production among exposed-uninfected infants of HIV-infected mothers given nevirapine prophylaxis, abstr. 861. Abstr. 10th Conf. Retrovir. Opportunistic Infect., Boston, MA, 10 to 14 February 2003.
- 15.Kuhn, L., S. Meddows-Taylor, G. Gray, and C. Tiemessen. 2002. Human immunodeficiency virus (HIV)-specific cellular immune responses in newborns exposed to HIV in utero. Clin. Infect. Dis. 34:267-276. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn, L., S. Meddows-Taylor, G. Gray, D. Trabattoni, M. Clerici, G. M. Shearer, and C. Tiemessen. 2001. Reduced HIV-stimulated T-helper cell reactivity in cord blood with short-course antiretroviral treatment for prevention of maternal-infant transmission. Clin. Exp. Immunol. 123:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez, X., C. Brandt, F. Saddallah, C. Tougne, C. Barrios, F. Wild, G. Dougan, P. H. Lambert, and C. A. Siegrist. 1997. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc. Natl. Acad. Sci. USA 94:8726-8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 19.Meddows-Taylor, S., S. L. Donninger, M. Paximadis, D. B. Schramm, F. S. Anthony, G. E. Gray, L. Kuhn, and C. T. Tiemessen. 2006. Reduced ability of newborns to produce CCL3 is associated with increased susceptibility to perinatal human immunodeficiency virus 1 transmission. J. Gen. Virol. 87:2055-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghupathy, R. 1997. Th1-type immunity is incompatible with successful pregnancy. Immunol. Today 18:478-482. [DOI] [PubMed] [Google Scholar]
- 21.Rogge, L., L. Barberis-Maino, M. Biffi, N. Passini, D. H. Presky, U. Gubler, and F. Sinigaglia. 1997. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 23.Salk, J., P. A. Bretscher, P. L. Salk, M. Clerici, and G. M. Shearer. 1993. A strategy for prophylactic vaccination against HIV. Science 260:1270-1272. [DOI] [PubMed] [Google Scholar]
- 24.Sarzotti, M., D. S. Robbins, and P. M. Hoffman. 1996. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science 271:1726-1728. [DOI] [PubMed] [Google Scholar]
- 25.Schramm, D. B., L. Kuhn, G. E. Gray, and C. T. Tiemessen. 2006. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J. Acquir. Immune Defic. Syndr. 42:545-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shearer, G. M., and M. Clerici. 1996. Protective immunity against HIV infection: has nature done the experiment for us? Immunol. Today 17:21-24. [DOI] [PubMed] [Google Scholar]
- 27.Somani, J., S. Lonial, H. Rosenthal, S. Resnick, I. Kakhniashvili, and E. K. Waller. 2002. A randomized, placebo-controlled trial of subcutaneous administration of GM-CSF as a vaccine adjuvant: effect on cellular and humoral immune responses. Vaccine 21:221-230. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson, M., S. Haggerty, C. A. Lamonica, C. M. Meier, S. K. Welch, and A. J. Wasiak. 1990. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J. Virol. 64:2421-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo, S. J., A. S. Dighe, U. Gubler, and K. M. Murphy. 1997. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 185:817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wabwire-Mangen, F., R. H. Gray, F. A. Mmiro, C. Ndugwa, C. Abramowsky, H. Wabinga, C. Whalen, C. Li, and A. J. Saah. 1999. Placental membrane inflammation and risks of maternal-to-child transmission of HIV-1 in Uganda. J. Acquir. Immune Defic. Syndr. 22:379-385. [DOI] [PubMed] [Google Scholar]
- 31.Wasik, T. J., J. Bratosiewicz, A. Wierzbicki, V. E. Whiteman, R. R. Rutstein, S. E. Starr, S. D. Douglas, D. Kaufman, A. V. Sison, M. Polansky, H. W. Lischner, and D. Kozbor. 1999. Protective role of beta-chemokines associated with HIV-specific Th responses against perinatal HIV transmission. J. Immunol. 162:4355-4364. [PubMed] [Google Scholar]