Abstract
Considerable effort has been made to elucidate the mechanism of Lyme arthritis. We focused on p19, a cell cycle-regulating molecule, because it is known to inhibit cell cycle division of T lymphocytes which may be responsible for the induction of arthritis. We show that anti-p19 antibody treatment enhances the inflammatory response normally detected at the tibiotarsal joints of Borrelia burgdorferi-vaccinated and Borrelia bissettii-challenged mice. Specifically, anti-p19 antibody treatment augmented the severity of inflammation within the synovial and subsynovial tissue. Moreover, treatment with anti-p19 antibody caused severe erosion of cartilage and bone with ankle joint destruction. In addition, anti-p19 antibody treatment of Borrelia-vaccinated and -challenged mice enhanced the borreliacidal antibody response, especially against the vaccine isolate. The novel activities of anti-p19 antibody show that p19 may be an important therapeutic site for the treatment of Lyme arthritis.
Arthritis is one of the most frequently reported clinical manifestations associated with humans infected with Borrelia burgdorferi sensu lato (1, 2, 46), the etiologic agent of Lyme borreliosis. Lyme arthritis presents with intermittent attacks of mono- or polyarticular arthritis within months to a few years after tick-transmitted infection in 60% of Lyme borreliosis patients (1, 2, 46). Approximately 10% of these patients develop persistent arthritis, despite extensive treatment with antimicrobial agents (1, 2, 46). In severe cases, chronic inflammatory Lyme arthritis (following a correct diagnosis) can lead to cartilage and bone erosion (46) with permanent joint destruction (46).
Considerable effort has been made to elucidate the immune agents and mechanisms responsible for Lyme arthritis (3, 5-7, 10, 20, 21, 40, 42). It is generally accepted that T cells, particularly CD4+ T cells (5, 7, 20, 51) and CD4+ CD25+ T cells (35-37), participate in the induction, resolution, and prevention of the arthritis. Similarly, accumulating evidence suggests that cytokines, especially interleukin-17 (IL-17) (11) and IL-15 (4), can also influence the activation and maintenance of the immune responses that cause Lyme arthritis. Other cellular immune mechanisms may also be involved (5, 7, 10, 20, 21). Moreover, a paradigm for the molecular regulation of arthritis involving CD4+ T cells, CD4+ CD25+ T cells, and cell-associated cytokine production has emerged in recent years. This involves cell cycle-regulating molecules (13, 22, 26) like protein (p) 19, also referred to as p19INK4D (22, 50). This 165-amino-acid protein participates in the G1 phase arrest of T cells in the cell cycle (13, 16, 29). Disruption of p19 activity has been associated with the development of acute T-cell lymphoblastic leukemia (15, 18, 44) and other cellular diseases (30, 48). This protein may also be important for controlling T-cell proliferation, differentiation, and activation and the release of cytokines that promote the pathology associated with Borrelia-induced arthritis.
We hypothesized that treatment with anti-p19 antibody of Borrelia-vaccinated and -challenged mice would enhance arthritis. In this report, we show that treatment of Borrelia-vaccinated and -challenged mice with anti-p19 antibody augments the arthritic response. In addition, anti-p19 antibody treatment caused an increase in borreliacidal antibody production.
MATERIALS AND METHODS
Mice.
Six- to 12-week-old inbred male and female C57BL/6 mice were obtained from William F. Dove (University of Wisconsin). The mice were bred at the animal facility located at the University of Wisconsin Medical School. All mice were kept in a climate-controlled environment at 21°C with 12-h light-dark cycles, and food and water were provided ad libitum. The experimental procedures performed on the mice were approved by the Animal Care and Use Committee for the University of Wisconsin Medical School.
Organisms.
Low-passage-number (<10) virulent B. burgdorferi senso stricto isolate 297 (human cerebrospinal fluid) and Borrelia bissettii (isolated by S.M.C. from Microtus pennsylvanicus) were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium to a concentration of 107 spirochetes/ml. Aliquots (500 μl) were then dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, NC) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, MO). The tubes were sealed and stored at −70°C.
Vaccine preparation.
Frozen aliquots (10 ml) of B. burgdorferi 297 culture were thawed and pelleted by centrifugation (10,000 × g at 23°C for 10 min) and washed three times with phosphate-buffered saline (PBS), pH 7.4 (Grand Island, NY). The washed pellet was then resuspended and mixed with 10% neutral buffered zinc formalin (Sigma Chemical Co.), incubated at ambient temperature with periodic mixing for 30 min, washed three times by centrifugation (10,000 × g at 23°C for 10 min) with PBS, and resuspended in PBS. Subsequently, the formalin-inactivated spirochetes were mixed with a sufficient volume of 3% aluminum hydroxide (Reheis, Berkeley Heights, NJ) to yield 2 × 107 spirochetes/ml.
Vaccination of mice.
