Abstract
Specific antibodies, available in unlimited quantities, have not been produced against Mycobacterium avium subsp. paratuberculosis, the bacterium that causes Johne's disease (JD). To fill this gap in JD research, monoclonal antibodies (MAbs) against M. avium subsp. paratuberculosis were produced from BALB/c mice immunized with a whole-cell extract of M. avium subsp. paratuberculosis. A total of 10 hybridomas producing MAbs to proteins ranging from 25 to 85 kDa were obtained. All MAbs showed some degree of cross-reactivity when they were analyzed against a panel of whole-cell protein lysates comprising seven different mycobacterial species. The MAbs were characterized by several methods, which included isotype analysis, specificity analysis, epitope analysis, reactivity in immunoblot assays, and electron microscopy. The identities of the antigens that bound to two selected MAbs were determined by screening an M. avium subsp. paratuberculosis lambda phage expression library. This approach revealed that MAb 9G10 detects MAP1643 (isocitrate lyase) and that MAb 11G4 detects MAP3840 (a 70-kDa heat shock protein), two proteins present in high relative abundance in M. avium subsp. paratuberculosis. The epitopes for MAb 11G4 were mapped to the N-terminal half of MAP3840, whereas MAb 9G10 bound to the C-terminal half of MAP1643. Aptamers, nucleic acids that bind to specific protein sequences, against the hypothetical protein encoded by MAP0105c were also generated and tested for their binding to M. avium subsp. paratuberculosis as well as other mycobacteria. These detection reagents may be beneficial in many JD research applications.
The genus Mycobacterium comprises a diverse group of animal and human pathogens as well as saprophytes, many of which are ubiquitous in the environment. Mycobacterium avium subsp. paratuberculosis is a member of the Mycobacterium avium complex (MAC) and an animal pathogen that is highlighted by the large financial burden that it places on the dairy industry due to Johne's disease (JD). Figures extrapolated from the 1996 NAHMS dairy survey suggest that the cost of this disease was over $200 million per year (25). The growing recognition of M. avium subsp. paratuberculosis infection in wildlife species is of considerable concern, since it may affect our ability to control or eradicate JD from domesticated animals (10, 11).
Despite the research difficulties and economic consequences of JD, very few reports have described specific, antigen-based detection reagents for M. avium subsp. paratuberculosis. With the exception of a single study published 10 years ago (24), the scientific literature is silent on the subject of M. avium subsp. paratuberculosis monoclonal antibodies (MAbs) and their use in JD research. Very recently, single-chain antibodies were selected by cloning heavy and light chains from sheep with JD (6). This effort has resulted in two very promising recombinant antibodies; however, the M. avium subsp. paratuberculosis proteins that these antibodies react with remain unknown. The overall lack of detection reagents for M. avium subsp. paratuberculosis is in stark contrast to the availability of detection reagents for other bacterial pathogens of cattle, such as Brucella or Mycobacterium bovis, for which scores of MAbs are available to researchers (7, 9, 19, 21, 23, 32).
Against this background, recent changes have modified the JD research landscape. Within the United States, a national consortium, entitled the Johne's Disease Integrated Program (JDIP; www.jdip.org), has identified the high research priorities and the knowledge gaps necessary to combat JD. Similar JD research consortiums have also recently formed in Europe and New Zealand. One of the priorities identified by JDIP is the development of specific detection reagents such as MAbs for M. avium subsp. paratuberculosis. More than just their obvious application for the diagnosis of JD, MAbs are critical reagents in cell biology and pathogenesis studies, including studies of macrophage-pathogen interactions, studies that use Luminex and magnetic bead technologies, as well as histopathology studies. MAbs that detect specific M. avium subsp. paratuberculosis proteins are ideal for incorporation into diagnostic assays such as those already developed for Campylobacter (8) and Escherichia coli (16). Furthermore, MAbs have application in the histopathological examination of infected tissues, typically the lamina propria of the intestine, where acid-fast staining has historically been used to demonstrate the presence of M. avium subsp. paratuberculosis, albeit with a low sensitivity and specificity (30).
The results of this study have identified and characterized novel MAbs against M. avium subsp. paratuberculosis with potential use in several JD-related research applications. We demonstrate here the specificity, subcellular location, and utility by electron microscopy for each MAb developed. In addition, we have identified the corresponding M. avium subsp. paratuberculosis proteins detected by two of these MAbs.
MATERIALS AND METHODS
Mycobacterial antigens.
The National Animal Disease Center's mycobacterial culture collection served as the source of all strains used in this study (Table 1). M. avium subsp. paratuberculosis ATCC 19698 is the type strain; M. avium subsp. paratuberculosis Linda is a human isolate; and all other M. avium subsp. paratuberculosis strains tested are cattle isolates, including K-10, the sequenced strain (20). All mycobacteria were cultivated in Middlebrook 7H9 medium supplemented with oleic acid-albumin-dextrose-catalase (Hardy Diagnostics, Santa Maria, CA). For the cultivation of M. avium subsp. paratuberculosis, mycobactin J (2 mg/liter; Allied Monitor, Fayette, MO) was added to the Middlebrook-oleic acid-albumin-dextrose-catalase medium. The whole-cell-sonicated extracts of mycobacterial species and isolates were prepared for use as antigens in immunoassays, as described previously (31). The sonicated extracts were centrifuged at 50,000 × g for 1 h. The pellet was resuspended in an equal volume of phosphate-buffered saline (PBS; 150 mM NaCl, 10 mM NaPO4, pH 7.4) and was thereafter used as the membrane-enriched fraction. The supernatant was collected and used as the cytosol-enriched fraction. All samples were assayed for protein content (Bio-Rad Laboratories [Richmond, CA] protein assay) and were stored at −20°C.
TABLE 1.
