Abstract
Children who have siblings and/or who attend day care have higher rates of nasopharyngeal colonization with pneumococci than lone children do. Pneumococcal colonization is usually asymptomatic but is a prerequisite for invasive disease. We studied the effect of social mixing with other children on immunity to a pneumococcal vaccine. One hundred sixty children aged 1 year were immunized with a 7-valent conjugate pneumococcal vaccine. A blood sample was obtained before and 9 to 11 days after the vaccine. The concentration and avidity of antibody against vaccine pneumococcal serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) were studied in relation to pneumococcal carriage rate and measures of social mixing. Children with increased social mixing had higher antibody concentrations against serotypes 4, 9V, 14, and 23F than lone children did. The least-carried serotype, serotype 4, was the one of the most immunogenic. This contrasts with serotype 6B, the most common nasopharyngeal isolate but the least immunogenic. Social mixing in infancy enhances the immune response to a Streptococcus pneumoniae polysaccharide-protein conjugate vaccine at 1 year of age. Exposure to pneumococci in the first year of life may induce immunological priming. An alternative explanation is that differences in immunological experience, such as increased exposure to respiratory viral infections in early childhood, alters the response to vaccines perhaps by affecting the balance between Th1 and Th2 cytokines. The low immunogenicity of serotype 6B polysaccharide might make conditions more favorable for carriage of the 6B organism and explain why 6B pneumococci were more frequently isolated than other serotypes.
Streptococcus pneumoniae is the leading bacterial cause of death in children under 5 years of age in the world with the greatest burden of severe disease (meningitis and pneumonia) occurring in low-income countries (42). Pneumococcus is one of the most frequently reported causes of bacteremia and meningitis in England and Wales (11) and is the leading cause of bacterial community-acquired pneumonia (3).
Vaccines containing capsular polysaccharides have been available for several decades but are poorly immunogenic in children under 2 years of age and provide very limited protection in this age group (36). Bacterial capsular polysaccharides are T-independent antigens that are not presented with major histocompatibility complex molecules and therefore do not recruit cognate T-cell help or induce immunological memory. Conjugation of bacterial capsular polysaccharides to a protein carrier overcomes the poor immunogenicity of these vaccines in infants by allowing recruitment of T-cell help to the polysaccharide-specific B-cell response (18). In 2000, a heptavalent pneumococcal conjugate vaccine was included in the United States infant immunization program, resulting in a substantial decline in pneumococcal infections in childhood (41).
Nasopharyngeal carriage of S. pneumoniae is very common in infancy and early childhood (5), and invasive disease is preceded by acquisition of pneumococci from a carrier (4). A longitudinal study of pneumococcal carriage in infants in Oxfordshire, United Kingdom, found that 60% of these children carried one or more pneumococcal serotypes in the first year of life. In this United Kingdom study, the acquisition rate was higher for infants with older siblings than for first children, with 50% more acquisitions occurring per additional sibling (37). Other studies have confirmed that exposure to other children in day care or within a family increases the rate of carriage of S. pneumoniae (32). Furthermore, day care attendance has been associated with invasive infection in children (29).
It is not known whether exposure to respiratory microorganisms, including nasopharyngeal carriage of pneumococci, in infancy affects the response to glycoconjugate vaccines. Several studies seem to indicate that nasopharyngeal carriage of encapsulated bacteria or exposure to cross-reacting antigens from other bacteria is responsible for the development of natural immunity. Repeated encounters with Haemophilus influenzae type b (Hib) or cross-reacting antigens from other organisms structurally related to Hib throughout childhood, lead to protective levels of capsular antibody. Escherichia coli K100 fed to volunteers induced bactericidal antibodies to Hib (35). In addition, Hib conjugate vaccines appear to boost the development of natural antibodies. In a Swedish study of 6-year-old children, significantly fewer vaccinated children (3%) than unvaccinated children (13%) had serum antibodies below the protective level of 0.15 μg/ml (8). The antibody response in sera of adults who were immunized with a serogroup C meningococcal polysaccharide vaccine showed avidity characteristics of a secondary immune response, which suggests that priming occurs either via carriage or previous exposure to cross-reacting antigens (13). Priming of the immune response by nasopharyngeal carriage of bacteria during childhood induces maturation to a high-avidity antibody that is reflected in an increase in functional activity of antibody with age, as shown in population studies of meningococcal serum bactericidal activity (31). Furthermore, studies of the immune response after meningococcal disease (30) found that the avidity of antibody produced in response to infection is low or absent in infancy and higher in later childhood. These studies suggest that carriage of encapsulated bacteria in the nasopharynx may stimulate a T-dependent immune response to the polysaccharides in the same way as a conjugate vaccine, since plain polysaccharides do not induce avidity maturation (1).
In this investigation we examined whether social mixing in the home or in day care affected the immune responses to a heptavalent pneumococcal conjugate vaccine administered when the infant was 1 year of age.
