Abstract
We previously described a simplified quantitative hemolytic assay for classical pathway (CP) hemolytic function in serum that has been shown to correlate with the 50% hemolytic complement (CH50) assay. In the present study, we used this assay to compare CP functions; plasma levels of C3, C4, and C3dg; and ratios of C3dg to C3 in healthy individuals and patients with systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) with different degrees of complement activation. A significant depression in CP function and levels of C4 and C3 and increased C3dg levels and C3dg/C3 ratios were observed in the SLE patients. In patients with RA, CP function was normal, whereas C3, C4, and C3dg levels and the C3dg/C3 ratio were elevated. The SLE results are compatible with systemic complement consumption, whereas the RA data suggest an acute-phase reaction with a normal C3 catabolic rate. To facilitate the handling of patient samples, we also developed a method to restore the hemolytic function of EDTA-plasma by transferring it to Veronal-buffered saline containing the thrombin inhibitor lepirudin. This process inhibits coagulation and enables complement activation, allowing a longer time lag between sample harvesting and testing. These results, combined with previous correlation studies, suggest that the CP hemolytic assay can effectively replace the CH50 assay for routine SLE differential diagnosis and monitoring of disease activity.
There are two major reasons for analyzing complement function in immune complex-mediated autoimmune diseases. The first is that the complement system is a key inflammatory mediator in immune complex disease. As a consequence, activation of complement leads to its consumption and to decreased complement function as well as to the generation of various activation products. These changes are most pronounced in immune complex diseases such as systemic lupus erythematosus (SLE) and urticarial vasculitis, in which complement-related activity varies in relation to disease activity. The second reason is that hereditary deficiencies of complement components are not uncommon in SLE patients and may be associated with predisposition to this disease (26, 43).
Two analytical approaches have generally been used to assess complement function, namely, immunochemical and functional approaches. Reduced levels of circulating, nonactivated, intact complement proteins and indications of ongoing consumption are often demonstrated in active stages of SLE (7, 35, 43). The levels of C3 and C4 are commonly used as benchmarks, but analysis of the level of C1q has been demonstrated to be particularly useful as a marker of severe SLE flares with kidney involvement (16, 18, 42). However, in less active forms of the disease, immunochemical analysis tends to fall short in terms of sensitivity. Instead, methods to measure activation products that are generated by the complement reaction and occur in normal plasma only at very low concentrations are generally preferred (2, 8, 14, 15).
Functional tests that monitor the complement sequence from initiation to the effector stage, such as hemolytic assays, have the advantage of detecting both consumption-related depression of complement activity and deficiencies in complement components. The first technique described, the 50% hemolytic complement (CH50) technique of Mayer (30), has not come into general clinical use because of the difficulty in adapting it for routine laboratory work. However, less demanding procedures, in the form of rapid lysis in-gel tests, are being used with considerable success to detect complement deficiencies (45), but these assays are not suitable for assessing complement consumption.
As a step toward automation, several classical pathway (CP) hemolytic complement assays have been developed that do not require centrifugation. Liu and Young described an assay performed in wells of microtiter plates in which the readout is the turbidity in the sample; a low turbidity is correlated with a high degree of lysis of the erythrocytes (27). More recently, alternative assays for evaluation of complement function have been introduced, but they have not yet assumed a full role in the clinic (7, 10, 20, 24, 29, 46).
In our laboratory, we have developed and previously described an alternative modification of the CH50 test that is inexpensive, rapid, and easy to perform; thus, it is able to meet clinicians' demand for a result within an hour (37). In the present paper, we report the long-term performance of this functional assay and demonstrate its ability to discriminate between patients with inactive and active disease and between patients with exacerbations of different severities. In addition, we introduce a buffer exchange procedure that makes it possible to restore the hemolytic function of EDTA-plasma samples and hence to use EDTA-plasma as an alternative to serum in this assay. The technique relies on the specific thrombin inhibitor lepirudin, which efficiently inhibits coagulation without compromising the complement system. The option to avoid using serum may be particularly useful when handling samples from SLE patients since great individual differences in coagulation rates for this patient group have been reported (11, 40). Because coagulation enzymes have been shown to activate C3, C4, and C5 (19, 23), a prolonged coagulation time during serum preparation may be expected to induce artifactual complement activation in vitro, which in turn will affect the results obtained in the hemolytic assay (17, 33, 41).
MATERIALS AND METHODS
Patients and controls. (i) Cross-sectional group of SLE patients.
The SLE patient group consisted of 74 consecutive SLE patients attending the outpatient clinic of the Department of Rheumatology, Sahlgren University Hospital, Gothenburg, Sweden, who fulfilled four or more of the American Rheumatism Association criteria for SLE (44). With the aim of obtaining patient groups with distinctive degrees of complement activation, these patients were classified according to the system of Morrow et al. (36) into one of the following four clinical disease activity groups: inactive, low active, moderately active, and severely active, referred to as SLE grades I, II, III, and IV, respectively. This classification is based on the number of the following symptoms exhibited: arthralgia, myalgia, vasculitis, pleuritis, pericarditis, and cerebral, skin, or renal involvement. Complement analysis was performed once to several times for each patient (mean, 2.5 times; range, 1 to 9 times).
