Abstract
The diagnosis of visceral leishmaniasis remains difficult in rural areas where the disease is endemic, and serologic methods still need assessment, as they are not very sensitive for the detection of asymptomatic infectious dogs. Here we present data on the development of enzyme-linked immunosorbent assay (ELISA)-based methods for the detection of antibodies against recombinant leishmanial antigens (namely, the recombinant K26 [rK26] and rK39 antigens from Leishmania infantum and the rA2 protein from Leishmania donovani) in comparison to ELISAs employing crude soluble antigen (CSA). The assays utilized sera from known negative controls (n = 25) and clinically asymptomatic (n = 50) and symptomatic (n = 50) dogs with confirmed L. infantum infections. Additional studies were also done using sera from animals harboring other infections (n = 14) for the evaluation of cross-reactivity. Our study indicated that rK26 and rK39 used in ELISAs provided very high sensitivities for the detection of symptomatic dogs (94% and 100%, respectively), followed by CSA (88%) and rA2 (70%). Conversely, rA2 was more sensitive for asymptomatic dogs (88%) than rK39 and rK26 (both 66%) and CSA (30%). Some cross-reactivity in sera from dogs with other infections (Leishmania braziliensis and Leptospira interrogans) was identified, but the rA2 protein provided the greatest specificity (98%). Data further indicate that all three recombinant proteins must be used in parallel to detect essentially all infected dogs. Efforts should be made to develop a cheap and reliable serologic test based on epitope selection from these diagnostic markers for the sensitive detection of L. infantum-infected dogs.
Zoonotic visceral leishmaniasis (VL) caused by Leishmania infantum (Leishmania chagasi) is an important emerging parasitic disease found in countries around the Mediterranean basin, in the Middle East, and in Latin America (www.who.int/tdr/diseases). In the Neotropics, the parasite is transmitted by the sand fly Lutzomyia longipalpis and wild canids (Cerdocyon thous and Lycalopex vetulus) serve as the major sylvatic reservoirs, and domestic dogs are the principal reservoir hosts in sites where the disease is endemic (18). Such animals are a potential source of infection for sand fly vectors (11). Hence, the prevalence and the incidence of canine visceral leishmaniasis (CVL) are important epidemiological parameters for controlling transmission, the estimation of which depends on the reliable identification of infected dogs (15, 16, 24). Control programs focus on the mass elimination of seropositive dogs (8). Mathematical modeling suggests that culling programs fail because of the high incidence of canine infection and infectiousness, the insensitivity of the diagnostic tests to detect infectious dogs, and time delays between diagnosis and culling used in practice by public health services (11).
Due to the limitations of direct methods to detect parasites in dogs (either by microscope examination, culture, or the inoculation of hamsters with biopsy specimens) (18), the presence of antiparasite antibodies is routinely used as a marker of infection, with the production of antibody as the definition of infection or challenge (16, 24). A wide variety of possible serologic techniques and antigens have been evaluated, but serology may be less specific than the assessment of parasites in biopsy specimens due to cross-reactions with other infectious agents, and the choice of a suitable cutoff may not be obvious (16). The currently available serologic tests include the indirect fluorescence antibody test (IFAT) (20), enzyme-linked immunosorbent assays (ELISAs) (4), dot-ELISA (32), the direct agglutination test (19), Western blotting (2), and the lateral immunochromatographic test (31). Overall, the reported performances of these tests indicate that in general they are suitable tools for the serodiagnosis of VL in both symptomatic and asymptomatic dogs. In addition to the serologic tests, PCR-based assays have also been used for detecting L. infantum DNA in diagnostic samples from humans and dogs (23, 25). However, results from both cross-sectional and longitudinal analyses of the PCR and serologic data suggest that PCR is most useful for detecting active infection while serology can be a more sensitive technique for the detection of all infected dogs (23).