Mice were anesthetized with ether (Sigma Chemical Co.) or 15% isoflurane in mineral oil (Sigma Chemical Co.) contained in a nose-and-mouth cup and injected subcutaneously in the inguinal regions with 0.25 ml of the formalin-inactivated whole-cell vaccine preparation. Whole cells of Borrelia are not recommended for vaccination of humans, based on concerns associated with other types of whole-cell vaccines (28). However, we have shown that whole cells of Borrelia can consistently induce arthritis in gamma interferon-deficient and wild-type C57BL/6 mice after Borrelia infection (4, 11, 37).
Infection of mice.
A frozen aliquot (1 ml) of B. bissettii culture was thawed and added to 4 ml of fresh BSK medium and incubated at 32°C for 24 h. Twenty-one or 28 days after vaccination of mice with B. burgdorferi 297 in alum, mice were anesthetized with ether or 15% isoflurane in mineral oil contained in a nose-and-mouth cup and were injected subcutaneously in both hind paws with 50 μl of BSK medium containing 106 viable B. bissettii organisms. Vaccinated mice were also challenged the following day with 106 viable B. bissettii organisms that had been incubating at 32°C in BSK medium.
It is necessary to infect B. burgdorferi 297-vaccinated mice with B. bissettii because vaccination induces protective antibodies that prevent a homologous infection from eliciting arthritis (17, 32, 43). Other infectious Borrelia isolates, besides B. bissettii, can also elicit the arthritis (32). Controls included vaccinated mice injected with only BSK medium, which does not induce arthritis.
Administration of anti-p19 antibody.
Purified mouse anti-p19INK4d monoclonal antibody (BD PharMingen, San Diego, CA) and nonspecific isotype immunoglobulin G1 (IgG1) antibody (BD PharMingen) were diluted in filter-sterilized (0.2-μm-pore-size Acrodisk filter; Gelman Sciences, Ann Arbor, MI) PBS (pH 7.4) to yield a final concentration of 20 μg/ml. Coomassie blue-stained gels and Western blot analysis showed the solution of anti-p19 antibody to be free of contaminating protein. The salts and preservatives present were identical to those found in the isotype control. One hour after infection of vaccinated mice, mice were anesthetized with ether or 15% isoflurane in mineral oil contained in a nose-and-mouth cup and were injected subcutaneously in both hind paws with 50 μl of anti-p19 antibody or isotype antibody. Treatment with anti-p19 antibody and isotype was administered daily thereafter for 6 or 9 days. In other studies, anti-p19 antibody was administered 7 or 15 days after challenge and continued daily for 6 days.
Flow cytometry.
The popliteal and inguinal lymph nodes were obtained from four groups of three mice vaccinated with or without challenge and with or without treatment with anti-p19 antibody. In addition, popliteal and inguinal lymph nodes were obtained from non-anti-p19 antibody-treated nonvaccinated and noninfected mice. Single-cell suspensions of the lymph node cells were prepared by teasing apart the lymph nodes with forceps and passing them through a sterile nylon mesh screen (Fisher, Hanover Park, IL) into cold filter-sterilized PBS. The total number of viable lymphocytes was determined. Cells (5 × 105) were then dispensed into chilled centrifuge tubes and mixed with 2.5 μl each of fluorescein isothiocyanate-conjugated rat anti-mouse CD4 antibody (BD PharMingen) and R-phycoerythrin-conjugated rat anti-mouse CD25 (BD PharMingen) and incubated at 4°C for 30 min under dark conditions. Subsequently, the cells were washed with 1% fetal calf serum (heat inactivated; Sigma Chemical Co.) in PBS at 4°C (500 × g for 5 min), and the pellets were resuspended in 300 μl of cold 1% fetal calf serum in PBS. The cells were then fixed in 1% methanol-free formaldehyde (Polysciences, Warrington, PA) for 24 h. Data were acquired using a FACSCalibur flow cytometer (Becton Dickinson) using CellQuest acquisition and FlowJo fluorescence-activated cell sorting data analysis software (Tree Star, Inc., Ashland, OR). Events were gated to include only the fixed lymphocytes. Twenty-five thousand gated events were collected and analyzed using a gated dot plot. The percentage of CD4+ CD25+ T cells was determined by comparing samples of lymph node cells stained with fluorescein isothiocyanate-conjugated rat anti-mouse CD4 antibody and R-phycoerythrin-conjugated rat anti-mouse CD25 with those stained with either R-phycoerythrin-conjugated rat anti-mouse CD25 or fluorescein isothiocyanate-conjugated rat anti-mouse CD4 antibody. Total cell populations for CD4+ and CD4+ CD25+ T cells in the lymph nodes were calculated by multiplying the percentage of occurrence in a dot plot of a cell population by the total number of cells counted in the node.
Detection of borreliacidal antibodies.