Mycobacterial isolates used in this study
| Isolate | Organism | Host | Location | Reference or source |
|---|---|---|---|---|
| K-10 | M. avium subsp. paratuberculosis | Bovine | Feces | 20 |
| 19698 | M. avium subsp. paratuberculosis | Bovine | Feces | ATCC 19698 |
| 6100 | M. avium subsp. paratuberculosis | Human | Ileum | ATCC 43015 |
| 187 | M. avium subsp. paratuberculosis | Bovine | Ileum | Recent clinical isolate, NADC |
| 523 | M. avium subsp. paratuberculosis | Bovine | Ileum | |
| 803 | M. avium subsp. paratuberculosis | Bovine | Ileum | |
| 6009 | M. avium subsp. avium | Bovine | ATCC 35716 (TMC715) | |
| 6003 | M. avium subsp. avium | Chicken | ATCC 35713 (TMC702) | |
| 6006 | M. avium subsp. silvaticum | Roe deer | V1-72 | |
| 6076 | M. abscessus | ATCC 19977 | ||
| M. bovis | Bovine | Lymph node | ATCC 19210 | |
| M. bovis BCG Pasteur | Bovine | Milk | ATCC 35734 | |
| 6081 | M. kansasii | Human | ATCC 12478 | |
| 6010 | M. intracellulare | Swine | ATCC 35773 | |
| 6083 | M. phlei | ATCC 11758 | ||
| 6077 | M. scrofulaceum | Human | Lymph node |
Expression clones producing recombinant M. avium subsp. paratuberculosis proteins were constructed by using the detailed methods described previously (4) and briefly mentioned below. All recombinant fusion proteins contained maltose-binding protein (MBP) as the tag for use in affinity purification. The MBP fusion was produced by cloning the M. avium subsp. paratuberculosis gene of interest into the pMAL-c2 expression vector (New England Biolabs, Beverly, MA). The entire reading frame or partial reading frame was amplified with AmpliTaq-Gold DNA polymerase (Applied Biosystems, Branchburg, NJ) and purified M. avium subsp. paratuberculosis K-10 genomic DNA as the template. The upstream and downstream oligonucleotides for each amplification are listed in Table 2. The vector and amplification product were each digested with XbaI and HindIII and purified from 1% agarose gels with Gene Clean (Bio101). Ligation of these products yielded in-frame fusions between the malE gene in the vector and the M. avium subsp. paratuberculosis reading frame. After overnight ligation at 16°C, the products were transformed into competent E. coli DH5α cells (Invitrogen). Constructs from selected transformants in each experiment were authenticated by DNA sequencing. Each fusion protein was overexpressed and purified by maltose affinity chromatography by using an amylose resin supplied by New England Biolabs. The detailed methods used for the induction and affinity purification of MBP/MAP fusion proteins have been described previously (4).
TABLE 2.
Oligonucleotide primers used to amplify MAP1643, MAP3840, and MAP0105c
| Primer name | Sequencea | Location within geneb |
|---|---|---|
| MAP1643F | ATCCTCTAGAATCGACAAAGAGACGCAAGTCCG | 10 to 32 |
| MAP1643R | GCGCAAGCTTCAACCCTCTTTGGTGATCAGCTT | 2266 to 2287 |
| MAP1643F2 | ATCCTCTAGACAGTCCATCGACGACCTGTTC | 1021 to 1041 |
| MAP1643R2 | GCGCAAGCTTCACTCGTCGGCGCTCATGTCAGC | 1153 to 1174 |
| MAP3840F | ATCCTCTAGAATGGCTCGTGCGGTCGGTATCGAC | 1 to 24 |
| MAP3840R | GCGCAAGCTTCACTTGGACTCCCGGTCATCGTC | 1849 to 1872 |
| MAP3840F2 | ATCCTCTAGAGACCGCACCCGTCAGCCGTTCAAG | 859 to 882 |
| MAP3840R2 | GCGCAAGCTTCACGGCTGACGGGTGCGGTCCAG | 856 to 876 |
| 218-4 F | CCTCTTCTAGAATGCGCGGAAACCGCAGCGAG | 163 to 183 |
| 218-4 R | GCGCAAGCTTCATTCGGTGAATGCGCGGAC | 1837 to 1856 |
| 218-9 F | CCTCTTCTAGACGCATGCGATCGAACAGT | 1609 to 1629 |
| 218-9 R | GCGCAAGCTTCAGGTGACGGCGCGTTTGAT | 2587 to 2606 |
Underlined nucleotides represent restriction endonuclease cut sites. The XbaI recognition sequence is TCTAGA, and AAGCTT is the HindIII recognition site.
The numbers represent the nucleotide position within the respective gene. The gene is indicated within the primer name, except that 218-4 F, 218-4 R, 218-9 F, and 218-9 R target MAP0105c.
Production of MAbs.
MAbs were produced by standard methods (13). Briefly, BALB/c mice were immunized intraperitoneally three times at 14-day intervals with a sonicated protein lysate of M. avium subsp. paratuberculosis K-10 (100 μg per injection) suspended in 0.5 ml of PBS (pH 7.3). The antigen was emulsified in Freund's incomplete adjuvant for all immunizations. The humoral immune responses of each mouse were evaluated by preparative immunoblot analysis with the sonicated antigen. Cell fusions with splenic lymphocytes and myeloma cells were performed with the cells from the best responder mouse. Positive antibody-secreting hybridomas were identified by immunoblot screening with culture supernatant. Stable secreting hybridomas were immunotyped by using isotype kit I from Pierce (Rockford, IL).
Immunoblot assay.
Polyacrylamide gel electrophoresis (PAGE) was performed with 12% (wt/vol) polyacrylamide gels. Electrophoretic transfer of proteins onto pure nitrocellulose was accomplished with a Trans Blot Cell (Bio-Rad) with sodium phosphate buffer (25 mM, pH 7.8) at 0.8 A for 90 min. After transfer, the filters were blocked with PBS (pH 7.4) plus 2% bovine serum albumin (BSA) and 0.1% Tween 20, termed PBS-BSA. Culture supernatants or MAbs were diluted in PBS-BSA and exposed to the blot at room temperature for 2 h. After three washes in PBS plus 0.1% Tween 20, the blots were incubated for 1.5 h in goat anti-mouse peroxidase (Pierce) diluted 1:20,000 in PBS-BSA. The blots were again washed three times as described above and developed for chemiluminesence with SuperSignal detection reagents (Pierce). For preparative immunoblots, 12% SDS-polyacrylamide gels were cast with only a single lane (trough) across the top of the gel, in addition to a notch for the protein size standards. A 100-μl aliquot of M. avium subsp. paratuberculosis whole-cell protein lysate (0.1 mg total) was loaded into the single, long lane and the gel was subjected to electrophoresis. Blotting was then carried out as described immediately above. The complete panel of MAbs was evaluated on preparative immunoblots placed in a slot-blot device (Bio-Rad), such that individual culture supernatants could be loaded into independent slots on the same blot. This method enabled the most direct comparison of the antigen sizes detected by the respective MAbs.
Aptamer selection against recombinant MAP0105c.
