Abstract
Recent clinical studies have suggested that, for certain strains of influenza virus, intradermal (i.d.) delivery may enable protective immune responses using a lower dose of vaccine than required by intramuscular (i.m.) injection. Here, we describe the first preclinical use of microneedle technology for i.d. administration of three different types of influenza vaccines: (i) a whole inactivated influenza virus, (ii) a trivalent split-virion human vaccine, and (iii) a plasmid DNA encoding the influenza virus hemagglutinin. In a rat model, i.d. delivery of the whole inactivated virus provided up to 100-fold dose sparing compared to i.m. injection. In addition, i.d. delivery of the trivalent human vaccine enabled at least 10-fold dose sparing for the H1N1 strain and elicited levels of response across the dose range similar to those of i.m. injection for the H3N2 and B strains. Furthermore, at least fivefold dose sparing from i.d. delivery was evident in animals treated with multiple doses of DNA plasmid vaccine, although such effects were not apparent after the first immunization. Altogether, the results demonstrate that microneedle-based i.d. delivery elicits antibody responses that are at least as strong as via i.m. injection and that, in many cases, dose sparing can be achieved by this new immunization method.
The recent shortages in influenza vaccine availability have highlighted the need for new technologies to increase or extend the supply of vaccine (21, 22, 25). In the United States, most influenza vaccines are currently supplied in multidose vials. These vials are typically overfilled, in part, to accommodate the dead space volume contained within conventional needles and syringes. In this respect, the conversion to syringes with reduced waste space volume can enable up to 19% additional vaccine to be recovered from each multidose vial (39, 46). Conversion from multidose vials to single-dose prefilled syringes could also result in reduced vaccine wastage. Another approach includes the conversion to cell-derived vaccine manufacturing methods that are more efficient than traditional approaches involving viral propagation in chicken eggs (5, 19).
An alternative strategy has been to explore new vaccine delivery routes such as intradermal (i.d.) and intranasal. The skin, in particular, is a potent immunostimulatory tissue with an abundance of professional antigen-presenting cells (7, 43, 48). As such, i.d. delivery has been suggested as a means of “stretching” the available supply of influenza vaccine by eliciting a protective immune response using less vaccine per dose (31). Over the years, i.d. delivery has been investigated for rabies, hepatitis B, influenza, and other vaccines (6, 8, 10, 11, 13, 20, 24, 29, 30, 37, 40-42, 44, 50). In several cases, dose-sparing benefits from i.d. delivery have been reported. Intradermal delivery of influenza vaccine was investigated in humans in the late 1970s using monovalent and bivalent vaccine preparations (10, 20, 24). In these early studies, i.d. delivery of one-fifth the conventional dose was shown, in some cases, to elicit levels of immune response similar to those elicited by intramuscular (i.m.) or subcutaneous (s.c.) administration of the full dose. Similar dose-sparing benefits provided by i.d. delivery have been observed in more recent studies using current trivalent split antigen vaccines (8, 29). Additional clinical trials comparing i.m. and i.d. routes for influenza vaccines are in progress (information found at http://www.clinicaltrials.gov [accessed 14 September 2006]). Clinical studies are also under way to investigate whether adjuvants such as aluminum hydroxide and MF59 may increase the immune response to annual influenza vaccines as well as vaccines directed against H5N1 virus (9, 16, 23, 45). Finally, investigators are also pursuing DNA plasmids, viral vectors, and protein subunits as possible alternatives to conventional influenza vaccines based on split-virion preparations (17, 38, 47, 49).
One factor that has precluded the widespread adoption of the i.d. route is the difficulty associated with performing i.d. injections using conventional needles and syringes. We have undertaken development of microneedles, at least in part, to overcome such difficulties. We have incorporated microneedles into reliable and easy-to-use delivery devices and have used such devices for vaccine delivery (1, 2, 4, 15, 33-36). We previously demonstrated that microneedle-based i.d. administration induces stronger immune responses than conventional injection for anthrax and Japanese encephalitis vaccines in preclinical animal studies (15, 36). The potential advantages of microneedles for drug and vaccine delivery, as well as the challenges associated with commercialization of such technology, are reviewed elsewhere (35).
The mouse has been extensively used as an early preclinical model for influenza vaccine research and has been previously used for early stage testing of microneedles. When available, however, it is preferable to test microneedles in species with thicker skin so as to more reliably deposit the vaccine into the i.d. tissue space. Thus, we have chosen to conduct our initial studies on influenza vaccine in a rat model since rat skin is thicker than the total length of the microneedle (i.e., 1 mm) employed in these studies. This rat model was initially developed for respiratory immunotoxicology and influenza virus host resistance studies (12, 28, 32) and has been recently used for preclinical immunogenicity studies of an influenza vaccine nasally administered as a dry powder (27). The results of the current study show that microneedle-based i.d. delivery of viral and DNA-based influenza vaccines elicits antibody responses that are at least as strong as i.m. injection and that, in many cases, dose sparing can be attained.
MATERIALS AND METHODS
Animals.
Female Brown Norway rats (Charles River, Raleigh, NC) 7 to 10 weeks of age were housed at BD Technologies. Studies were conducted in accordance with U.S. Department of Agriculture and National Institutes of Health guidelines for the care and use of animals and under protocols approved by an Institutional Animal Care and Use Committee. Rats were anesthetized prior to dosing and blood sampling by intraperitoneal (i.p.) injection of a cocktail containing ketamine, acepromazine maleate, and xylazine (Butler, Columbus, OH) at a ratio of 30:1:1.