(ii) RA patients.
The rheumatoid arthritis (RA) group consisted of 132 consecutive patients with rheumatoid factor-positive, definite or classical RA according to the criteria of the American Rheumatism Association (1). They were classified according to the system of McCarty (31) into the following three clinical disability score groups: low, moderate, and severe, referred to below as RA grades I, II, and III, respectively. Complement analysis was performed once for each patient.
(iii) Control subjects.
Control plasma/serum specimens were obtained from 50 healthy blood donors (25 men and 25 women) at the blood bank at the Sahlgren University Hospital, Gothenburg, Sweden.
(iv) Longitudinal group of SLE patients.
In addition, we recruited 20 patients with SLE who were taking part in a prospective control program at the Department of Rheumatology, University Hospital, Lund, Sweden (Table 1) (4). Each patient was monitored for one 8-month period, during which each experienced one exacerbation of the disease. During the observation period, the patients were seen five times at regular 2-month intervals for clinical evaluation and blood tests. A validated index (SLEDAI) was used for assessment of disease activity (5). The index was slightly modified in that laboratory items (complement activation and DNA binding) were excluded to allow comparisons of clinical disease activities with complement data. The maximum possible score for the SLEDAI was therefore reduced from 103 to 99, but in validation studies the scores rarely exceeded 25 (5). Five samples were analyzed from each patient. One of the 20 patients (patient 18) had a total C2 deficiency (22).
TABLE 1.
Characteristics of SLE patients in the longitudinal group
| Patient no. | Group no. | Maximum SLEDAI | Minimum CP function (%) | Presence or identification of indicated conditiona
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Arthritis | Dermatitis | Serositis | Kidney disease | Central nervous system disease | Blood disease | Miscellaneous | ||||
| 1 | 1 | 10 | 100 | + | + | Pulmonary embolus | ||||
| 2 | 1 | 7 | 84 | + | + | Myelitis | ||||
| 3 | 1 | 2 | 108 | + | + | |||||
| 4 | 1 | 16 | 102 | + | ||||||
| 5 | 1 | 4 | 98 | + | + | + | ||||
| 6 | 2 | 12 | 65 | + | + | + | ||||
| 7 | 2 | 13 | 4 | + | + | |||||
| 8 | 2 | 15 | 59 | + | Thrombocytopenia | Vasculitis | ||||
| 9 | 2 | 7 | 19 | + | Gastrointestinal vasculitis | |||||
| 10 | 2 | 12 | 2 | + | + | + | ||||
| 11 | 2 | 7 | 46 | + | + | |||||
| 12 | 2 | 10 | 7 | + | + | + | Myositis | |||
| 13 | 2 | 12 | 0 | + | + | + | ||||
| 14 | 2 | 16 | 1 | + | + | + | + | Thrombocytopenia | ||
| 15 | 3 | 7 | 62 | + | ||||||
| 16 | 3 | 16 | 75 | + | + | Polyneuropathy | ||||
| 17 | 3 | 2 | 49 | + | + | |||||
| 18 | 4 | 6 | 0 | + | + | |||||
| 19 | 4 | 25 | 3 | + | + | + | + | Thrombocytopenia | ||
| 20 | 4 | 9 | 0 | + | + | + | Thrombocytopenia | |||
+, indicated condition present.
(v) Complement-deficient patients.
To further evaluate the ability of the assay to identify patients with complement deficiencies, stored samples from previously diagnosed patients were analyzed. In addition to the C2-deficient patient in the longitudinal SLE group, samples were collected from another SLE patient totally lacking C4 (25) and from a patient with an SLE-like syndrome who had a C3 dysfunction (38). Further samples were collected from patients who were susceptible to bacterial infections and totally lacking C2, C3 (12), C6, C7, C9, or properdin (one patient with each deficiency). In each case, the deficiency was confirmed by immunochemical, functional, and/or genetic analysis. This study was performed after consent from the Ethical Committees at the University Hospitals of Gothenburg and Lund, respectively.
Specimens.
Unless stated otherwise, serum and EDTA-plasma samples were centrifuged, frozen, and stored at −70°C within 2 h of venipuncture.
Analysis of complement hemolytic function.
The CP hemolytic function of serum was determined according to the method of Nilsson and Nilsson (37). Duplicate aliquots of test serum (0.1 ml at a 1/5 dilution) were added to 0.1 ml of sensitized sheep erythrocytes (0.8 × 109 cells) and incubated at 37°C for 20 min. The degree of lysis was determined by spectrophotometric analysis of the supernatants after centrifugation. In the assay, the degree of lysis is a linear function of the complement dose and is expressed as a percentage of the lysis produced by a serum pool collected from 50 healthy blood donors. The test requires erythrocytes exclusively sensitized with immunoglobulin M. A suitable reagent was obtained by depleting a commercial preparation of anti-sheep erythrocyte (Forssman antigen) antiserum of immunoglobulin G by means of protein A affinity chromatography (GE Healthcare, Uppsala, Sweden). The long-term interassay coefficients of variation (CVs) for the test were 12% at the 33% level (24 months; n = 108) and 5% at the 106% level (37 months; n = 170). In addition, the alternative pathway (AP) hemolytic function, as monitored by the lysis of rabbit erythrocytes, was determined with sera from the complement-deficient patients (37). The CVs of the AP hemolytic assay were 16% at the 39% level and 13% at the 86% level (6 months; n = 60 [both levels]).
