Abstract
Dengue virus (DEN), the pathogen behind dengue hemorrhagic fever, remains a public health problem in Asia and South America. In this study, monoclonal antibodies (MAbs) against DEN serotype 1 (DEN-1) were generated by fusing NSI/1-Ag4-1 mouse myeloma cells with lymphocytes from BALB/c mice immunized with DEN-1. Twelve MAbs were found to react specifically to the DENs by enzyme-linked immunosorbent assay, immunofluorescence analysis, and immunoblotting analysis. Five MAbs, namely, DA4-7, DA6-7, DA9-5, DA10-2, and DA11-13, were found to react with envelope proteins of DEN-1. Two serotype-specific MAbs of DEN-1, DA6-7 and DA11-13, were further shown to neutralize DEN-1 infection by a plaque reduction neutralization test. The neutralizing epitopes of these MAbs were further identified from a random peptide library displayed on phage. Immunopositive phage clones reacted specifically with these MAbs and did not react with normal mouse serum. Epitope-based peptide antigens were proved able to detect antibodies in serum samples collected from DEN-1-infected patients but not in those taken from DEN-2-infected patients or healthy controls. We believe that these MAbs and neutralizing epitopes will provide information that will lead to the development of DEN-1 serotype-specific diagnostic reagents and vaccines.
Dengue virus (DEN) causes a variety of illnesses that range in severity from mild, in such syndromes as dengue fever (DF), to severe, in the syndromes dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (18, 19). DF is manifested as a typical biphasic fever, headache, body pain, and rash. DHF, however, is characterized by abnormalities of hemostasis and vascular permeability and is often fatal. Two-fifths of the world's population is at risk of infection, and it is estimated that 50 million people a year are infected with this virus. One percent of people infected with this virus will develop DHF (59).
Until now, no effective method has been reported to be capable of preventing the development of DHF/DSS because the pathogenic mechanisms of this disease are unclear (5, 6, 41). However, several relevant hypotheses exist, including antibody-dependent enhancement of infection and virus variation (20, 35, 37). DENs are divided into four serotypes, DEN-1, -2, -3, and -4, which have very similar genome sequences and envelope protein (E protein) antigenic properties. A secondary infection with a different DEN serotype may increase the risk for DHF (20). One possibility is that monocytes/macrophages take up the virus complexes by binding to nonneutralizing antibodies or subneutralizing cross-reactive antibodies (6, 19, 20). Antibody-dependent enhancement, in conjunction with activation of memory T-cell responses, is believed to contribute to the immunopathogenic disease process (50).
Virus variation may also account for differences in severity of dengue-related diseases (32, 47, 49). Moreover, since DEN infection is often accompanied by the production of cytokines or chemokines and the activation of complement or immune cells, these may also contribute to the pathogenesis of DHF/DSS (15, 16, 24, 28). The severity of disease also depends on the serotype of the infecting DEN, the degree of viremia, and the genetic background (51, 55). In summary, several complicated mechanisms have been hypothesized to be involved in the pathogenesis of DEN infection, though their relative roles need further investigation.
Because DEN is a major cause of pediatric morbidity and mortality in tropical regions (19), a safe vaccine and a simple reliable test for the serodiagnosis of DEN infection could significantly reduce morbidity and mortality. The ideal vaccine would protect against all four DEN serotypes and provide long-lasting immunity against DEN infection. Importantly, vaccination should not predispose patients to the development of DHF/DSS. Prerequisites to the development of such a vaccine are epitope mapping and the discovery of serotype-specific and neutralizing epitopes of DENs.
In addition to vaccine development, the identification of neutralizing epitopes is useful in the study of virus-host cell interactions and the pathogenesis of DHF. Measuring the ability of a monoclonal antibody (MAb) to bind to fragments of the E protein expressed in bacteria can give us an understanding of the antigenic map of the DEN-2 E protein (40, 48). Polyclonal sera from dengue patients and dengue-immune rabbits were also used to identify the linear serological epitopes (six to eight amino acids) in the DEN-2 E protein by overlapping synthetic peptides (PEPSCAN) (1, 23). However, oligopeptide antigens cannot be used to identify epitopes that are conformationally or discontinuously recognized by neutralizing antibodies. Only two epitopes have been found to be involved in neutralization in DEN-2, at E307 (34) and at E383 to -385 (22). However, there is no evidence yet that either epitope is recognized by serum samples from dengue patients.
Alternatively, through a selection process called biopanning, the phage display technique makes possible the rapid identification of linear epitopes (36, 60) or conformational epitopes (13, 61, 62). Phage-displayed random peptide libraries provide opportunities to map B-cell epitopes (11, 14, 52, 60, 61) and protein-protein contacts (3, 7, 42, 53), select bioactive peptides bound to receptors (26, 33) or proteins (7, 9, 10, 27, 44), search for disease-specific antigen mimics (13, 36, 46), and determine cell-specific (4, 31, 39) and organ-specific (2, 12, 45) peptides.
In the present study, two neutralizing MAbs against DEN-1 were generated. The neutralizing epitopes of both antibodies were further identified with a phage-displayed random peptide library. Nine serotype-specific antibodies (DA4-7, DA6-6, DA6-7, DA10-2, DA11-9, DA11-13, DA15-2, DA31-6, and DA32-9) and epitope-based peptide antigens can be used to develop a convenient, efficient serologic test and to address the role of antibodies in the pathogenesis of primary and secondary DEN-1 infections. The neutralizing MAbs and epitopes of DEN-1 may be useful for studying the mechanism of viral entry and may provide information for the development of vaccines.
MATERIALS AND METHODS
Cells and viruses.