Borreliacidal antibodies were detected by a flow cytometric procedure (12). Viable B. burgdorferi 297 or B. bissettii organisms in logarithmic growth phase were enumerated with a Petroff-Hausser counting chamber and diluted with fresh BSK medium to a concentration of approximately 5 × 105 organisms/ml. Concomitantly, serum samples were diluted 1:20 with BSK and filter sterilized by passage through a 0.2-μm-pore-size microcentrifuge filter (Costar, Cambridge, MA). The filtered serum samples were then transferred to sterile 1.5-ml screw-cap microcentrifuge tubes (Sarstedt, Newton, NC) and diluted serially (1:40 to 1:40,960) with BSK. Serum samples were heat inactivated at 56°C for 10 min, and a 100-μl aliquot of the spirochetes and 10 μl of sterile guinea pig serum (50% hemolytic component of ≥200 units/ml) were added. The assay mixtures were mixed thoroughly and incubated for 16 to 24 h at 35°C.
Following incubation, 100 μl of each assay suspension was transferred to a 12- by 75-mm polystyrene tube (Becton-Dickinson, Franklin Lakes, NJ) containing 400 μl of PBS and 1 μg of acridine orange (Sigma) per ml. A FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) was then used to detect borreliacidal activity. Spirochetes were isolated by gating (CellQuest software; Becton Dickinson) and analyzed for 1 to 2 min with the flow rate set at low. Borreliacidal antibodies kill the spirochete by inducing a complement cascade that disrupts the outer membrane and causes the membrane to bleb. Borreliacidal antibodies were detected indirectly by monitoring the increased fluorescence intensity that occurs when the acridine orange intercalates into blebbed, nonviable spirochetes. A shift of ≥13% in the mean fluorescence intensity compared to a normal serum control was considered positive (12). The presence of blebbed, nonmotile spirochetes was then confirmed by dark-field microscopy.
Assessment of arthritis.
Measurements of hind paw swelling were used to determine the level of the inflammatory response induced in Borrelia-vaccinated mice with or without treatment with anti-p19 antibody and challenge with B. bissettii. Paws were measured on day of challenge and every other day for approximately 3 weeks using a Vernifer digital caliper (Fisher Scientific, Pittsburgh, PA) with readout to 0.01 mm. Mice were anesthetized with ether or 15% isoflurane in mineral oil contained in a nose-and-mouth cup. The width and thickness of both hind tibiotarsal joints were measured. The caliper values within a group of mice were summed and divided by the number of measurements to obtain the mean value of the size of the joint for the day.
Preparation of tissues for histologic examination.
Twenty-one days after infection, mice were euthanized with ether or 15% isoflurane in mineral oil, and their hind paws were amputated at mid-femur. The paws were fixed in 10% neutral buffered zinc formalin solution for 24 h. Subsequently, the paws were placed in a decalcifying solution (Fisher Scientific, Kalamazoo, MI) for 3 h, followed by the addition of fresh decalcifying solution for an additional 48 h. Following decalcification, the legs were placed in tissue-embedding cassettes (Fisher Scientific), embedded in paraffin, and cut into 6 μm-thick sections. These sections were placed on glass slides and stained with hematoxylin and eosin. Sections were cryptically coded, and an unbiased histopathological examination was performed by a board-certified pathologist (T. F. Warner). The following word scale was used to define the neutrophilic infiltration in the tibiotarsal joint: mild (neutrophilic infiltration in the subsynovial tissues), moderate (neutrophilic infiltration of the subsynovial tissues, synovium, and synovial space), severe (profuse neutrophilic infiltration of the subsynovial tissues, synovium, and synovial space), and severe destructive (profuse neutrophilic infiltration of all tibiotarsal tissues along with bone and/or cartilage destruction).
Statistical analysis.
Swelling of the hind paws and flow cytometry data among groups were tested by analysis of variance (45). The alpha level was set at 0.05 before the experiments were started. The standard error for the experiment was then determined.
RESULTS
Effects of anti-p19 antibody treatment on development and progression of hind paw swelling.
Four groups of four mice each were vaccinated with B. burgdorferi isolate 297 in alum. Twenty-one days after vaccination, two of the four groups of vaccinated mice were challenged in the hind paw with B. bissettii. Concomitantly, one of the two groups of vaccinated and infected mice was administered anti-p19 antibody on the day of challenge and daily thereafter for 6 or 9 days. A group of four vaccinated but noninfected mice was also treated with anti-p19 antibody on day 21 of vaccination and daily thereafter for 6 or 9 days. A remaining group of four vaccinated but not infected mice was not treated with anti-p19 antibody. Additional controls included nonvaccinated and nonchallenged mice and vaccinated and challenged mice treated with an IgG1 isotype antibody.
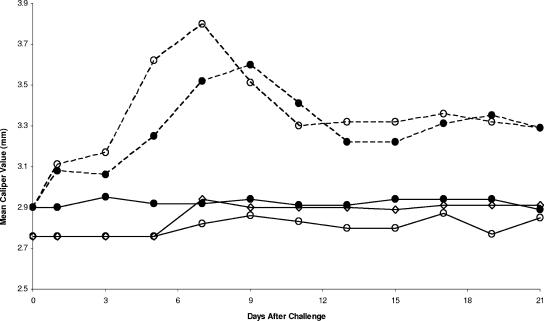
Similar, significant (P < 0.05) swelling of the right and left hind paws was detected in vaccinated and infected mice with or without treatment with anti-p19 antibody (Fig. 1) or with treatment with an isotype IgG1 antibody. The hind paw swelling of untreated and anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice peaked on days 7 and 9, respectively, and gradually decreased. In contrast, no swelling of the hind paws occurred with vaccinated, nonchallenged mice with or without treatment with anti-p19 antibody. Likewise, no swelling was detected in the nonvaccinated and nonchallenged mice. Similar results were obtained when these experiments were repeated four times with three or four mice per group.