Buffer exchange of EDTA-plasma for analysis of CP hemolytic function.
As an alternative procedure, 100-μl aliquots of EDTA-plasma were transferred to Veronal-buffered saline (1.8 mM sodium barbiturate, 3.1 mM barbituric acid, and 0.15 M NaCl, pH 7.4, containing 0.75 mM Ca2+ and 2.5 mM Mg2+) to enable complement activation, with 50 μg/ml lepirudin (32) used to replace EDTA as the anticoagulating agent (Refludan; Schering AG, Berlin, Germany), using Bio-Spin 6 chromatography columns (Bio-Rad, Hercules, CA). These samples were used to assess CP hemolytic function as described above. In order to investigate how long samples could be stored prior to analysis without compromising the results, samples of EDTA-blood were collected from SLE patients. Each sample was divided into two aliquots. One aliquot was stored either at 4°C as EDTA-plasma or at room temperature (approximately 22°C) as EDTA-blood for up to 72 h. The other aliquot was centrifuged and stored at −70°C immediately after being harvested and served as a control. These samples were then transferred to Veronal-buffered saline with lepirudin and analyzed in parallel in the CP hemolytic assay. Thereafter, the difference in measured CP activities between samples from the same individual was calculated.
Analysis of complement levels.
C3 and C4 were measured by single radial immunodiffusion (28), using anti-C3c and anti-C4c from Dako A/S (Glostrup, Denmark) and a commercial standard (Behring, Hoechst AG, Frankfurt, Germany). C3dg was analyzed by rocket immunoelectrophoresis after fractionation of the samples with polyethylene glycol (6). Serum stored at 37°C for 7 days in the presence of sodium azide served as a C3dg standard: complete degradation of C3 into C3c and C3dg occurs under these conditions (30). The C3dg concentration of the standard was calculated from the original C3 concentration of the serum. The interassay CVs of the test were 11% at 4.2 mg/liter and 7% at 13.5 mg/liter. C1q was determined by electroimmunoassay (21).
Statistical analyses.
The data are presented as means ± standard deviations (SD), compared using the nonparametric Mann-Whitney method. Simple regression analysis was used to determine correlations. The sensitivity (i.e., number of positive SLE samples/all measured SLE samples) and specificity (i.e., number of negative-control samples/all measured control samples) for each complement test (CP activity, levels of C4, C3, and C3dg, and ratio of C3dg to C3) were calculated. Calculations were made using the total numbers of samples from SLE patients (n = 183) in the cross-sectional group and from healthy controls (n = 50).
RESULTS
CP hemolytic function in buffer-changed EDTA-plasma.
The CP hemolytic function of SLE patients was measured in 33 samples of EDTA-plasma that had been transferred to Veronal-buffered saline containing cations to enable complement activation and lepirudin to inhibit coagulation. Serum samples collected from the same patients on the same occasions were analyzed in parallel. A linear correlation (R = 0.969) was found between values obtained using the two techniques (Fig. 1).
FIG. 1.
Correlation between CP functions analyzed in serum and buffer-changed EDTA-plasma. Samples of EDTA-plasma from 33 individuals were transferred to Veronal-buffered saline containing lepirudin, as described in Materials and Methods. CP hemolytic activity was then measured in these samples and in serum samples from the same individuals collected at the same time.
To test the stability of the samples, aliquots of EDTA-blood and EDTA-plasma from 52 SLE patients were stored for up to 3 days at room temperature or 22°C (blood) or for up to 2 days at 4°C (plasma). The samples were then transferred to Veronal-buffered saline with lepirudin, and the CP hemolytic activity was measured. EDTA-plasma samples that had been collected from the same individuals at the same time points, centrifuged, and then immediately stored at −70°C were analyzed in parallel. As shown in Table 2, the hemolytic activities in the samples did not change significantly under any of the tested storage conditions, but there was a tendency towards declined activity in the samples stored at room temperature for 2 and 3 days.
TABLE 2.
