Abstract
Adamantylamide l-alanyl-d-isoglutamine (AdDP) is a synthetic adjuvant which belongs to the family of the desmuramyl peptides. AdDP exerts its adjuvant properties when it is administered either by the parenteral or by the mucosal route, leading to the elicitation of strong humoral responses at both the systemic and the mucosal levels. However, very little is known about the effect of AdDP on cellular immunity. Here we demonstrate that AdDP is able to stimulate cellular responses, which are characterized by the release of gamma interferon by CD8+ T cells when they are restimulated with a major histocompatibility complex class I-restricted peptide and strong in vivo lymphocyte-mediated cytotoxic activity. The capacity of AdDP to stimulate the elicitation of both cellular and humoral adaptive responses makes this adjuvant a promising tool for the development of mucosal vaccine formulations.
Muramyl dipeptide (MDP; N-acetylmuramyl-l-alanyl-d-isoglutamine) is a synthetic derivative of a component present in cell wall peptidoglycans of many bacteria. It is also the minimal bioactive structure required to replace the whole mycobacteria present in the Freund's complete adjuvant. Experimental studies have demonstrated that the immunomodulatory properties of MDP and certain analogues, alone or in combination with other agents, can confer resistance against viruses (e.g., human immunodeficiency virus, influenza virus, herpes simplex virus, Sendai virus, Semliki Forest virus, vaccinia virus, and murine hepatitis virus), bacteria, and fungi (8, 12, 14, 19, 20). However, the pyrogenic and arthritogenic effects of MDP preclude its use in humans (2). In order to take advantage of its immunomodulatory properties but to minimize the risk of side effects, nontoxic MDP derivatives have been generated, such as the adamantylamide dipeptide (AdDP), MDP-Lys(L18), murabutide (ester derivate), and glucosaminylmuramyl dipeptide (1, 18).
In the 1980s, attempts to design new tools against the influenza virus led to the combination of amantadine, which has been extensively employed for the prophylaxis and chemotherapy of influenza (4), with the dipeptide from MDP. The new compound not only retains the antiviral characteristics of amantadine but also possesses immunomodulatory properties that might be desirable in a vaccine formulation. In fact, this novel synthetic compound, called AdDP, was successfully tested in preclinical studies alone or in combination with influenza virus antigens (21).
Recent studies have demonstrated that AdDP is a powerful adjuvant which is able to enhance humoral immune responses against coadministered antigens at both the systemic and the mucosal levels in different animal species (2). Additional work showed that a vaccine formulation based on the recombinant P6 protein and AdDP was able to confer protection against pulmonary and middle ear infections caused by nontypeable Haemophilus influenzae (5). However, the effects of AdDP and other desmuramyl peptides on cellular responses are poorly characterized, if they have any effects at all. Finally, there is still fragmentary information and a lack of consensus concerning the general mechanisms of action of this family of adjuvants. Thus, the major aim of this work was to provide insights on the in vivo immunomodulatory properties of AdDP, particularly at the level of stimulation of cellular immune responses. The results obtained demonstrated that AdDP is able to stimulate the release of gamma interferon (IFN-γ) by CD8+ T cells and lymphocyte-mediated cytotoxic activity in vivo.
MATERIALS AND METHODS
Antigens, peptides and adjuvant.
The model antigens β-galactosidase (β-Gal) and ovalbumin (OVA) were purchased from Boehringer (Mannheim, Germany) and Sigma, respectively. The peptides encompassing the major histocompatibility complex (MHC) class I immunodominant Ld-restricted β-Gal peptide (TPHPARIGL) and kb-restricted OVA peptide (SIINFEKL) (11, 15, 23) were synthesized at the Helmholtz Centre for Infection Research (Braunschweig, Germany). AdDP was synthesized by Bachem, Switzerland, under good manufacturing practice guidelines by a previously described procedure (10).
Animals and cell cultures.
Female BALB/c (H-2d) and C57BL/6 mice (ages, 6 to 8 weeks) were purchased from Harlam-Winkelmann GmbH (Borchen, Germany) and were treated in accordance with local and European Community guidelines. Spleen cells were grown in RPMI 1640 supplemented with 10% fetal calf serum, 100 U/ml of penicillin, 50 μg/ml of streptomycin, 5 × 10−5 M 2-mercaptoethanol, and 1 mM l-glutamine (GIBCO BRL, Karlsruhe, Germany) and were maintained at 37°C in a humidified 5% CO2 atmosphere.
