Abstract
We observed false-positive results in the Platelia Aspergillus enzyme-linked immunoassay (EIA) for specimens from patients with histoplasmosis and mice with experimental infection. Platelia Aspergillus EIA-positive specimens were negative in the second-generation Histoplasma antigen EIA. Care must be taken to exclude histoplasmosis for patients with positive Platelia Aspergillus EIA results.
The Platelia Aspergillus enzyme-linked immunoassay (EIA) detects a galactomannan antigen produced by several molds (5). However, studies to date have not included Histoplasma capsulatum. We recently observed false-positive results in the Platelia Aspergillus EIA for specimens from six patients with culture-proven histoplasmosis, as have others (4). Based upon these observations (Table 1), we conducted a laboratory-based study of specimens submitted for Platelia Aspergillus EIA or Histoplasma antigen testing and evaluated cross-reactivity in experimental models of histoplasmosis and aspergillosis.
TABLE 1.
Clinical specimens identified to have positive results in second-generation Histoplasma EIA and Platelia Aspergillus EIA
| Case no. | Second-generation Histoplasma EIA result (U)a
|
Platelia Aspergillus EIA result for serum (GMI) | Type of histoplasmosisb | |
|---|---|---|---|---|
| Serum | Urine | |||
| 1 | 42.6 | 31.4 | 6.2 | Pulmonary |
| 2 | QNS | 20.4 | 5.5 | Disseminated |
| 3 | 4.2 | 12.5 | 5.5 | Cavitary |
| 4 | 33.9 | 32.0 | 1.5 | Disseminated |
| 5 | 24.2 | ND | 4.7 | Disseminated |
| 6 | 98.7 | 66.8 | 7.8 | Disseminated |
QNS, quantity not sufficient to test; ND, not done.
None of these patients showed evidence of aspergillosis.
(Part of this work was presented at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, 27 to 30 September 2006.)
Residual serum and bronchoalveolar lavage (BAL) fluid specimens that were submitted to MiraVista Diagnostics for Histoplasma antigen testing or Platelia Aspergillus EIA and were positive were retested the following day in the other EIA. The second-generation Histoplasma antigen EIA (MiraVista Diagnostics, Indianapolis, IN) uses polyclonal antibodies to H. capsulatum and has been described elsewhere (6). Results of ≥1 unit were regarded as positive. The Platelia Aspergillus EIA (Bio-Rad Laboratories, Redmond, WA) uses monoclonal antibodies produced against Aspergillus fumigatus. Specimens were pretreated with EDTA for the Platelia Aspergillus EIA and boiled in accordance with the manufacturer's specifications. Results with a galactomannan index (GMI) of 0.5 or greater were reported as positive. Note that the Platelia Aspergillus EIA is not FDA cleared for specimens other than serum.
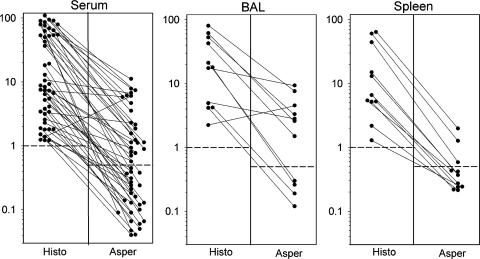
Twenty-three of 48 serum specimens positive for antigen in the second-generation Histoplasma antigen EIA were positive in the Platelia Aspergillus EIA (Fig. 1). Positive results were more frequent for specimens giving levels of 40 units or higher in the Histoplasma antigen EIA (12/17 [70.6%]) than for those giving levels below 40 units (11/31 [35.5%]) (P = 0.043 by chi-square test). As controls, 12 serum specimens that were negative in the Histoplasma antigen EIA were tested in the Platelia Aspergillus EIA, and all were negative. Seven of 11 (63.6%) BAL fluid specimens that were positive in the Histoplasma antigen EIA were positive in the Platelia Aspergillus EIA. Results for the Histoplasma antigen EIA ranged from 2.2 to 61.7 units for the BAL fluid specimens that were positive in the Platelia Aspergillus EIA, compared to 4.2 to 21.2 units for those that were negative. Ten control BAL fluid specimens that were negative in the Histoplasma antigen EIA were negative in the Platelia Aspergillus EIA.
FIG. 1.
Comparison of antigen levels in sera (left) and BAL fluids (middle) from patients with histoplasmosis and in spleen tissues from mice with histoplasmosis (right), tested in the Histoplasma EIA (Histo) and Platelia Aspergillus EIA (Asper). The vertical axis depicts antigen units (Histo) and GMI (Asper). The cutoffs for positivity are 1.0 unit for the second-generation Histoplasma EIA and 0.5 GMI for the Platelia Aspergillus EIA, as shown by broken horizontal lines. Results for the same specimens tested in both assays are connected by solid lines.
Twenty serum specimens that were positive in the Platelia Aspergillus EIA (GMI range, 0.54 to 9.08; median, 1.8) were negative in the Histoplasma antigen EIA. Eighteen BAL fluid specimens that were positive in the Platelia Aspergillus EIA (GMI range, 0.84 to 9.29; median, 6.1) were negative in the Histoplasma antigen EIA.