Influenza virus vaccines.
Influenza virus A/Puerto Rico/8/34 (H1N1), produced by Charles River SPAFAS (North Franklin, CT), was propagated in chicken eggs, purified via sucrose gradient, and inactivated by formalin prior to suspension in HEPES-saline. The commercial human vaccine (Fluzone 2003-2004 formulation, Sanofi-Pasteur, Swiftwater, PA) contained an H1N1 strain (A/New Caladonia/20/99), an H3N2 strain (A/Panama/2007/99), and a B strain (B/Hong Kong/1434/2002).
Preparation of plasmid DNA.
Plasmid DNA, driven by cytomegalovirus (CMV) promoter and expressing firefly luciferase or β-galactosidase (hereafter referred to as pCMV-Luc and pCMV-β, respectively) or the hemagglutinin (HA) of influenza A/Puerto Rico/8/34 (originally provided by Hariet Robinson, Emory University, Atlanta, GA, and hereafter referred to as pCMV-HA), was produced by Aldevron (Fargo, ND) and supplied suspended in phosphate-buffered saline.
Injections.
Hair was first removed from the i.d. and i.m. injection sites with electric clippers. For i.d. delivery, rats were injected on the lower dorsal surface using a 1-mm-long, 34-gauge (Ga) stainless steel microneedle (15, 34, 36) fitted to a 1-ml syringe (BD, Franklin Lakes, NJ) and inserted perpendicularly into the skin to control delivery depth (3). Intramuscular injections employed a 30-Ga needle (1/2 in. in length) and 1-ml syringe (BD) and were given into the quadriceps muscle.
Reporter gene expression.
Rats (n = 4 per group) were administered i.d. or i.m. injections of 50 μg or 5 μg of reporter plasmid DNA (pCMV-Luc) suspended in 50 μl phosphate-buffered saline. Twenty-four hours postdelivery, rats were euthanized and tissues of the delivery sites (muscle or skin) were excised. For i.m. sites, the entire quadriceps muscle was removed and processed. Skin injection sites were marked with an indelible marking pen for identification at the time of tissue collection. These skin sites were approximately 1 cm in diameter. We did not normalize based on total protein content or tissue weight due to the substantially higher protein content and weight of recovered muscle as compared to skin. Such normalization would artificially reduce the calculated relative light unit (RLU)/mg values from i.m.-treated groups as compared to i.d.-treated groups. Rather, we normalized based on luciferase background signal in tissue (muscle or skin) collected from sites injected with an unrelated plasmid (pCMV-β) on the contralateral side of the same animal. The negative control tissue was of similar size to tissue collected from sites injected with pCMV-Luc and was processed in the same manner. In addition, muscle and skin samples were homogenized in an equivalent volume (900 μl) of passive lysis buffer (Promega, Madison, WI) and an equivalent volume of supernatant (20 μl) was assayed from each sample. Homogenates were prepared using a hand-held homogenizer (Biospec Products, Bartlesville, OK) and then were centrifuged, and supernatants were assayed for luciferase activity using a luciferase assay kit (Promega, Madison, WI) and tube luminometer instrument (Analytical Luminescence Laboratory, San Diego, CA). Luciferase activity was recorded as mean increase in RLU over background signal from sites injected with the unrelated pCMV-β plasmid.
Immunizations.
For studies using whole, inactivated influenza virus, rats (n = 4 per group) were injected i.d. or i.m. on days 0, 21, and 42 with 1, 0.1, or 0.01 μg of vaccine (dosage levels based on total protein content in the viral suspension) in an injection volume of 50 μl. For studies using the human trivalent split-virion vaccine, rats (n = 10 per group) were given a single i.d. or i.m. injection with 100 μl of either undiluted vaccine (“high dose”) or vaccine diluted 1:10 (“low dose”). For DNA immunization, rats (n = 5 per group) were immunized by either i.d. or i.m. injection of 50, 10, 5, or 1 μg pCMV-HA in a total volume of 50 μl on days 0, 21, and 42.
Influenza virus ELISA.
For the influenza virus enzyme-linked immunosorbent assay (ELISA), blood was collected, under anesthesia, via the retro-orbital sinus. Serially diluted serum samples were added to 96-well MaxiSorp plates (Nalge Nunc, Rochester, NY) coated with 1 μg/ml of either whole inactivated influenza virus A/Puerto Rico/8/34, A/New Caladonia/20/99, or A/Panama/2007/99 (Biodesign International, Saco, ME) or recombinantly derived HA from influenza virus B/Hong Kong/1434/2002 (Protein Sciences Corp., Meriden, CT) in carbonate coating buffer (Sigma, St. Louis, MO). Bound antibody was detected using horseradish peroxidase-labeled secondary antibody (Southern Biotech, Birmingham, AL) followed by addition of tetramethylbenzidine substrate (Sigma). Absorbance readings for each sample (optical density at 450 nm) were obtained using a plate reader (Tecan USA, Research Triangle Park, NC) following the addition of 0.5 M H2SO4 (Sigma) to each well. Endpoint serum antibody titers were calculated as the highest serum dilution producing an absorbance reading at least 3 times that of the equivalent dilution of naive control serum.
HAI assay.