An aptamer library that consisted of a randomized 40-mer DNA sequence flanked by two known 28-mer primer binding sites (5′-TTTGGTCCTTGTCTTATGTCCAGAATGC-N40-ATTTCTCCTACTGGGATAGGTGGATTAT-3′, where N40 represents 40 random nucleotides with equimolar amounts of A, C, G, and T) was synthesized (Integrated DNA Technology, Inc., Coralville, IA). Recombinant MBPs/MBP fusion proteins 218-4 and 218-9, which represent the N-terminal and C-terminal halves of MAP0105c, respectively, served as targets for combinatorial library selection. The aptamer library was enriched for candidates unique to each protein by use of a counter SELEX protocol and standard lateral flow chromatography methods, as described previously (29). In brief, the aptamer library was exposed to MBP prior to exposure to 218-4 or 218-9 so as to exclude any candidates that bound to the MBP affinity tag. After six rounds of the counter SELEX protocol with each protein, the aptamer library was screened for cross-reactivity. All selected candidates appeared to bind to 218-4 and 218-9 alone. However, aptamers selected against each of these molecules cross-reacted. Thus, a second round of the counter SELEX protocol was applied, whereby aptamers exposed to 218-4 were subsequently exposed to 218-9. These manipulations were expected to remove any cross-reacting aptamers. After six additional rounds of the counter SELEX protocol, 50 clones from libraries derived from the two proteins were cloned and sequenced. Several candidate aptamers were identified for specificity analysis. Aptamers with little or no cross-reactivities between the two proteins were identified and were selected for use in further studies. Dot blots with the aptamers were performed as follows. A nitrocellulose membrane strip (5 by 5 in.; PROTRAN; Schleicher & Schuell Inc., Keene, NH) was cut and marked with a pencil for orientation. Positive and negative controls consisted of a 1-μl aliquot of 10 μM any biotinylated oligonucleotide spotted on the nitrocellulose membrane strip and cross-linked under UV light for 5 min (positive control) as well as 1 μl of purified BSA (0.05 mg/ml; negative control). Test dots of 1 μl of an E. coli cell lysate suspension (E. coli negative control), 1 μl of 1 mg/ml MBP-LacZ (MBP negative control), 1 μl of 1 mg/ml MBP-218-4, and 1 μl of 1 mg/ml MBP-218-9 were also spotted on the strip and allowed to air dry. The strips were processed for immunoblot analysis as described in the preceding section.
Epitope mapping of selected recombinant proteins.
The full-length proteins of MAP1643 (AceAb) and MAP3840 (DnaK) as well as the N-terminal and C-terminal halves of each were produced as a fusion with MBP by the method described under “Mycobacterial antigens” above. The primers for these truncated and full-length constructs are shown in Table 2. These recombinant proteins were used in immunoblot analysis to determine if the location of antibody binding was at the N-terminal or the C-terminal half of each protein.
Electron microscopy.
All fixation and staining procedures were conducted at room temperature. Mycobacterial bacilli were fixed for 2 to 4 h in 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4. Fixed cells were washed in the same buffer three times and were postfixed in 1% OsO4 in 0.1 M cacodylate buffer, pH 7.4, for 2 h. After the cells were washed in the same buffer, they were incubated with 30% ethanol for 10 min. The cells were further dehydrated with a graded series of ethanol and embedded in epoxy resin (Embed 812). Ultrathin sections for immunoelectron microscopy were washed in buffer three times for 15 min each time and etched with saturated sodium metaperiodate for 15 min. The cells were then blocked with 5% BSA for 30 min at room temperature. The cells were treated with each MAb (diluted 1:40) in the blocking solution for 2 h at room temperature. The cells were washed in Tris buffer containing 0.1% Tween 20 and 0.1% BSA four times for 10 min each time and were then incubated with goat anti-mouse immunoglobulin G (IgG) conjugated to colloidal gold (diameter, 10 nm) in Tris buffer for 2 h. The immunolabeled sections were washed in Tris buffer four times and fixed with 1% glutaraldehyde in Tris for 10 min. All ultrathin sections were double stained with uranyl acetate and Reynolds lead citrate and then observed under a Philips 410 microscope.
RESULTS
MAbs against M. avium subsp. paratuberculosis whole-cell homogenates.
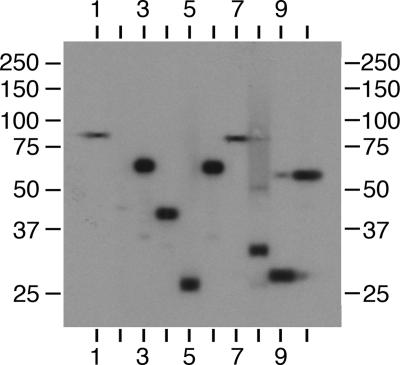
To obtain MAbs against M. avium subsp. paratuberculosis proteins, 6-week-old female BALB/c mice were immunized with a sonicated protein lysate of M. avium subsp. paratuberculosis K-10, as described in Materials and Methods. Ten hybridomas were identified by using this immunization regimen. By immunoblot analysis, the hybridomas reacted with a variety of proteins of different sizes from approximately 25 kDa to 85 kDa, as shown in Fig. 1. However, the MAbs designated 11F6 and 9G10 each reacted with a protein of a similar size at approximately 85 kDa (Fig. 1, lanes 1 and 7), and MAbs 13A4 and 11G4 also reacted with a similarly sized protein at approximately 66 kDa (Fig. 1, lanes 3 and 6). MAb 5A10 initially reacted with a single band in the 45-kDa region; however, a subsequent tissue culture supernatant obtained from this hybridoma showed no reactivity (Fig. 1, lane 2), indicating that this hybridoma had stopped secreting antibody. The rest of the culture supernatants reacted strongly with a single, well-defined band (Fig. 1, lanes 1, 3 to 7, and 10) or two bands (Fig. 1, lanes 8 and 9). Because MAb 5A10 was not a stably secreting hybridoma, only the remaining nine MAbs were used in further experiments. A preliminary characterization of each stable MAb was performed by defining the isotype and antigen size. The isotype specificity of each MAb is presented in Table 3, along with the estimated size of the M. avium subsp. paratuberculosis proteins to which they bind.
FIG. 1.
Immunoblot analysis of M. avium subsp. paratuberculosis whole-cell lysates with hybridoma culture supernatants containing MAbs. Ten hybridoma culture supernatants were loaded onto independent lanes or slots and analyzed in parallel on a preparative slot immunoblot containing M. avium subsp. paratuberculosis homogenates separated by SDS-PAGE. Antibodies bound to M. avium subsp. paratuberculosis proteins ranging in size from 25 kDa to 95 kDa. Lanes: 1, MAb 11F6; 2, MAb 5A10; 3, MAb 13A4; 4, MAb 4B6; 5, MAb 12C9; 6, MAb 11G4; 7, MAb 9G10; 8, MAb 14D4; 9, MAb 14G3; 10, MAb 14G11. Protein size standards are indicated in kilodaltons in the left and right margins.
TABLE 3.
Monoclonal antibodies developed in this study
| MAb | Isotype | M. avium subsp. paratuberculosis protein | Protein size (Da) |
|---|---|---|---|
| 11G4 | IgG1, κa | MAP3840 | 66,500 |
| 13A4 | IgG2b, κ | MAP3840 | 66,500 |
| 9G10 | IgG2a, κ | MAP1643 | 85,200 |
| 11F6 | IgG1, κ | MAP1643 | 85,200 |
| 14G11 | IgG1, κ | Unknown | 60,000 |
| 4B6 | IgG1, κ | Unknown | 42,000 |
| 14G3 | IgG2a, κ | Unknown | 28,000 |
| 12C9 | IgG1, κ | Unknown | 21,000 |
| 14D4 | IgG3, κ | Unknown | 35,000 |
κ, kappa chain (light chain of the antibody molecule).
Identification of M. avium subsp. paratuberculosis antigens that bind to selected MAbs.