Effects of storage conditions on CP hemolytic activity
| Blood samplesa | Storage temp (°C) | Storage time (days) | No. of samples | Mean CP activity (%) | Mean % difference in CP activity ±SEMb |
|---|---|---|---|---|---|
| Control EDTA-plasma | −70 | 10 | 77 | ||
| EDTA-plasma | +4 | 1 | 10 | 77 | 0.5 ± 0.9 |
| Control EDTA-plasma | −70 | 10 | 77 | ||
| EDTA-plasma | +4 | 2 | 10 | 75 | 1.5 ± 1.0 |
| Control EDTA-plasma | −70 | 22 | 91 | ||
| EDTA-blood | +22 | 1 | 22 | 92 | 1.4 ± 2.1 |
| Control EDTA-plasma | −70 | 5 | 111 | ||
| EDTA-blood | +22 | 2 | 5 | 95 | 16.4 ± 6.1 |
| Control EDTA-plasma | −70 | 5 | 97 | ||
| EDTA-blood | +22 | 3 | 5 | 80 | 17.4 ± 12.4 |
Samples of EDTA-blood were collected from SLE patients (n = 52), and each sample was divided into two aliquots, one of which was frozen immediately after being harvested (control EDTA-plasma) and one of which was stored either at 22°C (EDTA-blood) or at 4°C (EDTA-plasma). Prior to analysis, the samples were transferred to Veronal-buffered saline containing lepirudin as described in Materials and Methods, and the samples from each patient were analyzed in parallel in the CP hemolytic assay. The difference in recorded CP activities between samples from the same individual was calculated.
None of the differences was statistically significant.
Complement analyses for patients with SLE.
In SLE patient samples, CP hemolytic function and C3 and C4 levels were significantly lower, while C3dg levels and C3dg/C3 ratios were significantly higher than those of the controls (Fig. 2). These differences were apparent for the total group of samples (n = 74 [183 observations]) and for the subgroups, where covariance was seen between the disease activity grades and increasing complement aberrations. The differences in all parameters between the different grades of disease activity were strongly significant (P < 0.05 to P < 0.001), except between disease activity groups III and IV, most likely due to the smaller numbers of observations for these groups (26 and 5, respectively, compared with 61 and 91 for the other two groups).
FIG. 2.
Complement activation in SLE and RA. The graphs show the relationships between CP hemolytic function (A) and the immunochemical complement parameters C4 (B) and C3 (C) and the C3dg/C3dg ratio (D) in SLE patients with grade I (n = 40 [61 observations]), grade II (n = 25 [91 observations]), grade III (n = 13 [26 observations]), and grade IV (n = 3 [5 observations]) disease, RA patients with grade I (n = 76), grade II (n = 41), and grade III (n = 15) disease, and healthy controls (n = 50). The mean values ± SD are indicated in the figure. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (for comparisons between the controls and the other groups [Mann-Whitney test]).
Complement analyses for patients with RA.
The levels of C3 and C3dg in the RA patients were significantly higher than those in the controls (Fig. 2). Analysis of the clinical subgroups indicated that this elevation was significantly related to the clinical disability index. The CP hemolytic function, levels of C4, and C3dg/C3 ratios in RA patients were not significantly different from those of healthy controls.
Sensitivity and specificity of complement assays.
The sensitivity and specificity of each of the five tests of complement (CP function, levels of C4, C3, and C3dg, and ratio of C3dg to C3) could be deduced from the data for the total cross-sectional SLE material and for the healthy blood donors. The specificities were very high, ranging from 96 to 100% for all five tests, because of the selected reference interval (mean ± 2 SD). The sensitivity, on the other hand, was clearly higher for the C3dg/C3 ratio and for CP hemolytic function (57 and 55%, respectively) than for the other tests (27 to 41%) (Table 3).
TABLE 3.
Sensitivities and specificities of CP hemolytic function and other complement assays for SLE patients (cross-sectional study)a
| Assay | Sensitivity | Specificity |
|---|---|---|
| CP function | 0.55 | 1.00 |
| C4 | 0.27 | 0.98 |
| C3 | 0.29 | 0.98 |
| C3dg | 0.41 | 0.98 |
| C3dg/C3 | 0.57 | 0.96 |
Determined with data from healthy individuals versus those from SLE patients (all calls).
Longitudinal studies of CP hemolytic function in patients with SLE.
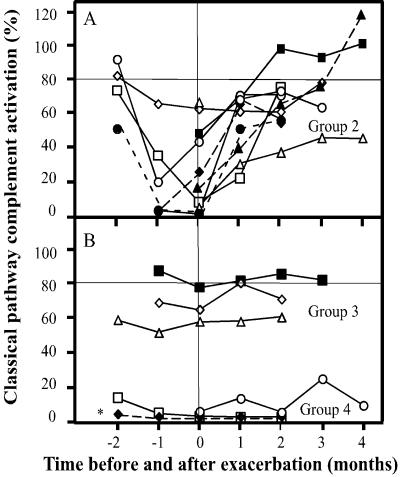
To further evaluate the hemolytic test with patients with different complement activation, CP hemolytic function was assessed in a series of five serum samples collected from each of 20 SLE patients during time periods when each of them suffered one exacerbation of disease (Table 1). Based on these results, the patients were divided into the following four groups: group 1 (n = 5), with normal CP activity which never fell below 80%; group 2 (n = 9), in which the CP activity fell below 80%, and in some patients down to <10%, on one or more occasions before or during exacerbation; group 3 (n = 3), in which the CP activity was moderate (80% or lower) but did not change during exacerbation; and group 4 (n = 3), which showed negligible activation (Fig. 3). When the whole group of patients was considered, there was no correlation (R2 = 0.09) between the maximal SLEDAI and minimal CP activity (not shown).