MATERIALS AND METHODS
Participants and immunizations.
Healthy infants aged 1 year residing in Oxfordshire, United Kingdom, were enrolled. Written informed parental consent was obtained in person. Ethical approval was obtained from the Oxfordshire Research Ethics Committee (C02.005) and complied with the World Medical Association Helsinki Declaration on human experimentation. All infants had been immunized according to the United Kingdom schedule with a combined diphtheria, tetanus, whole-cell pertussis, and Hib vaccine, a meningococcal C vaccine, and an oral polio vaccine, each given at 2, 3, and 4 months. The study was undertaken prior to the introduction of the pneumococcal conjugate vaccine for routine immunization. Infants were vaccinated with a pneumococcal 7-valent conjugate vaccine (Prevenar; Wyeth Vaccines) administered intramuscularly into the left anterolateral thigh at 12 to 13 months of age. Data on the number of siblings, day care attendance, and parental smoking was obtained at this first visit, and a nasopharyngeal swab was taken from the infants to identify S. pneumoniae colonization. Day care attendance was defined as any child care arrangement with other children ranging from one half-day session of 4 h to full-time attendance of 10 sessions. Household smoking was defined as either parent smoking in the home. Venipuncture was performed at the first visit and 9 to 11 days later. A second dose of the vaccine was given as recommended for pneumococcal immunization of children under 2 years of age in the United Kingdom 2 months later.
Isolation of S. pneumoniae from the nasopharynx.
Pernasal swabs were transported in vials of transport medium (STGG [11a]) and plated within 8 h onto blood agar and gentamicin blood agar plates. Three alpha-hemolytic colonies that were morphologically distinct were selected from the agar plates after overnight incubation (37°C in 5% CO2). S. pneumoniae was identified using colony morphology and optochin susceptibility. Serotyping was performed using the Quellung reaction with sera from the Statens Serum Institut, Copenhagen, Denmark. Isolates that were nontypeable but optochin susceptible and bile soluble were considered to be presumptive pneumococci.
Measurement of serum antibody and avidity.
Total immunoglobulin (Ig) and immunoglobulin G (IgG), specific to each of the seven vaccine serotypes was measured by enzyme-linked immunosorbent assay (ELISA) following the World Health Organization consensus protocol (40). Antibody avidity for each vaccine pneumococcal serotype was measured at a single time point after vaccination by adapting the serotype-specific pneumococcal ELISA to incorporate an elution step using ammonium thiocyanate as the chaotrophic agent. The avidity index measured was calculated as the molar concentration of ammonium thiocyanate required to elute 50% of the bound specific antibody in a given sample (12).
Statistical analysis.
Antibody levels were log transformed to achieve normality. The effects of siblings and day care attendance on antibody levels were analyzed using analysis of covariance, adjusting for parental smoking, pneumococcal carriage rate, and baseline values. Comparisons of avidity level after vaccination were performed using the Mann-Whitney test to determine differences in sera from children with or without siblings or attendance at day care. The level of significance for avidity analysis was set at 1% to account for multiple comparisons, while all other comparisons were at 5%. All analyses were carried out on all available data.
RESULTS
Demographics.
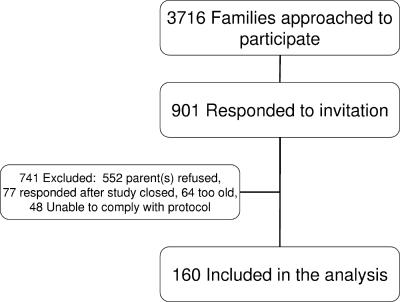
The study ran from February 2003 to September 2004. A total of 160 children were included in the study (Fig. 1), 91 female and 69 male, aged between 12 months and 13 months 4 days. Five children were premature at birth ranging from 33 to 36 weeks gestation. None of these children required intensive care, and all had uncomplicated neonatal courses. The numbers of children with siblings and/or who attended day care are outlined in Table 1. The parents of 40 children reported household smoking. Of the children who attended day care, 39 attended up to 6 sessions and 23 attended 7 to 10 sessions. There was no linear relationship between the number of sessions attended and geometric mean concentration following vaccination.
FIG. 1.
Summary of recruitment and enrollment of infants into the study.
TABLE 1.
Number of children in each group and pneumococcal carriage rates
| Groupa | Day care attendance | Siblings | No. of children | Pneumococcal carriage rate (%) |
|---|---|---|---|---|
| 1 | Yes | Yes | 33 | 88 |
| 2 | No | Yes | 53 | 62 |
| 3 | No | No | 43 | 35 |
| 4 | Yes | No | 31 | 77 |
Children are divided into groups on the basis of whether they have siblings and attend day care.
Antibody response to a pneumococcal 7-valent conjugate vaccine.