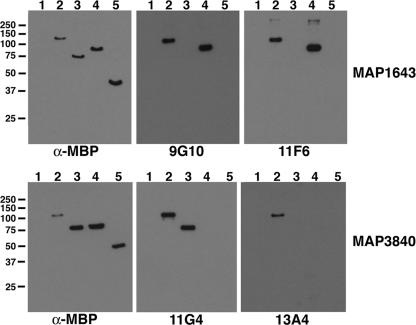
As shown in Fig. 1, several MAbs were obtained from mice immunized with a whole-cell homogenate of M. avium subsp. paratuberculosis. However, the M. avium subsp. paratuberculosis antigens that react with these MAbs are unknown. Therefore, four of the MAbs were used to screen an M. avium subsp. paratuberculosis lambda phage expression library developed previously (5). No positive plaques were obtained with MAbs 12C9 and 14G3; however, positive plaques were obtained when the library was screened with MAbs 11G4 and 9G10. DNA sequencing of the subcloned lambda phage inserts revealed open reading frames for MAP3840 (dnaK) and MAP1643 (aceAb) that reacted with MAbs 11G4 and 9G10, respectively. In order to conclusively demonstrate that these two MAbs reacted with the identified gene products, the MAP3840 and MAP1643 coding sequences were cloned and expressed in Escherichia coli. The purified recombinant fusion proteins representing MAP3840 and MAP1643 were analyzed by immunoblotting, which showed that these gene products were detected by the MAbs (Fig. 2). AceAb is a probable isocitrate lyase enzyme that is involved in the glyoxylate cycle (14). DnaK is the 70-kDa heat shock protein (34).
FIG. 2.
Identification of M. avium subsp. paratuberculosis proteins detected by selected MAbs and localization of epitopes to regions of the MAP1643 and MAP3840 gene products. For determination of the approximate locations of MAb epitopes in the primary sequence of these M. avium subsp. paratuberculosis proteins, purified recombinant peptides representing the full length (lane 2), N-terminal half (lane 3), and C-terminal half (lane 4) of each protein were probed by immunoblotting with selected MAbs, as indicated beneath each blot. These results show that MAbs 9G10 and 11F6 react with AceAb and that both MAbs detect an epitope on the C-terminal half of the protein. Similarly, MAbs 11G4 and 13A4 both react with DnaK; however, MAb 13A4 reacts only with the full-length protein and MAb 11G4 detects an epitope on the N-terminal half of DnaK. The blots probed with α-MBP (anti-MBP, a monoclonal antibody developed to the MBP affinity tag) detect all the proteins present and indicate their relative amounts and positions within each blot. The MBP-LacZ control protein is present in lane 5. No reactivity is observed with the protein size standards (lane 1). Kilodalton size standards are indicated in the left margin.
Mapping multiple MAbs to MAP3840 and MAP1643.
Once the identities of the antigens that reacted with MAbs 9G10 and 11G4 were discovered, further experimentation quickly revealed that additional independently isolated MAbs, 13A4 and 11F6, also detected MAP3840 and MAP1643, respectively (Table 3 and Fig. 2). One-dimensional sodium dodecyl sulfate (SDS)-PAGE separation of the fractionated lysates, followed by excision and mass spectroscopy of prominent Coomassie blue-stained bands, showed that both the MAP3840 and the MAP1643 gene products were present in high relative abundance in M. avium subsp. paratuberculosis (data not shown). Therefore, because of the antigenicity and high relative abundance of the MAP1643 and the MAP3840 gene products, it was not surprising to obtain multiple MAbs to these proteins. To determine if these MAbs detected the same epitopes or distinct epitopes within the same protein, the N-terminal and C-terminal halves of each protein were cloned and purified from the recombinant E. coli cells. These proteins, along with the corresponding full-length proteins, were then analyzed by immunoblotting with each of the respective MAbs. Both MAb 11F6 and MAb 9G10 bound to the C-terminal half of AceAb, as shown in Fig. 2. Furthermore, MAb 11G4 detected the N-terminal half of DnaK, but MAb 13A4 detected only the full-length protein (Fig. 2). These data suggest that MAbs 11G4 and 13A4 may bind to distinct epitopes within DnaK; however, because MAbs 11F6 and 9G10 both bound to the C-terminal half of AceAB, it remains inconclusive if the epitope is shared or distinct.
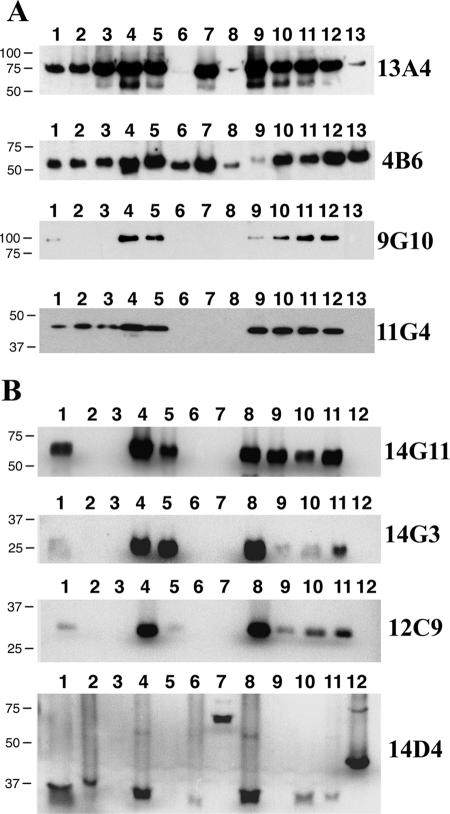
Specificities of antimycobacterial MAbs.
Antibodies were next screened by immunoblotting with whole-cell lysate preparations of nine mycobacterial species and subspecies, including M. avium subsp. avium, M. avium subsp. silvaticum, M. avium subsp. paratuberculosis, M. scrofulaceum, M. abscessus, M. bovis, M. phlei, M. intracellulare, and M. kansasii. All nine stable MAbs identified in Fig. 1 reacted to all three of the M. avium subsp. paratuberculosis isolates, which included K-10; ATCC 19698; and isolate Linda, which was recovered from a patient with Crohn's disease (Fig. 3). Although the MAb 11F6 immunoblot is not shown, reactivity identical to that observed for MAbs 9G10 and 14G11 was obtained by using this MAb. MAb 14D4 showed the most unexpected immunoblot reactivity, with bands of widely varying sizes detected among the different mycobacterial species (Fig. 3). Surprisingly, MAb 14D4 was also the only antibody that did not detect either of the M. avium subsp. avium isolates but reacted with the more distantly related mycobacteria, such as M. phlei and M. bovis. While all antibodies showed some degree of cross-reactivity with other mycobacterial species, MAb 12C9 showed strong reactivity with the two M. avium subsp. paratuberculosis bovine isolates (Fig. 3, lanes 5 and 9) and weaker reactivity with Crohn's disease isolate Linda (Fig. 3, lane 11). The 4B6 antibody detected a protein that was the most conserved among the mycobacteria, as a band of similar size was observed in every species and subspecies tested (Fig. 3).