The hemagglutination inhibition (HAI) assay was performed as described in the latest version of Current Protocols in Immunology (14). Briefly, sera were inactivated at 56°C for 10 min to destroy complement and HAI inhibitors. Samples (25 μl per well) were then added in serial dilution to wells containing 25 μl of 4 hemagglutinating units of either A/New Caladonia/20/99 or A/Panama/2007/99 whole inactivated influenza virus or recombinantly derived HA from influenza B/Hong Kong/1434/2002. Chicken red blood cells (50 μl of 0.5% cells) were then added and incubated at room temperature for 1 h. Endpoint HAI titers were defined as the reciprocal of the highest serum dilution that completely inhibited hemagglutination of the red blood cells.
Statistics.
Statistical analysis was performed with an analysis of variance model including dose, delivery route, and bleed (i.e., time point) and their interactions. ELISA titer values were natural logarithm transformed to improve normality. Contrasts were performed to characterize the nature of the factor effects, with a Bonferroni adjustment used to correct for multiple comparisons. In order to assess the repeated measures nature of the study, separate analysis of variance models including dose and delivery route were fit to data from the three bleeds to confirm that overall trends held for subsets of uncorrelated observations.
RESULTS
Microneedles.
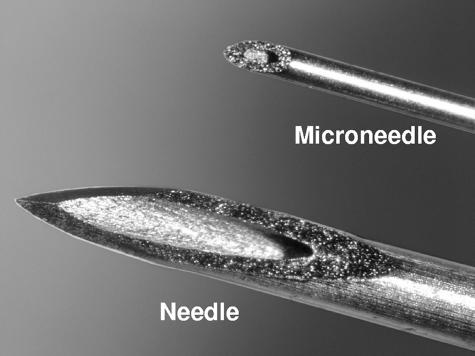
Figure 1 displays a 34-Ga, stainless steel microneedle as compared to a standard 27-Ga needle. The microneedles used to immunize rats in this study had an inner diameter of 76 μm, an outer diameter of 178 μm, and an exposed length of 1 mm. Microneedles were affixed to a conventional syringe and used according to a method whereby the microneedle is inserted perpendicularly to the skin surface to its full exposed length in order to control the dermal penetration depth (3).
FIG. 1.
Comparison of standard 27-Ga needle to 34-Ga microneedle. The displayed microneedle has an inner diameter of 76 μm, an outer diameter of 178 μm, and a total exposed length of 1.0 mm.
Immune response to whole inactivated influenza virus.
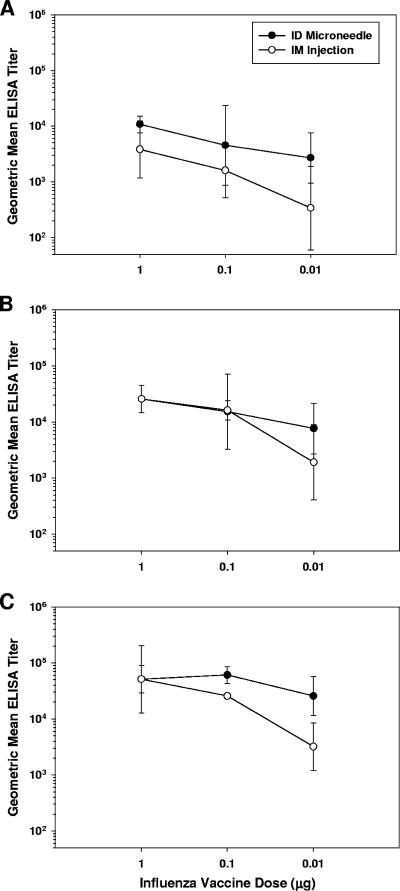
Groups of rats were immunized with graded doses of whole inactivated influenza virus A/Puerto Rico/8/34 via microneedle-based i.d. delivery or i.m. injection using a conventional needle. Serum antibody responses were measured by ELISA after one, two, or three inoculations of vaccine (Fig. 2A, B, and C, respectively). Intradermal delivery stimulated responses that were comparable to or greater than those elicited by i.m. injection at all doses and time points investigated. Notably, there was a less marked reduction in titer as the vaccine dose was reduced from 1 μg to 0.01 μg for the i.d. groups relative to the i.m. groups. After a single injection, the i.d.-induced geometric mean titer (GMT) dropped 4-fold across the dose range, while the corresponding i.m.-induced GMT dropped 11-fold (Fig. 2A). Similar results were also apparent after boosting; GMT elicited by i.d. injection dropped two- to threefold as the dose was reduced, while i.m.-induced GMT fell by 13- to 16-fold (Fig. 2B and C). At the completion of the study, i.d. delivery of 0.01 μg of vaccine stimulated antibody titers (GMT of 25,600) at levels not significantly different (P = 0.99) from those elicited using 100-fold-more vaccine (GMT of 51,200), while in contrast, the corresponding responses induced by i.m. injection differed significantly (P = 0.002) across this dose range (GMT at 0.01-μg dose of 3,200; GMT at 1-μg dose of 51,200) (Fig. 2C).
FIG. 2.
Serum antibody response in rats (n = 4 per group) following immunization with 1, 0.1, or 0.01 μg of whole inactivated influenza virus A/PR/8/34 on days 0, 21, and 42. Influenza virus-specific antibodies were measured by ELISA on (A) day 21, (B) day 42, and (C) day 56. Displayed are ELISA GMTs ± standard deviation.
Immune response to trivalent split-virion vaccine.