FIG. 1.
Development of swelling of the hind paws of vaccinated (solid line) and vaccinated and challenged (broken line) mice treated with (•) or without (○) anti-p19 antibody. Additional controls included nonvaccinated and nonchallenged mice (⋄) and vaccinated and challenged mice treated with an IgG1 isotype antibody (data not shown). The swelling detected in vaccinated and challenged mice treated with the isotype antibody was similar to the swelling observed in vaccinated and challenged mice. Data are means; standard errors for the experiment did not exceed 0.06.
Histopathologic effects of anti-p19 antibody treatment of vaccinated and challenged mice.
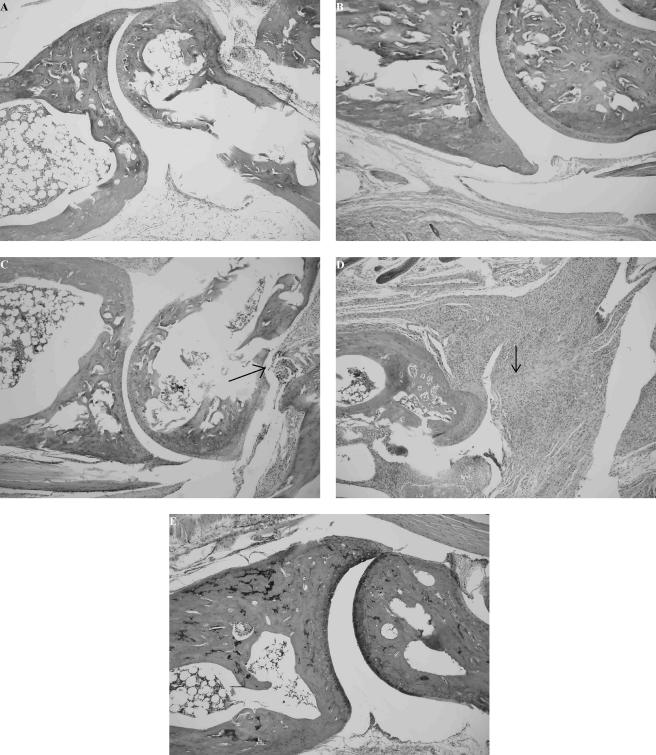
Although no differences in swelling of the hind paws were detected between vaccinated and challenged mice with or without treatment with anti-p19 antibody, there were differences in histopathology between the groups, especially 21 days after infection. Anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice showed severe inflammation of the periarticular soft tissue. Moreover, the ankle joint showed severe inflammation of the synovial and subsynovial tissue along with severe neutrophilic infiltration of the synovial space. Most importantly, severe erosion of cartilage and bone occurred, and the ankle joints were destroyed (Fig. 2D). In contrast, vaccinated and challenged mice with or without treatment with a nonspecific isotype IgG1 antibody showed moderate inflammation of the periarticular soft tissue, moderate inflammation of the synovial and subsynovial tissue, and no erosion of cartilage or bone (Fig. 2C). Vaccinated mice with (Fig. 2B) or without (Fig. 2A) treatment with anti-p19 antibody showed only mild or no inflammatory changes of the tibiotarsal joint or surrounding tissue. Likewise, no histopathological changes were observed with the tibiotarsal joints of nonvaccinated and nonchallenged mice (Fig. 2E). When these studies were repeated four times, anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice (total 16) always had more severe pathology than the vaccinated and challenged mice without anti-p19 antibody and the other control animals.
FIG. 2.
Histopathology of the tibiotarsal joints of vaccinated mice (A), vaccinated mice treated with anti-p19 antibody (B), vaccinated and challenged mice (C), and vaccinated and challenged mice treated with anti-p19 antibody (D) at day 21 after Borrelia infection or day 42 after vaccination. A fifth tibiotarsal joint is shown from a nonvaccinated and nonchallenged mouse (E). Arrows indicate inflammation. Additional controls (not shown) included vaccinated and challenged mice treated with an IgG1 isotype antibody. The histopathology detected in vaccinated and challenged mice treated with the isotype antibody was similar to the histopathology observed in vaccinated and challenged mice, shown in panel C. Magnification, ×100.
Effects of delayed administration of anti-p19 antibody.
In a separate set of experiments, Borrelia-vaccinated and -infected mice were administered anti-p19 antibody at the time of infection or at day 7 or day 15 after infection. The anti-p19 antibody treatment was continued daily for 6 days with each group of vaccinated and challenged mice. Controls included non-anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice. These latter mice developed moderate histopathology involving the tibiotarsal joint (Fig. 2C). Enhanced pathology involving the synovium, subsynovial tissue, and joint space developed only in vaccinated and challenged mice that received anti-p19 antibody at the time of infection. No significant differences in histopathology were detected among non-anti-p19 antibody-treated vaccinated and challenged mice and vaccinated and challenged mice that received anti-p19 antibody at day 7 or day 15 after challenge.