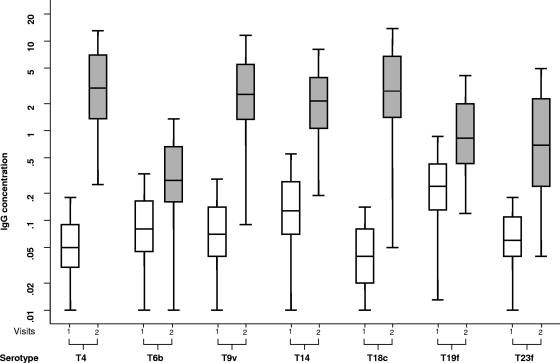
There was a rise in serotype-specific serum IgG, following vaccination with the 7-valent conjugate pneumococcal vaccine (Fig. 2). The serum IgG concentration rose above 0.2 μg/ml in 100%, 64%, 98%, 99%, 97%, 92%, and 81% of subjects against serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F, respectively.
FIG. 2.
Pneumococcal serotype-specific serum IgG concentrations before and after immunization with a 7-valent pneumococcal conjugate vaccine. The clear boxes represent the middle 50% spread before the vaccine, and the shaded boxes represents the middle 50% spread 9 to 11 days after the vaccine. The bars represent 95% spread. The lines in the boxes represent the median values. IgG concentration is measured in micrograms per milliliter. Serotypes are shown with a capital T (for type). (Stata and Microsoft Power Point were used to prepare this figure.)
Effect of social mixing.
The influence of exposure to other children (siblings and day care attendance), on an individual's IgG immune response to the vaccine was evaluated (Table 2). Serum total Ig concentration tended in the same direction as IgG concentration for most of the serotype responses (data not shown). Infants with siblings or who attended day care had significantly higher serotype 23F antibody responses. The antibody response was 67% higher in children who had siblings and 69% higher in children who attended day care. Day care attendance was associated with significantly higher antibody concentration for serotypes 4, 9V, and 14 after vaccination. Serotype 4, 9V, and 14 antibody responses were 95%, 53%, and 53% higher in children who attended day care, respectively. A smaller effect was seen for children with siblings for serotypes 4 and 9V, but this did not reach statistical significance. In contrast, serotype 6B antibody response was 9% lower in children with siblings and 18% lower in children who attended day care. Risk factors for pneumococcal carriage had very little effect on serotype 18C and 19F antibody responses.
TABLE 2.
Effect of exposure to siblings or day care attendance on specific antibody to S. pneumoniae vaccine serotypes following vaccination with 7-valent conjugate pneumococcal vaccinea
| Serotype | Risk factor | Estimated relative change in specific IgG antibody level (95% CI)b | P valuec |
|---|---|---|---|
| 4 | Siblings | 1.38 (0.95-2.02) | 0.09 |
| Day care | 1.95 (1.33-2.87) | 0.001 | |
| 6B | Siblings | 0.93 (0.64-1.34) | 0.7 |
| Day care | 0.86 (0.59-1.27) | 0.5 | |
| 9V | Siblings | 1.29 (0.96-1.74) | 0.09 |
| Day care | 1.47 (1.08-1.99) | 0.01 | |
| 14 | Siblings | 1.13 (0.79-1.62) | 0.5 |
| Day care | 1.55 (1.08-2.22) | 0.02 | |
| 18C | Siblings | 0.99 (0.64-1.54) | 0.9 |
| Day care | 1.26 (0.80-1.99) | 0.3 | |
| 19F | Siblings | 1.28 (0.89-1.86) | 0.2 |
| Day care | 1.38 (0.94-2.01) | 0.1 | |
| 23F | Siblings | 1.62 (1.02-2.28) | 0.04 |
| Day care | 1.69 (1.06-2.74) | 0.03 |
The effects of siblings and day care attendance on antibody levels were analyzed using analysis of covariance, adjusting for parental smoking, carriage of the specific pneumococcal serotype, and baseline values.
95% CI, 95% confidence interval.
A P value of <0.05 was taken as statistically significant and is shown in bold type.
Table 3 illustrates the effect risk factors for nasopharyngeal carriage of pneumococci have on vaccine serotype avidity following immunization with a 7-valent conjugate pneumococcal vaccine. There was a significantly higher avidity of serotype 4 antibody in the sera of children who had siblings, and anti-serotype 19F antibody avidity was higher in children who attended day care.
TABLE 3.