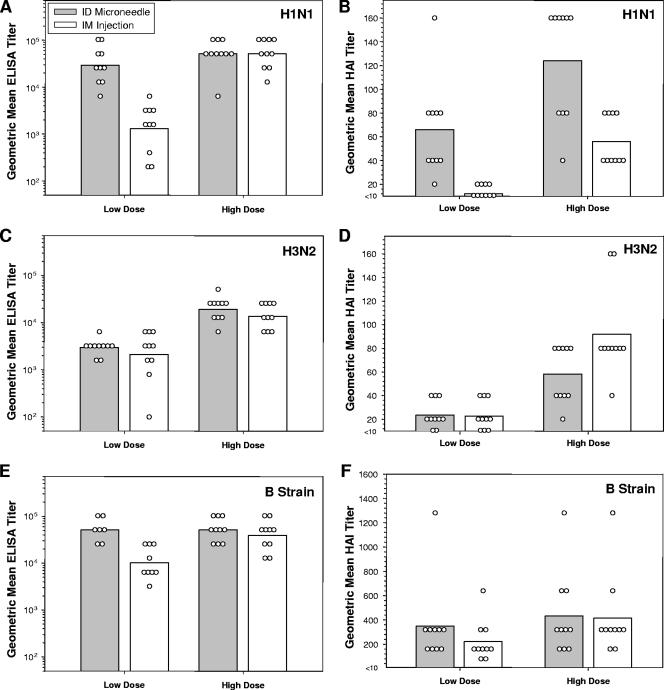
Currently approved influenza vaccines for human use consist of a trivalent split-virion preparation containing 15 μg HA from each of the three strains represented in the formulation (H1N1, H3N2, and B strains). Antibody responses to this vaccine are typically measured by HAI assay, in which a reciprocal titer of 40 is considered to be protective in humans. To compare microneedle-based i.d. delivery and i.m. injection, rats were given a single dose of the 2003-2004 Fluzone vaccine and monitored for serum antibody responses against each strain by ELISA and HAI assay 21 days after immunization. Rats were immunized with either a “high dose” (100 μl of undiluted vaccine) or a “low dose” (100 μl of vaccine diluted 1:10) of vaccine. Intradermally induced antibody titers against the H1N1 strain dropped less markedly as the dose was reduced than the corresponding titers elicited by i.m. injection (Fig. 3A and B). Responses induced by i.d. delivery of a low dose of vaccine were not significantly different in magnitude from the corresponding responses elicited by either i.d. or i.m. injection of a high dose, as measured both by ELISA (Fig. 3A; P = 0.19) and HAI assay (Fig. 3B; P = 0.85). All animals immunized with a high dose of vaccine responded with HAI titers against the H1N1 strain of at least 40 regardless of the method of administration. In contrast, none (0/10) of the rats immunized with the low dose of vaccine by i.m. injection raised an HAI titer against the H1N1 strain of at least 40, while 90% (9/10) of rats immunized with the low dose of vaccine by i.d. delivery responded with an HAI titer of at least 40 (Fig. 3B).
FIG. 3.
Rats (n = 10 per group) were immunized with either a high dose (100 μl of undiluted vaccine) or a low dose (100 μl of vaccine diluted 1:10) of trivalent split-virion influenza vaccine (Fluzone 2003-2004 formulation) and analyzed for serum antibody response 21 days later. Bars represent group GMT, and open symbols represent the responses from individual animals. (A) ELISA response against the H1N1 (A/NC/20/99) strain. (B) HAI response against the H1N1 (A/NC/20/99) strain. (C) ELISA response against the H3N2 (A/Pan/2007/99) strain. (D) HAI response against the H3N2 (A/Pan/2007/99) strain. (E) ELISA response against the B (B/HK/1434/02) strain. Due to insufficient quantities of sera from some animals, for analysis of ELISA titers against the B strain, only seven individuals from the i.d. “low-dose” group and nine animals from the i.m. “low-dose” group were analyzed. (F) HAI response against the B (B/HK/1434/02) strain.
There were less marked differences between i.d.- and i.m.-induced titers raised against the H3N2 and B strains (Fig. 3C to F). The responses against the H3N2 strain dropped to a similar extent in both i.d. and i.m. groups as the dose was reduced 10-fold, and response levels were similar between i.d. and i.m. routes at each dosage level (Fig. 3C and D). There was some evidence of i.d.-enabled dose sparing for the B strain by ELISA (Fig. 3E), although such an effect was not evident by the HAI assay, in which results were more variable (Fig. 3F).
Reporter gene expression.
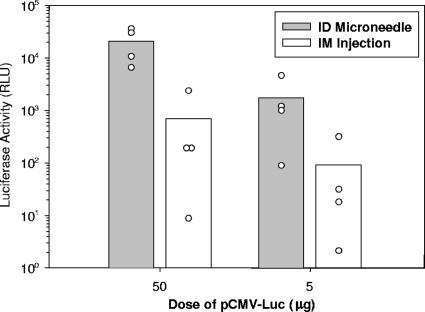
In order to examine the feasibility of using microneedles to administer plasmid DNA, we initially compared levels of reporter gene expression following i.d. or i.m. injection of a luciferase-encoding plasmid (Fig. 4). Mean relative levels of luciferase activity in skin following i.d. delivery of 50 μg of pCMV-Luc were approximately 30-fold greater than corresponding reporter gene activity in muscle following i.m. injection. A similar enhancement was also observed at the lower 5-μg dosage level, with i.d. delivery inducing 20-fold-greater relative luciferase activity than i.m. injection (Fig. 4).
FIG. 4.
Luciferase activity in skin or muscle following i.d. or i.m. administration of either 50 μg or 5 μg of pCMV-Luc (n = 4 rats per group). As a negative control, rats were injected with the unrelated reporter plasmid, pCMV-β. Tissues were collected 24 h postdelivery and analyzed by luciferase assay for reporter gene expression. Luciferase activity is expressed as mean increase in RLU over background luciferase activity from tissue sites that had been injected with pCMV-β. Bars represent group means, and open symbols represent responses from individual animals.