The serodiagnosis of CVL remains problematic because the current diagnostic tests lack sufficient sensitivity or specificity, require technological expertise and specialized laboratory apparatuses, and can be labor-intensive and time-consuming. However, rapid tests like the immunochromatographic-dipstick and gel tests using the recombinant K39 (rK39) and rK26 proteins of L. infantum (5, 9) as antigens seem to be most suited for point-of-care diagnosis of symptomatic cases of CVL but lack sensitivity for asymptomatic dogs (21, 22, 25, 31). Hence, efforts should be made to develop a more sensitive and specific recombinant protein-based immunoassay capable of detecting asymptomatic carriers in mass screening surveys. Here we report a study in which the diagnostic potentials of parasite-specific recombinant antigens rK39, rK26, and rA2 in comparison with that of crude soluble antigen (CSA) in ELISAs were evaluated. The findings indicate that these markers complement one another, thus increasing the overall sensitivity of the antibody detection test for symptomatic and asymptomatic dogs with confirmed L. infantum visceral infections.
MATERIALS AND METHODS
Dogs and infection status.
Four groups of serum samples from dogs were established. Group 1 contained negative control sera from 25 healthy pets of various ages and breeds that had attended a veterinary clinic in Rio de Janeiro, Brazil. Group 2 contained cross-reaction control sera from 14 dogs with either Leishmania (Viannia) braziliensis dermal leishmaniasis (n = 9; parasites isolated from the lesions were characterized by multilocus enzyme electrophoresis), leptospiroses (n = 2; dogs were seropositive for Leptospira interrogans by ELISA), or toxoplasmosis (n = 3; dogs were seropositive by ELISA) that had attended a veterinary clinic at the municipality of Vitoria in Espírito Santo State (a VL-free area of Brazil). Group 3 contained sera (previously classified as having IFAT antileishmanial antibody titers of ≤1:80) from 50 asymptomatic dogs naturally infected with L. infantum. Group 4 contained sera (with IFAT-determined titers of ≥1:160) from 50 L. infantum-infected dogs with active disease (clinically classified as either oligosymptomatic [n = 9] or polysymptomatic [n = 41]). Infections were proven by the demonstration of the presence of the parasite in Giemsa-stained smears and/or in vitro cultures of the dogs' bone marrow aspirates. The necropsy findings also showed parasite-containing macrophages in the liver, spleen, and lymph nodes. All infected dogs enrolled in the study were selected based on their serologic results from IFAT (which was performed at the Federal University of Espírito Santo) during a cross-sectional serodiagnosis survey of L. infantum CVL carried out in a rural area of endemicity (northwest Espírito Santo State) in southeast Brazil.
Sampling and parasitology.
Permission was obtained from all householders to use their dogs. Positive control sera were samples taken from study dogs at any time when Leishmania parasites were found in the bone marrow biopsy material by culture or microscopy. In addition, postmortem culturing or histological examinations of lymphoid tissues (such as lymph node, spleen, and liver tissues) allowed for the assessment of subclinical infection. Prior to each sampling, dogs were anesthetized with 20 mg of ketamine hydrochloride (Vetalar)/kg of body weight injected intramuscularly. Then 10 ml of venous blood was taken by venipuncture. Bone marrow was aspirated from the iliac crest with a 16-by-25-mm needle into a 20-ml syringe containing 0.5% EDTA. The sample was used to make one to four thin smears for culture in vitro and/or inoculated into hamsters. The examination of cultures and smears of bone marrow specimens was done by standard techniques (24). Paraffin sections from necropsy tissue samples (fixed in 10% neutral buffered formalin) were stained with hematoxylin and eosin. All infected dogs were euthanized with sodium pentobarbital (Euthanol), and the necropsies were performed for the assessment of parasites in the skin, bone marrow, liver, and spleen. Leishmanial isolates from studied dogs were typed as L. infantum by multilocus enzyme electrophoresis in our laboratory as described previously (12). This research has complied with all relevant Brazilian federal guidelines (Projeto de lei 3.964/97; www.planalto.gov.br).
Clinical examination.
All infected dogs underwent gross physical examination by veterinary practitioners in the field. Animals were scored clinically for six typical signs of CVL (alopecia, dermatitis, chancres, conjunctivitis, lymphadenopathy, and onychogryposis) on a semiquantitative scale as described by Quinnell and coworkers (23). Dogs with total scores of 0 to 2 were classified as asymptomatic, those with scores of 3 to 6 were classified as oligosymptomatic, and those with scores of 7 to 18 were classified as polysymptomatic.
Antigens.