Effect of exposure to siblings or day care attendance on avidity to S. pneumoniae vaccine serotypes following vaccination with 7-valent conjugate pneumococcal vaccine
| Serotype | Risk factor | Median difference in absolute avidity (99% CI)a | Mann-Whitney P valueb |
|---|---|---|---|
| 4 | Siblings | 11 (0.25) | 0.01 |
| Day care | −4 (−20 to 8) | 0.39 | |
| 6B | Siblings | −3 (−66 to 21) | 0.73 |
| Day care | 12 (−16 to 74) | 0.25 | |
| 9V | Siblings | −0.5 (−96 to 92) | 0.97 |
| Day care | −23 (−217 to 24) | 0.2 | |
| 14 | Siblings | 44 (−33 to 134) | 0.16 |
| Day care | −16 (−99 to 71) | 0.6 | |
| 18C | Siblings | 8 (−109 to 121) | 0.74 |
| Day care | −12 (−143 to 87) | 0.59 | |
| 19F | Siblings | 6 (−26 to 89) | 0.63 |
| Day care | 68.5 (−1 to 195) | 0.01 | |
| 23F | Siblings | −5 (−22 to 11) | 0.27 |
| Day care | 7 (−9 to 23) | 0.4 |
Comparisons of the avidity level on the second visit between children with or without siblings and between children who did or did not attend day care, using the Mann-Whitney test. 95% CI, 95% confidence interval.
A P value of ≤0.01 was taken as statistically significant and is shown in bold type.
Nasopharyngeal carriage.
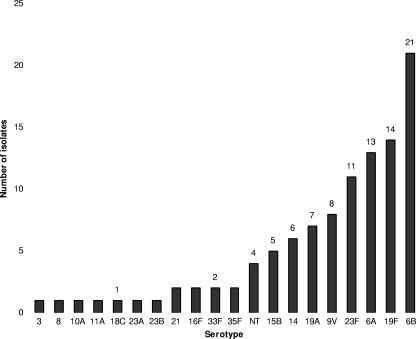
The distribution of nasopharyngeal serotypes of S. pneumoniae is shown in Fig. 3. A positive nasopharyngeal swab was obtained from 99 out of 159 children (62.3%) of whom 95% were carrying one serotype and 5% were carrying two serotypes. Serotypes 6B, 19F, 6A, 23F, 9V, 19A, 14, and 15B were the most frequently isolated serotypes, accounting for 81.7% of all isolates. S. pneumoniae was isolated from 29 children (88%) in group 1, 33 (62%) in group 2, 15 (35%) in group 3, and 24 (77%) in group 4 (Table 1).
FIG. 3.
Serotypes of Streptococcus pneumoniae isolated from the nasopharynx of 1-year-old children at enrollment into the study. NT, nontypeable.
Children who were not carrying serotype 9V on the day of vaccination had a significantly greater postimmunization 9V antibody concentration than the eight individuals who carried serotype 9V (data not shown). For carriers of other serotypes, the differences in the immune response were not different compared to noncarriers of that serotype, although noncarriers generally had higher antibody concentrations than carriers.
Relationship between nasopharyngeal carriage and immune response.
Serotype 4 was not isolated from the nasopharynx but was the one of the most immunogenic of the vaccine serotypes. By contrast, serotype 6B was the most common nasopharyngeal isolate but was the least immunogenic of the vaccine serotypes, and risk factors for pneumococcal carriage did not appear to have an effect on the antibody response.
DISCUSSION
We have shown that 1-year-old infants who engage in social mixing with other children have an enhanced immune response to a single dose of saccharides in a pneumococcal conjugate vaccine at 1 year of age than infants who have less exposure to the upper airway flora of other young children. There are several possible explanations for these data. For example, exposure to pneumococci in the first year of life induces immunological priming or those differences in immunological experience, such as increased exposure to respiratory viral infections in early childhood, alter the responses to vaccines.
Children who attended day care had significantly higher antibody responses to serotypes 4, 9V, 14, and 23F than did their contemporaries who did not attend day care, and the effect of day care exposure on antibody responses was greater than the impact of exposure to siblings. Day care attendance increases nasopharyngeal colonization with pneumococci (32) and contributes to the wide variation of pneumococcal carriage seen across communities (16), suggesting that these infants may have been primed through exposure to pneumococci acquired from their contemporaries. Both the rate and serotype distribution of carriage of pneumococci in our group of infants in Oxfordshire, United Kingdom, are consistent with previously published data from Oxford, United Kingdom (24) and elsewhere (15).
Colonization with encapsulated bacteria is thought to lead to the development of natural immunity to polysaccharides, and a recent study in adults found that levels of anticapsular IgG increased significantly after carriage of serotypes 9V, 14, 18C, 19F, and 23F by an individual or family member (14). However, in contrast to these adult data and in line with our low baseline antibody levels, Soininen et al. found that antibody production remained low in unvaccinated infants during carriage of serotype 6B, 19F, or 23F, suggesting that infants do not have a significant immune response to carriage of these serotypes (although antibodies did rise on contact with serotypes 11A and 14) (38). The low baseline levels in our study may indicate that any rise in antibody level following carriage during the first year of life is transient. Studies with Hib glycoconjugate vaccine in infancy have shown that immunological priming may occur with a single dose of vaccine, although the rise in antibody concentration is smaller than after two doses (19), and this may be analogous to the apparent priming that we observed from nasopharyngeal colonization. Vaccination with the 9-valent pneumococcal conjugate vaccine stimulated higher levels of antibody in South African children compared to children vaccinated with the 7-valent pneumococcal conjugate vaccine in Finland and the United States. This response may be due to the early exposure to pneumococcal antigen priming children in developing countries to respond more strongly to pneumococcal vaccines (23).