Immunization protocols.
Groups of five BALB/c mice each were immunized by the intraperitoneal route three times on days 1, 7, and 14 with either 50 μg of β-Gal alone or β-Gal coadministered with 200 μg of AdDP. Similarly, C57BL/6 mice were immunized by the intranasal route with 50 μg of OVA alone or OVA coadministered with 100 μg of AdDP. Negative controls received phosphate-buffered saline (PBS). Serum samples were collected from blood of the tail vein 1 day before each immunization and 1 week after the last immunization, when the mice were killed by CO2 inhalation. Sera were stored at −20°C prior to determination of specific antibodies. Bronchoalveolar lavage fluid samples were obtained by flushing the organs with PBS supplemented with 10 mM phenylmethylsulfonyl fluoride.
The spleens were removed aseptically and were pooled for analysis of cellular immune responses. Bone marrow (BM) cells were isolated from the femur and tibia, as described elsewhere (9). Briefly, the legs were separated, all muscle tissues were removed from the femur and tibia, and the epiphyses were cut off. The bone ends were punctured with a needle and the BM was flushed out with medium. The concentration of the cells was adjusted according to the specific experiment.
Detection of specific antibodies.
β-Gal- and OVA-specific antibodies were determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well Immuno MaxiSorp assay plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 100 μl of either β-Gal or OVA at 5 μg/ml in carbonate buffer (pH 9.6). After the wells were blocked, the plates were washed and further incubated with 100 μl of serial twofold dilutions of sera for 1 h at 37°C. After four washes, detection antibody, either biotinylated γ- or μ-chain-specific goat anti-mouse antibody (Sigma Chemie, Deisenhofen, Germany), was added. The plates were further incubated for 1 h at 37°C. After the plates were washed, peroxidase-conjugated streptavidin (PharMingen) was added and the plates were incubated at room temperature for 45 min. After another four washes, the reactions were developed by using 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid) in 0.1 M citrate-phosphate buffer (pH 4.35) containing 0.01% H2O2, and the absorbance was read at a wavelength of 405 nm. The immunoglobulin G (IgG) isotypes present in the serum samples were determined by an ELISA, as described previously (2), by using as secondary antibodies biotin-conjugated rat anti-mouse IgG1, IgG2a, IgG2b, or IgG3 (Southern Biotechnology Associates, Birmingham, AL). The absorbance values were plotted against the dilutions, and the endpoint titers were determined as the highest sample dilution that gave an A405 of ≥0.2 above the background values. The results were expressed as mean ± standard errors of the means (SEMs) for each group. The presence of OVA-specific secretory IgA in lung lavage fluid specimens was determined as described previously (3).
Detection of Ig-producing cells by ELISPOT assay.
The numbers of total and antigen-specific IgG secreting cells were determined by enzyme-linked immunospot (ELISPOT) assay. Briefly, polyvinylidene difluoride plates (Millipore, Bedford, MA) were coated with either 100 μl/well of isotype-specific capture antibodies (Sigma, Germany) at a concentration of 5 μg/ml or antigen (β-Gal or OVA at 5 μg/ml in carbonate buffer, pH 9.6) to determine total and antigen-specific antibodies, respectively. Different concentrations of BM cells were incubated in quadruplicate for 6 h. Then, the plates were washed, 100 μl of the corresponding biotinylated detection antibody (Sigma) was added, and the plates were further incubated overnight at 4°C. After several washes, the plates were incubated for 1 h with 100 μl/well of peroxidase-conjugated streptavidin (BD-Pharmingen, Germany). The spots were developed by using 3-amino-9-ethylcarbazole (Sigma) in 0.1 M acetate buffer (pH 5.0) and 0.05% H2O2 (30%). The reaction was stopped by rinsing the plates with tap water, and the plates were air dried. The spots were scanned with an ImmunoSpot series 3A analyzer and were counted by using ImmunoSpot image analyzer software (version 3.2; Cellular Technology, Ltd.).