Immune response to DNA vaccine.
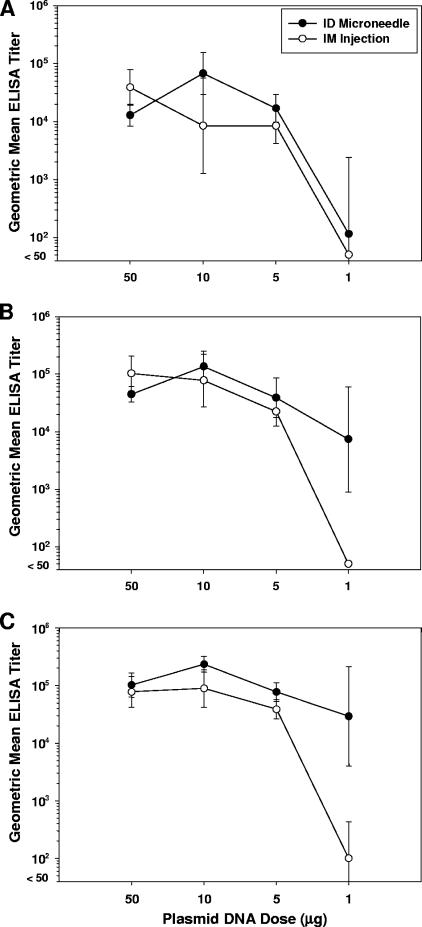
To examine whether greater plasmid gene expression translates into stronger immune responses, rats were immunized with graded doses of plasmid DNA encoding the HA of the influenza A/Puerto Rico/8/34 strain (pCMV-HA) by microneedle-based i.d. delivery or i.m. injection. Similar dose-response curves between i.d. and i.m. groups were observed after a single dose of DNA vaccine (Fig. 5A). ELISA titers remained elevated as the dose was reduced from 50 μg to 5 μg and then dropped precipitously as the dose of plasmid was further reduced to 1 μg. After boosting, however, dose-sparing benefits from i.d. delivery were apparent (Fig. 5B and C). Although titers were similar for i.d. and i.m. groups across the 50-μg to 5-μg dose range, titers dropped much less dramatically in the i.d. group as the dose was further reduced to 1 μg. The GMT for the i.m. group treated with two 1-μg doses of DNA plasmid was below the limit of detection (<50; Fig. 5B), with none of the animals (0/5) responding. In contrast, all of the animals (5/5) in the i.d. group responded with titers ranging from 800 to 51,200 (GMT = 7,352; Fig. 5B). Similar results were apparent after a third dose of vaccine; i.d.-induced GMT dropped 3-fold as the dose was reduced from 5 μg to 1 μg, while corresponding GMT elicited by i.m. injection dropped over 350-fold as the dose was reduced (Fig. 5C). Antibody titers from individual animals immunized by the i.d. route with three doses of 1 μg of DNA ranged from 3,200 to 409,600 (GMT = 29,407; Fig. 5C), while titers from the corresponding i.m. group ranged from <50 to 800 (GMT = 100; Fig. 5C).
FIG. 5.
Serum antibody response in rats (n = 5 per group) following immunization with 50, 10, 5, or 1 μg of pCMV-HA on days 0, 21, and 42. Antibodies against whole inactivated influenza virus A/PR/8/34 were measured by ELISA on (A) day 21, (B) day 42, and (C) day 56. Displayed are ELISA GMTs ± standard deviation.
DISCUSSION
Means of extending the available supply of influenza vaccine are of great interest given the recent shortages of vaccine availability (21, 22, 25) and the looming threat of a new influenza pandemic caused by the avian influenza virus (26, 51). The potential to spare dose by i.d. administration was suggested over 25 years ago (10, 20, 24) and has received renewed attention recently (8, 29, 31). However, the lack of a reproducible and easy-to-use i.d. delivery system has prevented the widespread conversion to this promising alternate mode of administration. Microneedles, when incorporated into a familiar and easy-to-use delivery platform such as the syringe, may offer a means to overcome these difficulties and render the i.d. delivery route as a preferred method of vaccination. In so doing, it may be possible to fully exploit the immunological benefits of vaccination via the upper layers of the skin.
Previous studies compared i.d. delivery of a low dose (e.g., 0.1 ml) of influenza vaccine to i.m. or subcutaneous injection of a high dose (e.g., 0.5 ml), but did not conduct direct head-to-head comparisons of the two methods of delivery at the lower dosage level (6, 8, 10, 20, 24, 29). Here, we conducted dose titration studies in animals in which i.m. delivery and i.d. delivery were directly compared using both viral and DNA-based vaccines. Dose sparing from i.d. delivery was evident for both types of viral vaccines (i.e., whole inactivated influenza virus and trivalent split virion) and the DNA plasmid construct. The extent of dose sparing, however, varied according to the vaccine employed and the strain of influenza virus. For example, while dose sparing was evident for the H1N1 strain in the trivalent vaccine, such an effect was not observed for the H3N2 and B strains (Fig. 3). There were some differences in responses observable by HAI assay as compared to ELISA (Fig. 3). We cannot rule out the possibility that such differences may have been due to residual nonspecific hemagglutination inhibitors present in the serum. Nonetheless, the dose-sparing effects from i.d. delivery against the H1N1 strain were apparent by both assays. Numerous clinical trials comparing i.d. administration and i.m. administration of influenza vaccines (both annual trivalent preparations and H5N1 strains) are in progress (information found at http://www.clinicaltrials.gov [accessed 14 September 2006]). Additional clinical testing of microneedles will be required in order to determine the extent to which microneedle-based i.d. delivery can spare dose in humans.