Promastigotes of L. infantum (strain MHOM/BR/2000/1669) provided the source of whole-parasite extracts. A preparation of CSA derived from promastigotes broken by ultrasonic treatment was made as described elsewhere (27). The two recombinant proteins of L. infantum, namely, rK39 (a 39-amino-acid-repetitive immunodominant B-cell epitope kinesin-related antigen) (9) and rK26 (a gene fragment product containing the repetitive sequence of K26) (5), were kindly provided by the Infectious Disease Research Institute, Seattle, WA. The L. donovani rA2 protein was prepared as described elsewhere (10).
ELISA.
Sera from the dogs were analyzed by standard micro-ELISA (21) to detect antigen-specific antibodies (by using a whole-molecule peroxidase-conjugated rabbit anti-dog immunoglobulin G [IgG]; Sigma, St. Louis, MO). The reaction was revealed with a biotin-avidin peroxidase system. The substrate consisted of 0.04% o-phenylenediamine dihydrochloride and 0.012% hydrogen peroxidase in phosphate-citrate buffer, pH 5.0. The absorbance at 492 nm was measured in an E max microplate reader (Molecular Devices, Ramsey, MN). A group of sera with previously known titers as control values, as well as negative control sera from healthy dogs, was included in each test. The lower limit of positivity (cutoff) was determined by using the mean plus 3 standard deviations of the A492 values for 25 normal controls (sera of group 1).
Data analysis.
The sensitivities for the samples from the L. infantum-infected dogs in groups 3 (asymptomatic; n = 50) and 4 (symptomatic; n = 50) were calculated separately by using the following equation: [number of true positives/(number of true positives + number of false negatives)] × 100. Test specificities were assessed with sera from group 1 (normal controls from a region of nonendemicity; n, 25 “true negatives”) and group 2 (dogs infected with L. braziliensis or other pathogens; n = 14). The percent specificity was calculated by using the following equation: [number of true negatives/(number of true negatives + number of cross-reactive sera, considered to be false positives)] × 100. Statistical analyses of all quantitative data were performed regardless of how significant the differences looked in the table or figure. Data were appropriately analyzed as parametric (normally distributed) and nonparametric data (by using Student's t test and Fisher's exact test, respectively), and a P value of <0.05 was considered statistically significant.
RESULTS
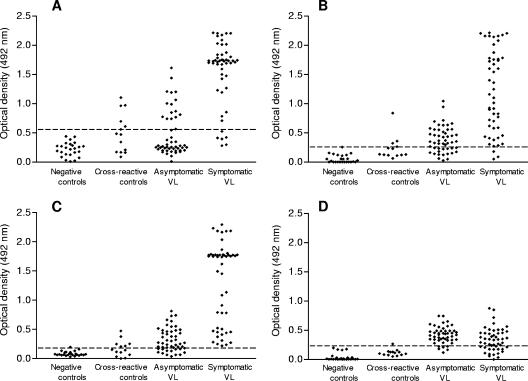
All ELISA procedures were optimized with regard to antigen concentrations and the serum dilution (data not shown). Optimal antigen concentrations were 2 μg/ml for CSA and 0.5 μg/ml for each recombinant protein. A clear separation between the parasite-positive dogs and healthy (uninfected) controls was possible by using a single serum dilution of 1:600. Using this serum dilution, we then compared the immunoreactivities of sera from symptomatic and asymptomatic L. infantum-infected dogs, healthy controls, and dogs harboring other infections for the evaluation of cross-reactivities. Figure 1 shows the results and indicates that positive ELISA signals (means ± standard deviations of the A492 values) given by IgG specific for rK39, rK26, and rA2 antigens were very variable in asymptomatic dogs (0.321 ± 0.098, 0.385 ± 0.221, and 0.418 ± 0.141, respectively) and, as expected, significantly higher (P = 0.019) in symptomatic dogs (1.319 ± 0.690, 1.191 ± 0.706, and 0.337 ± 0.195, respectively). In addition, the optical density readings produced by sera from symptomatic dogs with all antigens but rA2 were consistently higher than those produced by sera from oligosymptomatic animals. Moreover, the overall reactivity of the sera from symptomatic dogs with rA2 was significantly lower (P = 0.01) than that with either rK39 or rK26.
FIG. 1.