Detection of IFN-γ- and IL-4-producing cells by ELISPOT assay.
The number of IFN-γ and interleukin-4 (IL-4)-secreting cells was determined by ELISPOT assay, according to the manufacturer's instructions (Becton Dickinson). In brief, spleen cells were added at final concentrations of 1 × 106 and 5 × 105 cells/well and were incubated in quadruplicate in the absence or presence of different concentrations of the MHC class I-restricted peptide (IFN-γ) or OVA (IL-4). After 16 h of culture, the cells were removed and locally produced single-cell-derived IFN-γ or IL-4 was detected by using IFN-γ or IL-4 biotinylated antibodies, which were developed by addition of peroxidase-conjugated streptavidin and substrates, as described above.
Measurement of cellular proliferation.
Proliferation assays were performed in triplicate, as described previously (6). Briefly, spleen cells (5 × 105 cells/well) were incubated for 4 days in the presence of β-Gal or OVA. Eighteen hours before harvest, 1 μCi of [3H]thymidine (Amersham International, Freiburg, Germany) was added to each well. The cells were harvested on filter paper (Filtermat A; Wallac, Freiburg, Germany) by using a cell harvester (Inotech, Wohlen, Switzerland), and the amount of [3H]thymidine incorporated into the DNA of proliferating cells was determined with a γ-scintillation counter (Wallac 1450; Micro-Trilux).
Determination of lymphocyte-mediated cytotoxity in vivo.
Suspensions of splenocytes from naïve C57BL/6 mice were depleted of red cells and split into two equal portions. One portion was labeled with a high concentration (1 μM) of carboxyfluorescein-succinyl-ester (CFSEhi; Molecular Probes) and pulsed for 1 h at 37°C with the dominant OVA peptide (amino acids 257 to 264) at a concentration of 15 μg/ml. The other portion was labeled with a low concentration (0.1 μM) of CFSE (CFSElo) and was further incubated for 1 h at 37°C without peptide. Equal numbers of each cell population were mixed. A total amount of 2 × 107 cells was adoptively transferred by intravenous injection into the immunized mice. Cells from the spleen were analyzed by flow cytometry after 16 h with a FACScalibur instrument and BD Cell Quest Pro software. The percent OVA-specific lysis was determined by the loss of the peptide-pulsed CFSEhi population and compared to the control CFSElo population (13). The following formula was used to calculate the percentage of specific lysis: 100 − {[(percent CFSEhi in immunized mice/percent CFSElo in immunized mice)/(percent CFSEhi in control mice/percent CFSElo in control mice)] × 100}.
Statistical analysis.
The statistical significance of the differences between two groups was determined from the means and the SEMs by the Student's two-tailed t test, and the statistical significance of the differences between three or more groups was determined by one-way analysis of variance. Differences were considered significant at a P value of <0.05.
RESULTS
Immunization with AdDP as the adjuvant by either parenteral or mucosal route results in elicitation of strong antigen-specific antibody responses.
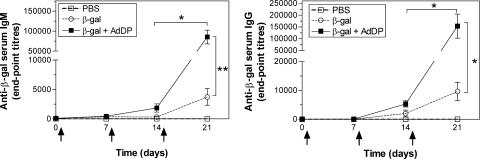
First, we evaluated if the use of AdDP results in the stimulation of strong antibody responses in BALB/c mice receiving the model antigen β-Gal, as was previously demonstrated with other immunogens, such as OVA. To this end, we measured the β-Gal-specific antibody responses before each immunization and 1 week after the last boost. In serum samples collected at day 6, 1 week after the first immunization, a weak response of β-Gal-specific IgM antibodies was observed in the groups receiving either β-Gal alone or β-Gal coadministered with AdDP (Fig. 1). However, at day 13, 1 week after the second immunization, the group that received β-Gal together with AdDP showed sixfold higher titers than the group immunized with β-Gal alone (Fig. 1). At the same time point, mice receiving AdDP as the adjuvant had β-Gal-specific IgG titers that were also slightly different from the titers in those receiving β-Gal alone (Fig. 1). At day 21, animals receiving β-Gal coadministered with AdDP showed 22-fold higher antigen-specific IgM titers than control animals immunized with β-Gal alone (P < 0.01). In addition, the β-Gal-specific IgG antibody titers were 16-fold higher in mice vaccinated with AdDP than in control mice receiving β-Gal (P < 0.05) (Fig. 1). The subclass profile of the β-Gal-specific serum Igs was measured at the last time point. BALB/c mice immunized with β-Gal coadministered with AdDP showed dominant specific IgG1 subclass antibodies (data not shown), which are generally associated with a Th2-type response (7, 24).