Effects of anti-p19 antibody treatment on the number of lymphocytes, CD4+ T cells and CD4+ CD25+ T cells in the lymph nodes.
Popliteal and inguinal lymph nodes were obtained from nonvaccinated mice, Borrelia-vaccinated mice with or without treatment with anti-p19 antibody, and Borrelia-vaccinated and -infected mice with or without treatment with anti-p19 antibody on day 8 after infection. Table 1 shows a slight increase in the total number of lymphocytes and CD4+ T cells in all groups of mice except vaccinated mice and vaccinated mice treated with anti-p19 antibody. Likewise, vaccinated mice treated with or without anti-p19 antibody had decreased numbers of CD4+ CD25+ T cells compared to the number of CD4+ CD25+ T cells in the control group. In contrast, Borrelia-vaccinated and -challenged mice treated with or without anti-p19 antibody had significantly (P ≤ 0.1) increased numbers of CD4+ CD25+ T cells (1.1 × 105 to 1.3 × 105). In summary, anti-p19 antibody treatment of Borrelia-vaccinated and -challenged mice slightly increased the number of lymphocytes, CD4+ T cells, and CD4+ CD25+ T cells compared to the other control groups. However, these results were not statistically significant. There were four control groups (Table 1).
TABLE 1.
Numbers of lymphocytes, CD4+ T cells, and CD4+ CD25+ T cells in the popliteal and inguinal lymph nodes of vaccinated and vaccinated and challenged mice with or without treatment with anti-p19 antibody
| Treatmenta | No. of lymphocytes/nodeb | No. of CD4+ lymphocytes/nodec | No. of CD4+ CD25+ lymphocytes/nodec |
|---|---|---|---|
| Nonvaccinated | 1.2 × 106 | 2.2 × 105 | 5.7 × 104 |
| Vaccinated | 1.9 × 106 | 2.1 × 105 | 4.4 × 104 |
| Vaccinated and treated with anti-p19 | 0.9 × 106 | 0.1 × 105 | 2.1 × 104 |
| Vaccinated and challenged | 6.1 × 106 | 3.7 × 105 | 1.1 × 105 |
| Vaccinated, challenged, and treated with anti-p19 | 7.4 × 106 | 5.5 × 105 | 1.3 × 105 |
Eighteen to 24 popliteal and inguinal nodes were collected from 5 to 6 mice per group 8 days after infection. Single-cell suspensions in PBS were obtained, and cells were counted with a Petroff-Hauser counting chamber.
Obtained by multiplying the percentage of lymphocytes by the average number of cells/node.
Obtained by multiplying the percentage of CD4+ or CD4+ CD25+ lymphocytes by the average number of lymphocytes/node.
Treatment with anti-p19 antibody enhances borreliacidal activity.
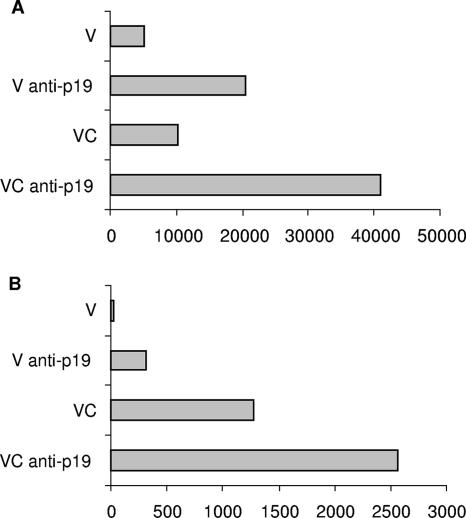
Sera were collected from B. burgdorferi isolate 297-vaccinated mice with or without B. bissettii challenge and with or without treatment with anti-p19 antibody 43 days after vaccination or 22 days after infection, respectively. Figure 3A shows that borreliacidal antibody directed against B. burgdorferi isolate 297 was enhanced fourfold or more in vaccinated mice or vaccinated and challenged mice treated with anti-p19 antibody. Likewise, borreliacidal antibody directed against the challenge agent, B. bissettii (Fig. 3B), was enhanced twofold or more in vaccinated and vaccinated and challenged mice after anti-p19 antibody treatment.
FIG. 3.
Borreliacidal antibody titers directed against B. burgdorferi isolate 297 (A) and B. bissettii (B) obtained with sera from vaccinated mice (V), vaccinated mice treated with anti-p19 antibody (V anti-p19), vaccinated and challenged mice (VC), and vaccinated and challenged mice treated with anti-p19 antibody (VC anti-p19). Sera were collected on day 22 after infection or day 43 after vaccination. The titer represents the reciprocal of the last dilution with significant borreliacidal activity. A shift of ≥13% in the mean fluorescence intensity compared to the control serum was considered positive by flow cytometry. In most cases (90%), the same titer was obtained when the study was repeated.