DEN-1 strain 766733 is a local Taiwanese strain isolated from patients with DF. Four prototype dengue viruses—DEN-1 (HI), DEN-2 (New Guinea C), DEN-3 (H87), and DEN-4 (H241)—were provided by Duane J. Gubler from the Centers for Disease Control and Prevention, Fort Collins, CO. All viral strains were used to infect mosquito C6/36 cells with growth medium containing 50% Mitsumashi and Maramorsch insect medium (Sigma) plus 50% Dulbecco's modified Eagle's minimal essential medium (DMEM; GIBCO). The DEN-infected C6/36 cells were incubated at 28°C for 7 to 9 days. The viruses were harvested from the supernatants and titrated in BHK cells by plaque assay, and aliquots were stored at −80°C in a deep freezer. BHK-21 cells were grown in minimal essential medium (MEM) containing 10% heat-inactivated fetal bovine serum (FBS).
Human serum samples.
An active physician-based dengue surveillance system has been established in both local hospitals in southern Taiwan and the National Taiwan University Hospital in northern Taiwan. Serum samples used in this study were all convalescent-phase sera collected from dengue patients between 1 and 2 months after the onset of disease. All serum samples collected from dengue patients were confirmed as DEN-1 or DEN-2 positive by serotype-specific reverse transcriptase PCR at the acute phase (30). Normal human serum (NHS) samples from healthy adults who tested negative for anti-DEN antibody by commercial capture enzyme-linked immunosorbent assay (ELISA) (Dengue Duo; PanBio, Queensland, Australia) (43, 57) served as references to establish cutoff levels.
Generation of MAbs against DEN-1.
Hybridomas secreting anti-DEN-1 antibodies were generated according to a standard procedure (25). Briefly, female BALB/c mice were immunized intraperitoneally with DEN-1 emulsified in Freund's adjuvant (Sigma, St. Louis, MO) four times at 3-week intervals. On day 4 after the final immunization, the spleen was removed and the cells fused with NSI/1-Ag4-1 myeloma cells, using 50% (vol/vol) polyethylene glycol (GIBCO BRL). The fused cell pellet was resuspended in DMEM supplemented with 15% FBS, hypoxanthine-aminopterin-thymidine medium, and hybridoma cloning factor (ICN, Aurora, OH). Hybridoma colonies were screened for secretion of MAbs that bound DEN-infected C6/36 cells by ELISA. Selected clones were subcloned by limiting dilution. Ascitic fluids were produced in pristane-primed BALB/c mice. Hybridoma cell lines were grown in DMEM with 10% heat-inactivated FBS. MAbs were affinity purified with protein G Sepharose 4B gel. ELISA and Western blotting measured the activity and specificity of antibodies.
Screening of MAbs against DEN by ELISA.
Serially diluted MAbs were added to the plates of DEN-1-infected cells and incubated at room temperature for 1 h. The plates were washed three times with phosphate-buffered saline containing 0.1% (wt/vol) Tween 20 (PBST0.1) and incubated with horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG; Jackson ImmunoResearch Laboratories, West Grove, PA). The plates were washed five times with PBST0.1 and incubated with the peroxidase substrate o-phenylenediamine dihydrochloride (OPD; Sigma). The reaction was stopped with 3 N HCl, and the plates were read using a microplate reader at 490 nm.
Preparation of DEN antigens.
C6/36 cells were infected with four serotypes of DEN. Infected cells were then lysed in lysis buffer (25 mM Tris-HCl, pH 7.4, 150 mM NaCl, and 1% Nonidet P-40) in the presence of protease inhibitors. Cell debris was removed by centrifugation at 3,000 × g for 10 min at 4°C, and protein was quantified using the protein dye binding method described by Bradford (8).
Western blot analysis.
Proteins or antigens were mixed with an equal volume of the native sample buffer (50 mM Tris-HCl, pH 6.8, 0.1% bromophenol blue, 10% glycerol), separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a nitrocellulose membrane (Hybond-C Super; Amersham, Little Chalfont, United Kingdom). Nonspecific antibody-binding sites were blocked with 5% skim milk in PBS, and membranes were incubated with primary antibodies and HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) and developed with chemiluminescence reagents (ECL; Amersham).
Plaque reduction neutralization test (PRNT).
MAbs were diluted with serum-free MEM, mixed with an equal volume of virus suspension, and incubated for 1 h at 37°C. The antibody-virus mixture was incubated in duplicate with BHK-21 cells in 12-well plates. After adsorption of viruses for 2 h, 2 ml of medium (MEM containing 2% FBS, antibiotic, and 1% carboxymethyl cellulose) was added to each well. Plates were incubated in 5% CO2 at 37°C for 5 to 7 days. Cells were stained with 0.5% crystal violet added directly to the medium and left for 60 min. After being washed three times with tap water, the plaques were counted.
Inhibition of DEN infection in BHK-21 cells.
Cells were seeded in monolayers on sterile glass slides. MAbs were diluted with serum-free MEM and mixed with virus at a multiplicity of infection of 0.1 and incubated for 1 h at 4°C. The antibody-virus mixture was incubated in duplicate with BHK-21 cells. After adsorption of virus for 2 h, fresh medium (MEM containing 2% FBS and antibiotic) was added to each well. Plates were incubated in 10% CO2 at 37°C for 2 days. The antienvelope MAb 4G2 was then incubated with cells at 4°C for 1 h. The glass slides were incubated in a humidified chamber. After three washings with PBS, cells were incubated with fluorescein isothiocyanate-conjugated goat anti-mouse IgG. The inhibition of DEN infection in BHK-21 cells by neutralizing MAbs was observed with a fluorescence microscope.
Identification of neutralizing epitopes by phage display.
An ELISA plate was coated with 100 μg/ml of neutralizing MAbs in 0.1 M sodium bicarbonate buffer (pH 8.6). The plate was then incubated with blocking buffer (1% bovine serum albumin in PBS) at 4°C overnight and washed with PBST0.5. A phage-displayed 12-mer peptide library was purchased from New England Biolabs, Inc. (Beverly, MA). Phage display biopanning procedures were performed according to our previously published method (61). The titer of the unamplified third-round phage particles was determined on Luria-Bertani medium-IPTG (isopropyl-β-d-thiogalactopyranoside)-X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) plates, and immunopositive phage clones were screened by ELISA.