FIG. 1.
Kinetic analysis of β-Gal-specific serum antibodies in vaccinated animals. BALB/c mice were immunized with PBS, β-Gal (50 μg) alone, or β-Gal coadministered with AdDP (200 μg) as the adjuvant. Immunizations are indicated by arrows (days 1, 8, and 15). Antibody titers were determined by endpoint dilution ELISA. Each point represents the group mean titer of total anti-β-Gal IgM (left panel) and IgG (right panel). The SEMs are indicated by vertical lines. Differences were statistically significant at P < 0.05 (*) and P < 0.001 (**).
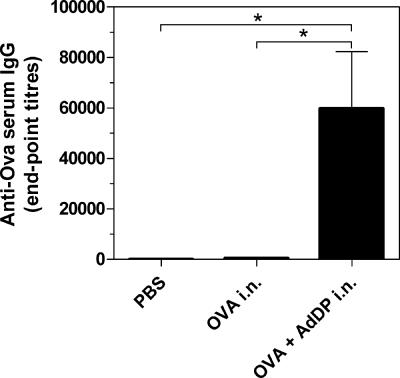
In all previous work, BALB/c mice were used to demonstrate the potency of AdDP as the adjuvant. Thus, we also used C57BL/6 mice in the present study to ensure that the immune responses observed are not restricted to the BALB/c strain. Therefore, the model antigen, OVA, was coadministered with AdDP to C57BL/6 mice by the intranasal route and the antibody responses were evaluated. C57BL/6 mice immunized with OVA plus AdDP showed strong IgG production in comparison with that for the animals receiving OVA alone (P < 0.05) (Fig. 2). As expected, AdDP was also able to promote the elicitation of a mucosal immune response in mice vaccinated by the intranasal route. A 10-fold increment in the levels of OVA-specific secretory IgA was observed in lung lavage fluid samples from animals to which OVA was coadministered with AdDP compared to that in mice receiving OVA alone. These results were in agreement with the observed increment in the number of IL-4-producing cells (98 versus 0 per 106 splenocytes in the group treated with OVA plus AdDP and the control group, respectively) and the presence of a dominant anti-OVA IgG1 response in sera.
FIG. 2.
Analysis of OVA-specific serum antibodies in C57BL/6 mice immunized by the intranasal (i.n.) route with AdDP as the adjuvant. OVA-specific IgG titers from serum samples were determined by endpoint dilution ELISA. Each bar represents the group mean endpoint titer. The SEMs are indicated by vertical lines. Differences were statistically significant at P < 0.05 (*).
Immunization with AdDP as adjuvant results in the accumulation of antigen-specific Ig-secreting cells in BM.
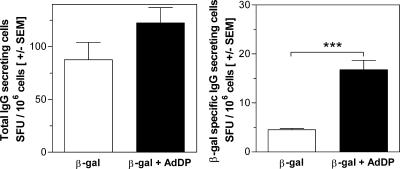
It is known that after primary contact with an antigen, a short antibody response characterized by the expansion of antibody-secreting plasmablasts is generated. However, most antibody-secreting cells (ASCs) produced during secondary immune responses leave the secondary lymphoid organs, with the main final destinations being BM, mucosa-associated tissues, and chronically inflamed tissues (16). These ASCs with the phenotype of mature plasma cells have a potential life span of more than 1 year. Because the IgG subclass has a half-life of 3 weeks, the maintenance of antibody titers over a long period is the responsibility of BM ASCs (22). Therefore, we examined the ability of BM cells to secrete total and β-Gal-specific IgG after 6 h of restimulation by the ELISPOT assay. This short incubation period is not enough for the differentiation of memory B cells into ASCs; thus, only actively secreting plasma cells are determined (17). We did not find any statistically significant difference in the number of total IgG-secreting cells between BALB/c mice receiving β-Gal alone or β-Gal in combination with AdDP (Fig. 3, left panel). In contrast, we observed a significant (P < 0.001) increase in the number of β-Gal-specific IgG-secreting cells in mice immunized with β-Gal together with AdDP in comparison with the number in those immunized with β-Gal alone (Fig. 3, right panel). Sixty days after the last immunization, C57BL/6 mice that were immunized with OVA plus AdDP also exhibited high titers of antibodies that correlated with the number of total OVA-specific IgG ASCs, with IgG1 being the major IgG subclass produced, as determined by the ELISPOT assay (data not shown). These findings agree with our previous results, wherein we showed the maintenance of high specific IgG titers for a period of 1 year (2).