FIG. 3.
Evaluation of MAbs against whole-cell homogenates from several mycobacterial species. Immunoblot analysis shows that the reactivity of each MAb is observed with more than just M. avium subsp. paratuberculosis lysates. (A) Lanes: 1, M. silvaticum; 2, M. scrofulaceum; 3, M. abscessus; 4, M. avium subsp. paratuberculosis K-10; 5, M. avium (strain TMC702); 6, M. bovis (strain 95-1315); 7, M phlei; 8, M. bovis BCG; 9, M. avium subsp. paratuberculosis ATCC 19698; 10, M. avium subsp. avium (strain TMC715); 11, M. avium subsp. paratuberculosis (Linda); 12, M. intracellulare; 13, M. kansasii. (B) Lanes: 1, M. silvaticum; 2, M. scrofulaceum; 3, M. abscessus; 4, M. avium subsp. paratuberculosis K-10; 5, M. avium subsp. avium (strain TMC702); 6, M. bovis (strain 95-1315); 7, M phlei; 8, M. avium subsp. paratuberculosis ATCC 19698; 9, M. avium subsp. avium (strain TMC715); 10, M. avium subsp. paratuberculosis (strain Linda); 11, M. intracellulare; 12, M. kansasii. Kilodalton size standards are indicated in the left margin, and the MAb used is indicated in the right margin.
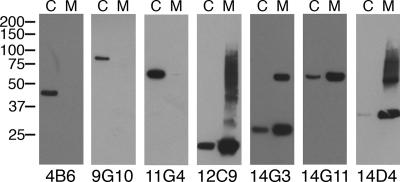
Location of M. avium subsp. paratuberculosis antigens that bind to MAbs.
Antigen localization was determined by immunoblot analysis of the MAbs against membrane-enriched and cytosol-enriched fractionated lysates, prepared as described in Materials and Methods. Note that MAbs 12C9, 14D4, 14G3, and 14G11 all bound to an unidentified M. avium subsp. paratuberculosis protein that is primarily localized in the cell membrane fraction (Fig. 4), whereas MAbs 4B6, 9G10, and 11G4 (Fig. 4) and MAbs 11F6 and 13A4 (data not shown) all detected proteins that were present predominantly in the cytoplasmic fraction. The protein detected by MAb 14G3 may form a dimer complex in the membrane-enriched fraction, as a protein band approximately twice the size of the monomer was also detected (Fig. 4).
FIG. 4.
Localization of antigens in fractionated M. avium subsp. paratuberculosis cell lysates. Equal amounts (0.5 μg/lane) of cell lysates from membrane-enriched M. avium subsp. paratuberculosis K-10 (lanes M) and cytoplasmic enriched fractions of M. avium subsp. paratuberculosis K-10 (lanes C) were loaded onto SDS-polyacrylamide gels and analyzed by immunoblotting with selected MAbs, which are indicated beneath each blot. Three MAbs detected proteins in the cytoplasmic enriched fraction, and four MAbs detected proteins present in the membrane-enriched fraction. Kilodalton size standards are indicated in the left margin.
Detection of M. avium subsp. paratuberculosis bacilli by immunoelectron microscopy with the MAbs developed in this study.
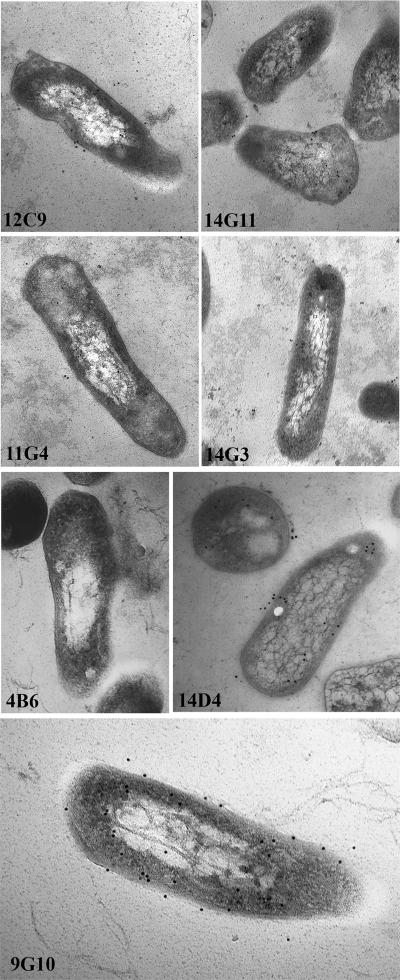
Each of the MAbs was next evaluated to determine reactivity levels with mycobacterial bacilli by immunoelectron microscopy. Of all the MAbs tested, MAbs 14D4 and 9G10 showed the most immunogold labeling, which indicates antibody binding to M. avium subsp. paratuberculosis (Fig. 5). Although MAb 4B6 reacted strongly with all mycobacteria tested by immunoblotting, no reactivity by immunoelectron microscopy was observed, suggesting that either the processing for microscopy damaged the epitope or the epitope is perhaps masked by another protein(s). None of the labeling patterns were distinct enough to confirm the subcellular locations of the antigens to which they bind, although MAb 14D4 seemed to label mostly at the periphery of the bacilli (Fig. 5). This labeling pattern is consistent with what was observed by immunoblotting of the fractionated lysates probed with MAb 14D4 (Fig. 4).
FIG. 5.
Immunogold labeling of M. avium subsp. paratuberculosis bacilli with selected MAbs. The bacilli were cultured and processed for immunoelectron microscopy as described in Materials and Methods. The MAb used is indicated in the lower left corner of each image. The magnification for all images except for that for MAb 9G10 is ×104,000. The magnification for the image for MAb 9G10 was ×112,000.
Development of aptamers to the M. avium subsp. paratuberculosis hypothetical protein encoded by MAP0105c.
