Abstract
Pneumonic plague is a severe, rapidly progressing disease for which there is no effective vaccine. Since the efficacy of new vaccines cannot be tested in humans, it is essential to develop in vitro surrogate assays that are valid predictors of immunity. The F1 capsule antigen stimulates a protective immune response to most strains of Yersinia pestis. However, strains of Y. pestis that are F1− but still virulent have been isolated, and an in vitro assay, the results which can predict protection against both F1+ and F1− strains, is needed. The virulence antigen (V) is an essential virulence factor of Y. pestis and stimulates protective antibodies. We investigated potential correlates of plague immunity that are based on anti-V antibody-mediated neutralization of Yersinia-induced macrophage cytotoxicity. The neutralizing activity of sera from mice vaccinated with an F1-V fusion candidate vaccine was determined. The decrease in the level of the apoptosis-specific enzyme caspase-3 significantly predicted survival in one- and two-dose vaccination experiments. Sera from F1-V-vaccinated nonhuman primates were evaluated with macrophage assays based on caspase-3 and on other markers manifested at the different stages in cell death. Using murine- and human-derived macrophages in microscopic and fluorescence-activated-cell-sorting-based live/dead staining assays of terminal necrosis, we demonstrated a strong association between in vitro neutralization of macrophage cytotoxicity induced by serum-treated Yersinia and in vivo protection against lethal infection. These results provide a strong base for the development of reliable in vitro correlate bioassays that are predictive of protective immunity to plague.
Pneumonic plague is a severe and rapidly progressing disease for which no fully effective vaccine exists. There is no licensed vaccine available that elicits complete immunity in animal models to pneumonic plague. Although the previously licensed vaccine for plague protected experimental animals against parenteral challenge, it was ineffective against pneumonic challenge (19, 27; M. L. Pitt, unpublished data). Furthermore, the former vaccine, which consisted of killed whole cells of virulent Yersinia pestis (27), did not protect against virulent nonencapsulated (F1−) strains (19) since the vaccine did not contain immunogenic quantities of the virulence antigen (V) or other potentially protective immunogens (8, 9, 21). It was previously demonstrated that a combination of both F1 capsular protein antigen and V effectively protects against both encapsulated (F1+) and nonencapsulated (F1−) strains of Y. pestis (3). New candidate plague vaccines containing a single recombinant F1-V fusion protein or a combination of these two proteins have been developed (19, 49a).
The protective efficacy against lethal challenge of these new candidate plague vaccines cannot be ethically tested in humans. Thus, it is essential that an in vitro surrogate marker that can reliably predict the level of protective immunity in sera from vaccinated individuals be developed. A competitive inhibition enzyme-linked immunosorbent assay (ELISA) of serum anti-F1 antibody (Ab) levels was recently developed and appears to provide a good in vitro correlation with immunity in mice to F1+ Y. pestis (T. C. Chanh et al., unpublished results). However, strains of Y. pestis that are nonencapsulated and F1− but retain full or nearly full virulence have been isolated from vaccinated and naive animals (2, 50). An in vitro assay, the results of which predict protection against both F1+ and F1− virulent strains, is clearly needed.
V antigen is an essential virulence factor of Y. pestis, is highly immunogenic, and confers protection by active vaccination (1, 8, 9, 21, 22, 31, 35). It stimulates production of V-specific Ab that confers protection passively. Passive transfer of both polyclonal and monoclonal anti-V Abs was shown previously to protect against lethal Y. pestis infection (2, 20, 30, 34, 40, 46). In studies with polyclonal anti-V antisera adsorbed with different fragments of V, Motin et al. showed that sera specific for the amino acids 168 to 275 (of the 326-amino-acid V) were protective (32). Hill et al. cloned truncated fragments of V to help identify its antigenic and protective regions (20). Vaccination with the truncated antigens identified regions of V that induce protective immunity. The region of V spanned by amino acids 135 to 275 contained a major protective region, although other regions of V probably contribute. A protective monoclonal Ab (MAb) recognizing this 135- to 275-amino-acid region was isolated (MAb 7.3). However, the results of endpoint ELISAs for anti-V titers and of ELISAs for total serum V antigen-specific immunoglobulin G (IgG) have not consistently correlated with protection (17, 18).
We initiated efforts to establish correlate bioassays of plague immunity based on anti-V Ab-mediated neutralization of macrophage (Mφ) cytotoxicity. The rationale behind these assays is the observation that Y. pestis induces apoptotic cell death in the mouse Mφ-like cell line J774.A1 and in primary Mφs (28, 47). In these assays, infection of the cultures with extracellular Y. pestis leads to Mφ cytotoxicity and apoptotic death. Pretreating the organisms with anti-V Ab can neutralize the in vitro cytotoxic and antiphagocytic activities of Y. pestis, probably by preventing the delivery of the effector Yersinia outer proteins (Yops) into target cells (38, 39, 47). The Ab-mediated inhibitory effect has been hypothesized to occur either directly by preventing the V-dependent assembly and function of the translocation apparatus (33, 47) or indirectly by stimulating phagocytosis of the organisms (12). An assay based on the detection of the apoptosis-associated enzyme caspase-3 was used to detect cytotoxicity-neutralizing anti-V Ab. Caspase-3 activation is considered an indication of very early apoptotic activity in cells (23, 45). This assay, as well as one analyzing lactate dehydrogenase release (LDH; a measure of late-stage apoptosis/necrosis), was described previously (47).
In efforts to establish an in vitro correlate assay of immunity to plague in vaccinated animals, the Mφ cytotoxicity-neutralizing activities of sera from mice and nonhuman primates (NHP) vaccinated with the F1-V fusion antigen and challenged with a lethal strain were tested. The neutralizing activities of sera from mice in caspase-3 assays were significantly predictive of survival. However, similar assays with sera from vaccinated NHP suggested, but did not establish, such a correlation. We evaluated Mφ assays based on other measurable markers that encompassed all of the stages in cell death from the very earliest events to the terminal stages of necrosis. Using human-derived Mφs in assays of the terminal stage of necrosis, we observed a clear association between survival of the vaccinated NHP and protective immunity.
MATERIALS AND METHODS
Bacterial strains and antibodies.
The Y. pestis C092 strain and its derivatives, including the virulent, nonencapsulated C12 strain and an attenuated pgm-, pPst-cured double mutant C092 [pgm−, pPst−], were used (47, 48, 51). Y. pseudotuberculosis strain YpIII(pIB19:pTrcV) (Y. pseudotuberculosis pTrcV) has an in-frame deletion mutation of the plasmid pLcr-encoded lcrV gene and is transformed with the recombinant pTrcV expression plasmid (38). Polyclonal anti-V IgG was purified by protein A-Sepharose chromatography from the sera of rabbits vaccinated with purified His-tagged recombinant Y. pestis V (rV) (49). MAbs to rV were IgG purified from hybridoma culture supernatants and assessed for binding of rV by ELISA. The anti-V MAbs 7.3, 101.3, and 104.3, kindly provided by J. Hill (Defence Science and Technology Laboratory, Porton Down, United Kingdom) and described previously (20), were tested for passive protection in mice challenged subcutaneously (s.c.) with Y. pestis (20); MAbs provided by T. C. Chanh (U.S. Army Medical Research Institute of Infectious Diseases) were evaluated for protective efficacy against Y. pestis strain C12 by using a murine aerosol challenge model.
Murine and human cell lines.
The J774.A1 cell line (ATCC TIB-67), a Mφ-like cell line derived from BALB/c mice with reticulum cell sarcoma, was maintained in Dulbecco minimum essential medium with 4 mM l-glutamine, 1.5 g of sodium bicarbonate/liter, and 4.5 g of glucose/liter supplemented with 10% fetal bovine serum (FBS) and is active in Ab-dependent phagocytosis. The KG-1 cell line (ATCC CCL-246), a human myeloblast cell line derived from a patient with acute myelogenous leukemia, was maintained in Iscove modified Dulbecco minimal essential medium (IMDM) supplemented with 20% FBS. The HL60 cell line (ATCC CCL-240), a human promyeloblast cell line derived from a patient with acute promyelocytic leukemia, was maintained in IMDM supplemented with 10% FBS; it displays phagocytic activity, responds to chemotactic stimuli, and exhibits a low rate of spontaneous differentiation. Both of the latter cell lines are nonadherent, in contrast to the adherent J774.A1 cells, and were used in suspension.
Vaccination of animals and collection of sera. (i) Mice.
Sera for testing the association between survival and the serum cytotoxicity neutralizing activity were obtained from mice vaccinated with either one or two doses of the F1-V fusion protein. These sera were obtained as part of our separate studies or were provided by T. C. Chanh. In one experiment, mice received two doses s.c. of F1-V (10, 5, 1, or 0.1 μg) and were challenged s.c. with a 50% lethal dose (LD50) of 5 × 107 of Y. pestis C092. In another experiment, mice were placed in seven dose groups. In six of them, each animal was vaccinated with a single dose ranging from 0.1 to 30 μg of F1-V/ml; the animals in the seventh group were negative controls that were vaccinated only with the adjuvant (Alhydrogel [HCL Biosector, Frederikssund, Denmark]). In a third experiment, mice were placed in five dose groups, and each was vaccinated with a single dose ranging from 0.1 to 10 μg of F1-V (0, 0.1, 1, 5, and 10 μg)/ml or with Alhydrogel alone. The mice in all of these experiments were challenged s.c. with the C092 strain of Y. pestis.