FIG. 3.
Determination of the number of antibody-secreting cells present in immunized animals. Spleen cells from BALB/c mice vaccinated with β-Gal were cultured for 6 h, and the number of IgG-secreting cells was evaluated by the ELISPOT assay. The results are presented as the total number of IgG spot-forming units (SFU)/106 cells (left panel) and β-Gal-specific IgG spot-forming units/106 cells (right panel). The SEMs of quadruplicate values are indicated by vertical lines. Differences were statistically significant at P < 0.0001 (***).
Immunization with AdDP as adjuvant stimulates strong cellular immune responses.
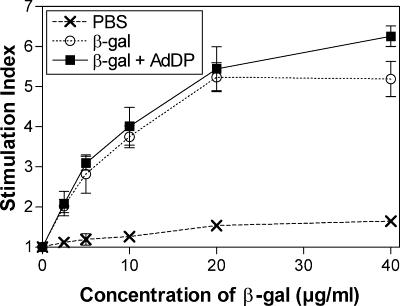
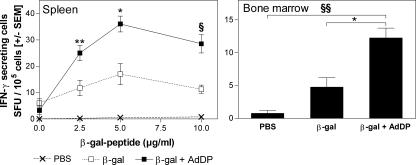
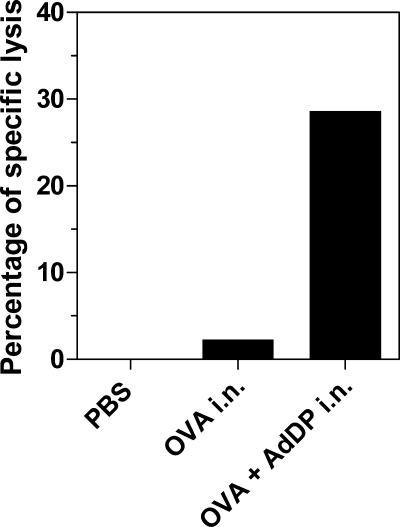
The cellular immune responses induced in BALB/c mice were evaluated by assessing the proliferative capacity of spleen cells after in vitro stimulation with β-Gal. A dose-dependent proliferative response was observed in mice immunized with either β-Gal alone or β-Gal coadministered with AdDP but not in control mice (Fig. 4). A slightly stronger response was observed in mice receiving β-Gal and AdDP compared to that observed in those receiving β-Gal alone, but only at the highest concentration of β-Gal tested (40 μl/ml). However, the number of CD8+ IFN-γ-producing cells consistently increased in mice vaccinated with AdDP as the adjuvant after restimulation with the peptide encompassing the immunodominant MHC class I-restricted epitope compared to the number of CD8+ IFN-γ-producing cells in those receiving β-Gal alone (Fig. 5). The difference in the number of IFN-γ-secreting cells detected after in vitro restimulation in animals receiving β-Gal alone and β-Gal coadministered with AdDP was statistically significant over the range of peptide concentrations tested (for 2.5, 5, and 10 μg/ml, P < 0.02, P < 0.05, and P < 0.005, respectively), with a maximum at 5 μg/ml (Fig. 5). Similar results were obtained when a similar analysis was performed with BM cells and the peptide at a concentration of 5 μg/ml (P < 0.01) (Fig. 5). On the other hand, C57BL/6 mice immunized with OVA plus AdDP by the intranasal route showed stronger in vivo cytotoxic T-lymphocyte activity than mice immunized with OVA alone (Fig. 6), suggesting that despite the observed Th2-biased response pattern, immunization with AdDP also induces a strong Th1 component.