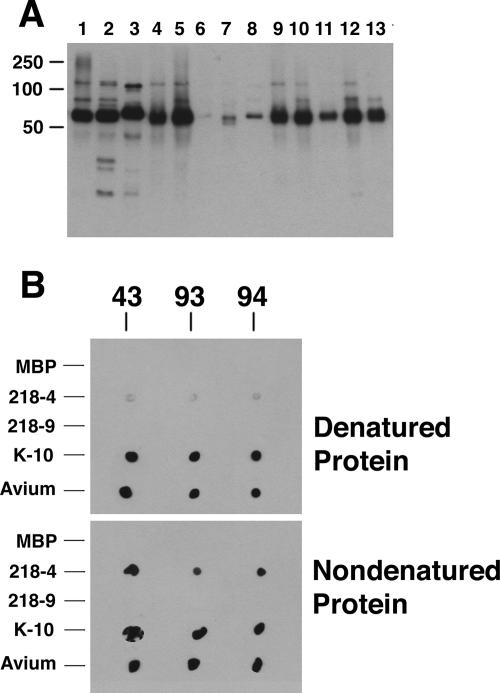
In an attempt to obtain a more specific reagent to detect M. avium subsp. paratuberculosis, aptamers were screened to identify those that bind to the MAP0105c gene product recombinantly produced in E. coli. Aptamers are short nucleic acid sequences that bind to specific protein epitopes with a high affinity (27). MAP0105c is a gene reported to be unique to M. avium subsp. paratuberculosis (2). In that study, MAP0105c was designated gene 218, because the project was published prior to annotation of the M. avium subsp. paratuberculosis K-10 genome (20). Three aptamers, designated 43, 93, and 94, were obtained following repeated screening and enrichments with the recombinant protein. Immunoblot analysis with these aptamers showed binding to the protein of the correct size, but reactivity was also seen in most of the mycobacteria analyzed (Fig. 6A). This cross-reactivity with other mycobacteria prompted a reexamination of MAP0105c by BLAST analysis. This analysis still suggested that the nucleotide sequence of MAP0105c is present only in M. avium subsp. paratuberculosis and not other bacteria, including mycobacteria; however, BLAST analysis with the translated sequence did show hits in Streptomyces (30% identity), Frankia (22% identity), and Rhodococcus (28% identity). MAP0105c was also produced as N-terminal and C-terminal halves (designated 218-4 and 218-9, respectively), and dot blot analysis with these truncated proteins shows that all three aptamers detected the N-terminal half (Fig. 6B). Furthermore, all aptamers reacted more strongly with the nondenatured recombinant protein than with the denatured recombinant protein.
FIG. 6.
Immunoblot (A) and dot blot (B) analyses of aptamers to MAP0105c. (A) The immunoblot containing mycobacterial whole-cell sonicated extracts was exposed to aptamer 94. Lane assignments: 1, M. silvaticum; 2, M. scrofulaceum; 3, M. abscessus; 4, M. avium subsp. paratuberculosis K-10; 5, M. avium subsp. avium (strain TMC702); 6, M. bovis; 7, M. phlei; 8, M. bovis BCG; 9, M. avium subsp. paratuberculosis ATCC 19698; 10, M. avium subsp. avium (strain TMC715); 11, M. avium subsp. paratuberculosis (strain Linda); 12, M. intracellulare; 13, M. kansasii. Size standards are indicated in kilodaltons in the left margin. (B) The dot blot was exposed to the three aptamers, which are indicated above the blots. Proteins spotted to the membrane are indicated in the left margin, and the state of the proteins is indicated in the right margin. Abbreviations: MBP, MBP fused to the α-peptide of LacZ; 218-4, an MBP fusion containing the N-terminal half of MAP0105c; 218-9, an MBP fusion containing the C-terminal half of MAP005c; K-10, a whole-cell lysate of M. avium subsp. paratuberculosis K-10; Avium, a whole-cell lysate of M. avium subsp. avium TMC715.
DISCUSSION
The predominant difficulties in working with M. avium subsp. paratuberculosis—the slow growth, the underdeveloped technology for genetic modification, and the intramacrophage location during infection, combined with a lack of knowledge regarding the 1,810 M. avium subsp. paratuberculosis hypothetical and unknown proteins—present challenges that require unique approaches. The production of MAbs, such as those produced in this study, is one critical tool currently lacking in JD research. The absence of these reagents has blocked the progress of unique research approaches directed at controlling this disease.
All MAbs and aptamers produced in this study cross-reacted with one or more species of mycobacteria. This result is not surprising, given the high degree of genetic similarity that M. avium subsp. paratuberculosis shares with other members of the MAC complex (26), and highlights the challenges encountered in the development of subspecies-specific detection reagents for this pathogen. However, what is surprising is that MAb 14D4 did not react with M. avium subsp. avium, which is most closely related to M. avium subsp. paratuberculosis, and yet this same MAb reacted with the more distantly related species M. bovis and even M. phlei. These findings highlight the need to broadly test any new detection reagent across a large number of mycobacterial species. Furthermore, even if specificity is demonstrated within the mycobacterial genus, additional tests with species outside the genus may be necessary.
In an effort to obtain a more specific detection reagent for M. avium subsp. paratuberculosis, aptamers that bind to the MAP0105c gene product were obtained. Nucleic acid similarity searches and PCR surveys have previously suggested that this gene is present uniquely in M. avium subsp. paratuberculosis (2). The three aptamers identified in these studies all detected the N-terminal half of MAP0105c. However, aptamer 94 bound to nearly all mycobacteria tested (Fig. 6A), suggesting that MAP0105c has conserved epitopes. Because the aptamers clearly reacted with the E. coli-expressed fusion protein representing MAP0105c (Fig. 6B), we are confident that they bind specifically to the native MAP0105c protein produced by M. avium subsp. paratuberculosis. The unexpected cross-reactivity prompted a BLAST analysis of the nonredundant protein database with MAP0105c, which shows that the nucleic acid sequence is present uniquely in M. avium subsp. paratuberculosis; however, the translated sequence has similarity to a hypothetical protein from Streptomyces avermitilis, with a 30% amino acid identity, and a hypothetical protein from Frankia species (22% identity). Nonetheless, there is still no bioinformatic evidence to explain the cross-reactivity with other mycobacterial species observed in this study (Fig. 6A). Taken together, these results suggest that MAP0105c does have conserved epitopes and that at least sections of the gene product should not be considered M. avium subsp. paratuberculosis specific.
To identify the corresponding antigens, four MAbs were chosen and used to screen an M. avium subsp. paratuberculosis lambda phage expression library. It is interesting to note that only MAbs 11G4 and 9G10, both of which detected proteins present in the cytoplasm of M. avium subsp. paratuberculosis, were identified during the screening of the expression library. The other two MAbs, 12C9 and 14D4, used in the screening experiments reacted with proteins in the mycobacterial cell membrane fraction (Fig. 4). It is likely that the membrane proteins detected by MAbs 12C9 and 14D4 are not readily cloned or expressed or are underrepresented in the lambda phage library. An alternative strategy that could be used to identify these membrane proteins is to combine affinity purification with the MAbs to capture the native M. avium subsp. paratuberculosis protein and analyze the antibody-antigen complex by tandem mass spectroscopy. This method would enable limited sequence identification of the peptides from the captured antigen, thus overcoming cloning and expression obstacles.
Heat shock proteins, which belong to families of widely conserved proteins found in prokaryotes and eukaryotes, are commonly immunodominant antigens recognized following infection with many bacterial pathogens (1, 17, 18, 35). The M. avium subsp. paratuberculosis DnaK, encoded by MAP3840, appears to be immunodominant as well, since two of nine immortalized B-cell cultures secreting antibody to this protein were obtained. While this cytoplasmically located antigen is not unique to M. avium subsp. paratuberculosis, it is present in high relative abundance in mycobacterial bacilli cultured in Middlebrook 7H9 medium, which may also account for the identification of more than one MAb. The same is also true for AceAb, encoded by MAP1643.