Previous studies using the gene gun for epidermal powder immunization have suggested a Th2 bias by this form of cutaneous delivery, while standard needles used for i.d. or i.m. delivery were reported to induce a predominantly Th1 response (18). In contrast, we have not observed such a bias from microneedle-based epidermal or dermal delivery for DNA or protein subunit vaccines (33, 34). For example, we previously showed that microneedle-based cutaneous delivery of a DNA plasmid encoding the hepatitis B surface antigen induced a mixed and balanced immunoglobulin G1/immunoglobulin G2a response in mice that was similar to that induced by conventional i.m. injection using a standard needle (33). The present study further demonstrates that microneedle-based i.d. delivery can induce HAI antibodies which are thought to be important for protection against influenza virus infection. Similarly, we previously showed that cutaneous delivery of a flavivirus vaccine using microneedles induces protective levels of virus-neutralizing antibodies in nonhuman primates (15). Additional studies are required in order to further reconcile the potential differences in Th1/Th2 induction following cutaneous delivery using microneedles compared to other methods.
Belshe et al. reported that i.d. delivery of a low dose of influenza vaccine induces slightly lower responses than i.m. injection of the full dose in subjects >60 years of age; however, seroprotection rates were still very high, ranging from 93 to 100% following i.d. vaccination (8). In a recent clinical study conducted in Thailand, it was reported that i.d. delivery of one-fifth the conventional dose elicited weaker responses than i.m. injection of the full dose in healthy young adults (6). Nonetheless, response levels from i.d. delivery exceeded levels required for influenza vaccine licensure by the European Committee for Proprietary Medicinal Products. One factor that may influence the magnitude of the i.d.-enabled dose-sparing effect is the level of preexisting antibodies at the time of immunization (6, 10, 24). Thus, it will be of interest to examine microneedle delivery systems in various age groups and in subjects both with and without preexisting antibodies against the vaccine strains. The successful clinical and commercial development of a microneedle-based i.d. delivery system for influenza vaccine has the potential to be of significant benefit to annual influenza vaccination programs as well as emergency vaccination against new emerging pandemic strains.
Acknowledgments
We thank Hariet Robinson of Emory University for providing pCMV-HA, Pat McCutchen for administrative assistance in preparing the manuscript, M. Ishaq Haider and Tommy Robinson for device fabrication, Richard Clarke for digital photography and image processing, and Joanne Huang for assay development and critical review of the manuscript. We also thank Elaine McVey and Perry Haaland for statistical analyses.
Footnotes
Published ahead of print on 28 February 2007.
REFERENCES
- 1.Alchas, P. G. December 2002. Intradermal delivery device including a needle assembly. U.S. patent 6,494,865.
- 2.Alchas, P. G., C. E. Guillermo, and M. S. Korisch. May 2003. Prefillable intradermal injector. U.S. patent 6,569,123.
- 3.Alchas, P. G., and P. Laurent. May 2003. Method of intradermally injecting substances. U.S. patent 6,569,143.
- 4.Alchas, P. G., P. Laurent, C. E. Guillermo, and M. S. Korisch. January 2005. Intradermal needle. U.S. patent 6,843,781.
- 5.Audsley, J. M., and G. A. Tannock. 2004. The role of cell culture vaccines in the control of the next influenza pandemic. Exp. Opin. Biol. Ther. 4:709-717. [DOI] [PubMed] [Google Scholar]
- 6.Auewarakul, P., U. Kositanont, P. Sornsathapornkul, P. Tothong, R. Kanyok, and P. Thongcharoen. 2007. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 25:659-663. [DOI] [PubMed] [Google Scholar]
- 7.Babiuk, S., M. Baca-Estrada, L. A. Babiuk, C. Ewen, and M. Foldvari. 2000. Cutaneous vaccination: the skin as an immunologically active tissue and the challenge of antigen delivery. J. Control. Rel. 66:199-214. [DOI] [PubMed] [Google Scholar]
- 8.Belshe, R. B., F. K. Newman, J. Cannon, C. Duane, J. Treanor, C. Van Hoecke, B. J. Howe, and G. Dubin. 2004. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 351:2286-2294. [DOI] [PubMed] [Google Scholar]
- 9.Bresson, J. L., C. Perronne, O. Launay, C. Gerdil, M. Saville, J. Wood, K. Hoschler, and M. C. Zambon. 2006. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367:1657-1664. [DOI] [PubMed] [Google Scholar]
- 10.Brown, H., J. A. Kasel, D. M. Freeman, L. D. Moise, N. P. Grose, and R. B. Couch. 1977. The immunizing effect of influenza A/New Jersey/76 (Hsw1N1) virus vaccine administered intradermally and intramuscularly to adults. J. Infect. Dis. 136(Suppl.):S466-S471. [DOI] [PubMed] [Google Scholar]
- 11.Bryan, J. P., M. H. Sjogren, P. Macarthy, E. Cox, L. J. Legters, and P. L. Perine. 1992. Persistence of antibody to hepatitis B surface antigen after low-dose, intradermal hepatitis B immunization and response to a booster dose. Vaccine 10:33-38. [DOI] [PubMed] [Google Scholar]
- 12.Burleson, G. R. 2000. Models of respiratory immunotoxicology and host resistance. Immunopharmacology 48:315-318. [DOI] [PubMed] [Google Scholar]