FIG. 3.
Alterations in CP activation in relation to disease activity in SLE. (A) Values from each time point for the patients in group 2 (n = 9), in which the CP activity decreased below 80% on one or more occasions. (B) Values for the patients in group 3 (n = 3), with moderate but stable activation, and group 4 (n = 3), with negligible activation. *, patient with a total C2 deficiency (patient 18 in Table 1).
In order to investigate whether there was a correlation between C1q values and CP function in SLE sera, these parameters were determined for samples collected at the time of maximal CP activity for each individual in the longitudinal study. An approximate linear correlation (R = 0.877) was found when these values were plotted against each other (Fig. 4). The exception was the C2-deficient individual (highlighted by an asterisk), who totally lacked activity but had an apparently normal level of C1q and whose values were omitted from the calculation.
FIG. 4.
Correlation between the levels of C1q and the maximal values for CP activation in SLE patients. The numbers refer to the groups defined in the legend to Fig. 3, and the asterisk indicates the same C2-deficient patient as that indicated in Fig. 3.
Complement analyses for complement-deficient patients.
Since the CP hemolytic assay was first introduced as an initial complement screening technique in our laboratory, a number of patients with different complement deficiencies have been identified. In each case, the deficiency has been confirmed by immunochemical, functional, and/or genetic assay of the deficient factor (Table 4).
TABLE 4.
Complement activation in serum samples from patients with complement deficiencies, identified by the hemolytic assay
| Deficient factor (no. of patients) | % Complement activation
|
Concn of the deficient factor (%)a | |
|---|---|---|---|
| CP | AP | ||
| C2 (2) | 0,* 9b | 128,* 143b | 0,* 0b |
| C3 (1) | 7 | 0 | 1 |
| C3 (1) (dysfunction) | 24 | 3 | 53 |
| C4 (1) | 1 | 128 | 0 |
| C6 (1) | 2 | 6 | 0 |
| C7 (1) | 1 | 0 | 0 |
| C9 (1) | 37 | 50 | 0 |
| Properdin (1) | 101 | 25 | 0 |
The concentration of the lacking or (in one case) dysfunctional complement component, as determined by immunochemical techniques, is given for each patient.
DISCUSSION
In the present study, we have evaluated a previously described hemolytic assay of CP function (37) whose output correlates strongly with that of the traditional classical CH50 test described by Mayer (30). This functional test is simple and can be performed within 30 min; it also screens for complement deficiencies within the classical and terminal pathways, which otherwise must be analyzed separately. Deficiencies within the AP, e.g., properdin, must be analyzed by the AP hemolytic assay. Monitoring of the interassay CV over a long period of time showed that the assay was very stable and that there were only minor variations in the results obtained. The interassay CV within the reference interval was only 3.3%. The sensitivity and specificity were also comparable to those of sensitive assays for complement activation products. A specificity of nearly 100% and a sensitivity of 55% were obtained.
Samples from SLE and RA patients with various degrees of disease activity were analyzed for C3, C4, and C3dg by conventional quantitative immunochemical techniques, and the results were compared to those for the CP hemolytic function test. Both the functional and the immunochemical parameters indicated a significant complement involvement in SLE. A reduction in CP function and a moderate reduction in C3 and C4 concentrations were found, combined with an increase in the C3dg level and the C3dg/C3 ratio. This combination of results points to ongoing systemic complement consumption. These results were in contrast to those obtained for RA patients, which also deviated from the control results in that the C3, C4, and C3dg levels were elevated while the CP function was normal. This profile is not characteristic of ongoing systemic complement consumption but rather suggests an acute-phase reaction resulting in an increase in the synthetic rates of complement components such as C3 and C4. The results also confirmed, as already pointed out by Nürnberger and Bhakdi (39), that the C3dg level per se is a less sensitive indicator of complement consumption than the C3dg/C3 ratio. This distinction is explained by the fact that the in vivo level of C3dg reflects a slow ongoing turnover of C3 to C3(H2O) and activation of C3 to C3b. Both processes finally generate C3c and C3dg. Thus, the level of C3dg may be affected by both the rate of protein synthesis and the rate of catabolism. The first process is affected by the concentration of C3 in extracellular fluids, i.e., a high C3 concentration also correlates with high levels of C3dg and vice versa. Without complement activation and at a normal rate of extracellular C3 degradation, the increased C3dg levels are expected to be proportional to the C3 concentration, as observed for the RA patients. The differences in C3dg/C3 ratios between SLE and RA patients are therefore in agreement with increased complement consumption in SLE but not in RA, as reviewed by Dalmasso (9) and Morgan (35).