Identification of immunopositive phage clones by ELISA.
An ELISA plate was coated with 100 μg/ml of antibody in 0.1 M sodium bicarbonate buffer (pH 8.6) and blocked with blocking buffer. Serially diluted phage was added to the antibody-coated plate and incubated at room temperature for 1 hour. The plate was washed with PBST0.5, and HRP-conjugated anti-M13 antibody (Pharmacia) diluted in blocking buffer was added. The plate was incubated and washed with PBST0.5, and then the same procedures were followed as those described in “Screening of MAbs against DEN by ELISA.”
DNA sequencing and computer analysis.
Immunopositive phage clones were further characterized by DNA sequencing. DNA sequences of purified phages were determined by the dideoxynucleotide chain termination method with an automated DNA sequencer (ABI PRISM 377; Perkin-Elmer, CA). The primer used for phage DNA sequencing was 5′-CCCTCATAGTTAGCGTAA-3′. The phage-displayed peptide sequences were translated and aligned with the Genetics Computer Group program.
Phage competitive inhibition assay.
Antigens were mixed with an equal volume of native sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under denaturing conditions, and transferred to a nitrocellulose membrane. Nonspecific antibody-binding sites were blocked with 5% skim milk in PBS. MAbs were diluted with 5% skim milk in PBS, mixed with different concentrations of phage, and incubated for 1 h at 4°C. The membranes were incubated with MAbs and the phage mixture for 1 h at 4°C. HRP-conjugated goat anti-mouse immunoglobulin was added, and the membranes were developed with chemiluminescence reagents.
Detection of IgG antibodies in DEN-infected patient serum samples by capture ELISA.
Plates were coated with 10 μg/ml of DA6-7 or DA11-13 in 0.1 M sodium bicarbonate buffer (pH 8.6). After incubation at room temperature for 2 h, the microplates were washed with PBS (pH 7.2) and blocked with blocking buffer (1% bovine serum albumin in PBS). After being blocked, the plates were washed and then incubated with diluted DEN-1. The plates were then washed with PBST0.1 and incubated with diluted (1:200) DEN-1-infected patient serum or NHS in blocking buffer. They were then washed and incubated with HRP-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). They then underwent the same procedures as those described in “Screening of MAbs against DEN by ELISA.” The mean optical density of NHS at 490 nm (A490) plus three times the standard deviation was used to determine the cutoff value for this assay.
Detection of DEN-infected patient serum samples by immunopositive phage clones.
ELISA plates were coated with 10 μg/ml purified anti-human IgG capture antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA), blocked with blocking solution, and then incubated with the diluted (1:200) tested serum samples. Immunopositive phage particles (109) were added to antibody-coated plates, and the same procedures as those described in “Identification of immunopositive phage clones by ELISA” were followed. The mean optical density of NHS at 490 nm (A490) plus three times the standard deviation was used to determine the cutoff value for this assay.
RESULTS
Generation and identification of MAbs against DEN-1.
In this study, we generated serotype-specific and neutralizing MAbs against DEN-1 to aid in our exploration of the immunopathogenesis of DHF and development of serologic diagnostic reagents and therapeutic antibodies. Immunoblotting and ELISA were used to test the specificities of MAbs for four serotypes of DEN (Table 1). We generated five MAbs against E proteins of DENs. DA4-7, DA6-7, DA10-2, and DA11-13 reacted only with E proteins of DEN-1. DA9-5 reacted with E proteins of both DEN-1 and DEN-2 (Fig. 1A). We also generated seven MAbs against nonstructural protein 1 (NS1 protein) specific to DENs. They were DA6-6, DA11-9, DA15-2, DA31-6, and DA32-9, which reacted specifically with NS1 protein of DEN-1, and DA8-4 and DA11-1, which reacted with NS1 proteins of DEN-1 and DEN-3 (Fig. 1B). These MAbs recognized only the viral proteins of DEN-infected cells, not mock-infected cells, by immunoblotting analysis (data not shown). The specificities of these MAbs for viral proteins were further confirmed with MAbs 4G2 (ATCC HB197; against E proteins of four serotypes of DENs) (21) and 15F3 (ATCC HB47; against NS1 proteins of DEN-1) (21, 60) by an immunoblotting assay (data not shown). ELISA revealed C6/36 cells to be infected with DEN-1, -2, -3, and -4. Infected cells were fixed and incubated with MAbs against DEN, normal mouse IgG (NM-IgG), and normal mouse serum (NMS). All MAbs recognized DEN-1-infected C6/36 cells (Table 1).
TABLE 1.
Generation and characterization of MAbs against DEN-1 by Western blotting, ELISA, and PRNT assay
| MAb | Western blot result
|
ELISA result
|
DEN-1 PRNT50 (μg/ml) | Specificitya | ||||
|---|---|---|---|---|---|---|---|---|
| DEN-1 | DEN-2 | DEN-3 | DEN-4 | DEN-1 | DEN-3 | |||
| DA4-7 | + | − | − | − | + | − | E | |
| DA6-6 | + | − | − | − | + | − | NS1 | |
| DA6-7 | + | − | − | − | + | − | <1.25 | E |
| DA8-4 | + | − | + | − | + | − | NS1 | |
| DA9-5 | + | + | − | − | + | − | E | |
| DA10-2 | + | − | − | − | + | − | E | |
| DA11-1 | + | − | + | − | + | + | NS1 | |
| DA11-9 | + | − | − | − | + | − | NS1 | |
| DA11-13 | + | − | − | − | + | − | <1.25 | E |
| DA15-2 | + | − | − | − | + | − | NS1 | |
| DA31-6 | + | − | − | − | + | − | NS1 | |
| DA32-9 | + | − | − | − | + | − | NS1 | |
E, envelope proteins; NS1, nonstructural protein 1.