Comparison of Leishmania-specific IgG antibodies in different groups of dogs. Antigen-specific antibody was assessed by ELISA using either CSA derived from promastigotes (A) or the rK26 (B), rK39 (C), and rA2 (D) proteins. The recombinant antigens were evaluated for diagnostic potential by using a panel of sera from dogs with proven L. infantum infections grouped by their clinical statuses. Normal control (uninfected) diagnostic samples and cross-reaction control sera were included to achieve adequate precision for estimates of the specificities of the ELISAs. Results are expressed as the optical densities at 492 nm. Dashed lines, cutoff optical densities (mean + 3 standard deviations for normal controls) for each antigen.
Table 1 summarizes the results of the different ELISAs used in this comparative study.
TABLE 1.
Sensitivity of ELISAs using crude and recombinant leishmanial antigens for the diagnosis of asymptomatic and symptomatic canine visceral leishmaniasisa
| Antigen | No. of positive sera/no. of tested sera (% sensitivity)b from:
|
Asymptomatic/symptomatic index | |
|---|---|---|---|
| Asymptomatic dogs | Symptomatic dogs | ||
| CSA | 15/50 (30) | 44/50 (88) | 0.34 |
| K26 | 33/50 (66) | 47/50 (94) | 0.70 |
| K39 | 33/50 (66) | 50/50 (100) | 0.66 |
| A2 | 42/48 (88) | 35/50 (70) | 1.2 |
| Totalc | 50/50 (100) | 50/50 (100) | |
Infections were proven by parasitological examination (using microscopy, in vitro culture, and/or inoculation of hamsters with biopsy material). At sampling times, dogs were examined for six typical clinical signs of VL and then classified on a semiquantitative scale as described in Materials and Methods.
The percent sensitivity was calculated as indicated in Materials and Methods.
Dog sera that tested positive with at least one of the recombinant antigens assayed by ELISA.
Sensitivities.
The sensitivity of the widely used CSA-based ELISA was 88% for the detection of dogs with symptomatic disease (previously having IFAT antileishmanial antibody titers of ≤1:160), but this assay missed 35 of 50 of the asymptomatic dogs with CVL (having IFAT-determined titers of ≤1:80), resulting in an extremely decreased sensitivity (30%; P < 0.0001). ELISAs with rK39 and rK26 antigens gave the best results in terms of sensitivity (100 and 94%, respectively) for symptomatic dogs but detected considerably fewer asymptomatic cases (both 66%; P < 0.0008). Conversely, the rA2 ELISA was 88% sensitive for asymptomatic dogs and performed less well for symptomatic cases (70% sensitivity; P < 0.048), yielding the highest number of false-negative results in this group. Of note, each of the recombinant antigens used in the ELISAs detected some positive sera that others missed (data not shown). The data indicated that all three recombinant antigens must be used in parallel to obtain an assay with 100% sensitivity (Table 1).
Specificities.
To define potential cross-reactions with antigens for the diagnosis of CVL, we tested L. infantum-derived CSA and the recombinant antigens against sera from dogs infected with other pathogens (Table 2). As anticipated, most of the L. braziliensis-infected dogs (five of nine) were positive as determined by the CSA ELISA (77.8% cross-reactivity). Surprisingly, however, anti-rK39 and anti-rK36 antibodies were detected in three and five dogs, respectively. In addition, one dog with leptospiroses was also positive with rK39 and rK36. In contrast, specific anti-rA2 antibodies were found in only one dog with active L. braziliensis dermal infection, thus showing the excellent overall specificity of the rA2 ELISA of 98%, as assessed with healthy dogs and dogs with other infections. Finally, sera from three dogs with toxoplasmosis and those from 25 healthy dogs from an area of nonendemicity were all negative with all tested antigens.
TABLE 2.
Specificity of ELISAs using crude and recombinant leishmanial antigens for the diagnosis of canine visceral leishmaniasis
| Antigen | No. of positive sera/no. of tested sera from dogs with:
|
% Specificityc | |||
|---|---|---|---|---|---|
| L. braziliensis dermal leishmaniasisa | Leptospira interrogans leptospirosesa | Toxo- plasmosisa | No infection (controls)b | ||
| CSA | 5/9 | 0/2 | 0/3 | 0/25 | 87 |
| K26 | 3/9 | 1/2 | 0/3 | 0/25 | 90 |
| K39 | 5/9 | 1/2 | 0/3 | 0/25 | 85 |
| A2 | 1/9 | 0/2 | 0/3 | 0/25 | 96 |
Infections were proven by parasitological or serologic examination.