Sera for testing the association between survival of vaccinated mice challenged with F1-negative Y. pestis and the serum cytotoxicity neutralizing activity were obtained. These samples were from an experiment comparing the F1-V fusion protein with vaccines composed of either a mixture of F1 and V or of V alone. Mice were given two s.c. doses of antigen in Alhydrogel 6 weeks apart (35.2 μg of F1-V, 25.2 μg of V, 20 μg of V plus 10 μg of F1, or Alhydrogel alone for the controls). Mice were challenged s.c. with one of three doses of the virulent C12 strain 6 weeks after the boost. Mice were in groups of 15 per challenge dose of 107, 108, or 109 CFU.
(ii) Nonhuman primates.
Sera from cynomolgus macaques (CM) and African green (Chlorocebus aethiops) monkeys (AGM) vaccinated s.c. three times at 4-week intervals with the F1-V fusion protein, 150 μg/dose, were also tested. These sera were obtained from separate studies, two experiments (experiments 1 and 2) with CM and one experiment with AGM, and were provided in part by L. Pitt. The animals were challenged by aerosol 6 to 7 weeks after the last dose with Y. pestis strain C092 (CM and AGM group 2) or strain C12 (AGM group 1).
Sera for use in the in vitro assays were collected from mice and NHP just before vaccination, 2 weeks after each vaccine dose, and before challenge. The Ab titers to V and F1 were determined by endpoint dilution ELISA (1-3, 14, 21).
In vitro assays of Ab neutralization of macrophage cytotoxicity.
The J774.A1 Mφ cell line was used except as indicated in assays below. A total per well of 106 Mφs in Dulbecco modified Eagle medium with 9% FBS were seeded into microtiter plates 24 h before their use in the assay (47). Inocula were prepared by inoculating a slant of tryptose blood agar base and 100 μg of ampicillin and 50 μg of kanamycin/ml with Y. pseudotuberculosis pTrcV or a sheep blood plate with Y. pestis, using a small loopful from a thawed single-use stock freezer vial. The cultures were incubated for 2 to 3 days at room temperature. The growth was then harvested in 5-ml heart infusion broth (HIB) for Y. pestis or in brain heart infusion broth (BHI) for Y. pseudotuberculosis, and the turbidity was adjusted to an optical density at 620 nm of 1.0. The suspension was next diluted 100-fold into HIB with 0.2% xylose (Y. pestis) or BHI with 20 mM MgCl2 and 5 mM EGTA (BHI+) (Y. pseudotuberculosis) and incubated at room temperature on an orbital shaker with gentle agitation for 16 to 24 h. The following day, the cultures were diluted into fresh Eagle basal medium with Earle's salts containing HEPES (EBME) and 7.5% FBS, centrifuged for 10 min at 1,500 rpm, and resuspended in 10 ml of EBME with 7.5% FBS. A 9.5-ml volume of EBME was inoculated with 0.5 ml of a resuspended culture and incubated at 37°C for 2 h with gentle agitation. After 2 h of incubation, the cultures were centrifuged for 10 min at 1,500 rpm, and the pellet was resuspended in prewarmed (37°C) EBME with 2 mM Glutamax without antibiotics and adjusted to an A620 of 0.5 in EBME (47). Diluted sera or Abs (10 μl) were added to bacterial suspensions (500 μl) and incubated at 38°C with shaking for 5 min. The seeded plate was washed, fresh medium was added, and the bacterial samples were added. The plate was centrifuged for 5 min at 50 × g and then incubated at 37°C with 5% CO2 for 2 h. The cells were washed with phosphate-buffered saline (PBS), medium containing 50 μg of gentamicin/ml was added, and the plate was reincubated for 1 h. After a wash with Ca2+/Mg2+-free 10 mM PBS, the cells were treated as described for the individual protocols. Exceptions to this procedure are indicated in each protocol below. All assays included three controls: uninfected cells, cells infected with rabbit polyclonal anti-V Ab-pretreated Yersinia, or cells infected with untreated Yersinia.
Caspase-3 microtiter apoptosis assay.
The assay is a modification of one described previously (47). The plates were prepared and incubated as described above to induce apoptosis and included the indicated controls. Caspase-3 activity was measured with an EnzChek caspase-3 assay kit (Molecular Probes) as detailed by the manufacturer. The percent decrease in caspase-3 produced by Mφs exposed to bacteria pretreated with sera collected during the course of vaccination was assessed statistically as a predictor of survival. The results reported for the caspase-3 assays are the mean of the relative fluorescent units for three replicates. In instances in which an individual value differed by more than 10% from the mean, the value was discarded as an outlier, and the test for that sample was repeated (a total of 6 samples out of about 1,000). In the one-dose mouse experiment (four groups receiving 0.1 to 10 μg of F1-V fusion), for 2 of 64 naive samples and 1 of 64 samples from day 28, one of the three replicates was an outlier; in the two-dose mouse experiment, for 3 of 124 naive samples, one of three replicates was an outlier. For all of these samples, the entire sample set was repeated and, upon repeat, no outliers were detected. For analysis purposes, the percent apoptosis was determined by dividing the value for each sample by the value for the cells plus untreated bacteria (Y. pseudotuberculosis pTrcV) control sample, which was set as 100%.
DNA fragmentation (TUNEL [terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling]) assay by fluorescence-activated cell sorting (FACS).
The human-derived KG-1 cells were grown in suspension, and wells of a 24-well plate were seeded with 106 cells. The assay (DeadEnd Fluorometric TUNEL System; Promega) was performed according to the manufacturer's instructions. In brief, the cell cultures were treated as described above to induce apoptosis and included the indicated controls. At 30 min before infection, the cells were refed with medium containing 0.5 μg of cytochalasin D/ml. Serum- or MAb-treated bacterial suspensions were added in medium containing cytochalasin D, and the plates were incubated for 2 h at 37°C. Suspensions were then transferred to microfuge tubes, washed with PBS, resuspended in 1 ml of IMDM with FBS and 50 μg of gentamicin/ml, and incubated an additional 46 h at 37°C. After a wash in PBS, 1% formaldehyde (Tousimis) was added, and the cells were incubated on ice, washed, and resuspended in 0.2% Triton X-100. After 5 min of incubation, they were labeled with fluorescein isothiocyanate (FITC) according to the kit's instructions, and the samples were read and analyzed on a FACSCalibur flow cytometer (BD Biosciences).
Annexin V apoptosis assay by FACS.
KG-1 cells were prepared and exposed to Ab-pretreated or untreated Yersinia as described for the TUNEL assay. The cultures were incubated at 37°C as specified by the supplier and resuspended in binding buffer. Annexin V-FITC (20 μg/ml) and propidium iodide (PI; 50 μg/ml) were added, and the samples were incubated 15 min. Analysis was performed by FACS using FL1 (FITC green, 488-nm) and FL2 (PI red, >620-nm) channel profiles.
L/D viability staining for microscopy.
J774.A1 cells were seeded and grown in LabTek chambers (Nunc) and treated to induce apoptosis as described above. After the slide was washed five times with PBS, 500 μl of live/dead (L/D) stain (PBS with 5 μg of PI/ml and 2.5 mM Syto-13) per well was added, and the slides were incubated for 30 min at 37°C. The chambers were removed, and the slides were observed on a Nikon E800 fluorescence microscope using a triple-bypass filter to detect Syto-13 and PI staining. Syto-13 is a green fluorescent cell-permeable dye that stains DNA, and PI is a red fluorescent dye that also stains DNA but is impermeable to intact cell membranes. Live cells stain green, and apoptotic cells, which have compromised membrane integrity, stain with both dyes, with the red dye being predominant microscopically. Viable and dead cells were counted from photomicrographs (≥3 fields and/or 200 cells were counted per sample). The results for the L/D microscopy assay were displayed as the percent live or percent dead. These values were calculated from the number of cells with the staining pattern of interest divided by the total number of cells counted.
L/D viability assay by FACS.