Although aceAb is considered a metabolic gene encoding the isocitrate lyase enzyme used in the glyoxylate cycle, it has been shown to be upregulated in M. avium subsp. avium-infected macrophages (28), implicating it in virulence as well. In M. tuberculosis H37Rv, the isocitrate lyase gene actually consists of two overlapping genes (aceAa at 1,104 bp and aceAb at 1,197 bp) that share a single base pair at the 3′ end of aceAa and the 5′ end of aceAb but is a single 2,300-bp gene in M. tuberculosis CDC1551 (12). In M. avium subsp. paratuberculosis, aceAb appears to be a single open reading frame 2,289 bp in length sandwiched by two hypothetical genes, MAP1642 and MAP1644 (20).
As an initial step in defining the antigenic structure of DnaK and AceAb, the epitopes were mapped to either the N-terminal or the C-terminal halves of the proteins. Both MAbs 11F6 and 9G10 detected the C-terminal half of AceAb, suggesting that this half may contain more B-cell epitopes or perhaps a single dominant epitope. In contrast, MAb 11G4 detected the N-terminal half of DnaK, while MAb 13A4 did not react with either half of DnaK and detected only the full-length protein. These data suggest that MAbs 11G4 and 13A4 recognize distinct epitopes. The exact reason why MAb 13A4 detects only the full-length protein is unclear; however, one possibility is that the epitope is at the center of the protein and that by producing the two halves, the epitope is no longer intact. A more likely possibility is that the MAb detects a conformational or discontinuous epitope that is disrupted when only one half of the protein is represented.
From this study, four MAbs that react with proteins present in the membrane fraction of M. avium subsp. paratuberculosis were identified. Thus, these proteins may even be surface exposed and, hence, immune targets for the host. Although these potential surface proteins remain to be identified, the MAbs are nonetheless useful in many applications. Pathogenesis studies should include MAbs that bind to surface molecules because they may block infection of cultured epithelial cells (3) or facilitate uptake and entry into macrophages (15). The results from these studies would determine if the proteins are important in adherence or invasion of epithelial cells that line the bovine or ovine intestine. More practically, these novel MAbs may be used to purify and concentrate M. avium subsp. paratuberculosis from environmental samples, such as water or bulk milk tank samples, by immunomagnetic separation technologies (22, 33). Confident identification of M. avium subsp. paratuberculosis in tissues from Crohn's disease patients might also be obtained with these MAbs. Furthermore, the MAbs developed in this study can be used to identify the potential surface proteins on M. avium subsp. paratuberculosis, making them strong candidates for subunit vaccines. Finally, they also provide a way to check the quality of fractionated protein preparations, such as membrane-enriched or cytoplasm-enriched lysates used in proteomic studies.
It is important to have MAbs against proteins that are located on the cell surface or cell membrane for ease of bacillus detection in downstream applications. While MAbs to a cytoplasmic protein may be important in studies highly focused on particular proteins, they may not be as effective at detecting bacilli in diagnostic or general research settings. Not all MAbs are used solely for detection purposes, however. Further delineation of the epitopes recognized by both antibodies as well as T cells will be important in understanding the immunopathological conditions caused by infection with M. avium subsp. paratuberculosis. The MAbs described here may be extremely useful reagents for such studies.
Acknowledgments
We thank Janis K. Hansen (NADC) and Paul Kapke (Iowa State University) for excellent technical assistance.
This work was supported by a USDA-Agricultural Research Service and USDA-NRI-CAP grant (JDIP).
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Ausiello, C. M., R. Palazzo, F. Spensieri, G. Fedele, R. Lande, A. Ciervo, G. Fioroni, and A. Cassone. 2005. 60-kDa heat shock protein of Chlamydia pneumoniae is a target of T-cell immune response. J. Biol. Regul. Homeost. Agents 19:136-140. [PubMed] [Google Scholar]
- 2.Bannantine, J. P., E. Baechler, Q. Zhang, L. Li, and V. Kapur. 2002. Genome scale comparison of Mycobacterium avium subsp. paratuberculosis with Mycobacterium avium subsp. avium reveals potential diagnostic sequences. J. Clin. Microbiol. 40:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannantine, J. P., J. F. Huntley, E. Miltner, J. R. Stabel, and L. E. Bermudez. 2003. The Mycobacterium avium subsp. paratuberculosis 35 kDa protein plays a role in invasion of bovine epithelial cells. Microbiology 149:2061-2069. [DOI] [PubMed] [Google Scholar]
- 4.Bannantine, J. P., and M. L. Paustian. 2006. Identification of diagnostic proteins in Mycobacterium avium subsp. paratuberculosis by a whole genome analysis approach, p. 185-196. In L. O'Connor (ed.), Diagnostic bacteriology protocols, 2nd ed. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 5.Bannantine, J. P., and J. R. Stabel. 2001. Identification of two Mycobacterium avium subsp. paratuberculosis gene products differentially recognised by sera from rabbits immunised with live mycobacteria but not heat-killed mycobacteria. J. Med. Microbiol. 50:795-804. [DOI] [PubMed] [Google Scholar]
- 6.Berger, S., D. Hinz, J. P. Bannantine, and J. F. Griffin. 2006. Isolation of high-affinity single-chain antibodies against Mycobacterium avium subsp. paratuberculosis surface proteins from sheep with Johne's disease. Clin. Vaccine Immunol. 13:1022-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowden, R. A., J. M. Verger, M. Grayon, and A. Cloeckaert. 1997. Rapid identification of rough Brucella isolates by a latex coagglutination assay with the 25-kilodalton outer membrane protein and rough-lipopolysaccharide-specific monoclonal antibodies. Clin. Diagn. Lab. Immunol. 4:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks, B. W., J. Devenish, C. L. Lutze-Wallace, D. Milnes, R. H. Robertson, and G. Berlie-Surujballi. 2004. Evaluation of a monoclonal antibody-based enzyme-linked immunosorbent assay for detection of Campylobacter fetus in bovine preputial washing and vaginal mucus samples. Vet. Microbiol. 103:77-84. [DOI] [PubMed] [Google Scholar]
- 9.Cloeckaert, A., H. S. Debbarh, M. S. Zygmunt, and G. Dubray. 1996. Production and characterisation of monoclonal antibodies to Brucella melitensis cytosoluble proteins that are able to differentiate antibody responses of infected sheep from Rev. 1 vaccinated sheep. J. Med. Microbiol. 45:206-213. [DOI] [PubMed] [Google Scholar]