FIG. 4.
Proliferative responses stimulated in immunized animals. The spleen cells of vaccinated mice were restimulated with different concentrations of β-Gal for 4 days. Proliferation was assessed by measurement of [3H]thymidine incorporation. The results are expressed as the ratio between values (average of triplicates) from stimulated or nonstimulated samples (stimulation index). SEMs are indicated by vertical lines.
FIG. 5.
Stimulation of IFN-γ-secreting cells in immunized animals. The number of IFN-γ-secreting cells was determined by the ELISPOT assay. Spleen cells were incubated for 16 h in the presence or the absence of a peptide corresponding to the immunodominant Ld-restricted β-Gal epitope (TPHPARIGL), which is specific for MHC class I presentation. The results are presented as the numbers of IFN-γ spot-forming units (SFU)/105 cells. The SEMs of quadruplicate values are indicated by vertical lines. Differences were statistically significant at P < 0.05 (*), P < 0.02 (**), P < 0.01 (***), P < 0.005 (§), and P < 0.001 (§§).
FIG. 6.
Analysis of the lymphocyte-mediated cytotoxic activity stimulated in vivo in C57BL/6 mice immunized with AdDP as the adjuvant. Spleen cells from naïve C57BL/6 mice labeled with 0.1 μM and 1 μM CFSE were incubated alone or with a peptide encompassing the dominant MHC class I-restricted epitope from OVA (SIINFEKL). After the cells were washed, equal numbers of cells of each type were mixed. A total of 2 × 107 cells were adoptively transferred by intravenous injection into immunized mice. After 16 h, the spleens were harvested and CFSEhi and CFSElo cells were detected by flow cytometry. Results are expressed as the percentage of specific lysis of peptide-pulsed cells. i.n., intranasal.
DISCUSSION
During recent decades, the increasing interest in the development of mucosal vaccines has led to the active search for new effective adjuvants. Several molecules from different microorganisms have been proposed for this purpose. MDPs, bacterial toxins (e.g., cholera toxin and Escherichia coli heat-labile toxin), and Toll-like receptor agonists (e.g., CpG motifs and MALP-2) have been among the most effective adjuvants described up to now. However, some of them have shown side effects that preclude their use in humans. In addition, to achieve optimal results following vaccination, it would be necessary to stimulate different types of immune responses, according to the specific needs. It is extremely unlikely that this will be achieved by the use of a single adjuvant molecule. Thus, there is an urgent need for new adjuvant compounds.
Previous studies suggested that muramyl peptides and their derivatives are promising adjuvants (18). However, only a few muramyl peptides, particularly desmuramyl peptides, have been characterized. We have previously shown that antigen coadministration with AdDP results in the stimulation of strong humoral responses both at the systemic level and at the mucosal level (2, 5). Preclinical studies also demonstrated that incorporation of AdDP into a P6-based vaccine formulation resulted in the stimulation of a protective response against nontypeable H. influenzae (5). However, up to now there has been almost no knowledge about the cellular immune responses evoked by AdDP. Hence, we performed a thorough characterization of the cellular immune responses stimulated by AdDP after immunization with the well-characterized model antigens β-Gal and OVA.
In agreement with what was previously reported, mice immunized with AdDP as the adjuvant showed strong humoral responses. AdDP was able to enhance IgM production and to induce the switch to IgG (3, 5). A significant increment in the number of antigen-specific ASCs was observed when splenocytes and BM cells were tested. Sixty days after immunization with AdDP as the adjuvant, antigen-specific ASCs were found in BM. Because antibody titers are maintained for long periods after immunization with AdDP and the IgG subclass has a half-life of 3 weeks, these results provide an explanation for our previous findings. Thus, immunization with AdDP as the adjuvant improves the migration of plasmablasts from the follicles of secondary lymph nodes to the BM and their differentiation in plasma cells.