HL60 cells were prepared, exposed to Ab-pretreated or untreated Yersinia in a 24-well tray, and resuspended in medium with FBS and 50 μg of gentamicin/ml, as was done for the TUNEL assay, except that cytochalasin D was not included. After incubation for an additional 46 h at 37°C, the samples were transferred to microfuge tubes, washed, and resuspended in the L/D stain described above. The cells were incubated with the stain for 30 min at 37°C, 1 ml of PBS was added, and the samples were then read within 1 h by FACS. All assays included the three controls described above. Settings for the FACS assay were as follows: a total of at least 10,000 events were counted with FL-1 and FL-2 (green and red channels) and displayed as logarithmic values. The intensity of Syto-13 staining was displayed on the x axis, and the intensity of PI staining is shown on the y axis. The histogram was acquired and viewed as a four-color image of FL-1 versus FL-2, and the threshold and compensation were adjusted to obtain the optimal separation. For testing animal sera the bacteria were pretreated with sera from animals collected during the vaccination period to determine their cytotoxicity-neutralizing activity. The collection days were as follows: day 0 (baseline for animal prior to vaccination), 2 weeks after each vaccination, and a day or two before challenge. The results reported for the FACS L/D assays are the total number of events present in each stained population for three replicates of each sample as determined by FACS counting. In instances in which an individual value differed by >10% from the mean, the value was discarded as an outlier and the test for that sample was repeated; the results of the latter were all usable (i.e., within 10% of the mean). For the experiment with the 12 C092-challenged AGM, one of the three replicates of one of the naive serum samples was an outlier; for CM experiment 1 involving 12 animals, one of the three replicates of one serum collected on day 42 was an outlier. The results for the FACS L/D assay are displayed as the percent live or percent dead. These values were calculated from the number of cells with the staining pattern of interest divided by the total number of cells counted.
Statistical analysis.
Ab titers were log10 transformed before analysis. The influences of Ab titers, the percent decrease in caspase-3, and the percent live cells in L/D staining assays on survival were tested by logistic regression and the Cox proportional hazards regression model. The statistical relationship between in vitro assays was determined by Pearson product-moment correlations. Differences in assays and titers between doses and over time were evaluated by analysis of variance with Tukey's and Dunnett's post-hoc tests. Survival rates were compared between doses and groups by the Fisher exact tests with step-down bootstrap adjustment. Mean times to death between doses were compared by using the Student t tests with step-down bootstrap adjustment. Survival curves were estimated by Kaplan-Meier analysis and compared between groups by using log-rank tests.
All research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). The facility where this research was conducted is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
RESULTS
Correlates of immunity in mice. (i) Anti-V MAb neutralization of Mφ apoptosis and necrosis in vitro correlates with protective immunity.
In previous studies, Weeks et al. showed that anti-V antigen Ab could inhibit the Y. pestis-induced cytotoxicity of J774.A1 Mφs in vitro (47). To verify the Mφ protective effects of anti-V Ab, anti-V MAbs that had been shown previously to be passively protective or nonprotective in mice challenged with Y. pestis were tested. Three in vitro assays of Mφ cytotoxicity—one that measures the apoptosis-associated caspase-3 enzyme and two L/D staining assays for necrosis—were used to test the association between in vitro MAb-mediated cytotoxicity neutralization and in vivo protection. The three assays are described in more detail below. In all three assays, MAb 7.3, shown to protect mice against lethal challenge with Y. pestis (20), was associated with significantly enhanced protection of Mφs compared to several nonprotective anti-V MAbs or to an unrelated irrelevant MAb specific for Burkholderia mallei (P < 0.05). Neither the nonprotective MAbs nor the unrelated MAb significantly reduced levels of necrosis or apoptosis as measured by either the L/D assay or the caspase-3 assay (data not shown). The results of all three assays correlated with each other (P < 0.0001 in a combined analysis). These results confirmed our previous comparisons of the protective MAb 7.3 and two nonprotective anti-V MAbs (MAb 101.3 and MAb 104.3) using an in vitro LDH release assay of Mφ necrosis (47).
(ii) Neutralization of Mφ apoptosis by Ab in vitro correlates with immunity in vaccinated mice.
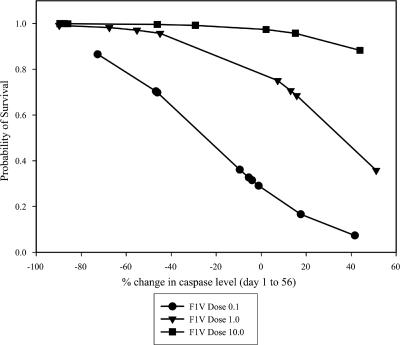
Five groups of mice were vaccinated s.c. with two doses of F1-V fusion and challenged s.c. with Y. pestis C092 as described in Materials and Methods and in Fig. 1. All of the sera collected from vaccinated animals during the course of the vaccination period (days 0 to 56) were titrated and assayed for their ability to neutralize the production of caspase-3 by Yersinia-infected Mφ cultures. The association between survival and two parameters—serum cytotoxicity neutralizing activity as determined by the level of Mφ caspase-3 and dose of vaccine—was assessed. The decline in Mφ caspase-3 levels in assays determined using sera from days 1 to 56, together with the vaccine dose, was significantly associated with survival (Fig. 1). The change in amount of caspase-3 produced by Mφs in response to infection with serum-pretreated yersiniae is shown; a negative value indicates that the level of caspase-3 in Mφ cultures infected with the treated bacteria decreased from day 1 to day 56. Survivors tended to have negative values; an increase in the Mφ protective activity of the sera with time was associated with a reduction in apoptosis and thus also in the production of caspase-3. The development of increasing amounts of serum caspase-3-neutralizing activity during vaccination was associated with survival. The positive association between survival and vaccine dose is also illustrated in Fig. 1.
FIG. 1.
Serum caspase-3 neutralizing activity and survival after challenge of mice vaccinated twice with F1-V. Mφs were exposed to Y. pseudotuberculosis pTrcV that had been pretreated with serum from vaccinated mice as described in Materials and Methods. The infected cells were lysed, and the caspase-3 apoptosis-specific enzyme was measured by immunoassay. The association between the percent change in caspase levels in Mφs incubated with organisms and sera and the probability of survival is shown; the sera were collected over the course of the vaccination period. The caspase levels in Mφs were determined for the day 0, 14, and 35 sera and for the prechallenge day 56 sera, and the percent changes in caspase from day 0 to days 14, 35, and 56 were calculated. The decline in Mφ caspase-3 levels in assays determined using sera from days 1 to 56, together with the vaccine dose, was significantly associated with survival (P = 0.0014 by regression analysis). The F1-V vaccine doses were as follows: 10 μg (▾, 9 of 9 survived), 5 μg (5 of 10 survived), 1 μg (▪, 12 of 15 survived), 0.1 μg (•, 6 of 14 survived), and 0 μg (Alhydrogel-only control, 0 of 9 survived).
In a separate dose-ranging study, seven groups of mice (15 mice/group except 5 in the alhydrogel control group) were vaccinated s.c. with a single dose of F1-V as described in Materials and Methods. Sera were assayed just before challenge on day 28. There was no significant association between mouse survival outcome and vaccine dose (P = 0.64), anti-V titers (P = 0.23), or anti-F1 titers (P = 0.06), although the latter approached significance. In contrast, survival outcome was significantly associated with the caspase-3 levels present in Mφs infected with serum-pretreated bacteria. Both the MΦ caspase-3 levels on day 28 and the percent change in caspase-3 levels from days 0 to 28 were significantly associated with survival (P = 0.031 and P = 0.007, respectively; data not shown).
(iii) Serum samples tested in the caspase-3 and L/D cytotoxicity assays demonstrate comparable correlation.
Correlate assays of immunity should be capable of predicting protection in all animal models of plague. Caspase-3 assays of Mφ cytotoxicity neutralization were able to predict survival in mice; however, similar assays with sera from vaccinated NHP did not establish such a correlation. As detailed below, viability assays that detected the terminal stage of apoptosis (necrosis) and which were done using human-derived Mφs were promising candidates for an in vitro correlate of protection. To evaluate these assays (L/D staining) as predictors of immunity in both species, we tested them using sera from mice vaccinated with different V-containing vaccines. In the first experiment, sera from a total of 64 mice that had been vaccinated once with one of four doses of F1-V (0.1, 1, 5, or 10 μg) or were sham vaccinated and then challenged s.c. 28 days later with the Y. pestis C092 strain were tested as described in Materials and Methods and in Table 1. The sera from these mice were assayed for their Mφ cytotoxicity activity and Ab titers; for the former, they were tested in both HL60 cells (L/D FACS) and J774.A1 cells (L/D microscopy and caspase-3 assays). Although only 2 of the 64 mice challenged survived, the mice differed in their survival times, and statistical analyses of the predictive capacity of the three MΦ assays were performed using the Cox proportional hazards regression model. When the results of the assays with the day 14 sera or the day 0 and 14 sera combined were evaluated, all three correlated significantly with each other despite the lack of a consistent correlation with either anti-V Ab titer or vaccine dose (Table 1 and data not shown); the results with naive sera (day 0) did not yield a significant correlation. All three Mφ assays were also found to be significant predictors of survival time (P < 0.0001; data not shown). In contrast, neither the vaccine dose nor the anti-V Ab titers correlated with the Mφ assay results, nor were they significantly associated with survival time (P = 0.544; Table 1 and data not shown). Thus, even in the absence of overall protection, the in vitro Mφ assays could detect a vaccination-associated effect on survival time, and the data supported the conclusion that both the caspase-3 and L/D assays are reliable predictors of the relative immunity of vaccinated mice.
TABLE 1.