- 13.Carcaboso, A. M., R. M. Hernandez, M. Igartua, J. E. Rosas, M. E. Patarroyo, and J. L. Pedraz. 2004. Enhancing immunogenicity and reducing dose of microparticulated synthetic vaccines: single intradermal administration. Pharmacol. Res. 21:121-126. [DOI] [PubMed] [Google Scholar]
- 14.Cottey, R., C. A. Rowe, and B. S. Bender. 2006. Influenza virus. Hemagglutination inhibition assay (HAI) using mouse serum, p. 19.11.19-19.11.20. In R. Cioco (ed.), Current protocols in immunology. Wiley Press, New York, NY. [DOI] [PubMed]
- 15.Dean, C. H., J. B. Alarcon, A. M. Waterston, K. Draper, R. Early, F. Guirakhoo, T. P. Monath, and J. A. Mikszta. 2005. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax-JE) in non-human primates. Hum. Vaccines 1:106-111. [DOI] [PubMed] [Google Scholar]
- 16.Del Giudice, G., A. K. Hilbert, R. Bugarini, A. Minutello, O. Popova, D. Toneatto, I. Schoendorf, A. Borkowski, R. Rappuoli, and A. Podda. 2006. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine 24:3063-3065. [DOI] [PubMed] [Google Scholar]
- 17.Drape, R. J., M. D. Macklin, L. J. Barr, S. Jones, J. R. Haynes, and H. J. Dean. 2006. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 24:4475-4481. [DOI] [PubMed] [Google Scholar]
- 18.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 19.Halperin, S. A., B. Smith, T. Mabrouk, M. Germain, P. Trepanier, T. Hassell, J. Treanor, R. Gauthier, and E. L. Mills. 2002. Safety and immunogenicity of a trivalent, inactivated, mammalian cell culture-derived influenza vaccine in healthy adults, seniors, and children. Vaccine 20:1240-1247. [DOI] [PubMed] [Google Scholar]
- 20.Halperin, W., W. I. Weiss, R. Altman, M. A. Diamond, K. J. Black, A. W. Iaci, H. C. Black, and M. Goldfield. 1979. A comparison of the intradermal and subcutaneous routes of influenza vaccination with A/New Jersey/76 (swine flu) and A/Victoria/75: report of a study and review of the literature. Am. J. Public Health 69:1247-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper, S. A., K. Fukuda, T. M. Uyeki, N. J. Cox, and C. B. Bridges. 2005. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 54:1-40. [PubMed] [Google Scholar]
- 22.Harper, S. A., K. Fukuda, T. M. Uyeki, N. J. Cox, and C. B. Bridges. 2004. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 53:1-40. [PubMed] [Google Scholar]
- 23.Hehme, N., H. Engelmann, W. Kuenzel, E. Neumeier, and R. Saenger. 2004. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 103:163-171. [DOI] [PubMed] [Google Scholar]
- 24.Herbert, F. A., R. P. Larke, and E. L. Markstad. 1979. Comparison of responses to influenza A/New Jersey/76-A/Victoria/75 virus vaccine administered intradermally or subcutaneously to adults with chronic respiratory disease. J. Infect. Dis. 140:234-238. [DOI] [PubMed] [Google Scholar]
- 25.Hinman, A. R., W. A. Orenstein, J. M. Santoli, L. E. Rodewald, and S. L. Cochi. 2006. Vaccine shortages: history, impact, and prospects for the future. Annu. Rev. Public Health 27:235-259. [DOI] [PubMed] [Google Scholar]
- 26.Horimoto, T., and Y. Kawaoka. 2005. Influenza: lessons from past pandemics, warnings from current incidents. Nat. Rev. Microbiol. 3:591-600. [DOI] [PubMed] [Google Scholar]
- 27.Huang, J., R. J. Garmise, T. M. Crowder, K. Mar, C. R. Hwang, A. J. Hickey, J. A. Mikszta, and V. J. Sullivan. 2004. A novel dry powder influenza vaccine and intranasal delivery technology: induction of systemic and mucosal immune responses in rats. Vaccine 23:794-801. [DOI] [PubMed] [Google Scholar]
- 28.Ibanes, J. D., K. T. Morgan, and G. R. Burleson. 1996. Histopathological changes in the upper respiratory tract of F344 rats following infection with a rat-adapted influenza virus. Vet. Pathol. 33:412-418. [DOI] [PubMed] [Google Scholar]
- 29.Kenney, R. T., S. A. Frech, L. R. Muenz, C. P. Villar, and G. M. Glenn. 2004. Dose sparing with intradermal injection of influenza vaccine. N. Engl. J. Med. 351:2295-2301. [DOI] [PubMed] [Google Scholar]
- 30.Kurugol, Z., S. Erensoy, S. Aksit, A. Egemen, and A. Bilgic. 2001. Low-dose intradermal administration of recombinant hepatitis B vaccine in children: 5-year follow-up study. Vaccine 19:3936-3939. [DOI] [PubMed] [Google Scholar]
- 31.La Montagne, J. R., and A. S. Fauci. 2004. Intradermal influenza vaccination—can less be more? N. Engl. J. Med. 351:2330-2332. [DOI] [PubMed] [Google Scholar]
- 32.Lebrec, H., K. Sarlo, and G. R. Burleson. 1996. Effect of influenza virus infection on ovalbumin-specific IgE responses to inhaled antigen in the rat. J. Toxicol. Environ. Health 49:619-630. [DOI] [PubMed] [Google Scholar]
- 33.Mikszta, J. A., J. B. Alarcon, J. M. Brittingham, D. E. Sutter, R. J. Pettis, and N. G. Harvey. 2002. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 8:415-419. [DOI] [PubMed] [Google Scholar]
- 34.Mikszta, J. A., J. P. Dekker III, N. G. Harvey, C. H. Dean, J. M. Brittingham, J. Huang, V. J. Sullivan, B. Dyas, C. J. Roy, and R. G. Ulrich. 2006. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect. Immun. 74:6806-6810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikszta, J. A., M. I. Haider, and R. J. Pettis. 2006. Microneedles for drug and vaccine delivery: when will the dream become a reality? p. 309-325. In J. Wille (ed.), Skin delivery systems. Transdermals, dermatologicals, and cosmetic actives. Blackwell Press, Ames, IA.