Serum samples were obtained from healthy blood donor pets born in a VL-free area of Brazil.
The percent specificity was calculated from an equation as indicated in Materials and Methods.
DISCUSSION
In general, a seroprevalence evaluation of VL may underestimate the true prevalence of infection (16), because (i) the sensitivity of antibody detection is generally lower for early or asymptomatic infections (21, 23, 25), (ii) there is a significant prepatent period before seroconversion (1), (iii) a fraction of infected dogs may never convert (16), and (iv) dogs may revert to seronegativity but remain parasite positive (24). Previous studies have reported various levels of L. infantum-specific antibodies of the IgG1 or IgG2 isotype in sera of infected dogs, sometimes with conflicting results (14, 21, 29). The genetic variability of canine immune responses may account for this controversy, but problems due to the specificities and sensitivities of serologic tests also occur (16). A reference test with suboptimal sensitivity can substantially affect the validity estimates for new tests under scrutiny. Thus, the published sensitivity and specificity estimates for serologic tests may be biased to some extent, depending on the controls and reference test used (7). On the other hand, parasite detection tests, despite being very specific, cannot be the gold standard because their sensitivities are defined by parasite burdens (16, 24, 33).
In this investigation, we compared the effectiveness of using different recombinant antigens in an ELISA format to evaluate a panel of sera from dogs considered to be truly parasite positive, along with negative and cross-reaction control sera. Although the results show great variation in levels of antigen-specific IgG antibodies, titers of antibodies to the rK26 and rK39 antigens were higher in symptomatic than in asymptomatic animals (P < 0.01). The results corroborated previous data showing that serologic test performance depends on many factors such as infection status (23, 25) and the type of diagnostic antigen or conjugate used (21, 27, 31).
The rK39 and rK26 antigens of L. infantum have proven to be of reliable sensitivities when used in ELISAs for the diagnosis of human VL (3-5). The rK39 ELISA is highly sensitive (reported sensitivities, 93 to 100%) for symptomatic dogs (3, 26-28, 34) but fails to detect or detects fewer asymptomatic cases (sensitivities, 52.9 to 64.7%) of proven infections (21, 26). In contrast, the rK26 ELISA has very high sensitivities for both symptomatic (n = 21; 100%) and asymptomatic (n = 48; 98%) dogs (31). Moreover, it has been suggested that the presence of specific anti-rK39 (3, 26) and anti-A2 (10, 17) antibodies can in fact be considered as a diagnostic marker for active disease in humans and dogs. In our assays, the rK39 and rK26 antigens were the most sensitive (100% and 94%, respectively), followed by CSA (88%) and rA2 (70%), for symptomatic cases of L. infantum CVL. Conversely, the rA2 antigen detected more asymptomatic cases (88%) than rK39 and rK26 (both 66%) and CSA (30%). When we calculated an index based on the ratio of asymptomatic to symptomatic cases that were identified as positive by the respective antigen used in the ELISAs, A2 presented the highest score (1.20), followed by K39 (0.66), K26 (0.70), and CSA (0.34), suggesting an association between the response to A2 and protective immunity. However, this possibility needs further confirmation with larger populations of dogs from other areas of endemicity. In a previous rA2 ELISA assay, responsiveness was found in 93.3% of 15 symptomatic dogs (10). Taken together, our data indicate that serologic tests employing rK39 will identify the symptomatic dogs (the most infectious animals) while rK26 and rA2 antigens seem to be most effective for the serodiagnosis of both asymptomatic and symptomatic CVL.
Previous studies reported high specificities (ranging from 94 to 100%) of ELISAs using the rK39, rK26, and A2 antigens (3, 10, 17, 27, 28, 31). Our study indicates lower specificities (85 to 98%) as assessed with sera from healthy blood donor dogs and cross-reactive sera obtained from dogs infected with either L. braziliensis or Leptospira interrogans (Table 2). Interestingly, previous reports have suggested that the rK39 and rK26 antigens when used for the diagnosis of human VL do not cross-react with L. braziliensis (3, 9, 13). In addition, in previous studies with human VL (10), we found that the antigen rA2 is recognized by 60% of sera from patients with mucosal leishmaniasis, which is caused by L. braziliensis. Although to a lesser extent, the present results indicate that such cross-reaction also occurs in CVL, as specific anti-rA2 antibodies were detected in one out of nine L. braziliensis-infected dogs. The reasons for the unexpected cross-reactivity of the antigens rK26 and rK39 in contrast to previous observations are unknown at the present time. The small number of animals as well as the different geographic regions of endemicity from which the dogs were enrolled in these various studies may account for this controversy. It is worth mentioning that taxonomic studies have shown genetic polymorphism in natural populations of L. braziliensis (12). Whether strain variants of this parasite modulate their antigen expression patterns differently remains to be determined.