FIG. 1.
Identification of MAbs against E and NS1 proteins of DENs by immunoblot analysis. Four serotypes of DEN antigens (D1 to D4) from DEN-infected C6/36 cell lysates were size fractionated in polyacrylamide gels. The blots were incubated with MAbs. (A) MAbs DA4-7, DA6-7, DA9-5, DA10-2, and DA11-13, recognizing E proteins (55 kDa) of DENs, were identified by immunoblot analysis using a nonreducing gel. (B) MAbs DA6-6, DA8-4, DA11-1, DA11-9, DA15-2, DA31-6, and DA32-9 against the dimeric form of NS1 proteins (75 kDa) were identified by immunoblot analysis using a nonreducing gel.
Inhibition of DEN-1 entry into BHK-21 cells by neutralizing antibodies.
In screening for neutralizing MAbs, we tested the neutralizing activity of all MAbs against DEN-1 by PRNT assay (see Materials and Methods for details). We identified two neutralizing MAbs, DA6-7 and DA11-13. Both had a 50% reduction in plaque formation at 1.25 μg/ml. NM-IgG (10 μg/ml) did not inhibit the formation of plaques (Fig. 2A).
FIG. 2.
In vitro neutralization of DEN strains by neutralizing MAbs DA6-7 and DA11-13. The ascitic fluids containing DA6-7 and DA11-13 were purified using a protein G Sepharose column. (A) The neutralizing activities of the purified MAbs were tested by PRNT against DEN-1 strain 766733. (B) Inhibition of DEN infection by neutralizing MAbs, using an immunofluorescence assay. DEN-1 strain 766733 was incubated with 100 μg/ml of neutralizing or control MAb for 1 hour and then used to infect BHK-21 cells. The DEN-infected cells were detected with the 4G2 MAb.
To further confirm the neutralizing ability of DA6-7 and DA11-13, the antibodies were incubated with DEN-1 before being used to infect BHK-21 cells. E proteins of DEN-1 in BHK-21 cells were detected by an indirect immunofluorescence assay. E proteins were found on BHK-21 cells infected with nonneutralized virions (Fig. 2B, no antibody and NM-IgG). Fluorescence staining for DEN E proteins was detected most intensely in the cytoplasm of the cell. In contrast, cells infected with DEN pretreated with DA6-7 and DA11-13 MAbs showed consistently negative staining (Fig. 2B). DEN entry into BHK-21 cells was inhibited by these neutralizing MAbs, and the viruses stopped replicating.
Screening of phage-displayed peptide library with neutralizing antibodies against DEN-1.
Neutralizing epitopes of DA6-7 and DA11-13 were identified by the phage display technique. To select immunopositive phage clones binding to neutralizing MAbs, DA6-7 and DA11-13 ascitic fluids were purified using a protein G affinity column. Purified antibodies were immobilized on ELISA plates, and bound phage clones were selected after biopanning three times. Further screening of immunopositive phage clones was performed by single-phage clone isolation and amplification for MAb screening by ELISA.
Of the 30 phage clones selected by DA6-7, 15 (DA6-7-C2, -C3, -C4, -C10, -C12, -C14, -C16, -C17, -C18, -C25, -C26, -C27, -C28, -C29, and -C30) were highly reactive with antibody DA6-7 and did not bind to NMS (Fig. 3A). Of the 36 phage clones selected by DA11-13, 32 (DA11-13-C1, -C2, -C3, -C4, -C5, -C6, -C7, -C8, -C9, -C10, -C11, -C12, -C13, -C14, -C15, -C16, -C17, -C19, -C20, -C21, -C22, -C24, -C25, -C27, -C28, -C29, -C30, -C31, -C32, -C33, -C34, and -C36) were highly reactive with antibody DA11-13 and did not bind to NMS (Fig. 3B).
FIG. 3.
Identification of neutralizing epitopes of DA6-7 and DA11-13 by phage display. (A) A phage-displayed random peptide library was screened by DA6-7. After three rounds of biopanning, 15 of 30 selected phage clones showed significant reactivity to antibody DA6-7 but not to control NMS. (B) A phage-displayed random peptide library was screened by DA11-13. After three rounds of biopanning, 32 of 36 selected phage clones showed significant reactivity to antibody DA11-13 but not to NMS.
Characterization of neutralizing B-cell epitopes.
Fifteen immunopositive phage clones (DA6-7-C2, -C3, -C4, -C10, -C12, -C14, -C16, -C17, -C18, -C25, -C26, -C27, -C28, -C29, and -C30) that were highly reactive with DA6-7 were amplified, and phage DNAs were isolated for DNA sequencing. All of the phage clones displayed the 12 amino acid residues NTYFTAFLDGPK.
Similarly, 17 immunopositive phage clones (DA11-13-C1, -C2, -C3, -C4, -C5, -C6, -C8, -C11, -C12, -C13, -C14, -C20, -C24, -C28, -C30, -C33, and -C36) that were highly reactive with DA11-13 were amplified, and phage DNAs were isolated for DNA sequencing. These immunopositive phage clones displayed four different peptide sequences. Phage clones DA11-13-C2, -C3, -C8, -C11, -C13, -C20, -C28, and -C30 displayed the same amino acid sequence, QVPSSLSLLQSR, clones DA11-13-C5 and -C36 displayed the sequence HKYSSLDLLQQR, and clone DA11-13-C12 displayed the sequence TAPSSISLIHAR. Phage clones DA11-13-C1, -C4, -C6, -C14, -C24, and -C33 displayed the sequence DPLTSLHAMQRR. Three amino acid residues, i.e., serine (S)/threonine (T)-S-leucine (L)/isoleucine (I) (shown in bold), were highly conserved in all of these immunopositive phage clones.