Summary of the correlation between results of three in vitro assays using sera from mice vaccinated with one dose of F1-Va
| Serum sample(s) | Correlation variable (assay)b | Correlation (P)
|
||||
|---|---|---|---|---|---|---|
| Vaccine dose | % Liveb
|
Caspase | Anti-V titer | |||
| FACS | Micro | |||||
| Combined days 0 + 14 | Caspase | 0.0262 | <0.0001 | <0.0001 | - | - |
| % Live (FACS) | NS | - | <0.0001 | <0.0001 | - | |
| % Live (Micro) | NS | <0.0001 | - | <0.0001 | - | |
| Day 0 | Caspase | <0.0001 | 0.0003 | NS | - | - |
| % Live (FACS) | 0.0002 | - | NS | 0.0003 | - | |
| % Live (Micro) | NS | NS | - | NS | - | |
| Day 14 | Anti-V titer | 0.0169 | NS | NS | NS | - |
| Caspase | 0.0157 | <0.0001 | <0.0001 | - | NS | |
| % Live (FACS) | NS | - | <0.0001 | <0.0001 | NS | |
| % Live (Micro) | NS | <0.0001 | - | <0.0001 | NS | |
The sera tested were from mice vaccinated once with one of five doses of F1-V (from 0.1 to 10 μg) and challenged s.c. 28 days after vaccination with Y. pestis C092. Statistical comparisons of the results of assays using sera collected just before vaccination (day 0) and at day 14 were done as described in the text. NS, not significant (P > 0.05). -, cells for which a correlation was not determined or was not applicable.
Micro, microscopy. FACS-based and microscopy staining assays were used to determine L/D viability, and the percentages of live cells observed were used for correlation analyses.
(iv) Ab neutralization of Mφ cytotoxicity in vitro correlates with immunity to virulent F1 capsule-negative Y. pestis.
To further evaluate the candidate in vitro correlate assays, sera from mice challenged with the virulent F1-negative C12 strain were tested. Animals were vaccinated twice with one of three different vaccines (V alone, F-V fusion, or F1+V). They were challenged s.c. with one of three different doses of the C12 strain, in groups of 15 mice each (150 mice total), as shown in Table 2. The challenge dose was a significant predictor of survival outcome (P < 0.0001). Each vaccine elicited significant protection against C12 (all three challenge doses combined and 107 LD50 alone) compared to the alhydrogel control group (P ≤ 0.029 for the three vaccines combined; P = 0.0018 for the F1-V fusion and V vaccines alone; and P = 0.043 for the F1+V component vaccine). The F1-V fusion vaccine (only) also resulted in a significantly greater mean survival rate than the control group against a 108 LD50 challenge dose (P = 0.043). None of the vaccines afforded overall protection of animals challenged with a dose of 109 LD50 Y. pestis C12; however, each resulted in significantly extended survival in comparison to the control group (P ≤ 0.003) by log-rank tests of survival curves.
TABLE 2.
Results of cytotoxicity assays with sera from mice challenged with Y. pestis strain C12a
| Vaccine (challenge dose [LD50]) | Control sample or TTDb | Antibody neutralizationc
|
FACS L/D
|
Microscopy L/D
|
Caspase-3
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Anti-F1 titer | Anti-V titer | n | % Lived | n | % Lived | n | % Apoptotice | ||
| None | Cells alone | 3 | 95.61 | 1 | 99.83 | 1 | 1.68 | |||
| Cells + pTrcV | 3 | 12.96 | 1 | 35.18 | 1 | 100.00 | ||||
| Cells + pTrcV + anti-V | 3 | 87.40 | 1 | 80.65 | 1 | 3.86 | ||||
| Adjuvant Alhydrogel (107) | 1-3 days | 9 | 320 | 320 | 9 | 13.43 | 6 | 46.45 | 1 | 83.27 |
| 4-6 days | 3 | 320 | 427 | 3 | 16.55 | 3 | 47.74 | 1 | 78.29 | |
| V (107) | 1-3 days | 2 | 320 | 20,800 | 2 | 12.48 | 2 | 36.50 | 1 | 79.08 |
| 4-6 days | 2 | 320 | 21,760 | 2 | 20.95 | |||||
| 7-9 days | 1 | 320 | 81,920 | 1 | 49.17 | |||||
| Survivor | 5 | 320 | 47,104 | 5 | 73.51 | 3 | 76.70 | 2 | 8.44 | |
| V (108) | 1-3 days | 3 | 427 | 34,133 | 3 | 10.89 | 1 | 38.94 | 1 | 84.43 |
| 4-6 days | 3 | 427 | 69,973 | 2 | 28.03 | 2 | 52.53 | 1 | 48.99 | |
| 7-9 days | 3 | 320 | 54,613 | 3 | 54.89 | 1 | 69.56 | |||
| Survivor | 1 | 640 | 40,960 | 1 | 75.07 | 1 | 83.62 | 1 | 9.38 | |
| V (109) | 1-3 days | 3 | 320 | 81,933 | 3 | 11.81 | 2 | 33.09 | ||
| 4-6 days | 8 | 320 | 21,120 | 8 | 23.81 | 6 | 47.27 | 2 | 67.18 | |
| 7-9 days | 2 | 320 | 46,080 | 2 | 48.41 | 2 | 63.77 | 1 | 34.86 | |
| F1-V (107) | 1-3 days | 2 | 2,560 | 204,800 | 2 | 7.64 | 2 | 37.02 | 1 | 78.82 |
| 4-6 days | 4 | 9,600 | 107,520 | 4 | 21.25 | 2 | 53.78 | 1 | 46.88 | |
| Survivor | 9 | 15,076 | 197,973 | 8 | 73.54 | 6 | 76.62 | 1 | 9.23 | |
| F1-V (108) | 1-3 days | 1 | 5,120 | 20,420 | 1 | 6.60 | 1 | 36.16 | 1 | 78.34 |
| 4-6 days | 5 | 3,968 | 36,864 | 5 | 26.40 | 5 | 50.18 | |||
| 7-9 days | 4 | 10,240 | 46,080 | 4 | 49.97 | 4 | 67.45 | 1 | 37.35 | |
| Survivor | 4 | 20,480 | 56,320 | 4 | 79.27 | 4 | 80.34 | 1 | 9.44 | |
| F1-V (109) | 1-3 days | 5 | 9,344 | 75,776 | 5 | 11.54 | 4 | 37.81 | 1 | 81.49 |
| 4-6 days | 5 | 5,780 | 245,760 | 5 | 34.41 | 4 | 53.41 | |||
| 7-9 days | 5 | 2,816 | 139,263 | 4 | 62.95 | 5 | 73.11 | 2 | 16.74 | |
| F1+V (107) | 1-3 days | 3 | 3,947 | 218,667 | 3 | 14.56 | 2 | 37.85 | 1 | 72.75 |
| 4-6 days | 2 | 61,440 | 368,640 | 2 | 26.68 | 2 | 47.42 | |||
| 7-9 days | 2 | 5,280 | 21,760 | 2 | 65.71 | 2 | 72.45 | 1 | 26.92 | |
| Survivor | 3 | 68,480 | 225,280 | 3 | 84.75 | 3 | 83.79 | 1 | 9.94 | |
| F1+V (108) | 1-3 days | 3 | 28,373 | 13,760 | 2 | 12.91 | 3 | 33.89 | 1 | 76.10 |
| 4-6 days | 5 | 92,160 | 25,600 | 5 | 30.60 | 3 | 53.43 | 1 | 43.58 | |
| 7-9 days | 4 | 128,000 | 57,600 | 4 | 46.75 | 3 | 73.61 | |||
| Survivor | 3 | 273,460 | 47,787 | 3 | 81.53 | 3 | 82.75 | 1 | 8.79 | |
| F1+V (109) | 1-3 days | 4 | 11,360 | 43,200 | 4 | 14.11 | 3 | 37.09 | 1 | 72.57 |
| 4-6 days | 6 | 335,787 | 276,480 | 5 | 21.06 | 6 | 48.52 | |||
| 7-9 days | 4 | 176,640 | 327,655 | 4 | 64.08 | 3 | 69.78 | 1 | 33.04 | |
| Survivor | 1 | 655,360 | 655,360 | 1 | 93.11 | 1 | 93.78 | 1 | 5.65 | |
Sera were collected prechallenge from a total of 124 mice vaccinated twice with one of three vaccines (V alone, F-V fusion, or F1+V) and assayed for their Mφ cytotoxicity activity and Ab titers. Three Mφ assays were compared: a caspase-3 microtiter assay with J774.A1 cells, an L/D microscopy assay with J774.A1 Mφs, and an L/D FACS assay of infected HL60 cells. The results using sera obtained 1 week before s.c. challenge with the F1-negative C12 strain of Y. pestis are shown. Controls included Mφs incubated in the absence of bacteria or Mφs incubated with bacteria alone or with bacteria pretreated with protective rabbit anti-V Ab, as well as a group that received adjuvant (Alhydrogel) but no vaccine before challenge. n, number of sample sets averaged to obtain the titer or percent results. Each sample set for the FACS, microscopic, and caspase-3 assays included three replicates.
TTD, time to death postchallenge (in days).