A major limitation to the study of the epidemiology of and hence control programs for CVL is the inability to identify and count asymptomatic carriers (15, 24) because classic diagnostic tests are insufficiently sensitive (16, 25). Thus, a rapid, sensitive, and specific tool for the detection of L. infantum infection in dogs would be highly desirable because it would allow for effective control interventions in areas where zoonotic VL is endemic. Recent studies have evaluated multiple-epitope chimerical antigens as diagnostic markers for the serodiagnosis of CVL (6, 30). ELISAs performed with these interesting chimerical antigens had sensitivities ranging from 79 to 96% for CVL and specificities ranging from 96 to 100%, depending on the negative control panel used. The main finding in our study was that the three parasite-specific recombinant antigens showed independent and complementary immunoreactivies and that assays reached an overall sensitivity of 100% when these antigens were used in parallel. These data reinforce the idea (6, 31) that the combination of these antigens in a multiple-epitope format could further improve the performance of a single-well test for the routine serodiagnosis of CVL.
Acknowledgments
The recombinant antigens K26 and K39 were kindly supplied by the Infectious Disease Research Institute (Seattle, WA). We thank Daniel Kiefer, Fundação Nacional da Saúde, for assistance in the field studies.
Financial support was received from Fiocruz, PRONEX 3 (CNPq-66.1037/1998-3), and the Millennium Institute for Vaccine Development and Technology (CNPq-420067/2005-1), National Council for Scientific and Technological Development of the Ministry of Science and Technology (Brazil).
We have no conflicts of interest concerning the work reported in this paper.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Abranches, P., G. Santos-Gomes, N. Rachamim, L. Campino, L. F. Schnur, and C. L. Jaffe. 1991. An experimental model for canine visceral leishmaniasis. Parasite Immunol. 13:537-550. [DOI] [PubMed] [Google Scholar]
- 2.Aisa, M. J., S. Castillejo, M. Gallego, R. Fisa, M. C. Riera, M. De Colmenares, S. Torras, X. Roura, J. Sentis, and M. Portus. 1998. Diagnostic potential of Western blot analysis of sera from dogs with leishmaniasis in endemic areas and significance of the pattern. Am. J. Trop. Med. Hyg. 58:154-159. [DOI] [PubMed] [Google Scholar]
- 3.Badaró, R., D. Benson, M. C. Eulálio, M. Freire, S. Cunha, E. M. Neto, D. Pedral-Sampaio, C. Madureira, J. M. Burns, R. L. Houghton, J. R. David, and S. G. Reed. 1996. rK39: a cloned antigen of Leishmania chagasi that predicts active visceral leishmaniasis. J. Infect. Dis. 173:758-761. [DOI] [PubMed] [Google Scholar]
- 4.Badaró, R., M. C. Eulálio, D. Benson, M. Freire, J. C. Miranda, D. Pedral-Sampaio, J. M. Burns, J. R. David, Jr., W. J. Johnson, and S. G. Reed. 1993. Sensitivity and specificity of a recombinant Leishmania chagasi antigen in the serodiagnosis of visceral leishmaniasis. Arch. Inst. Pasteur (Tunis) 70:331-332. [PubMed] [Google Scholar]
- 5.Bhatia, A., N. S. Daifalla, S. Jen, R. Badaro, S. G. Reed, and Y. A. Skeike. 1999. Cloning, characterization and serological evaluation of K9 and K26: two related hydrophilic antigens of Leishmania chagasi. Mol. Biochem. Parasitol. 102:249-261. [DOI] [PubMed] [Google Scholar]
- 6.Boarino, A., A. Scalone, L. Gradoni, E. Ferroglio, F. Vitale, R. Zanatta, M. G. Giuffrida, and S. Rasati. 2005. Development of recombinant chimeric antigen expressing immunodominant B epitopes of Leishmania infantum for serodiagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 12:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boelaert, M., S. Rijal, S. Regmi, R. Singh, B. Karki, D. Jacquet, F. Chappuis, L. Campino, P. Desjeux, D. Le Ray, S. Koirala, and P. Van der Stuyft. 2004. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. Am. J. Trop. Med. Hyg. 70:72-77. [PubMed] [Google Scholar]