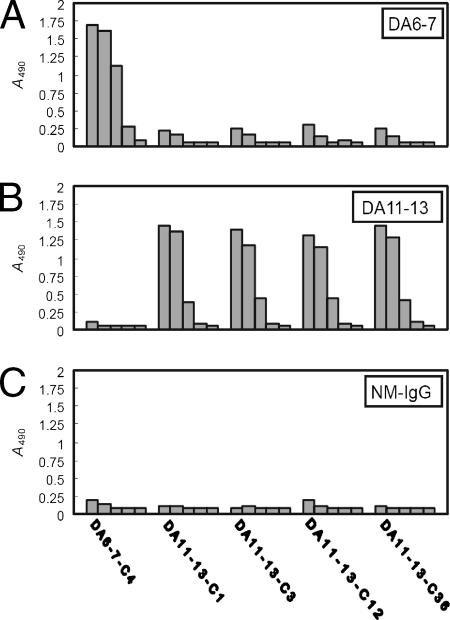
To confirm that the peptides displayed on immunopositive phage clones bound DA6-7 and DA11-13 specifically, a serial-dilution ELISA binding assay was performed. The DA6-7-selected phage clone (DA6-7-C4) reacted with DA6-7 specifically and dose dependently but not with DA11-13 or NM-IgG (Fig. 4). DA11-13-selected phage clones (DA11-13-C1, -C3, -C12, and -C36) reacted with DA11-13 specifically and dose dependently but not with DA6-7 or NM-IgG (Fig. 4).
FIG. 4.
Specific reactivities of selected phage clones to neutralizing MAbs. An ELISA plate was coated with DA6-7 (A), DA11-13 (B), or NM-IgG (C). The antibodies were then incubated with 10-fold serially diluted phage clones (109, 108, 107, 106, and 0 PFU). The DA6-7-selected phage clone (DA6-7-C4) bound to DA6-7 specifically but did not react with DA11-13 and NM-IgG. DA11-13-selected phage clones (DA11-13-C1, -C3, -C12, and -C36) bound to DA11-13 specifically but did not react with DA6-7 and NM-IgG.
To further confirm that the phage-displayed peptide sequences were the B-cell epitopes of neutralizing MAbs, phage competitive inhibition assays were performed to determine whether the immunopositive phage competed with E proteins for reactivity with DA6-7 and DA11-13. The reactivity of DA6-7 with E proteins was inhibited markedly by DA6-7-C4 at 1011 and 1010 PFU/ml of phage (Fig. 5A). Similarly, the reactivity of DA11-13 with E proteins was inhibited completely by DA11-13-C2 and -C4 at 1010 and 109 PFU/ml of phage (Fig. 5B and C). The binding activities of these neutralizing MAbs to E proteins were inhibited by immunopositive phage clones. These findings strongly suggest that the phage-displayed peptide sequences are indeed the B-cell epitopes of DA6-7 and DA11-13.
FIG. 5.
Phage competitive inhibition assay by immunoblot analysis. (A) The reactivity of DA6-7 with E protein was inhibited by phage clone DA6-7-C4. The reactivity of DA11-13 with E protein was inhibited by phage clones DA11-13-C2 (B) and DA11-13-C4 (C).
Detection of serum samples from DEN-infected patients by using immunopositive phage clones.
We tried to evaluate whether the phage-displayed epitopes could be used as a diagnostic tool in the detection of antibodies in serum samples from dengue patients. Six of eight serum samples from DEN-1-infected patients had positive ELISA antibody reactivity with DA6-7-C4 (Fig. 6C). Five of eight serum samples from DEN-1-infected patients had positive ELISA antibody reactivity with DA11-13-C1 (Fig. 6D). All serum samples obtained from DEN-2-infected patients were seronegative, and none of the control NHS samples had positive responses with these two phage clones (Fig. 6C and D).
FIG. 6.
(A and B) Capture ELISA for serum samples from patients with DEN-1 infection. Serum samples (200-fold dilution) from DEN-infected patients were analyzed. Representative data are shown to illustrate the MAb responses. All of the serum samples from DEN-1- and DEN-2-infected patients could be detected by DA6-7-captured DEN-1 (A) and DA11-13-captured DEN-1 (B). (C and D) ELISA reactivities of phage clones with serum samples (200-fold dilution) from DEN-infected patients. (C) Six of eight serum samples from DEN-1-infected patients could be identified by DA6-7-C4, but all serum samples from DEN-2-infected patients and NHS from healthy control subjects did not reveal such reactivity. (D) Five of eight serum samples from DEN-1-infected patients could be identified by DA11-13-C1, but eight serum samples from DEN-2-infected patients and NHS revealed no such reactivity. Cutoff values are represented by solid lines.
DISCUSSION
The identification of viral B-cell epitopes is important for the development of subunit vaccines and virus-specific serological diagnostic reagents as well as for studying virus-antibody interactions at the molecular level. In this study, we generated 12 MAbs, including two neutralizing antibodies against DEN-1. We also identified and characterized two neutralizing epitopes of DEN-1 by using a phage-displayed random peptide library. We found that the neutralizing MAb DA11-13-selected phage clones displayed 12-mer peptide sequences that had a consensus motif, S/TSL/I. The reactivities of the neutralizing MAbs with E proteins were inhibited markedly and dose dependently by the epitopes displayed by immunopositive phage clones (Fig. 5). Our data strongly suggest that the phage-displayed peptides are mimic epitopes of DA11-13 and DA6-7. We also used phage-displayed epitopes to detect antibodies in serum samples from DEN-infected patients (Fig. 6).