In other studies, anti-p19 antibody was administered at day 7 or day 15 after infection of Borrelia-vaccinated mice. Anti-p19 antibody treatment was continued daily for 6 or 9 days. Treatment with anti-p19 antibody enhanced borreliacidal antibody directed against B. burgdorferi isolate 297. A two- to fourfold increase in borreliacidal antibody was detected in sera from B. burgdorferi isolate 297-vaccinated and B. bissettii-challenged mice that received anti-p19 antibody at day 7 or day 15 after infection, respectively. By contrast, no significant increases in borreliacidal antibody against B. bissettii were detected when vaccinated and challenged mice were administered anti-p19 antibody at day 7 or day 15 after infection.
DISCUSSION
Several cytoplasmic proteins are required to inhibit cell division of T cells (13, 22, 26). We focused on p19 because it inhibits cyclin-dependent kinases (CDKs), especially CDK6, the earliest inducible member of the CDK family involved in cell cycle progression of T lymphocytes (13, 15, 22, 25, 26). Increasing amounts of intracellular p19 have also been shown to inhibit the kinase activity of CDK4 (13, 47). Therefore, p19 may restrict the number of T cells that can be produced following antigenic stimulation or infection (13, 15). This characteristic of p19 may also prevent tumor development (9, 30) and T-cell-driven or -associated diseases (18, 48). Little information, however, is available as to whether inhibitors of p19 will augment immune responses associated with Borrelia vaccination and challenge. Selective enhancement or inhibition of p19 could point the way to prevention of Lyme arthritis or enhancement of the quality of the protective borreliacidal response in future Lyme vaccines.
We showed previously that B. burgdorferi-vaccinated mice challenged with B. bissettii develop arthritis at the tibiotarsal joint (4, 11, 35-37). This finding is confirmed in the present report. The synovial space, synovium, and subsynovial tissue of the tibiotarsal joint of Borrelia-vaccinated and -challenged mice showed a severe inflammatory response involving a profuse infiltration of neutrophils. Subsequently, we hypothesized that the inflammatory arthritic response could be exacerbated by neutralizing p19 in Borrelia-vaccinated and -challenged mice. We speculated that in the absence of the p19 inhibitor, the responding immune cells in the periarticular tissues would continue to proliferate and promote the inflammatory response in the tibiotarsal joint.
Here, we show that anti-p19 antibody treatment did augment the inflammatory response normally detected at the tibiotarsal joints of Borrelia-vaccinated and -challenged mice. Although the synovial space, synovium, and subsynovial tissues contained a profuse infiltration of neutrophils, the most distinctive feature detected in anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice was erosion of cartilage and bone at the tibiotarsal joint. In fact, the joints were destroyed in most of the anti-p19-treated Borrelia-vaccinated and -challenged mice compared to the non-anti-p19-treated Borrelia-vaccinated and -challenged control mice. These results show that treatment with anti-p19 antibody exacerbates the arthritic response.
Treatment with anti-p19 antibody also induced several other effects in Borrelia-vaccinated and -challenged mice. Anti-p19 antibody treatment enhanced the borreliacidal antibody response of Borrelia-vaccinated and -challenged mice. Borreliacidal antibody increased two- to fourfold against both the vaccine and challenge agent compared to the borreliacidal activity detected in non-p19 antibody-treated Borrelia-vaccinated and -challenged mice. In addition, anti-p19 treatment was effective in enhancing borreliacidal antibody even when administered several days after Borrelia-vaccinated mice were challenged, especially against the vaccine agent. These results support our claim that anti-p19 treatment can affect the immune response to Borrelia vaccination and challenge.
In an attempt to elucidate the mechanism by which treatment with anti-p19 antibody exacerbated arthritis, we determined the number of lymphocytes, CD4+ T cells, and CD4+ CD25+ T cells in the lymph nodes (popliteal and inguinal) draining the site of the arthritic response. In general, the number of these cells was increased in anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice compared to the controls. This suggests that treatment with anti-p19 antibody prolongs or amplifies the immune response. In support of this hypothesis, it is known that increases in the number of lymph node cells and CD4+ T cells are associated with antigenic stimulation, cell proliferation, and activation (39). Of special interest is the number of CD4+ CD25+ T cells in the anti-p19-treated Borrelia-vaccinated and -challenged mice. Although CD4+ CD25+ T cells may play a major role in suppressing immune reactivity (8, 14, 27, 31, 41), initially CD4+ T cells expressing CD25, the IL-2α receptor, are active participants in developing the immune response (19, 23, 52). The increase (13%) in CD4+ CD25+ T cells found in the lymph nodes of anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice may have aided the arthritis-inducing effector CD4+ T cells by binding the T-cell growth-promoting factor, IL-2, to the CD25 receptor. This would further stimulate proliferation of arthritic reactive CD4+ T cells (19) and account for the enhanced histopathology of the tibiotarsal joint found in anti-p19 antibody-treated Borrelia-vaccinated and -challenged mice.