- 8.Braga, M. D., I. C. Coelho, M. M. Pompeu, T. G. Evans, I. T. MacAullife, M. J. Teixeira, and J. W. Lima. 1998. Control of canine visceral leishmaniasis: comparison of results from a rapid elimination program of serum-reactive dogs using an immunoenzyme assay and slower elimination of serum-reactive dogs using filter paper elution indirect immunofluorescence. Rev. Soc. Bras. Med. Trop. 31:419-424. [DOI] [PubMed] [Google Scholar]
- 9.Burns, J. M., Jr., W. G. Shreffler, D. R. Benson, H. W. Ghalib, R. Badaro, and S. G. Reed. 1993. Molecular characterization of a kinesin-related antigen of Leishmania chagasi that detects specific antibody in African and American visceral leishmaniasis. Proc. Natl. Acad. Sci. USA 90:775-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho, F. A. A., H. Charest, C. A. P. Tavares, G. Matlashewski, E. P. Valente, A. Rabello, R. T. Gazinelli, and A. P. Fernández. 2002. Diagnosis of American visceral leishmaniasis in humans and dogs using the recombinant Leishmania donovani A2 antigen. Diagn. Microbiol. Infect. Dis. 43:289-295. [DOI] [PubMed] [Google Scholar]
- 11.Courtenay, O., R. J. Quinnell, L. M. Garcez, J. J. Shaw, and C. Dye. 2002. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J. Infect. Dis. 186:1314-1320. [DOI] [PubMed] [Google Scholar]
- 12.Cupolillo, E., L. R. Brahim, C. B. Toaldo, M. P. Oliveira-Neto, M. E. Felinto de Brito, A. Falqueto, M. F. Naiff, and G. Grimaldi, Jr. 2003. Genetic polymorphism and molecular epidemiology of Leishmania (Viannia) braziliensis from different hosts and geographic areas in Brazil. J. Clin. Microbiol. 41:3126-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado, O., M. D. Feliciangeli, V. Coraspe, S. Silva, A. Perez, and J. Arias. 2001. Value of a dipstick based on recombinant rK39 antigen for differential diagnosis of American visceral leishmaniasis from other sympatric endemic diseases in Venezuela. Parasite 8:355-357. [DOI] [PubMed] [Google Scholar]
- 14.Desplazes, P., N. C. Smith, P. Arnold, H. Lutz, and J. Eckert. 1995. Specific IgG1 and IgG2 antibody responses of dogs to Leishmania infantum and other parasites. Parasite Immunol. 17:451-458. [DOI] [PubMed] [Google Scholar]
- 15.Dye, C., R. Killick-Kendrick, M. M. Vitutia, R. Walton, M. Killick-Kendrick, A. E. Harith, M. W. Guy, M.-C. Cañavate, and G. Hasibeder. 1992. Epidemiology of canine leishmaniasis: prevalence, incidence and basic reproduction number calculated from a cross-sectional serological survey on the island of Gozo, Malta. Parasitology 105:35-41. [DOI] [PubMed] [Google Scholar]
- 16.Dye, C., E. Vidor, and J. Deneure. 1993. Serological diagnosis of leishmaniasis: on detecting infection as well as disease. Epidemiol. Infect. 103:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghedin, E., W. W. Zhang, H. Charest, S. Sundar, R. T. Kenney, and G. Matlashewski. 1997. Antibody response against Leishmania donovani amastigote-stage-specific protein in patients with visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 4:530-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimaldi, G., Jr., and R. B. Tesh. 1993. Leishmaniasis of the New World: current concepts and implications for future research. Clin. Microbiol. Rev. 6:230-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harith, A., A. H. Kolk, P. A. Kager, J. Leeuwenburg, R. Muigai, S. Kiugu, and J. J. Laarman. 1986. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 80:583-587. [DOI] [PubMed] [Google Scholar]