Nonimmunosuppressed mice were infected intranasally with 106 H. capsulatum yeast cells, and spleens obtained 10 days later were homogenized in 2.0 ml of sterile RPMI (1). The spleen homogenates were tested at a 1:10 dilution in the Histoplasma antigen EIA and a 1:1 dilution in the Platelia Aspergillus EIA to reduce the chance of overlooking low-level cross-reactivity. All animal experiments were done according to institutional guidelines. Spleens from 3 of 11 (27.3%) mice were positive in the Platelia Aspergillus EIA. These three specimens exhibited the highest results in the Histoplasma antigen EIA (44.4 units, 60.5 units, and 64.0 units). Results for the eight spleen homogenates that were negative in the Platelia Aspergillus EIA ranged from 1.3 to 15.0 units in the Histoplasma antigen EIA.
In an experimental model of invasive pulmonary aspergillosis, 1.25 × 108 A. fumigatus conidia (NIH isolate 4215; ATCC MYA-1163) were administered intratracheally to profoundly neutropenic New Zealand White rabbits (n = 9) (Hazelton Research Products, Inc., Denver, PA) (3). Plasma (n = 32) and BAL fluid (n = 7) samples, which were used in another project and had been stored at −70°C for about 2 years, were all positive in the Platelia Aspergillus EIA (plasma GMI range, 0.5 to 5.9 [median, 1.1]; BAL fluid GMI range, 1.5 to 6.8 [median, 6.4]). All were negative in the Histoplasma antigen EIA.
These findings indicate that the antigen detected in body fluids from patients with histoplasmosis is detected in the Platelia Aspergillus EIA. Cross-reactivity correlated with the level of positivity in the Histoplasma antigen EIA, occurring twice as often in specimens with Histoplasma antigen levels of 40 units or more. Cross-reactivity also was observed in spleen tissues from mice with experimental histoplasmosis. Others have reported a false-positive Platelia Aspergillus EIA result for a patient with blastomycosis (2), and we have observed a false-positive Platelia Aspergillus EIA result for a patient with coccidioidomycosis (L. J. Wheat, unpublished data).
Surprisingly, specimens that were positive in the Platelia Aspergillus EIA, including those from rabbits with aspergillosis and very high Aspergillus antigen levels in BAL fluid, were negative in the Histoplasma antigen EIA. The reasons for this discrepancy are not fully understood but may relate to differences in the magnitudes of antigenemia in the two mycoses.
Recognition of false-positive results in the Platelia Aspergillus EIA for specimens from patients with histoplasmosis is important because treatment for aspergillosis may not be effective for histoplasmosis. For example, the echinocandins are neither active nor recommended for histoplasmosis, and experience using voriconazole is insufficient to recommend it. If only the Platelia Aspergillus EIA is ordered and the result is positive, the patient may be treated for aspergillosis without consideration that the result may be due to histoplasmosis.
Care must be taken to exclude histoplasmosis in patients with a positive Platelia Aspergillus EIA if histoplasmosis is deemed more likely than aspergillosis. An absence of severe neutropenia, congenital neutrophil functional impairment, or allogeneic stem cell transplantation makes aspergillosis less likely. Immune deficiency states favoring histoplasmosis include AIDS, solid organ transplantation, and corticosteroid or tumor necrosis factor inhibitor therapy. Disseminated histoplasmosis may also occur in individuals without known causes of immunosuppression, which is very rare for invasive aspergillosis. In patients with recent or past exposure to areas where histoplasmosis is endemic, histoplasmosis should be excluded by antigen testing, serology, and culture of relevant tissue or fluid samples. Although it is rare, dual infection with Aspergillus spp. and H. capsulatum is possible and should be considered for patients with risk factors and clinical findings compatible with both infections.
Acknowledgments
This work was conducted in part through the intramural program of the National Cancer Institute (T.J.W. and R.P.).
We disclose that L.J.W., E.H., M.D., and P.C. are employees of MiraVista Diagnostics, the laboratory that developed the second-generation Histoplasma antigen EIA and performs the Platelia Aspergillus EIA.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Connolly, P., J. Wheat, C. Schnizlein-Bick, M. Durkin, S. Kohler, M. Smedema, J. Goldberg, E. Brizendine, and D. Loebenberg. 1999. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob. Agents Chemother. 43:322-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings, J., G. Jamison, J. Boudreaux, M. Howles, T. Walsh, and R. Hayden. 2006. Abstr. 16th Congr. Int. Soc. Hum. Anim. Mycol., abstr. P-0376.
- 3.Petraitis, V., R. Petraitiene, A. A. Sarafandi, A. M. Kelaher, C. A. Lyman, H. E. Casler, T. Sein, A. H. Groll, J. Bacher, N. A. Avila, and T. J. Walsh. 2003. Combination therapy in treatment of experimental pulmonary aspergillosis: synergistic interaction between an antifungal triazole and an echinocandin. J. Infect. Dis. 187:1834-1843. [DOI] [PubMed] [Google Scholar]
- 4.Ranque, S., F. Dromer, S. Genot, A. Michel-Ngyen, F. Faraut, A. Stein, H. Tissot-Dupont, and H. Dumon. 2006. Abstr. 16th Congr. Int. Soc. Hum. Anim. Mycol., abstr. P-0299.
- 5.Verdaguer, V., T. J. Walsh, W. Hope, and K. J. Cortez. 2007. Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert Rev. Mol. Diagn. 7:21-32. [DOI] [PubMed] [Google Scholar]
- 6.Wheat, L. J., P. Connolly, M. Durkin, B. K. Book, and M. D. Pescovitz. 2006. Elimination of false-positive Histoplasma antigenemia caused by human anti-rabbit antibodies in the second-generation Histoplasma antigen assay. Transplant. Infect. Dis. 8:219-221. [DOI] [PubMed] [Google Scholar]