C1q levels have been shown to correlate well with the disease activity in SLE (3). In the present study, a clear linear correlation was found between the hemolytic activity and the concentration of C1q in the tested SLE group. In order to further evaluate the hemolytic assay, the relationship between disease activity, as reflected in the SLEDAI, and the level of hemolytic function was also documented over time for the same group. Three-fourths of the patients had depressed levels of hemolytic function before or at the time of the exacerbation (Fig. 3). The finding that the depression existed before the maximum SLEDAI suggests that hemolytic function may be used in many cases to anticipate the actual exacerbation (Fig. 3A). This illustrates that the assay is useful for monitoring SLE patients regardless of the underlying scoring system, which may vary between different care institutions.
A general disadvantage of quantitative hemolytic assays and other functional tests is that only blood serum can be used. This is a major drawback, since serum does not contain any inhibitors of in vitro complement activation. As a consequence, samples have to be tested within 4 h (B. Nilsson and K. N. Ekdahl, unpublished observation) unless the sample is separated; furthermore, if the sample is to be shipped from a distant site, it must be transported on dry ice. The method described here for transferring plasma to a buffer is therefore a major advance in that it allows unseparated samples of EDTA-blood to be sent by ordinary post without ice or other cooling devices. It also allows immunochemical determination of individual complement components and activation products in the same sample that is used for buffer exchange.
A number of assays have been described to measure the complement fragments or products that are generated during activation of the complement system (e.g., iC3b, C3a, and sC5b-9) (2, 8, 13-15). These assays are very sensitive, and in particular, the assay for the detection of the sC5b-9 complex is useful for evaluating disease activity in SLE patients (34). However, some of these assays have the disadvantages of being expensive, time-consuming, and perhaps too sensitive. The last disadvantage is associated with the special handling of the samples that is necessary in order to avoid erroneous complement activation in vitro. Some activation is unavoidable in daily clinical practice and probably rules out the use of the most sensitive assays for routine analysis. We now suggest that the performance of the assay in our routine clinical immunology laboratory indicates that the CP hemolytic function assay, combined with the C3dg/C3 ratio, offers a rapid and inexpensive alternative for diagnosis and monitoring of the course of disease in SLE patients.
Acknowledgments
This work was supported by grants from The Swedish National Association against Rheumatism, the King Gustaf V, Nanna Swarts, and Magn Bergvalls Research Foundations, the Alfred Österlund Foundation, The Crafoord Foundation, and the Greta and Johan Kock Foundation, by faculty grants from the Universities of Kalmar and Lund, and by grants 5647, 13489, and 15244 from the Swedish Research Council.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Arnett, F. C., S. M. Edworthy, D. A. Bloch, D. J. McShane, J. F. Fries, N. S. Cooper, L. A. Healey, S. R. Kaplan, M. H. Liang, H. S. Luthra, et al. 1988. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 31:315-324. [DOI] [PubMed] [Google Scholar]
- 2.Belmont, H. M., P. Hopkins, H. S. Edelson, H. B. Kaplan, R. Ludewig, G. Weissmann, and S. Abramson. 1986. Complement activation during systemic lupus erythematosus. C3a and C5a anaphylatoxins circulate during exacerbations of disease. Arthritis Rheum. 29:1085-1089. [DOI] [PubMed] [Google Scholar]
- 3.Bengtsson, A., R. Nezlin, Y. Shoenfeld, and G. Sturfelt. 1999. DNA levels in circulating immune complexes decrease at severe SLE flares—correlation with complement component C1q. J. Autoimmun. 13:111-119. [DOI] [PubMed] [Google Scholar]
- 4.Bengtsson, A. A., G. Sturfelt, L. Truedsson, J. Blomberg, G. Alm, H. Vallin, and L. Ronnblom. 2000. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus 9:664-671. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier, C., D. D. Gladman, M. B. Urowitz, D. Caron, and C. H. Chang. 1992. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 35:630-640. [DOI] [PubMed] [Google Scholar]
- 6.Bourke, B. E., I. K. Moss, and R. N. Maini. 1982. Measurement of the complement C3 breakdown product C3d by rocket immunoelectrophoresis. J. Immunol. Methods 48:97-108. [DOI] [PubMed] [Google Scholar]
- 7.Calano, S. J., P. A. Shih, C. C. Liu, A. H. Kao, J. S. Navratil, S. Manzi, and J. M. Ahearn. 2006. Cell-bound complement activation products (CB-CAPs) as a source of lupus biomarkers. Adv. Exp. Med. Biol. 586:381-390. [DOI] [PubMed] [Google Scholar]