Anti-F1 and anti-V titers are shown as the reciprocal titer in endpoint dilution ELISAs.
Values are percentages of live cells.
Values are percentages of apoptotic cells (normalized to the percentage of apoptotic cells in the untreated infected control).
A total of 124 sera collected one week before challenge were available for testing in the Mφ cytotoxicity assays. As illustrated in Table 2, in L/D FACS tests there was an increasing reduction in the levels of cytotoxicity as the time to death increased, with sera from nonsurvivors that succumbed early showing a much higher level of in vitro cytotoxicity than sera from animals that survived longer. These results suggested that protection of animals against challenge with C12 can be predicted using results of the in vitro Mφ assays (Table 2 and data not shown). Using logistic analysis, the correlation of the L/D cytotoxicity assays with survival was significant. The percentages of live cells in both the microscopy and the FACS L/D assays were significant predictors of survival (P = 0.0024 and P = 0.0065, respectively). There were insufficient numbers of samples available for testing in the caspase-3 assay to allow analysis of association of this assay with survival. However, the results of the caspase-3 data were clearly consistent with those of the viability assay (data not shown). In addition, the results of the three Mφ cytotoxicity neutralization assays overall correlated well with each other, as shown in Table 3. The correlation included all vaccine and challenge dose groups. These results again confirmed the validity of all three Mφ assays and also confirm that the assays are valid for predicting resistance to challenge by an F1-negative strain.
TABLE 3.
Correlation between results of in vitro assays of sera from mice challenged with Y. pestis strain C12a
| Vaccine | Challenge dose | Correlation variable (assay)c | Correlation (P)b
|
||||
|---|---|---|---|---|---|---|---|
| % Livec
|
Caspase | Anti-F1 titer | Anti-V titer | ||||
| FACS | Micro | ||||||
| F1+V | All | Anti-V titer | 0.005 | NS | 0.007 | 0.010 | - |
| Anti-F1 titer | NS | NS | NS | - | 0.010 | ||
| Caspase | 0.0004 | <0.0001 | - | NS | 0.007 | ||
| % Live (FACS) | - | <0.0001 | <0.0001 | NS | 0.005 | ||
| % Live (Micro) | <0.0001 | - | <0.0001 | NS | NS | ||
| F1-V | All | Anti-V titer | NS | 0.048 | 0.043 | NS | - |
| Anti-F1 titer | 0.015 | 0.028 | NS | - | NS | ||
| Caspase | 0.0002 | <0.0001 | - | NS | 0.043 | ||
| % Live (FACS) | - | <0.0001 | 0.0002 | 0.015 | NS | ||
| % Live (Micro) | <0.0001 | - | <0.0001 | 0.028 | 0.048 | ||
| V | All | Anti-V titer | NS | NS | NS | NS | - |
| Anti-F1 titer | NS | NS | NS | - | NS | ||
| Caspase | <0.0001 | <0.0001 | - | NS | NS | ||
| % Live (FACS) | - | <0.0001 | <0.0001 | NS | NS | ||
| % Live (Micro) | <0.0001 | - | <0.0001 | NS | NS | ||
| All (combined) | 107 | Anti-V titer | NS | NS | NS | 0.004 | - |
| Anti-F1 titer | NS | NS | NS | - | 0.004 | ||
| Caspase | 0.0002 | 0.0002 | - | NS | NS | ||
| % Live (FACS) | - | <0.0001 | 0.0002 | NS | NS | ||
| % Live (Micro) | <0.0001 | - | 0.0002 | NS | NS | ||
| All | 108 | Anti-V titer | 0.0137 | NS | NS | NS | - |
| Anti-F1 titer | NS | NS | NS | - | NS | ||
| Caspase | <0.0001 | <0.0001 | - | NS | NS | ||
| % Live (FACS) | - | <0.0001 | <0.0001 | NS | 0.014 | ||
| % Live (Micro) | <0.0001 | - | <0.0001 | NS | NS | ||
| All | 109 | Anti-V titer | 0.001 | 0.0031 | 0.0040 | 0.001 | - |
| Anti-F1 titer | 0.002 | NS | 0.0050 | - | 0.001 | ||
| Caspase | 0.001 | <0.0001 | - | 0.005 | 0.004 | ||
| % Live (FACS) | - | <0.0001 | 0.001 | 0.002 | 0.001 | ||
| % Live (Micro) | <0.0001 | - | <0.0001 | NS | 0.003 | ||
| All | All (combined) | Anti-V titer | 0.0002 | 0.006 | 0.0004 | 0.0012 | - |
| Anti-F1 titer | 0.003 | 0.012 | NS | - | 0.001 | ||
| Caspase | <0.0001 | <0.0001 | - | NS | 0.0004 | ||
| % Live (FACS) | - | <0.0001 | <0.0001 | 0.003 | 0.0002 | ||
| % Live (Micro) | <0.0001 | - | <0.0001 | 0.012 | 0.006 | ||
Sera tested were collected prechallenge from a total of 124 mice vaccinated twice with one of three vaccines (V alone, F1-V fusion, or F1+V). The mice were challenged s.c. with Y. pestis C12 (107, 108, or 109 LD50s).
Correlations were determined to be significant (P < 0.05) or not significant (NS; P > 0.05) by Pearson product-modified correlation analysis. -, the correlation was not determined or was not applicable.
Micro, microscopy. FACS-based and microscopy staining assays were used to determine L/D viability, and the percentages of live cells observed were used for correlation analyses.
In contrast, the anti-V Ab titers correlated consistently with results of the Mφ assays and with the anti-F1 Ab titers only when analysis was done with samples from the very highest challenge dose or from all challenge dose groups combined and using sera from all three vaccine groups combined (Table 3). The association of the anti-F1 Ab titers with results of the Mφ assays varied (Table 3). Nevertheless, both the anti-V and the anti-F1 Ab titers significantly predicted survival (P = 0.0449 and P = 0.023, respectively). Thus, in these experiments, both the Mφ and the Ab titration assays appeared to serve as significant predictors of mouse survival. These results are in contrast to those obtained in the other one- and two-dose F1-V mouse studies described above and in the studies involving both strains of NHP (detailed below).
Correlates of immunity in nonhuman primates.
The caspase-3 assay of Mφ cytotoxicity neutralization did not consistently predict survival in NHP when performed with either the murine- or human-derived cell lines (data not shown). The negative results suggested that the caspase-3 marker did not optimally reflect the cytotoxicity-neutralizing activity of the NHP Abs. Assays that measure activities that span all of the stages in cell death were evaluated. They included assays for combined caspase-3 and caspase-7 levels (early stage); annexin V binding or loss of mitochondrial potential (late early); DNA fragmentation (TUNEL assay) (intermediate); and differential L/D fluorescence staining, LDH release, or ATP detection (late, terminal).
In preliminary studies with both mouse- and human-derived cells, we found several of the assays to be unsuitable for reasons that include the lack of reproducibility, insensitivity, an unacceptable background signal, or failure to detect protection by control anti-V Abs. In contrast, the results of a FACS-based L/D assay with HL60 human-derived cells and NHP sera and utilizing the Syto-13 and PI dye combination suggested a positive correlation.
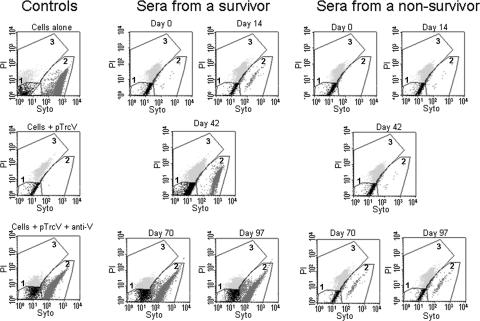
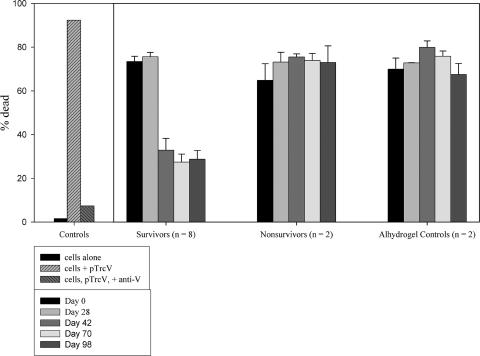
The potential correlation between protection and the results of the L/D FACS assays with HL60 Mφs and NHP sera was examined further in several experiments. The sample FACS profiles shown in Fig. 2 illustrate the use of the L/D FACS assay to demonstrate protection by NHP sera of HL60 cells from Yersinia-induced cell death; the assay parameters are detailed in the legend of Fig. 2. Pretreating the bacteria with anti-V Abs and sera from F1-V-vaccinated CM surviving challenge resulted in a notably reduced level of detectable cell death compared to that associated with day 0 sera or sera from vaccinated nonsurvivors (Fig. 2). The results with sera from CM which had been vaccinated s.c. three times with F1-V and challenged by aerosol with Y. pestis strain C092 (experiment 1, 12 animals) are presented in Fig. 3. Incubation of the bacteria with the control protective rabbit anti-V Ab suppressed the ability of the organisms to induce apoptosis in the Mφs (Fig. 3). Samples treated with sera from survivors exhibited an apparent reduction in the levels of necrosis after the second vaccine dose, relative to samples treated with day 0 sera or sera from nonsurvivor or control unvaccinated animals. Significance determinations could not be performed; however, later analyses of results from combined experiments (and thus a larger number of samples) yielded significant associations between the L/D assay results and protection, as will be shown.