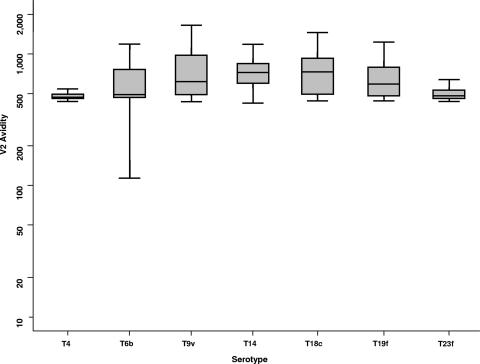
High antibody avidity may be a correlate of protection against pneumonia, since adults presenting at hospitals with pneumococcal pneumonia have lower polysaccharide-specific antibody avidity than healthy controls do (26), although in vitro studies have produced conflicting results about the relationship of high-avidity antibody with opsonophagoctyic activity and in vivo protection after immunization. In infants, a tendency to negative correlation was found between the concentration needed for killing of bacteria and avidity, but no such correlation was seen in adults (2, 33). After immunization with a pneumococcal conjugate vaccine using a three-dose primary schedule in infants at 2, 4, and 6 months of age, antibody avidity rose between the ages of 7 and 12 months, and a further increase in avidity was seen after boosting at 12 months of age (43). Using our thiocyanate elution ELISA, antibody avidity for all serotypes was lower than the avidity we expect for infants of the same age who have been primed with three doses during the first 4 months of life and similar to the avidity that we have observed immediately after priming in infants at 5 months of age (Fig. 4). The avidity of the antibody was similar to pneumococcal antibody avidity that we have observed in the sera of unvaccinated adult volunteers. The geometric mean avidity of the sera in this study was comparable to the avidity of a nonprimed cohort of infants (Oxford Vaccine Group [data not shown]).
FIG. 4.
Pneumococcal serotype-specific IgG avidity after a 7-valent conjugate pneumococcal vaccine. The shaded box represents the middle 50% spread, and the bars represent 95% spread. The line in the box represents the median value. Avidity is measured as the molar concentration of ammonium thiocyanate required to elute 50% of the bound specific antibody in a given sample. V2, second visit. Serotypes are shown with a capital T (for type). (Stata and Microsoft Power Point were used to prepare this figure.)
It is not known whether nasopharyngeal colonization with pneumococci in infancy induces somatic mutation in the polysaccharide-specific B cells and avidity maturation. We hypothesized that if the infants in our study had been primed through carriage, we would find a higher-avidity antibody present in those babies who had risk factors for higher rates of carriage of pneumococci. The data presented in Table 3 suggest that this may indeed be the case for serotypes 4 and 19F, but there is no convincing trend for other serotypes, and this limited but significant outcome is difficult to interpret.
An alternative explanation for our finding that infants who engage in social mixing have higher antibody levels after pneumococcal conjugate immunization is that other factors associated with social mixing among children affect the immune response to this vaccine. It is well recognized that having older siblings (22) and/or attendance at day care (17) results in an increased microbial burden in early childhood with higher carriage rates of other encapsulated bacteria and exposure to viral infections. Exposure to viral or bacterial infection may affect the cytokine milieu and influence the immune response to subsequent immunization or infection. For example, severe respiratory syncytial virus infection in one study resulted in higher production of type 2 cytokines in Gambian children at 5 years of age (39), and another study found that infection with Bordetella pertussis or immunization with the whole-cell pertussis vaccine at 2 months of age caused a shift to a more Th1 cytokine profile (21). Ota et al. found that Mycobacterium bovis BCG vaccine influenced the immune responses to hepatitis B vaccine resulting in higher antibody levels (28). In another study, a higher frequency of viral infections in the first year of life increased mitogen-induced gamma interferon (IFN-γ) responses at 1 year of age (9). Exposure to viral infections may therefore enhance antibody responses to pneumococcal conjugate vaccines, perhaps by affecting the balance between Th1 and Th2 cytokines. In fact, we measured the T-cell responses in some of our subjects and found that infants who had reduced exposure to respiratory infections (no siblings or day care [n = 12]) produced IFN-γ and interleukin 5 in response to the carrier protein (diphtheria toxoid) following vaccination with a pneumococcal-CRM conjugate vaccine (Th0 phenotype). Conversely, T cells from infants with an increased risk of respiratory infection (with siblings and/or attend day care [n = 8]) showed a decrease in IFN-γ secretion and continuing production of interleukin 5 (Th2 phenotype) (data not shown). Although the numbers were small and need confirmation, it would suggest that exposure to respiratory infections during early infancy may prime the infant T-cell response to production of a Th2 cytokine pattern.