- 8.Clough, J. D., and R. K. Chang. 1990. Effectiveness of testing for anti-DNA and the complement components iC3b, Bb, and C4 in the assessment of activity of systemic lupus erythematosus. J. Clin. Lab. Anal. 4:268-273. [DOI] [PubMed] [Google Scholar]
- 9.Dalmasso, A. P. 1989. Complement in laboratory medicine. Complement Inflamm. 6:5-7. [PubMed] [Google Scholar]
- 10.Davies, K. A., V. Hird, S. Stewart, G. B. Sivolapenko, P. Jose, A. A. Epenetos, and M. J. Walport. 1990. A study of in vivo immune complex formation and clearance in man. J. Immunol. 144:4613-4620. [PubMed] [Google Scholar]
- 11.de Pablo, P., A. Ramirez, E. Cortina, A. de la Pena, J. Zamora, R. Izaguirre, and M. C. Amigo. 2003. Increased fibrin polymerization rate in patients with primary antiphospholipid syndrome and systemic lupus erythematosus. Clin. Appl. Thromb. Hemost. 9:221-225. [DOI] [PubMed] [Google Scholar]
- 12.Ekdahl, K. N., and B. Nilsson. 1999. Alterations in C3 activation and binding caused by phosphorylation by a casein kinase released from activated human platelets. J. Immunol. 162:7426-7433. [PubMed] [Google Scholar]
- 13.Ekdahl, K. N., B. Nilsson, M. Pekna, and U. R. Nilsson. 1992. Generation of iC3 at the interface between blood and gas. Scand. J. Immunol. 35:85-91. [DOI] [PubMed] [Google Scholar]
- 14.Falk, R. J., A. P. Dalmasso, Y. Kim, S. Lam, and A. Michael. 1985. Radioimmunoassay of the attack complex of complement in serum from patients with systemic lupus erythematosus. N. Engl. J. Med. 312:1594-1599. [DOI] [PubMed] [Google Scholar]
- 15.Gawryl, M. S., D. S. Chudwin, P. F. Langlois, and T. F. Lint. 1988. The terminal complement complex, C5b-9, a marker of disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 31:188-195. [DOI] [PubMed] [Google Scholar]
- 16.Gunnarsson, I., B. Sundelin, M. Heimburger, J. Forslid, R. van Vollenhoven, I. Lundberg, and S. H. Jacobson. 2002. Repeated renal biopsy in proliferative lupus nephritis—predictive role of serum C1q and albuminuria. J. Rheumatol. 29:693-699. [PubMed] [Google Scholar]
- 17.Hong, J., A. Larsson, K. N. Ekdahl, G. Elgue, R. Larsson, and B. Nilsson. 2001. Contact between a polymer and whole blood: sequence of events leading to thrombin generation. J. Lab. Clin. Med. 138:139-145. [DOI] [PubMed] [Google Scholar]
- 18.Horak, P., Z. Hermanova, J. Zadrazil, H. Ciferska, M. Ordeltova, L. Kusa, M. Zurek, and T. Tichy. 2006. C1q complement component and antibodies reflect SLE activity and kidney involvement. Clin. Rheumatol. 25:532-536. [DOI] [PubMed] [Google Scholar]
- 19.Huber-Lang, M., J. V. Sarma, F. S. Zetoune, D. Rittirsch, T. A. Neff, S. R. McGuire, J. D. Lambris, R. L. Warner, M. A. Flierl, L. M. Hoesel, F. Gebhard, J. G. Younger, S. M. Drouin, R. A. Wetsel, and P. A. Ward. 2006. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 12:682-687. [DOI] [PubMed] [Google Scholar]
- 20.Jaskowski, T. D., T. B. Martins, C. M. Litwin, and H. R. Hill. 1999. Comparison of three different methods for measuring classical pathway complement activity. Clin. Diagn. Lab. Immunol. 6:137-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnsson, U., L. Truedsson, and B. Gustavii. 1983. Complement component in 100 newborns and their mothers determined by electroimmunoassay. Acta Pathol. Microbiol. Immunol. Scand. J. C 91:147-150. [PubMed] [Google Scholar]
- 22.Jönsson, G., L. Truedsson, G. Sturfelt, V. A. Oxelius, J. H. Braconier, and A. G. Sjoholm. 2005. Hereditary C2 deficiency in Sweden: frequent occurrence of invasive infection, atherosclerosis, and rheumatic disease. Medicine (Baltimore) 84:23-34. [DOI] [PubMed] [Google Scholar]
- 23.Kirschfink, M., and T. Borsos. 1988. Binding and activation of C4 and C3 on the red cell surface by non-complement enzymes. Mol. Immunol. 25:505-512. [DOI] [PubMed] [Google Scholar]
- 24.Kirschfink, M., and T. E. Mollnes. 2003. Modern complement analysis. Clin. Diagn. Lab. Immunol. 10:982-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjellman, M., A. B. Laurell, B. Low, and A. G. Sjoholm. 1982. Homozygous deficiency of C4 in a child with a lupus erythematosus syndrome. Clin. Genet. 22:331-339. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, M. J., and M. Botto. 2006. Complement deficiencies in humans and animals: links to autoimmunity. Autoimmunity 39:367-378. [DOI] [PubMed] [Google Scholar]
- 27.Liu, C. C., and J. D. Young. 1988. A semi-automated microassay for complement activity. J. Immunol. Methods 114:33-39. [DOI] [PubMed] [Google Scholar]
- 28.Mancini, G., A. O. Carbonara, and J. F. Heremans. 1965. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry 2:235-254. [DOI] [PubMed] [Google Scholar]
- 29.Manzi, S., J. S. Navratil, M. J. Ruffing, C. C. Liu, N. Danchenko, S. E. Nilson, S. Krishnaswami, D. E. King, A. H. Kao, and J. M. Ahearn. 2004. Measurement of erythrocyte C4d and complement receptor 1 in systemic lupus erythematosus. Arthritis Rheum. 50:3596-3604. [DOI] [PubMed] [Google Scholar]
- 30.Mayer, M. M. 1961. Complement and complement fixation, p. 133-240. In E. A. Kabat (ed.), Experimental immunochemistry. Thomas, Springfield, IL.