FIG. 2.
FACS L/D assay results from two vaccinated CM. The results are representative of 12 animals included in CM experiment 1 (8 survivors, 2 nonsurvivors, and 2 alhydrogel-only controls) as described in Fig. 3. L/D FACS assays were performed with HL60 cells infected with Y. pseudotuberculosis pTrcV that were pretreated with sera from the NHP. CM were vaccinated with F1-V and exposed to a lethal aerosol challenge with Y. pestis, and the results of tests with sera from a survivor and a nonsurvivor are shown. The bacteria were pretreated with sera collected 2 weeks after each vaccine dose, specifically on the following days: 0 (before vaccination), 14, 42, 70, and 97 (the day before challenge). The graphs display the differential staining of cells by Syto-13 and PI as described in Materials and Methods. The regions drawn on the graphs outline the following populations of cells: unstained background (black, gate 1), Syto-13-only stained (live; medium gray, gate 2), and Syto-13/PI stained (dead; light gray, gate 3). These regions were determined using unstained, Syto 13-stained, and PI-stained cell samples (data not shown). The control samples included uninfected (cells alone), infected (cells + pTrcV), and Ab-treated (cells + pTrcV + anti-V) samples.
FIG. 3.
L/D FACS assay for the effects of CM sera on the survival of cells infected with Yersinia. The sera were obtained from animals included in experiment 1 (see Fig. 2). The number in parentheses (n [x axis]) indicates the number of samples. Serum collection times are indicated in the box insert. All assays included control samples of Mφs incubated in the absence of bacteria, Mφs incubated with bacteria alone, and Mφs infected with bacteria pretreated with the protective polyclonal rabbit Abs. The Alhydrogel control CM received adjuvant but no vaccine before challenge. The percent dead was determined as indicated in Materials and Methods; the error bars indicate the standard errors of the mean. The results of statistical analyses of the NHP data are discussed in the text.
Similar results were obtained from an experiment in which 10 AGM were vaccinated with F1-V and aerosol-challenged with C092. Two of the vaccinated animals survived, and eight succumbed to infection. Although the numbers of animals precluded a statistical evaluation, the data clearly suggested an association between the L/D assay results and protection. The Mφ cytotoxicity assays and anti-V Ab titers were determined on sera from each prechallenge collection day (0, 14, 42, 70, and 105), and the day 70 sera had the highest mean anti-V titers. The anti-V titers exhibited no association with survival and did not correlate with the percent L/D assay values (P = 0.42, Pearson product correlation). In contrast, whereas the sera from the nonsurvivors resulted in a mean of 21.5% (4.6% standard error) live cells in the FACS assay, the survivor sera were associated with 88.6% (10.7% standard error) live cells (data not shown). In analyses of the combined experiments with all of the NHP (described below), these data resulted in percent live cell values in the FACS assay that were predictive of survival. As observed for vaccinated CM (Fig. 3), the AGM data suggest that the L/D assay measures the protection of HL60 cells by NHP sera.
Extensive statistical analyses of the NHP data resulted in several observations.
(i) First, we observed a change in percentage of live cells over vaccination time. There was a significant change in the percentage of live cells in cultures exposed to sera collected during the vaccination period as determined by FACS. In evaluations of CM sera from experiment 1, the mean percent live cells in the assay ranged from 28.3% for the day 0 sera to 62.4% for the day 98 sera (P < 0.0001). The number of nonsurvivors in CM experiment 2 was small (2 of the 10 vaccinated animals), and the assay did not achieve significance for the serum samples. However, an analysis of the data from the combined experiments (16 survivors, 4 nonsurvivors, and 4 Alhydrogel controls) showed that the overall increase in the percentage of live cells was significantly associated with the prechallenge time (P = 0.0003 [analysis of variance]). For survivors, the change in viability of infected Mφs using the FACS L/D assay was positively associated with the time of serum collection during the course of vaccination (data not shown).
(ii) Second, we observed a correlation between the percentage of live cells and the anti-V Ab titer. Using data from CM experiment 1, the increase in anti-V titer was significantly associated with viability of HL60 cells, as measured by the FACS assay, in tests of sera collected during the vaccination period. Statistical analysis of anti-V titers and the percentage of live cells using all of the combined sera collected prechallenge showed that the titers were significantly correlated with the percentage of live cells, with a P of <0.0001 for the results from CM experiment 1 (Fig. 4) for the data from experiments 1 and 2 combined. Specifically, for the combined experiment CM data, the anti-V titers of sera collected on day 14 were associated with the percentage of live cells (P = 0.0427). Ab titers of sera collected on other days did not correlate with the FACS L/D data. Similarly, for F1-V-vaccinated AGM challenged with F1-negative Y. pestis C12, the percentage of live cells and the anti-V Ab titers for samples from the day 0 and 70 time points combined were significantly correlated (P = 0.0214). Finally, for all NHP experiments (CM and AGM) involving both Y. pestis C092- and C12-challenged animals, the percentage of live cells and the anti-V Ab titers for samples obtained on day 70 were significantly associated (P = 0.019); anti-V Ab titers at all other time points were not significantly associated with the percentage of live cells. Despite the correlation observed for some samples between anti-V Ab endpoint dilution titers and L/D FACs assay results, the Ab titers were not significantly associated with survival outcome in the combined or individual analyses, as illustrated in Fig. 4.
FIG. 4.
Correlation between the percent live cells, as measured by the L/D FACS assay, and the anti-V Ab titers of sera from vaccinated CM. The increase in anti-V titer was compared to the viability of cells as measured by the FACS L/D assay in tests of sera collected over time during the vaccination period. The serum samples were collected during experiment 1 from the NHP vaccinated three times with F1-V vaccine. Analysis of the results with all of the combined sera collected prechallenge revealed that the titers were significantly correlated with the percent live cells (P < 0.0001). However, the Ab titers were not significantly associated with survival outcome (P > 0.05).
(iii) Third, we observed an association between the percentage of live cells (FACS) and survival. The association between the percentage of live cells (FACS) and survival of F1-V vaccinated NHP (CM and AGM) challenged with C092 or C12 was analyzed by logistic regression and Cox proportional hazards regression. The percentages of live cells after treatment with sera collected at days 0, 14, 28, 42, 63, 70, 98, and 105 in the FACS assays were used in the analysis. For vaccinated CM challenged with C092 (two experiments), the probability of survival relative to the percentage of live cells in assays done with sera obtained on each of three collection days (0, 42, and 98), as measured by the FACS L/D assay, was determined. The change in the percentage of live cells from day 0 to day 42 in the FACS assay was able to significantly predict survival outcome (P = 0.049; data not shown). For each 1% increase in live cells, the odds of survival increased by 6.3%. The change in the percentage of live FACS cells from day 0 to day 98 approached significance (P = 0.0630). Analyses were then performed on results from combined NHP experimental groups (CM and AGM) challenged with C092. The results indicated that the percentages of live cells at three time points could predict survival outcome (day 14, P = 0.0319; day 42, P = 0.0065; day 70, P = 0.0191). A similar analysis of serum samples from all experiments (CM and AGM; C092- and C12-challenged animals) yielded essentially identical results (data not shown).
DISCUSSION
The low calcium response plasmid (pLcr)-encoded V antigen of Yersinia has multiple roles in the pathogenesis of infection, and the host response to V appears to be a significant component of immunity. Passive and active vaccination studies in mice confirmed that anti-V Abs can protect against Yersinia infection (1, 2, 8, 9, 20-22, 30, 31, 34, 35, 40, 46). Activated Mφs are important constituents of a protective immune response, and it is suggested that they at least partially mediate the protective effect of anti-V Abs by several potential mechanisms (4, 7, 10, 24, 25, 35, 52). The anti-V Abs can promote Mφ phagocytosis and thus inhibit production by Y. pestis of the apoptosis-inducing and antiphagocytic Yops (12, 47); the Abs may also interact directly with V to prevent the type III secretion system-mediated translocation of the Yops, as suggested both for LcrV and for the homologous PcrV of P. aeruginosa (15, 33, 38, 39, 42, 43, 47), and anti-V can prevent production of the Y. pestis- and V-induced, immunosuppressive interleukin-10 cytokine (7, 36, 44). Although the relative roles of these various activities remain unclear, anti-V Ab-mediated effects on Mφs are likely to play a significant role in the protection against lethal infection in animals vaccinated with a V-containing vaccine.