Another observation from our data is that there was heterogeneity in the immune responses between different serotypes. Previous authors have commented that the most common pneumococcal serotypes that cause infections in children are the poorest immunogens in this age group (10, 20). The poor immunogenicity of serotype 6B polysaccharide for example has been observed in previous studies (27, 44) and might make conditions more favorable for persistent carriage of pneumococci bearing the 6B capsule. In carriage studies, including the data presented here, serotype 6B pneumococci are more frequently isolated than many other serotypes (6, 24). The lack of enhanced serotype 6B immune responses in the sera of our “carrier” children probably reflects the poor immunogenicity of this polysaccharide in those who are exposed and indicates that it does not prime. Alternatively, the high rate of carriage among young children may preclude distinction between the groups who are more or less likely to carry the organism.
By contrast, serotype 4 was very rarely carried in our study (and other carriage studies) but is one of the most immunogenic polysaccharides. Presumably, the enhanced immune response in our “carrier” group relates to increased acquisition risk from mixing with other children. Our carriage data represent a single point in time, which limits our ability to fully interpret the data. Without repeated nasopharyngeal samples over time, it is difficult to say whether lack of serotype 4 carriage indicates a lack of exposure or previous exposure to an immunogenic serotype that has since been cleared.
The differences in carriage and immunogenicity of serotypes 4 and 6B are in keeping with the theory raised by Sandgren et al. (34) who proposed that invasive disease is caused by two different groups of pneumococcal clones, one group of bacteria that appear primarily among invasive isolates (efficient transmitters but poor colonizers) and another group that are highly efficient colonizers with increased opportunity for invasion. In support of this thesis, Brueggemann et al. (7) found an inverse correlation between invasive disease and carriage prevalence; serotype 4 is a more invasive serotype than serotype 6B and is less commonly carried.
In addition, there are many bacterial virulence factors that influence carriage potential and the ability to “escape” immune surveillance (25).
This study has documented enhanced immune responses to pneumococcal conjugate vaccine in 1-year-old infants as a result of social mixing with other children at home or in day care. This observation suggests either that exposure to pneumococci in infancy primes the immune response at an age when plain polysaccharides are not immunogenic or that exposure to viral infections in infancy alters the immune response to vaccines.
Acknowledgments
We are grateful to the families of participating children, Shirley Ashmore for administrative assistance, Elizabeth Clutterbuck for comments on the manuscript, and the research nurses at the Oxford Vaccine Group for clinical assistance. Andrew J. Pollard is a Jenner Institute Investigator.
This study was an investigator-originated project funded through a grant to the University of Oxford from Wyeth Vaccines (Wyeth reference number 101325; registered in England and Wales; registration no. 135 937; registered office, Huntercombe Lane South, Taplow, Maidenhead, Berkshire SL6 0PH, United Kingdom).
A.J.P. acts as chief investigator for clinical trials conducted on behalf of Oxford University, sponsored by vaccine manufacturers (Chiron Vaccines, GlaxoSmithKline, Sanofi-Aventis, Sanofi-Pasteur MSD, and Wyeth Vaccines), and has received assistance from Aventis Pasteur MSD, Chiron Vaccines, and Wyeth Vaccines to attend scientific meetings. Industry-sourced honoraria for lecturing or writing are paid directly to an independent charity or an educational fund held by the Department of Paediatrics, University of Oxford. A.J.P. does not hold any paid consultancies with vaccine manufacturers.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Anttila, M., J. Eskola, H. Ahman, and H. Kayhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 2.Anttila, M., M. Voutilainen, V. Jantti, J. Eskola, and H. Kayhty. 1999. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., and L. M. Mundy. 1995. Community-acquired pneumonia. N. Engl. J. Med. 333:1618-1624. [DOI] [PubMed] [Google Scholar]
- 4.Bedos, J. P., S. Chevret, C. Chastang, P. Geslin, and B. Regnier. 1996. Epidemiological features of and risk factors for infection by Streptococcus pneumoniae strains with diminished susceptibility to penicillin: findings of a French survey. Clin. Infect. Dis. 22:63-72. [DOI] [PubMed] [Google Scholar]
- 5.Black, S., and H. Shinefield. 1997. Issues and challenges: pneumococcal vaccination in pediatrics. Pediatr. Ann. 26:355-360. [DOI] [PubMed] [Google Scholar]
- 6.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 7.Brueggemann, A. B., T. E. Peto, D. W. Crook, J. C. Butler, K. G. Kristinsson, and B. G. Spratt. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203-1211. [DOI] [PubMed] [Google Scholar]
- 8.Claesson, B. A., B. Trollfors, P. W. Anderson, J. Johansson, J. Taranger, R. Schneerson, and J. B. Robbins. 1996. Serum antibodies in six-year-old children vaccinated in infancy with a Haemophilus influenzae type b-tetanus toxoid conjugate vaccine. Pediatr. Infect. Dis. J. 15:170-172. [DOI] [PubMed] [Google Scholar]
- 9.Copenhaver, C. C., J. E. Gern, Z. Li, P. A. Shult, L. A. Rosenthal, L. D. Mikus, C. J. Kirk, K. A. Roberg, E. L. Anderson, C. J. Tisler, D. F. DaSilva, H. J. Hiemke, K. Gentile, R. E. Gangnon, and R. F. Lemanske, Jr. 2004. Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am. J. Respir. Crit. Care Med. 170:175-180. [DOI] [PubMed] [Google Scholar]
- 10.Douglas, R. M., J. C. Paton, S. J. Duncan, and D. J. Hansman. 1983. Antibody response to pneumococcal vaccination in children younger than five years of age. J. Infect. Dis. 148:131-137. [DOI] [PubMed] [Google Scholar]
- 11.George, A., and A. Melegaro. 2003. Invasive pneumococcal infection England and Wales 2000. CDR Wkly. 21:7-10. [Google Scholar]
- 11a.Gherna, R. L. 1981. Preservation, p. 208-217. In P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester, W. A. Wood, N. R. Krieg, and G. B. Phillips (ed.), Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC.