The similarities of amino acid sequences for the four serotypes of DEN range from 63.2 to 78.7% (58). The high similarity of amino acid sequences within the four serotypes of DEN makes them difficult to distinguish when using antigens obtained from overlapping synthetic peptides (1, 23, 48) or from recombinant or enzyme cleavage antigen fragments (38, 40, 54). Using epitope-based peptide antigens displayed on phage makes it easier to identify the antibodies in serum samples from DEN-1-infected patients. Furthermore, the serotype-specific epitopes are useful for differentiating between serum samples from DEN-1- and DEN-2-infected patients (Fig. 6). The sensitivities of ELISA for detecting serum samples from DEN-1-infected patients by using phage-displayed serotype-specific epitopes of DEN-1, namely, DA6-7-C4 and DA11-13-C1, were 75% (6/8) and 62.5% (5/8), respectively (Fig. 6C and D). Using the same serologic test, we found that serum samples from all eight healthy adults were seronegative, yielding a specificity of 100% for healthy donors for these two serotype-specific epitopes (Fig. 6C and D). The identification of more serotype-specific epitopes and combination of these epitope-based peptide antigens for serological diagnosis will increase the sensitivity of serotype-specific detection of DEN-infected patients.
Severe and sometimes fatal DHF and DSS often occur in regions where more than one DEN serotype is circulating (17, 19). It has been hypothesized that nonneutralizing cross-reactive antibodies acquired during the first DEN infection enhance a second infection by a different DEN serotype (19, 20). Two new dengue diagnostic tests, the MRL Diagnostics dengue fever virus IgM capture ELISA and the PanBio rapid immunochromatographic test, have been used to detect DEN antibodies. However, they cannot distinguish between the four serotypes of DEN and have 45 to 50% cross-reactivity with Japanese encephalitis virus-infected samples (29, 56, 57). In the present study, we generated serotype-specific MAbs recognizing DEN-1. We also identified the serotype-specific epitopes of DEN-1 and used epitope-based peptides as antigens to detect DEN-1 antibodies in serum samples from DEN-1-infected patients. Our serotype-specific MAbs and epitope-based peptide antigens may be useful in the development of diagnostic laboratory tests for DEN-1 infection.
Acknowledgments
This work was supported by grant NSC 95-2320-B-001-035 from the National Science Council, Taiwan, to H.-C.W.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Aaskov, J. G., H. M. Geysen, and T. J. Mason. 1989. Serologically defined linear epitopes in the envelope protein of dengue 2 (Jamaica strain 1409). Arch. Virol. 105:209-221. [DOI] [PubMed] [Google Scholar]
- 2.Arap, W., R. Pasqualini, and E. Ruoslahti. 1998. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377-380. [DOI] [PubMed] [Google Scholar]
- 3.Atwell, S., M. Ultsch, A. M. De Vos, and J. A. Wells. 1997. Structural plasticity in a remodeled protein-protein interface. Science 278:1125-1128. [DOI] [PubMed] [Google Scholar]
- 4.Barry, M. A., W. J. Dower, and S. A. Johnston. 1996. Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat. Med. 2:299-305. [DOI] [PubMed] [Google Scholar]
- 5.Bhakdi, S., and M. D. Kazatchkine. 1990. Pathogenesis of dengue: an alternative hypothesis. Southeast Asian J. Trop. Med. Public Health 21:652-657. [PubMed] [Google Scholar]
- 6.Bielefeldt-Ohmann, H. 1997. Pathogenesis of dengue virus diseases: missing pieces in the jigsaw. Trends Microbiol. 5:409-413. [DOI] [PubMed] [Google Scholar]
- 7.Bottger, V., A. Bottger, S. F. Howard, S. M. Picksley, P. Chene, C. Garcia-Echeverria, H. K. Hochkeppel, and D. P. Lane. 1996. Identification of novel mdm2 binding peptides by phage display. Oncogene 13:2141-2147. [PubMed] [Google Scholar]
- 8.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 9.Castano, A. R., S. Tangri, J. E. Miller, H. R. Holcombe, M. R. Jackson, W. D. Huse, M. Kronenberg, and P. A. Peterson. 1995. Peptide binding and presentation by mouse CD1. Science 269:223-226. [DOI] [PubMed] [Google Scholar]
- 10.DeLeo, F. R., L. Yu, J. B. Burritt, L. R. Loetterle, C. W. Bond, A. J. Jesaitis, and M. T. Quinn. 1995. Mapping sites of interaction of p47-phox and flavocytochrome b with random-sequence peptide phage display libraries. Proc. Natl. Acad. Sci. USA 92:7110-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Mello, F., C. D. Partidos, M. W. Steward, and C. R. Howard. 1997. Definition of the primary structure of hepatitis B virus (HBV) pre-S hepatocyte binding domain using random peptide libraries. Virology 237:319-326. [DOI] [PubMed] [Google Scholar]
- 12.Essler, M., and E. Ruoslahti. 2002. Molecular specialization of breast vasculature: a breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc. Natl. Acad. Sci. USA 99:2252-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folgori, A., R. Tafi, A. Meola, F. Felici, G. Galfre, R. Cortese, P. Monaci, and A. Nicosia. 1994. A general strategy to identify mimotopes of pathological antigens using only random peptide libraries and human sera. EMBO J. 13:2236-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu, Y., L. N. Shearing, S. Haynes, P. Crewther, L. Tilley, R. F. Anders, and M. Foley. 1997. Isolation from phage display libraries of single chain variable fragment antibodies that recognize conformational epitopes in the malaria vaccine candidate, apical membrane antigen-1. J. Biol. Chem. 272:25678-25684. [DOI] [PubMed] [Google Scholar]