Despite the protection associated with anti-V Abs, it has proven difficult to establish a reliable anti-V Ab-based in vitro correlate assay of plague immunity. In a study of mice vaccinated with recombinant V (1), the total IgG anti-V ELISA titers and titers of IgG2a and IgG2b between survivors and nonsurvivors were not statistically significant. In other studies of vaccinated mice, survivors and nonsurvivors both exhibited a wide range of prechallenge anti-V titers, and the mean anti-V titers were not consistently associated with survival time (J. Adamovicz, G. Anderson, and G. Andrews, unpublished results). Thus, the results suggested that direct anti-V Ab titers might not be a reliable in vitro correlate indicator of protection.
In more recent studies of mice vaccinated with live V recombinant Salmonella or V-DNA, there was no significant association between survival and the titers of total anti-V IgG or of Abs that could compete with a protective MAb (17, 18). Our results demonstrated a similar overall lack of association between anti-V or anti-F1 Ab titers and protection. The anti-V and anti-F1Ab titers often did not correlate with the results of the Mφ cytotoxicity assays, and their correlation with each other was variable (Tables 1 to 3). As described for the two studies with mice vaccinated with one dose of F1-V (Table 2 and data not shown) and in the CM and AGM studies (Fig. 4 and data not shown), the Ab titers overall were inconsistently associated with the rate or time of survival of F1-V-vaccinated mice and NHP. This lack of association between Ab titer and survival was observed even in the CM studies in which the Ab titers did correlate with the FACS L/D assay results (Fig. 4).
In previous studies (47), we observed that Y. pestis, like the other pathogenic species of Yersinia, is both cytotoxic for and resistant to phagocyotosis by J774.A1 Mφs. Treating Y. pestis with protective polyclonal and monoclonal anti-V Abs prevented Y. pestis-induced cell death and promoted phagocytosis of the organisms (47). We hypothesized that anti-V Ab cytotoxicity neutralizing activity potentially serves as an in vitro correlate of protective immunity to plague. In the present investigation, sera from vaccinated and challenged mice and NHP were used to evaluate assays that measured the anti-V Ab-mediated neutralization of Mφ cytotoxicity as candidate in vitro correlates of plague immunity. Several potential surrogate in vitro markers of protection were identified. A correlation between in vitro neutralization of caspase-3, an enzyme produced only during apoptosis, was established using the murine Mφ cell line and sera from vaccinated mice from four vaccine trials. However, a correlation using this assay in tests with sera from F1-V vaccinated NHP was not observed. Using human-derived Mφs in FACS-based L/D staining assays of the terminal stage (necrosis), an association with protection using the NHP sera was shown. The correlation between survival after aerosol challenge with Y. pestis C092 and the percentage of live cells infected in vitro with serum-pretreated Y. pestis was statistically significant, as determined from analyses of the combined FACS L/D data. Also, the results of these assays using sera from F1-V-vaccinated mice challenged with the C12 strain of Y. pestis confirmed that the Mφ protection observed in the L/D assays reflected the responses to the V and not the F1 component of the fusion vaccine. In addition, the results of evaluations of sera from vaccinated mice in the microscopy and FACS L/D assays agreed well with the predictions of immune protection obtained using the caspase-3 assay. Thus, the L/D assays exhibited no discernible animal species- or bacterial strain-associated specificity.
The basis for the animal species-associated differences we observed in the caspase-3 assays of Yersinia-infected Mφs is unknown. The disparate correlation results obtained in tests with immune sera from mouse and NHP sources might be related to the use of mouse-derived J774.A1 Mφs in the caspase assays. However, caspase-3 assays with human-derived monocyte-like cell lines and NHP sera again failed to provide a correlation with immunity in NHP. Possibly, the cells that ultimately provided the most consistent results in subsequent assays, the human HL60 monocytes, have intrinsic qualitative or quantitative differences in their cell death pathway.
Alternately, the lack of correlation in assays using NHP sera might have stemmed from the use of a marker that does not optimally measure the cytotoxicity neutralizing activity of the NHP Abs. Cell death mediated by bacterial pathogens has been shown to occur by several different mechanisms, many of which have some but not all of the features of apoptosis (16). Apoptosis is a noninflammatory form of programmed cell death mediated by several caspase enzymes and exhibiting specific morphological changes such as nuclear condensation and DNA damage. Other death pathways (i.e., autophagy, oncosis, pyroptosis, and necrosis) differ in the extent of the inflammation induced, the nature of the cytoplasmic degradation events that occur, and the dependence of the pathway on enzymes, such as caspase-1, that are not involved in apoptosis (5, 6, 13, 16). It should be noted that necrosis is often used to indicate forms of cell death that do not involve apoptosis. However, because apoptotic bodies can also proceed to necrosis, the latter term is most accurately used to describe dead cells without implying the path leading to the terminal cell death. The determination of the mode of cell death is further complicated by the observation that some pathogens can induce different forms of cell death depending on the multiplicity of infection or on the host cell infected (26), or they can activate more than one death pathway in one cell (37). Also, the various features of cell death measured in vitro are not necessarily specific to one death pathway. For example, condensed chromatin is observed in both apoptotic and oncotic cells (11), DNA fragmentation can occur in both necrotic and apoptotic cells (16), and annexin V, which stains membrane phosphatidylserine early in apoptosis, can also stain necrotic cells. Lastly, the destruction of the plasma membrane with release of cytoplasmic enzymes such as LDH and the uptake of a membrane-impermeant dye such as PI is the basis of many assays for necrosis, but these markers cannot be used to implicate the pathway to cell death.
As a consequence of these findings, in vitro cytotoxicity assays should be viewed as measures of specific features of cell death and not as predictors of the pathway taken or of the cytotoxic mechanism. Thus, although the effector Yops of the pathogenic Yersinia strains have been associated with features of host cell apoptosis both in vitro (41) and in vivo (29), the specific type and number of pathways of cell death induced in vivo by pathogenic yersiniae are not known. A complete knowledge of the mechanism(s) involved in Y. pestis-induced cell cytotoxicity could certainly guide the development of an in vitro assay, the results of which could correlate with protective immunity. However, although the mechanisms of V-mediated virulence are not entirely known, analyses of samples from different vaccine trials and animal models allowed us to develop promising in vitro surrogate assays of protective immunity.
In conclusion, using sera collected from vaccinated and challenged mice and NHP, we demonstrated a significant correlation between immune protection against aerosol and parenteral challenge with Y. pestis and the overall results of the FACS L/D assay. In tests with samples from mice, the results of an L/D microscopy assay and of a caspase-3 apoptosis assay also were strong predictors of protection. These assays also distinguished a protective MAb from nonprotective mouse MAbs and accurately predicted protection in mice challenged with F1-negative Y. pestis. In contrast, although anti-V Ab titers sometimes correlated with the results of the FACs assay, they were not significantly associated with survival. The relatively small number of NHP available for testing and the consequent difficulty in obtaining an adequate number and range of survivor and nonsurvivor sera precluded demonstration of a significant correlation between protection and FACS assay data within individual experiments. However, tests with sera from a larger number of vaccinated NHP are being done and will be reported separately. Further studies are also planned to determine the correlation between protection and the L/D assay using sera from vaccinated NHP challenged by aerosol with C12 or challenged intradermally with Y. pestis, in a model of bubonic plague. In conclusion, the results of the L/D assays provide a foundation for the final development of a broadly applicable test for Ab-mediated protective immunity against plague in humans.
Acknowledgments
We thank the individuals who kindly provided sera and information from several vaccination experiments with mice, specifically Tran C. Chanh, Christopher Bolt, and John Lee, and with nonhuman primates, Louise Pitt. We also thank Kelly Rea and Paula Austin for excellent technical assistance.
The research described here was sponsored by the Medical Biological Defense Research Program, U.S. Army Medical Research and Materiel Command under project 05-4-5A-00R.