- 12.Goldblatt, D. 1997. Simple solid phase assays of avidity, p. 31-51. In A. P. Johnstone and M. W. Turner (ed.), Immunochemistry 2: a practical approach. Oxford University Press, Oxford, United Kingdom.
- 13.Goldblatt, D., R. Borrow, and E. Miller. 2002. Natural and vaccine-induced immunity and immunologic memory to Neisseria meningitidis serogroup C in young adults. J. Infect. Dis. 185:397-400. [DOI] [PubMed] [Google Scholar]
- 14.Goldblatt, D., M. Hussain, N. Andrews, L. Ashton, C. Virta, A. Melegaro, R. Pebody, R. George, A. Soininen, J. Edmunds, N. Gay, H. Kayhty, and E. Miller. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192:387-393. [DOI] [PubMed] [Google Scholar]
- 15.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83-93. [DOI] [PubMed] [Google Scholar]
- 16.Huang, S. S., J. A. Finkelstein, and M. Lipsitch. 2005. Modeling community- and individual-level effects of child-care center attendance on pneumococcal carriage. Clin. Infect. Dis. 40:1215-1222. [DOI] [PubMed] [Google Scholar]
- 17.Hurwitz, E. S., W. J. Gunn, P. F. Pinsky, and L. B. Schonberger. 1991. Risk of respiratory illness associated with day-care attendance: a nationwide study. Pediatrics 87:62-69. [PubMed] [Google Scholar]
- 18.Jennings, H. J., and C. Lugowski. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127:1011-1018. [PubMed] [Google Scholar]
- 19.Kurikka, S., H. Kayhty, L. Saarinen, P. R. Ronnberg, J. Eskola, and P. H. Makela. 1995. Immunologic priming by one dose of Haemophilus influenzae type b conjugate vaccine in infancy. J. Infect. Dis. 172:1268-1272. [DOI] [PubMed] [Google Scholar]
- 20.Makela, P. H., M. Sibakov, E. Herva, J. Henrichsen, J. Luotonen, M. Timonen, M. Leinonen, M. Koskela, J. Pukander, S. Pontynen, P. Gronroos, and P. Karma. 1980. Pneumococcal vaccine and otitis media. Lancet ii:547-551. [DOI] [PubMed] [Google Scholar]
- 21.Mascart, F., V. Verscheure, A. Malfroot, M. Hainaut, D. Pierard, S. Temerman, A. Peltier, A. S. Debrie, J. Levy, G. Del Giudice, and C. Locht. 2003. Bordetella pertussis infection in 2-month-old infants promotes type 1 T cell responses. J. Immunol. 170:1504-1509. [DOI] [PubMed] [Google Scholar]
- 22.Matricardi, P. M., F. Franzinelli, A. Franco, G. Caprio, F. Murru, D. Cioffi, L. Ferrigno, A. Palermo, N. Ciccarelli, and F. Rosmini. 1998. Sibship size, birth order, and atopy in 11,371 Italian young men. J. Allergy Clin. Immunol. 101:439-444. [DOI] [PubMed] [Google Scholar]
- 23.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 24.Meats, E., A. B. Brueggemann, M. C. Enright, K. Sleeman, D. T. Griffiths, D. W. Crook, and B. G. Spratt. 2003. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. J. Clin. Microbiol. 41:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moine, P., and E. Abraham. 2004. Immunomodulation and sepsis: impact of the pathogen. Shock 22:297-308. [DOI] [PubMed] [Google Scholar]
- 26.Musher, D. M., H. M. Phan, D. A. Watson, and R. E. Baughn. 2000. Antibody to capsular polysaccharide of Streptococcus pneumoniae at the time of hospital admission for pneumococcal pneumonia. J. Infect. Dis. 182:158-167. [DOI] [PubMed] [Google Scholar]
- 27.Obaro, S. K., Z. Huo, W. A. Banya, D. C. Henderson, M. A. Monteil, A. Leach, and B. M. Greenwood. 1997. A glycoprotein pneumococcal conjugate vaccine primes for antibody responses to a pneumococcal polysaccharide vaccine in Gambian children. Pediatr. Infect. Dis. J. 16:1135-1140. [DOI] [PubMed] [Google Scholar]
- 28.Ota, M. O., J. Vekemans, S. E. Schlegel-Haueter, K. Fielding, M. Sanneh, M. Kidd, M. J. Newport, P. Aaby, H. Whittle, P. H. Lambert, K. P. McAdam, C. A. Siegrist, and A. Marchant. 2002. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J. Immunol. 168:919-925. [DOI] [PubMed] [Google Scholar]