- 15.Gagnon, S. J., F. A. Ennis, and A. L. Rothman. 1999. Bystander target cell lysis and cytokine production by dengue virus-specific human CD4(+) cytotoxic T-lymphocyte clones. J. Virol. 73:3623-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B. L. Innis, I. Kurane, A. L. Rothman, and F. A. Ennis. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755-762. [DOI] [PubMed] [Google Scholar]
- 17.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gubler, D. J., and G. G. Clark. 1995. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerg. Infect. Dis. 1:55-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 20.Halstead, S. B., C. N. Venkateshan, M. K. Gentry, and L. K. Larsen. 1984. Heterogeneity of infection enhancement of dengue 2 strains by monoclonal antibodies. J. Immunol. 132:1529-1532. [PubMed] [Google Scholar]
- 21.Henchal, E. A., M. K. Gentry, J. M. McCown, and W. E. Brandt. 1982. Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am. J. Trop. Med. Hyg. 31:830-836. [DOI] [PubMed] [Google Scholar]
- 22.Hiramatsu, K., M. Tadano, R. Men, and C. J. Lai. 1996. Mutational analysis of a neutralization epitope on the dengue type 2 virus (DEN2) envelope protein: monoclonal antibody resistant DEN2/DEN4 chimeras exhibit reduced mouse neurovirulence. Virology 224:437-445. [DOI] [PubMed] [Google Scholar]
- 23.Innis, B. L., V. Thirawuth, and C. Hemachudha. 1989. Identification of continuous epitopes of the envelope glycoprotein of dengue type 2 virus. Am. J. Trop. Med. Hyg. 40:676-687. [DOI] [PubMed] [Google Scholar]
- 24.King, C. A., J. S. Marshall, H. Alshurafa, and R. Anderson. 2000. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J. Virol. 74:7146-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 26.Koivunen, E., W. Arap, D. Rajotte, J. Lahdenranta, and R. Pasqualini. 1999. Identification of receptor ligands with phage display peptide libraries. J. Nucl. Med. 40:883-888. [PubMed] [Google Scholar]
- 27.Kraft, S., B. Diefenbach, R. Mehta, A. Jonczyk, G. A. Luckenbach, and S. L. Goodman. 1999. Definition of an unexpected ligand recognition motif for alphav beta6 integrin. J. Biol. Chem. 274:1979-1985. [DOI] [PubMed] [Google Scholar]
- 28.Kurane, I., B. L. Innis, A. Nisalak, C. Hoke, S. Nimmannitya, A. Meager, and F. A. Ennis. 1989. Human T cell responses to dengue virus antigens. Proliferative responses and interferon gamma production. J. Clin. Investig. 83:506-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lam, S. K., and P. L. Devine. 1998. Evaluation of capture ELISA and rapid immunochromatographic test for the determination of IgM and IgG antibodies produced during dengue infection. Clin. Diagn. Virol. 10:75-81. [DOI] [PubMed] [Google Scholar]
- 30.Lanciotti, R. S., C. H. Calisher, D. J. Gubler, G. J. Chang, and A. V. Vorndam. 1992. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee, T. Y., H. C. Wu, Y. L. Tseng, and C. T. Lin. 2004. A novel peptide specifically binding to nasopharyngeal carcinoma for targeted drug delivery. Cancer Res. 64:8002-8008. [DOI] [PubMed] [Google Scholar]
- 32.Leitmeyer, K. C., D. W. Vaughn, D. M. Watts, R. Salas, I. Villalobos, C. de Ramos, and R. Rico-Hesse. 1999. Dengue virus structural differences that correlate with pathogenesis. J. Virol. 73:4738-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, B., J. Y. Tom, D. Oare, R. Yen, W. J. Fairbrother, J. A. Wells, and B. C. Cunningham. 1995. Minimization of a polypeptide hormone. Science 270:1657-1660. [DOI] [PubMed] [Google Scholar]
- 34.Lin, B., C. R. Parrish, J. M. Murray, and P. J. Wright. 1994. Localization of a neutralizing epitope on the envelope protein of dengue virus type 2. Virology 202:885-890. [DOI] [PubMed] [Google Scholar]
- 35.Littaua, R., I. Kurane, and F. A. Ennis. 1990. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 144:3183-3186. [PubMed] [Google Scholar]
- 36.Liu, I. J., P. R. Hsueh, C. T. Lin, C. Y. Chiu, C. L. Kao, M. Y. Liao, and H. C. Wu. 2004. Disease-specific B cell epitopes for serum antibodies from patients with severe acute respiratory syndrome (SARS) and serologic detection of SARS antibodies by epitope-based peptide antigens. J. Infect. Dis. 190:797-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mady, B. J., D. V. Erbe, I. Kurane, M. W. Fanger, and F. A. Ennis. 1991. Antibody-dependent enhancement of dengue virus infection mediated by bispecific antibodies against cell surface molecules other than Fc gamma receptors. J. Immunol. 147:3139-3144. [PubMed] [Google Scholar]
- 38.Mason, P. W., M. U. Zugel, A. R. Semproni, M. J. Fournier, and T. L. Mason. 1990. The antigenic structure of dengue type 1 virus envelope and NS1 proteins expressed in Escherichia coli. J. Gen. Virol. 71:2107-2114. [DOI] [PubMed] [Google Scholar]
- 39.Mazzucchelli, L., J. B. Burritt, A. J. Jesaitis, A. Nusrat, T. W. Liang, A. T. Gewirtz, F. J. Schnell, and C. A. Parkos. 1999. Cell-specific peptide binding by human neutrophils. Blood 93:1738-1748. [PubMed] [Google Scholar]
- 40.Megret, F., J. P. Hugnot, A. Falconar, M. K. Gentry, D. M. Morens, J. M. Murray, J. J. Schlesinger, P. J. Wright, P. Young, M. H. Van Regenmortel, et al. 1992. Use of recombinant fusion proteins and monoclonal antibodies to define linear and discontinuous antigenic sites on the dengue virus envelope glycoprotein. Virology 187:480-491. [DOI] [PubMed] [Google Scholar]