- 20.Mancianti, F., and N. Meciani. 1988. Specific serodiagnosis of canine leishmaniasis by indirect immunofluorescence, indirect hemagglutination, and counter-immunoelectrophoresis. Am. J. Vet. Res. 49:1409-1411. [PubMed] [Google Scholar]
- 21.Mettler, M., F. Grimm, G. Capelli, and H. Camp. 2005. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J. Clin. Microbiol. 43:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otranto, D., P. Paradies, M. Sasanelli, R. Spinelli, and O. Brandonisio. 2004. Rapid immunochromatographic test for serodiagnosis of canine leishmaniasis. J. Clin. Microbiol. 42:2769-2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinnell, R. J., O. Courtenay, S. Davidson, L. Garcez, B. Lambson, P. Ramos, J. J. Shaw, M. A. Shaw, and C. Dye. 2001. Detection of Leishmania infantum by PCR, serology and immune response in a cohort study of Brazilian dogs. Parasitology 122:253-261. [DOI] [PubMed] [Google Scholar]
- 24.Quinnell, R. J., O. Courtenay, L. Garcez, and C. Dye. 1997. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology 115:143-156. [DOI] [PubMed] [Google Scholar]
- 25.Reithinger, R., R. J. Quinnell, B. Alexander, and C. R. Davies. 2002. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J. Clin. Microbiol. 40:2352-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhalem, A., H. Sahibi, N. Gueeous-Idrissi, S. Lasri, A. Natami, M. Riyad, and B. Berrag. 1999. Immune response against Leishmania antigens in dogs naturally and experimentally infected with Leishmania infantum. Vet. Parasitol. 81:173-184. [DOI] [PubMed] [Google Scholar]
- 27.Rosario, E. Y., O. Genaro, J. C. França-Silva, and R. T. Da Costa. 2005. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem. Inst. Oswaldo Cruz 100:197-203. [DOI] [PubMed] [Google Scholar]
- 28.Scalone, A., R. De Luna, G. Oliva, L. Balde, G. Satta, G. Vesco, W. Mignone, C. Turilli, R. R. Mondesire, D. Simpson, A. R. Donoghue, G. R. Frank, and L. Gradoni. 2002. Evaluation of the Leishmania recombinant K39 antigen as a diagnostic marker for canine leishmaniasis and validation of a standardized enzyme-linked immunosorbent assay. Vet. Parasitol. 104:275-285. [DOI] [PubMed] [Google Scholar]
- 29.Solano-Gallego, L., C. Riera, X. Roura, L. Iniesta, M. Gallego, J. E. Valladares, R. Fisa, S. Castiççejo, J. Alberola, L. Ferrer, M. Arboix, and M. Portús. 2001. Leishmania infantum-specific IgG, IgG1 and IgG2 antibody responses in healthy and ill dogs from endemic areas. Evolution in the course of infection and after treatment. Vet. Parasitol. 96:265-276. [DOI] [PubMed] [Google Scholar]
- 30.Soto, M., J. M. Requena, L. Quijada, and C. Alonso. 1998. Multicomponent chimeric antigen for serodiagnosis of canine visceral leishmaniasis. J. Clin. Microbiol. 36:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teodoro da Costa, R., J. C. França, W. Mayrink, E. Nascimento, O. Genaro, and A. Campos-Neto. 2003. Standardization of a rapid immunochromatographic test with the recombinant antigens K39 and K26 for the diagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 97:678-682. [DOI] [PubMed] [Google Scholar]
- 32.Vercammen, F., D. Berkvens, J. Brandt, and W. Vansteenkiste. 1998. A sensitive and specific 30-min Dot-ELISA for the detection of anti-leishmania antibodies in the dog. Vet. Parasitol. 79:221-228. [DOI] [PubMed] [Google Scholar]
- 33.Zijlstra, E. E., M. S. Ali, A. M. El Hassan, I. A. El Toum, M. Satti, H. W. Ghalib, and P. A. Kager. 1992. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans. R. Soc. Trop. Med. Hyg. 86:505-507. [DOI] [PubMed] [Google Scholar]
- 34.Zijlstra, E. E., N. S. Daifalla, P. A. Pager, E. A. Khalil, A. M. El-Hassan, S. G. Reed, and H. W. Ghalib. 1998. rK39 enzyme-linked immunosorbent assay for diagnosis of Leishmania donovani infection. Clin. Diagn. Lab. Immunol. 5:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]