- 31.McCarty, D. J. 1973. Measurement of arthritic disease disability. IMS Ind. Med. Surg. 42:9-11. [PubMed] [Google Scholar]
- 32.Mollnes, T. E., O. L. Brekke, M. Fung, H. Fure, D. Christiansen, G. Bergseth, V. Videm, K. T. Lappegard, J. Kohl, and J. D. Lambris. 2002. Essential role of the C5a receptor in E. coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 100:1869-1877. [PubMed] [Google Scholar]
- 33.Mollnes, T. E., P. Garred, and G. Bergseth. 1988. Effect of time, temperature and anticoagulants on in vitro complement activation: consequences for collection and preservation of samples to be examined for complement activation. Clin. Exp. Immunol. 73:484-488. [PMC free article] [PubMed] [Google Scholar]
- 34.Mollnes, T. E., H. J. Haga, J. G. Brun, E. W. Nielsen, A. Sjoholm, G. Sturfeldt, U. Martensson, K. Bergh, and O. P. Rekvig. 1999. Complement activation in patients with systemic lupus erythematosus without nephritis. Rheumatology (Oxford) 38:933-940. [DOI] [PubMed] [Google Scholar]
- 35.Morgan, P. 1990. The biological effects of complement activation, p. 37-55. In P. Morgan (ed.), Complement. Clinical aspects and relevance to disease. Academic Press, London, United Kingdom.
- 36.Morrow, W. J., D. J. Williams, C. Ferec, R. Casburn-Budd, D. A. Isenberg, E. Paice, M. L. Snaith, P. Youinou, and P. Le Goff. 1983. The use of C3d as a means of monitoring clinical activity in systemic lupus erythematosus and rheumatoid arthritis. Ann. Rheum. Dis. 42:668-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nilsson, U. R., and B. Nilsson. 1984. Simplified assays of hemolytic activity of the classical and alternative complement pathways. J. Immunol. Methods 72:49-59. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson, U. R., B. Nilsson, K. E. Storm, G. Sjolin-Forsberg, and R. Hallgren. 1992. Hereditary dysfunction of the third component of complement associated with a systemic lupus erythematosus-like syndrome and meningococcal meningitis. Arthritis Rheum. 35:580-586. [DOI] [PubMed] [Google Scholar]
- 39.Nürnberger, W., and S. Bhakdi. 1984. Plasma C3d/C3 quotient as a parameter for in vivo complement activation. J. Immunol. Methods 74:87-91. [DOI] [PubMed] [Google Scholar]
- 40.Pereira, J., G. Alfaro, M. Goycoolea, T. Quiroga, M. Ocqueteau, L. Massardo, C. Perez, C. Saez, O. Panes, V. Matus, and D. Mezzano. 2006. Circulating platelet-derived microparticles in systemic lupus erythematosus. Association with increased thrombin generation and procoagulant state. Thromb. Haemost. 95:94-99. [PubMed] [Google Scholar]
- 41.Stove, S., A. Klos, W. Bautsch, and J. Kohl. 1995. Re-evaluation of the storage conditions for blood samples which are used for determination of complement activation. J. Immunol. Methods 182:1-5. [DOI] [PubMed] [Google Scholar]
- 42.Sturfelt, G., U. Johnson, and A. G. Sjoholm. 1985. Sequential studies of complement activation in systemic lupus erythematosus. Scand. J. Rheumatol. 14:184-196. [DOI] [PubMed] [Google Scholar]
- 43.Sturfelt, G., and L. Truedsson. 2005. Complement and its breakdown products in SLE. Rheumatology (Oxford) 44:1227-1232. [DOI] [PubMed] [Google Scholar]
- 44.Tan, E. M., A. S. Cohen, J. F. Fries, A. T. Masi, D. J. McShane, N. F. Rothfield, J. G. Schaller, N. Talal, and R. J. Winchester. 1982. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271-1277. [DOI] [PubMed] [Google Scholar]
- 45.Truedsson, L., and A. G. Sjoholm. 1984. Inhibition of complement-mediated hemolysis in gel by rheumatoid factors. Acta Pathol. Microbiol. Immunol. Scand. C 92:1-4. [DOI] [PubMed] [Google Scholar]
- 46.Walport, M., Y. C. Ng, and P. J. Lachmann. 1987. Erythrocytes transfused into patients with SLE and haemolytic anaemia lose complement receptor type 1 from their cell surface. Clin. Exp. Immunol. 69:501-507. [PMC free article] [PubMed] [Google Scholar]