- 41.Morens, D. M. 1994. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin. Infect. Dis. 19:500-512. [DOI] [PubMed] [Google Scholar]
- 42.Nord, K., E. Gunneriusson, J. Ringdahl, S. Stahl, M. Uhlen, and P. A. Nygren. 1997. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 15:772-777. [DOI] [PubMed] [Google Scholar]
- 43.Palmer, C. J., S. D. King, R. R. Cuadrado, E. Perez, M. Baum, and A. L. Ager. 1999. Evaluation of the MRL Diagnostics Dengue Fever Virus IgM Capture ELISA and the PanBio Rapid Immunochromatographic Test for diagnosis of dengue fever in Jamaica. J. Clin. Microbiol. 37:1600-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pasqualini, R., E. Koivunen, and E. Ruoslahti. 1995. A peptide isolated from phage display libraries is a structural and functional mimic of an RGD-binding site on integrins. J. Cell Biol. 130:1189-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasqualini, R., and E. Ruoslahti. 1996. Organ targeting in vivo using phage display peptide libraries. Nature 380:364-366. [DOI] [PubMed] [Google Scholar]
- 46.Prezzi, C., M. Nuzzo, A. Meola, P. Delmastro, G. Galfre, R. Cortese, A. Nicosia, and P. Monaci. 1996. Selection of antigenic and immunogenic mimics of hepatitis C virus using sera from patients. J. Immunol. 156:4504-4513. [PubMed] [Google Scholar]
- 47.Rico-Hesse, R., L. M. Harrison, R. A. Salas, D. Tovar, A. Nisalak, C. Ramos, J. Boshell, M. T. de Mesa, R. M. Nogueira, and A. T. da Rosa. 1997. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 230:244-251. [DOI] [PubMed] [Google Scholar]
- 48.Roehrig, J. T., R. A. Bolin, and R. G. Kelly. 1998. Monoclonal antibody mapping of the envelope glycoprotein of the dengue 2 virus, Jamaica. Virology 246:317-328. [DOI] [PubMed] [Google Scholar]
- 49.Rosen, L. 1977. The Emperor's New Clothes revisited, or reflections on the pathogenesis of dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 26:337-343. [DOI] [PubMed] [Google Scholar]
- 50.Rothman, A. L., and F. A. Ennis. 1999. Immunopathogenesis of dengue hemorrhagic fever. Virology 257:1-6. [DOI] [PubMed] [Google Scholar]
- 51.Sakuntabhai, A., C. Turbpaiboon, I. Casademont, A. Chuansumrit, T. Lowhnoo, A. Kajaste-Rudnitski, S. M. Kalayanarooj, K. Tangnararatchakit, N. Tangthawornchaikul, S. Vasanawathana, W. Chaiyaratana, P. T. Yenchitsomanus, P. Suriyaphol, P. Avirutnan, K. Chokephaibulkit, F. Matsuda, S. Yoksan, Y. Jacob, G. M. Lathrop, P. Malasit, P. Despres, and C. Julier. 2005. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 37:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott, J. K., and G. P. Smith. 1990. Searching for peptide ligands with an epitope library. Science 249:386-390. [DOI] [PubMed] [Google Scholar]
- 53.Smith, W. C., J. H. McDowell, D. R. Dugger, R. Miller, A. Arendt, M. P. Popp, and P. A. Hargrave. 1999. Identification of regions of arrestin that bind to rhodopsin. Biochemistry 38:2752-2761. [DOI] [PubMed] [Google Scholar]
- 54.Trirawatanapong, T., B. Chandran, R. Putnak, and R. Padmanabhan. 1992. Mapping of a region of dengue virus type-2 glycoprotein required for binding by a neutralizing monoclonal antibody. Gene 116:139-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaughn, D. W., S. Green, S. Kalayanarooj, B. L. Innis, S. Nimmannitya, S. Suntayakorn, T. P. Endy, B. Raengsakulrach, A. L. Rothman, F. A. Ennis, and A. Nisalak. 2000. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 181:2-9. [DOI] [PubMed] [Google Scholar]
- 56.Vaughn, D. W., A. Nisalak, S. Kalayanarooj, T. Solomon, N. M. Dung, A. Cuzzubbo, and P. L. Devine. 1998. Evaluation of a rapid immunochromatographic test for diagnosis of dengue virus infection. J. Clin. Microbiol. 36:234-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaughn, D. W., A. Nisalak, T. Solomon, S. Kalayanarooj, M. D. Nguyen, R. Kneen, A. Cuzzubbo, and P. L. Devine. 1999. Rapid serologic diagnosis of dengue virus infection using a commercial capture ELISA that distinguishes primary and secondary infections. Am. J. Trop. Med. Hyg. 60:693-698. [DOI] [PubMed] [Google Scholar]
- 58.Westaway, E. G., and J. Blok. 1997. Taxonomy and evolutionary relationships of flaviviruses. CAB International, New York, NY.
- 59.Wilder-Smith, A., and E. Schwartz. 2005. Dengue in travelers. N. Engl. J. Med. 353:924-932. [DOI] [PubMed] [Google Scholar]
- 60.Wu, H. C., Y. L. Huang, T. T. Chao, J. T. Jan, J. L. Huang, H. Y. Chiang, C. C. King, and M. F. Shaio. 2001. Identification of B-cell epitope of dengue virus type 1 and its application in diagnosis of patients. J. Clin. Microbiol. 39:977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, H. C., M. Y. Jung, C. Y. Chiu, T. T. Chao, S. C. Lai, J. T. Jan, and M. F. Shaio. 2003. Identification of a dengue virus type 2 (DEN-2) serotype-specific B-cell epitope and detection of DEN-2-immunized animal serum samples using an epitope-based peptide antigen. J. Gen. Virol. 84:2771-2779. [DOI] [PubMed] [Google Scholar]
- 62.Wu, H. C., C. T. Yeh, Y. L. Huang, L. J. Tarn, and C. C. Lung. 2001. Characterization of neutralizing antibodies and identification of neutralizing epitope mimics on the Clostridium botulinum neurotoxin type A. Appl. Environ. Microbiol. 67:3201-3207. [DOI] [PMC free article] [PubMed] [Google Scholar]