- 29.Pereiro, I., J. Diez-Domingo, L. Segarra, A. Ballester, A. Albert, and A. Morant. 2004. Risk factors for invasive disease among children in Spain. J. Infect. 48:320-329. [DOI] [PubMed] [Google Scholar]
- 30.Pollard, A. J., R. Galassini, E. M. van der Voort, R. Booy, P. Langford, S. Nadel, C. Ison, J. S. Kroll, J. Poolman, and M. Levin. 1999. Humoral immune responses to Neisseria meningitidis in children. Infect. Immun. 67:2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollard, A. J., J. Ochnio, M. Ho, M. Callaghan, M. Bigham, and S. Dobsong. 2004. Disease susceptibility to ST11 complex meningococci bearing serogroup C or W135 polysaccharide capsules, North America. Emerg. Infect. Dis. 10:1812-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Principi, N., P. Marchisio, G. C. Schito, S. Mannelli, and The Ascanius Project Collaborative Group. 1999. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Pediatr. Infect. Dis. J. 18:517-523. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. E. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 34.Sandgren, A., K. Sjostrom, B. Olsson-Liljequist, B. Christensson, A. Samuelsson, G. Kronvall, and N. B. Henriques. 2004. Effect of clonal and serotype-specific properties on the invasive capacity of Streptococcus pneumoniae. J. Infect. Dis. 189:785-796. [DOI] [PubMed] [Google Scholar]
- 35.Schneerson, R., and J. B. Robbins. 1975. Induction of serum Haemophilus influenzae type B capsular antibodies in adult volunteers fed cross-reacting Escherichia coli 075:K100:H5. N. Engl. J. Med. 292:1093-1096. [DOI] [PubMed] [Google Scholar]
- 36.Shapiro, E. D., A. T. Berg, R. Austrian, D. Schroeder, V. Parcells, A. Margolis, R. K. Adair, and J. D. Clemens. 1991. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N. Engl. J. Med. 325:1453-1460. [DOI] [PubMed] [Google Scholar]
- 37.Sleeman, K. L., L. Daniels, M. Gardiner, D. Griffiths, J. J. Deeks, R. Dagan, S. Gupta, E. R. Moxon, T. E. Peto, and D. W. Crook. 2005. Acquisition of Streptococcus pneumoniae and nonspecific morbidity in infants and their families: a cohort study. Pediatr. Infect. Dis. J. 24:121-127. [DOI] [PubMed] [Google Scholar]
- 38.Soininen, A., H. Pursiainen, T. Kilpi, and H. Kayhty. 2001. Natural development of antibodies to pneumococcal capsular polysaccharides depends on the serotype: association with pneumococcal carriage and acute otitis media in young children. J. Infect. Dis. 184:569-576. [DOI] [PubMed] [Google Scholar]
- 39.van der Sande, M. A., I. M. Kidd, T. Goetghebuer, R. A. Martynoga, A. Magnusen, S. Allen, M. W. Weber, K. L. Fielding, A. Marchant, and H. C. Whittle. 2002. Severe respiratory syncytial virus infection in early life is associated with increased type 2 cytokine production in Gambian children. Clin. Exp. Allergy 32:1430-1435. [DOI] [PubMed] [Google Scholar]
- 40.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab. Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. 1999. Pneumococcal vaccines. WHO position paper. Wkly. Epidemiol. Rec. 74:177-184. [PubMed] [Google Scholar]
- 43.Wuorimaa, T., R. Dagan, M. Vakevainen, F. Bailleux, R. Haikala, M. Yaich, J. Eskola, and H. Kayhty. 2001. Avidity and subclasses of IgG after immunization of infants with an 11-valent pneumococcal conjugate vaccine with or without aluminum adjuvant. J. Infect. Dis. 184:1211-1215. [DOI] [PubMed] [Google Scholar]
- 44.Zielen, S., I. Buhring, N. Strnad, J. Reichenbach, and D. Hofmann. 2000. Immunogenicity and tolerance of a 7-valent pneumococcal conjugate vaccine in nonresponders to the 23-valent pneumococcal vaccine. Infect. Immun. 68:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]