Another important characteristic of an adjuvant is the ability to induce a cellular response. In the case of muramyl peptides and derivatives, most of the studies showed that they are able to stimulate humoral immune responses. However, the results obtained concerning the development of cellular immune responses are either unclear or contradictory (25). Therefore, a detailed analysis of the cellular immune responses stimulated in the presence of AdDP was performed. In accordance with the observed IgG isotype pattern (i.e., dominant IgG1 response) and the presence of IL-4-secreting cells, mice immunized with AdDP exhibited a major Th2 response pattern. Despite the modest cellular proliferation observed after restimulation in vitro of cells from AdDP-immunized mice, strong MHC class I-restricted responses were also present in these animals. In fact, a significant increment in the number of IFN-γ-secreting cells, as well as a strong in vivo cytotoxic T-lymphocyte response, was observed in mice immunized by the intranasal route.
Considering our previous results and the new data that have emerged from the present study, we conclude that AdDP it is a promising adjuvant able to stimulate not only the humoral immune response but also strong cellular immune responses, which can be exploited for the development of new and more efficient vaccine formulations to prevent or treat human or veterinary diseases.
Acknowledgments
We thank S. Borsutzky for helpful discussions and I. Conte for critical reading of the manuscript.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Azuma, I., and T. Otani. 1994. Potentiation of host defense mechanism against infection by a cytokine inducer, an acyl-MDP derivative, MDP-Lys(L18) (romurtide) in mice and humans. Med. Res. Rev. 14:401-414. [DOI] [PubMed] [Google Scholar]
- 2.Becker, P. D., R. S. Corral, C. A. Guzman, and S. Grinstein. 2001. Adamantylamide dipeptide as effective immunoadjuvant in rabbits and mice. Vaccine 19:4603-4609. [DOI] [PubMed] [Google Scholar]
- 3.Becker, P. D., S. Fiorentini, C. Link, G. Tosti, T. Ebensen, A. Caruso, and C. A. Guzman. 2006. The HIV-1 matrix protein p17 can be efficiently delivered by intranasal route in mice using the TLR 2/6 agonist MALP-2 as mucosal adjuvant. Vaccine 24:5269-5276. [DOI] [PubMed] [Google Scholar]
- 4.Bektimirov, T. A. 1985. Current status of amantadine and rimantadine as anti-influenza-A agents. Bull. W. H. O. 63:51-56. [PMC free article] [PubMed] [Google Scholar]
- 5.Bertot, G. M., P. D. Becker, C. A. Guzman, and S. Grinstein. 2004. Intranasal vaccination with recombinant P6 protein and adamantylamide dipeptide as mucosal adjuvant confers efficient protection against otitis media and lung infection by nontypeable Haemophilus influenzae. J. Infect. Dis. 189:1304-1312. [DOI] [PubMed] [Google Scholar]
- 6.Borsutzky, S., V. Fiorelli, T. Ebensen, A. Tripiciano, F. Rharbaoui, A. Scoglio, C. Link, F. Nappi, M. Morr, S. Butto, A. Cafaro, P. F. Muhlradt, B. Ensoli, and C. A. Guzman. 2003. Efficient mucosal delivery of the HIV-1 Tat protein using the synthetic lipopeptide MALP-2 as adjuvant. Eur. J. Immunol. 33:1548-1556. [DOI] [PubMed] [Google Scholar]
- 7.Coffman, R. L., B. W. Seymour, D. A. Lebman, D. D. Hiraki, J. A. Christiansen, B. Shrader, H. M. Cherwinski, H. F. Savelkoul, F. D. Finkelman, M. W. Bond, et al. 1988. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 102:5-28. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich, F. M., H. K. Hochkeppel, and B. Lukas. 1986. Enhancement of host resistance against virus infections by MTP-PE, a synthetic lipophilic muramyl peptide. I. Increased survival in mice and guinea pigs after single drug administration prior to infection, and the effect of MTP-PE on interferon levels in sera and lungs. Int. J. Immunopharmacol. 8:931-942. [DOI] [PubMed] [Google Scholar]
- 9.Ebensen, T., S. Paukner, C. Link, P. Kudela, C. de Domenico, W. Lubitz, and C. A. Guzman. 2004. Bacterial ghosts are an efficient delivery system for DNA vaccines. J. Immunol. 172:6858-6865. [DOI] [PubMed] [Google Scholar]
- 10.Flegel, M., J. Seifert, H. Farghali, K. Masek, and M. Krojidlo. 1986. Synthesis and pharmacological properties of adamantylamide analogs of muramyl-dipeptide, p. 561-564. In D. Theodoropoulos (ed.), Peptides 1986. Walter de Gruyter and Co., Berlin, Germany.