The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Anderson, G. W., S. E. C. Leary, E. D. Williamson, R. W. Titball, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, G. W., P. L. Worsham, C. R. Bolt, G. P. Andrews, S. L. Welkos, A. M. Friedlander, and J. P. Burans. 1997. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am. J. Trop. Med. Hyg. 56:471-473. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis C092 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 64:2180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autenrieth, I. B., and J. Heeseman. 1993. In vivo neutralization of tumor necrosis factor alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica in mice. Med. Microbiol. Immunol. 181:333-338. [DOI] [PubMed] [Google Scholar]
- 5.Boise, L. H., and C. M. Collins. 2001. Salmonella-induced cell lysis: apoptosis, necrosis, or programmed cell death? Trends Microbiol. 9:64-67. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, M. A., and B. T. Cookson. 2000. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38:31-40. [DOI] [PubMed] [Google Scholar]
- 7.Brubaker, R. R. 2003. Interleukin-10 and inhibition of innate immunity to yersiniae: roles of Yops and LcrV (V antigen). Infect. Immun. 71:3673-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burrows, T. W., and G. A. Bacon. 1956. The basis of virulence of Pasteurella pestis: antigen determining virulence. Br. J. Exp. Pathol. 37:481-493. [PMC free article] [PubMed] [Google Scholar]
- 9.Burrows, T. W., and G. A. Bacon. 1958. The effects of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br. J. Exp. Pathol. 39:278-291. [PMC free article] [PubMed] [Google Scholar]
- 10.Cavanaugh, D. C., and R. Randall. 1959. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of fleaborne plague. J. Immunol. 85:348-363. [PubMed] [Google Scholar]
- 11.Columbano, A. 1995. Cell death: current difficulties in discriminating apoptosis from necrosis in the context of pathological processes in vivo. J. Cell Biochem. 58:181-190. [DOI] [PubMed] [Google Scholar]
- 12.Cowan, C., A. V. Philipovskiy, C. R. Wulff-Strobel, Z. Ye, and S. C. Straley. 2005. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect. Immun. 73:6127-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dacheux, D., B. Toussant, M. Richard, G., Brochier, J. Croize, and I. Attree. 2000. Pseudomonas aeruginosa cystic fibrosis isolates induce rapid, type III secretion-dependent, but ExoU-independent, oncosis of macrophages and polymorphonuclear neutrophils. Infect. Immun. 68:2916-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis, K. J., P. Vogel, D. L. Fritz, K. E. Steele, M. L. Pitt, S. L. Welkos, A. M. Friedlander, and W. R. Byrne. 1996. Pathology of experimental pneumonic plague produced by F1-positive and F1-negative Yersinia pestis in African green monkeys. Arch. Pathol. Lab. Med. 120:156-163. [PubMed] [Google Scholar]
- 15.Faure, K., J. Fujimoto, D. W. Shimabukuro, T. Ajayi, N. Shime, K. Moriyama, E. G. Spack, J. P. Wiener-Kronish, and T. Sawa. 2003. Effects of monoclonal anti-PcrV antibody on Pseudomonas aeruginosa-induced acute lung injury in a rat model. J. Immune Based Ther. Vaccines 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fink, S. L., and B. T. Cookson. 2005. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying cells. Infect. Immun. 73:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garmory, H. S., D. Freeman, K. A. Brown, and R. Titball. 2004. Protection against plague afforded by immunization with DNA vaccines optimized for expression of the Yersinia pestis V antigen. Vaccine 22:947-957. [DOI] [PubMed] [Google Scholar]
- 18.Garmory, H. S., K. F. Griffin, K. A. Brown, and R. W. Titball. 2003. Oral immunization with live aroA attenuated Salmonella enterica serovar Typhimurium expressing the Yersinia pestis V antigen protects mice against plague. Vaccine 21:3051-3057. [DOI] [PubMed] [Google Scholar]
- 19.Heath, D. G., G. W. Anderson, Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 20.Hill, J., S. E. Leary, K. F. Griffin, E. D. Williamson, and R. W. Titball. 1997. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 65:4476-4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawton, W. D., R. I. Erdman, and M. J. Surgalla. 1963. Biosynthesis and purification of V and W antigens in Pasteurella pestis. J. Immunol. 91:179-194. [DOI] [PubMed] [Google Scholar]
- 22.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 63:2854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lincz, L. F. 1988. Deciphering the apoptotic pathway: all roads lead to death. Immunol. Cell Biol. 76:1-19. [DOI] [PubMed] [Google Scholar]
- 24.Lukaszewski, R. A., D. J. Kenny, R. Taylor, D. G. Rees, M. G. Hartley, and P. C. Oyston. 2005. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 73:7142-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 309:1739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuda, T., H. Saito, T. Inoue, K. Fukatsu, M. T. Lin, I. Han, S. Furukawa, S. Ikeda, and T. Muto. 1999. Ratio of bacteria to polymorphonuclear neutrophils (PMNs) determines PMN fate. Shock 12:365-372. [PubMed] [Google Scholar]
- 27.Meyer, K. F. 1970. Effectiveness of live or killed plague vaccines in man. Bull. W. H. O. 42:653-666. [PMC free article] [PubMed] [Google Scholar]
- 28.Mills, S. D., A. Boland, M. P. Sory, P. van der Smissen, C. Kerbourch, B. B. Finlay, and G. R. Cornelis. 1997. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc. Natl. Acad. Sci. USA 94:12638-12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monack, D. M., J. Mecsas, D. Bouley, and S. Falkow. 1998. Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection. J. Exp. Med. 188:2127-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 62:4192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motin, V. L., Y. A. Nedialkov, and R. R. Brubaker. 1996. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect. Immun. 64:4313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motin, V. L., M. S. Pokrovskaya, M. V. Telepnev, V. V. Kutyrev, N. A. Vidyaeva, A. A. Filippov, and G. B. Smirnov. 1992. The difference in the LcrV sequences between Y. pestis and Y. pseudotuberculosis and its application for characterization of Y. pseudotuberculosis strains. Microb. Pathog. 12:165-175. [DOI] [PubMed] [Google Scholar]
- 33.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310:674-676. [DOI] [PubMed] [Google Scholar]
- 34.Nakajima, R., and R. R. Brubaker. 1993. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect. Immun. 61:23-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima, R., V. L. Motin, and R. R. Brubaker. 1995. Suppression of cytokines in mice by protein A-V antigen fusion peptide and restoration of synthesis by active immunization. Infect. Immun. 63:3021-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nedialkov, Y. A., V. L. Motin, and R. R. Brubaker. 1997. Resistance to lipopolysaccharide mediated by Yersinia pestis V antigen-polyhistidine fusion peptide: amplification of interleukin-10. Infect. Immun. 65:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nonaka, T., A. Kuwae, C. Sasakawa, and S. Imajoh-Ohmi. 1999. Shigella flexneri YSH6000 induces two types of cell death, apoptosis and oncosis, in the differentiated human monoblastic cell line U937. FEMS Microbiol. Lett. 174:89-95. [DOI] [PubMed] [Google Scholar]
- 38.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32:961-976. [DOI] [PubMed] [Google Scholar]
- 39.Philipovskiy, A. V., C. Cowan, C. R. Wulff-Strobel, S. H. Burnett, E. J. Kerschen, D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2005. Antibody against V antigen prevents Yop-dependent growth of Yersinia pestis. Infect. Immun. 73:1532-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roggenkamp, A., A. M. Geiger, and L. Leitritz. 1997. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect. Immun. 65:446-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruckdeschel, K., A. Roggenkamp, V. Lafont, P. Mangeat, J. Heesemann, and B. Rouot. 1997. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65:4813-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sawa, T., T. L. Yahr, and M. Ohara. 1999. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 5:392-398. [DOI] [PubMed] [Google Scholar]
- 43.Shime, N., T. Sawa, J. Fujimoto, K. Faure, L. R. Allmond, T. Karaca, B. O. Swanson, E. G. Spack, and J. P. Weiner-Kronish. 2001. Therapeutic administration of anti-PcrV F(ab′)2 in sepsis associated with Pseudomonas aeruginosa. J. Immunol. 167:5880-5886. [DOI] [PubMed] [Google Scholar]
- 44.Sodhi, A., R. K. Sharma, and H. V. Batra. 2005. Yersinia rLcrV and rYopB inhibits the activation of murine peritoneal macrophages in vitro. Immunol. Lett. 99:146-152. [DOI] [PubMed] [Google Scholar]
- 45.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:227-237. [DOI] [PubMed] [Google Scholar]
- 46.Une, T., and R. R. Brubaker. 1984. Role of V antigen in promoting virulence and immunity in yersiniae. J. Immunol. 133:2226-2230. [PubMed] [Google Scholar]
- 47.Weeks, S., J. Hill, A. Friedlander, and S. Welkos. 2002. Anti-V antigen antibody protects macrophages from Yersinia pestis-induced cell death and promotes phagocytosis. Microb. Pathog. 32:227-237. [DOI] [PubMed] [Google Scholar]
- 48.Welkos, S., M. L. M. Pitt, M. Martinez, A. Friedlander, P. Vogel, and R. Tammariello. 2002. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in non-human primate and mouse models of pneumonic plague. Vaccine 20:2206-2214. [DOI] [PubMed] [Google Scholar]
- 49.Welkos, S. L., A. M. Friedlander, D. McDowell, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24:185-196. [DOI] [PubMed] [Google Scholar]
- 49a.Williamson, E. D., S. M. Eley, K. F. Griffin, M. Green, P. Russell, S. E. C. Leary, P. C. F. Oyston, T. Easterbrook, K. M. Reddin, A. Robinson, and R. W. Titball. 1995. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol. Med. Microbiol. 12:223-230. [DOI] [PubMed] [Google Scholar]
- 50.Winter, C. C., W. B. Cherry, and M. D. Moody. 1960. An unusual strain of Pasteurella pestis isolated from a fatal human case of plague. Bull. W. H. O. 23:408-409. [PMC free article] [PubMed] [Google Scholar]
- 51.Worsham, P. L., M. P. Stein, and S. L. Welkos. 1995. Construction of defined F1 negative mutants of virulent Yersinia pestis. Contrib. Microbiol. Immunol. 13:325-328. [PubMed] [Google Scholar]
- 52.Zhang, Y., and J. B. Bliska. 2005. Role of macrophage apoptosis in the pathogenesis of Yersinia. Curr. Top. Microb. Immunol. 289:151-174. [DOI] [PubMed] [Google Scholar]