- 36.Mikszta, J. A., V. J. Sullivan, C. Dean, A. M. Waterston, J. B. Alarcon, J. P. Dekker, J. M. Brittingham, J. Huang, C. R. Hwang, M. Ferriter, G. Jiang, K. Mar, K. U. Saikh, B. G. Stiles, C. J. Roy, R. G. Ulrich, and N. G. Harvey. 2005. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J. Infect. Dis. 191:278-288. [DOI] [PubMed] [Google Scholar]
- 37.Nagafuchi, S., S. Kashiwagi, S. Imayama, J. Hayashi, and Y. Niho. 1998. Intradermal administration of viral vaccines. Rev. Med. Virol. 8:97-111. [DOI] [PubMed] [Google Scholar]
- 38.Neirynck, S., T. Deroo, X. Saelens, P. Vanlandschoot, W. M. Jou, and W. Fiers. 1999. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat. Med. 5:1157-1163. [DOI] [PubMed] [Google Scholar]
- 39.Nelson, C. M., A. Sutanto, and I. G. P. Suradana. 1999. Use of SoloShot autodestruct syringes compared with disposable syringes, in a national immunization campaign in Indonesia. Bull. W. H. O. 77:29-33. [PMC free article] [PubMed] [Google Scholar]
- 40.Pancharoen, C., J. Mekmullica, U. Thisyakorn, S. Kasempimolporn, H. Wilde, and C. Herzog. 2005. Reduced-dose intradermal vaccination against hepatitis A with an aluminum-free vaccine is immunogenic and can lower costs. Clin. Infect. Dis. 41:1537-1540. [DOI] [PubMed] [Google Scholar]
- 41.Playford, E. G., P. G. Hogan, A. S. Bansal, K. Harrison, D. Drummond, D. F. M. Looke, and M. Whitby. 2002. Intradermal recombinant hepatitis B vaccine for healthcare workers who fail to respond to intramuscular vaccine. Infect. Control Hosp. Epidemiol. 23:87-90. [DOI] [PubMed] [Google Scholar]
- 42.Propst, T., A. Propst, K. Lhotta, W. Vogel, and P. Konig. 1998. Reinforced intradermal hepatitis B vaccination in hemodialysis patients is superior in antibody response to intramuscular or subcutaneous vaccination. Am. J. Kidney Dis. 32:1041-1045. [DOI] [PubMed] [Google Scholar]
- 43.Randolph, G. J., V. Angeli, and M. A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5:617-628. [DOI] [PubMed] [Google Scholar]
- 44.Redfield, R. R., B. L. Innis, R. M. Scott, H. G. Cannon, and W. H. Bancroft. 1985. Clinical evaluation of low-dose intradermally administered hepatitis B virus vaccine. A cost reduction strategy. JAMA 254:3203-3206. [PubMed] [Google Scholar]
- 45.Stephenson, I., K. G. Nicholson, A. Colegate, A. Podda, J. Wood, E. Ypma, and M. Zambon. 2003. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 21:1687-1693. [DOI] [PubMed] [Google Scholar]
- 46.Strauss, K., A. van Zundert, A. Frid, and V. Costigliola. 2006. Pandemic influenza preparedness: the critical role of the syringe. Vaccine 24:4874-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ulmer, J. B., U. Valley, and R. Rappuoli. 2006. Vaccine manufacturing: challenges and solutions. Nat. Biotechnol. 24:1377-1383. [DOI] [PubMed] [Google Scholar]
- 48.Valladeau, J., and S. Saeland. 2005. Cutaneous dendritic cells. Semin. Immunol. 17:273-283. [DOI] [PubMed] [Google Scholar]
- 49.Wang, S., J. Taaffe, C. Parker, A. Solórzano, H. Cao, A. Garcia-Sastre, and S. Lu. 2006. Hemagglutinin (HA) proteins from H1 and H3 serotypes of influenza A viruses require different antigen designs for the induction of optimal protective antibody responses as studied by codon-optimized HA DNA vaccines. J. Virol. 80:11628-11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warrell, M. J., D. A. Warrell, P. Suntharasamai, C. Viravan, A. Sinhaseni, D. Udomsakdi, R. Phanfung, C. Xueref, J. C. Vincent-Falquet, K. G. Nicholson, D. Bunnag, and T. Harinasuta. 1983. An economical regimen of human diploid cell strain anti-rabies vaccine for post-exposure prophylaxis. Lancet i:301-304. [DOI] [PubMed] [Google Scholar]
- 51.Webby, R. J., and R. G. Webster. 2003. Are we ready for pandemic influenza? Science 302:1519-1522. [DOI] [PubMed] [Google Scholar]