The ability of anti-p19 antibody treatment to augment the borreliacidal antibody response of Borrelia-vaccinated and Borrelia-vaccinated and -challenged mice, especially against the vaccine isolate, was a surprise. Sustained high levels of borreliacidal antibody is an important feature for establishing the efficacy of the next generation of Lyme vaccines. A major concern, however, for vaccine manufacturers is how to stimulate production of a strong and long-lived borreliacidal antibody response that has minimal side effects. Our results show that considerable enhancement of borreliacidal antibody can be achieved with anti-p19 antibody treatment; however, the mechanism of enhancement needs to be determined. By contrast, Borrelia-vaccinated and -challenged mice treated with anti-p19 antibody exhibited enhanced lymphocyte responses and borreliacidal activity to the vaccine isolate. Anti-p19 antibody treatment of Borrelia-vaccinated mice and Borrelia-vaccinated and -challenged mice may have caused a restricted clonal expansion of T cells specifically dedicated to stimulate production of anti-297 borreliacidal antibody (vaccine isolate). This restriction may have limited the borreliacidal antibody response to the challenge agent, B. bissettii. Our results show that anti-19 antibody treatment of vaccinated mice can enhance borreliacidal antibody which kills the homologous challenge agent, even when anti-p19 antibody treatment is delayed for 7 or 15 days. Additional studies with anti-p19 antibody are needed to determine the mechanism of antibody enhancement.
The novel activities of anti-p19 antibody are difficult to explain. How was the intracellular protein, p19, neutralized by treatment with anti-p19 antibody? It is generally accepted that antibodies cannot cross the cell membrane of eukaryotic cells (33, 39). However, there are antibodies called intrabodies that can function within a cell (24, 33, 49), and these can inhibit protein function (24, 33, 49). Intrabodies contain the antigen-binding regions and are generally small fragments (light chain) of the antibody molecule (24, 33). Presumably, anti-p19 antibody was altered in Borrelia-vaccinated and -challenged mice to form intrabodies that crossed the cell membrane and neutralized p19. Another explanation may be that p19 is expressed transiently on the surface of T cells and that interaction with anti-p19 antibody inactivates or down-regulates production of intracellular p19. A similar mechanism has been described for down-regulation or prevention of the production of cytokines (19, 39). Finally, anti-p19 antibody may be cross-reactive and bind to the cell receptors of alpha interferon or IL-10. These cytokines have been shown to increase the CDK inhibitor p19 (34, 38) and inhibit proliferation of immune cells (34, 38). Blockage of the receptors would prevent production of p19. Presently, we are investigating this latter hypothesis.
In conclusion, we show that treatment with anti-p19 antibody can alter the immune response, especially the severity of the arthritis induced in Borrelia-vaccinated and -challenged mice.
Acknowledgments
This study was supported by the Wisconsin State Laboratory of Hygiene, the public health laboratory for the state of Wisconsin, Madison, WI, and the Gundersen Medical Foundation, La Crosse, WI.
We also thank Dean Jobe and Krista Asp for their assistance in performing the borreliacidal assays and the Flow Cytometry Facility at the University of Wisconsin Hospital Comprehensive Cancer Center, Madison, WI.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Agger, W., K. L. Case, G. L. Bryant, and S. M. Callister. 1991. Lyme disease: clinical features, classification, and epidemiology in the upper Midwest. Medicine (Baltimore) 70:83-90. [PubMed] [Google Scholar]
- 2.Aguero-Rosenfeld, M. E., G. Wang, I. Schwartz, and G. P. Wormser. 2005. Diagnosis of Lyme borreliosis. Clin. Microbiol. Rev. 18:484-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amlong, C. A., D. T. Nardelli, S. Heil Peterson, T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-interleukin-15 prevents arthritis in Borrelia-vaccinated and -infected mice. Clin. Vaccine Immunol. 13:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anguita, J., M. Rincón, S. Samanta, S. W. Barthold, R. A. Flavell, and E. Fikrig. 1998. Borrelia burgdorferi-infected, interleukin-6-deficient mice have decreased Th2 responses and increased Lyme arthritis. J. Infect. Dis. 178:1512-1515. [DOI] [PubMed] [Google Scholar]
- 6.Anguita, J., S. W. Barthold, R. Persinski, M. N. Hedrick, C. A. Huy, R. J. Davis, R. A. Flavell, and E. Fikrig. 2002. Murine Lyme arthritis development mediated by p38 mitogen-activated protein kinase activity. J. Immunol. 168:6352-6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anguita, J., S. W. Barthold, S. Samanta, J. Ryan, and E. Fikrig. 1999. Selective anti-inflammatory action of interleukin-11 in murine Lyme disease: arthritis decreases while carditis persists. J. Infect. Dis. 179:734-737. [DOI] [PubMed] [Google Scholar]