- 11.Fremont, D. H., E. A. Stura, M. Matsumura, P. A. Peterson, and I. A. Wilson. 1995. Crystal structure of an H-2Kb-ovalbumin peptide complex reveals the interplay of primary and secondary anchor positions in the major histocompatibility complex binding groove. Proc. Natl. Acad. Sci. USA 92:2479-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George, C. X., R. K. Jain, C. M. Gupta, and N. Anand. 1986. Enhancement in anti-Semliki Forest virus activity of ds RNA by a muramyl dipeptide. FEBS Lett. 200:37-41. [DOI] [PubMed] [Google Scholar]
- 13.Hermans, I. F., J. D. Silk, J. Yang, M. J. Palmowski, U. Gileadi, C. McCarthy, M. Salio, F. Ronchese, and V. Cerundolo. 2004. The VITAL assay: a versatile fluorometric technique for assessing CTL- and NKT-mediated cytotoxicity against multiple targets in vitro and in vivo. J. Immunol. Methods 285:25-40. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda, S., T. Negishi, and C. Nishimura. 1985. Enhancement of non-specific resistance to viral infection by muramyldipeptide and its analogs. Antivir. Res. 5:207-215. [DOI] [PubMed] [Google Scholar]
- 15.Inoue, N., S. Kanaoka, S. Yamasaki, S. Arii, and M. Imamura. 1999. Production of specific antibody and T helper 1-dominant cytokine elicited by dendritic cells genetically modified with an adenovirus vector. Immunol. Lett. 70:77-81. [DOI] [PubMed] [Google Scholar]
- 16.Manz, R. A., and A. Radbruch. 2002. Plasma cells for a lifetime? Eur. J. Immunol. 32:923-927. [DOI] [PubMed] [Google Scholar]
- 17.Manz, R. A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature 388:133-134. [DOI] [PubMed] [Google Scholar]
- 18.Masihi, K. N. 2000. Immunomodulators in infectious diseases: panoply of possibilities. Int. J. Immunopharmacol. 22:1083-1091. [DOI] [PubMed] [Google Scholar]
- 19.Masihi, K. N., W. Brehmer, W. Lange, and E. Ribi. 1983. Effects of mycobacterial fractions and muramyl dipeptide on the resistance of mice to aerogenic influenza virus infection. Int. J. Immunopharmacol. 5:403-410. [DOI] [PubMed] [Google Scholar]
- 20.Masihi, K. N., W. Lange, B. Rohde-Schulz, and L. Chedid. 1990. Muramyl dipeptide inhibits replication of human immunodeficiency virus in vitro. AIDS Res. Hum. Retrovir. 6:393-399. [DOI] [PubMed] [Google Scholar]
- 21.Masihi, K. N., W. Lange, S. Schwenke, G. Gast, P. Huchshorn, A. Palache, and K. Masek. 1990. Effect of immunomodulator adamantylamide dipeptide on antibody response to influenza subunit vaccines and protection against aerosol influenza infection. Vaccine 8:159-163. [DOI] [PubMed] [Google Scholar]
- 22.Moser, K., K. Tokoyoda, A. Radbruch, I. MacLennan, and R. A. Manz. 2006. Stromal niches, plasma cell differentiation and survival. Curr. Opin. Immunol. 18:265-270. [DOI] [PubMed] [Google Scholar]
- 23.Rotzschke, O., K. Falk, S. Stevanovic, G. Jung, P. Walden, and H. G. Rammensee. 1991. Exact prediction of a natural T cell epitope. Eur. J. Immunol. 21:2891-2894. [DOI] [PubMed] [Google Scholar]
- 24.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 25.Zidek, Z. 1994. Immune-related edemagenic activity of glutamines and glutamic acid, components of immunomodulatory agents. Agents Actions 42:163-166. [DOI] [PubMed] [Google Scholar]