- 10.Corn, J. L., E. J. Manning, S. Sreevatsan, and J. R. Fischer. 2005. Isolation of Mycobacterium avium subsp. paratuberculosis from free-ranging birds and mammals on livestock premises. Appl. Environ. Microbiol. 71:6963-6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daniels, M. J., M. R. Hutchings, and A. Greig. 2003. The risk of disease transmission to livestock posed by contamination of farm stored feed by wildlife excreta. Epidemiol. Infect. 130:561-568. [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischmann, R. D., D. Alland, J. A. Eisen, L. Carpenter, O. White, J. Peterson, R. DeBoy, R. Dodson, M. Gwinn, D. Haft, E. Hickey, J. F. Kolonay, W. C. Nelson, L. A. Umayam, M. Ermolaeva, S. L. Salzberg, A. Delcher, T. Utterback, J. Weidman, H. Khouri, J. Gill, A. Mikula, W. Bishai, W. R. Jacobs, Jr., J. C. Venter, and C. M. Fraser. 2002. Whole-genome comparison of Mycobacterium tuberculosis clinical and laboratory strains. J. Bacteriol. 184:5479-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harlow, E., and D. Lane (ed.). 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 14.Honer Zu Bentrup, K., A. Miczak, D. L. Swenson, and D. G. Russell. 1999. Characterization of activity and expression of isocitrate lyase in Mycobacterium avium and Mycobacterium tuberculosis. J. Bacteriol. 181:7161-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hostetter, J., R. Kagan, and E. Steadham. 2005. Opsonization effects on Mycobacterium avium subsp. paratuberculosis-macrophage interactions. Clin. Diagn. Lab. Immunol. 12:793-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kerr, P., H. Chart, D. Finlay, D. A. Pollock, D. P. MacKie, and H. J. Ball. 2001. Development of a monoclonal sandwich ELISA for the detection of animal and human Escherichia coli O157 strains. J. Appl. Microbiol. 90:543-549. [DOI] [PubMed] [Google Scholar]
- 17.Koets, A., A. Hoek, M. Langelaar, M. Overdijk, W. Santema, P. Franken, W. Eden, and V. Rutten. 2006. Mycobacterial 70 kD heat-shock protein is an effective subunit vaccine against bovine paratuberculosis. Vaccine 24:2550-2559. [DOI] [PubMed] [Google Scholar]
- 18.Koets, A. P., V. P. Rutten, M. de Boer, D. Bakker, P. Valentin-Weigand, and W. van Eden. 2001. Differential changes in heat shock protein-, lipoarabinomannan-, and purified protein derivative-specific immunoglobulin G1 and G2 isotype responses during bovine Mycobacterium avium subsp. paratuberculosis infection. Infect. Immun. 69:1492-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuchinka, G. D., C. O. Thoen, and V. Moennig. 1990. Production and partial characterization of monoclonal antibodies to the neotype strain of Mycobacterium bovis. Am. J. Vet. Res. 51:1608-1615. [PubMed] [Google Scholar]
- 20.Li, L., J. P. Bannantine, Q. Zhang, A. Amonsin, B. J. May, D. Alt, N. Banerji, S. Kanjilal, and V. Kapur. 2005. The complete genome sequence of Mycobacterium avium subsp. paratuberculosis. Proc. Natl. Acad. Sci. USA 102:12344-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mertens, P., D. Walgraffe, T. Laurent, N. Deschrevel, J. J. Letesson, and X. De Bolle. 2001. Selection of phage-displayed peptides recognised by monoclonal antibodies directed against the lipopolysaccharide of Brucella. Int. Rev. Immunol. 20:181-199. [DOI] [PubMed] [Google Scholar]
- 22.Metzger-Boddien, C., D. Khaschabi, M. Schonbauer, S. Boddien, T. Schlederer, and J. Kehle. 2006. Automated high-throughput immunomagnetic separation-PCR for detection of Mycobacterium avium subsp. paratuberculosis in bovine milk. Int. J. Food Microbiol. 110:201-208. [DOI] [PubMed] [Google Scholar]
- 23.Morris, J. A., C. J. Thorns, and J. Woolley. 1985. The identification of antigenic determinants on Mycobacterium bovis using monoclonal antibodies. J. Gen. Microbiol. 131:1825-1831. [DOI] [PubMed] [Google Scholar]
- 24.Mutharia, L. M., W. Moreno, and M. Raymond. 1997. Analysis of culture filtrate and cell wall-associated antigens of Mycobacterium paratuberculosis with monoclonal antibodies. Infect. Immun. 65:387-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ott, S. L., S. J. Wells, and B. A. Wagner. 1999. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev. Vet. Med. 40:179-192. [DOI] [PubMed] [Google Scholar]
- 26.Paustian, M. L., V. Kapur, and J. P. Bannantine. 2005. Comparative genomic hybridizations reveal genetic regions within the Mycobacterium avium complex that are divergent from Mycobacterium avium subsp. paratuberculosis isolates. J. Bacteriol. 187:2406-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Proske, D., M. Blank, R. Buhmann, and A. Resch. 2005. Aptamers—basic research, drug development, and clinical applications. Appl. Microbiol. Biotechnol. 69:367-374. [DOI] [PubMed] [Google Scholar]
- 28.Sturgill-Koszycki, S., P. L. Haddix, and D. G. Russell. 1997. The interaction between Mycobacterium and the macrophage analyzed by two-dimensional polyacrylamide gel electrophoresis. Electrophoresis 18:2558-2565. [DOI] [PubMed] [Google Scholar]
- 29.Takemura, K., P. Wang, I. Vorberg, W. Surewicz, S. A. Priola, A. Kanthasamy, R. Pottathil, S. G. Chen, and S. Sreevatsan. 2006. DNA aptamers that bind to PrP(C) and not PrP(Sc) show sequence and structure specificity. Exp. Biol. Med. (Maywood) 231:204-214. [DOI] [PubMed] [Google Scholar]
- 30.Thoresen, O. F., K. Falk, and O. Evensen. 1994. Comparison of immunohistochemistry, acid-fast staining, and cultivation for detection of Mycobacterium paratuberculosis in goats. J. Vet. Diagn. Investig. 6:195-199. [DOI] [PubMed] [Google Scholar]
- 31.Waters, W. R., J. M. Miller, M. V. Palmer, J. R. Stabel, D. E. Jones, K. A. Koistinen, E. M. Steadham, M. J. Hamilton, W. C. Davis, and J. P. Bannantine. 2003. Early induction of humoral and cellular immune responses during experimental Mycobacterium avium subsp. paratuberculosis infection of calves. Infect. Immun. 71:5130-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weynants, V., D. Gilson, A. Cloeckaert, A. Tibor, P. A. Denoel, F. Godfroid, J. N. Limet, and J. J. Letesson. 1997. Characterization of smooth lipopolysaccharides and O polysaccharides of Brucella species by competition binding assays with monoclonal antibodies. Infect. Immun. 65:1939-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whan, L., H. J. Ball, I. R. Grant, and M. T. Rowe. 2005. Development of an IMS-PCR assay for the detection of Mycobacterium avium ssp. paratuberculosis in water. Lett. Appl. Microbiol. 40:269-273. [DOI] [PubMed] [Google Scholar]
- 34.Young, D. B., and T. R. Garbe. 1991. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect. Immun. 59:3086-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zugel, U., and S. H. Kaufmann. 1999. Immune response against heat shock proteins in infectious diseases. Immunobiology 201:22-35. [DOI] [PubMed] [Google Scholar]