- 8.Asano, M., M. Toda, N. Sakaguchi, and S. Sakaguchi. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartkova, J., E. Rajpert-De Meyts, N. E. Skakkebaek, J. Lukas, and J. Bartek. 2003. Deregulation of the G1/S-phase control in human testicular germ cell tumours. APMIS 111:252-265. [DOI] [PubMed] [Google Scholar]
- 10.Brown, J. P., J. F. Zachary, C. Teuscher, J. J. Weis, and R. M. Wooten. 1999. Dual role of interleukin-10 in murine Lyme disease: regulation of arthritis severity and host defense. Infect. Immun. 67:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burchill, M. A., D. T. Nardelli, D. M. England, D. J. DeCoster, J. A. Christopherson, S. M. Callister, and R. F. Schell. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan, F. K. M., J. Zhang, L. Cheng, D. N. Shapiro, and A. Winoto. 1995. Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol. Cell. Biol. 15:2682-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, X., J. J. Oppenheim, R. T. Winkler-Pickett, J. R. Ortaldo, and O. M. Howard. 2006. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+ CD4+ CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 36:2139-2149 [DOI] [PubMed] [Google Scholar]
- 15.Chilosi, M., C. Doglioni, Z. Yan, M. Lestani, F. Menestrina, C. Sorio, A. Benedetti, F. Vinante, G. Pizzolo, and G. Inghirami. 1998. Differential expression of cyclin-dependent kinase 6 in cortical thymocytes and T-cell lymphoblastic lymphoma/leukemia. Am. J. Pathol. 152:209-217. [PMC free article] [PubMed] [Google Scholar]
- 16.Coskun, V., and M. B. Luskin. 2001. The expression pattern of the cell cycle inhibitor p19INK4D by progenitor cells of the rat embryonic telencephalon and neonatal anterior subventricular zone. J. Neurosci. 21:3092-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernàndez, V., E. Hartmann, G. Ott, E. Campo, and A. Rosenwald. 2005. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J. Clin. Oncol. 23:6364-6369. [DOI] [PubMed] [Google Scholar]
- 19.Gaffen, S. L. 2001. Signaling domains of the interleukin 2 receptor. Cytokine 14:63-77. [DOI] [PubMed] [Google Scholar]
- 20.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 22.Guan, K. L., C. W. Jenkins, Y. Li, C. L. O'Keefe, S. Noh, X. Wu, M. Zariwala, A. G. Matera, and Y. Xiong. 1996. Isolation and characterization of p19INK4d, a p16 related inhibitor specific to CDK6 and CDK4. Mol. Biol. Cell 7:57-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumy, A., A. Aseffa, N. Rachinel, M. Breton, L. Otten, F. Tacchini-Cottier, M. Rocken, N. Doyen, H. Acha-Orbea, R. M. Locksley, H. R. MacDonald, P. Launois, and J. Louis. 2006. LACK-reactive CD4+ T cells require autocrine IL-2 to mediate susceptibility to Leishmania major. Eur. J. Immunol. 36:1465-1473. [DOI] [PubMed] [Google Scholar]
- 24.Heng, B. C., and T. Cao. 2005. Making cell-permeable antibodies (transbody) through fusion of protein transduction domains (PTD) with single chain variable fragment (scFv) antibodies: potential advantages over antibodies expressed within the intracellular environment (intrabody). Med. Hypotheses 64:1105-1108. [DOI] [PubMed] [Google Scholar]
- 25.Hirai, H., M. F. Roussel, J. Y. Kato, R. A. Ashmun, and C. J. Sherr. 1995. Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol. Cell. Biol. 15:2672-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter, T., and J. Pines. 1994. Cyclins and cancer II: cyclin D and CDK inhibitors come of age. Cell 79:573-582. [DOI] [PubMed] [Google Scholar]
- 27.Itoh, M., T. Takahashi, N. Sakaguchi, Y. Kuniyasu, J. Shimizu, F. Otsuka, and S. Sakaguchi. 1999. Thymus and autoimmunity: production of CD25+ CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 162:5317-5326. [PubMed] [Google Scholar]
- 28.Keitel, W. A. 1999. Cellular and acellular pertussis vaccines in adults. Clin. Infect. Dis. 28(Suppl. 2):S118-S123. [DOI] [PubMed] [Google Scholar]
- 29.Kodama, S., I. Mori, K. Roy, Z. Yang, K. Suzuki, and M. Watanabe. 2001. Culture condition-dependent senescence-like growth arrest and immortalization in rodent embryo cells. Radiat. Res. 155:254-262. [DOI] [PubMed] [Google Scholar]
- 30.Komata, T., T. Kanzawa, H. Takeuchi, I. M. Germano, M. Schreiber, Y. Kondo, and S. Kondo. 2003. Antitumour effect of cyclin-dependent kinase inhibitors (p16(INK4A), p18(INK4C), p19(INK4D), p21(WAF1/CIP1) and p27(KIP1)) on malignant glioma cells. Br. J. Cancer 88:1277-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.La Cava, A., L. Van Kaer, and Shi Fu-Dong. 2006. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 27:322-327. [DOI] [PubMed] [Google Scholar]
- 32.Lim, L. C., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobato, M. N., and T. H. Rabbitts. 2004. Intracellular antibodies as specific reagents for functional ablation: future therapeutic molecules. Curr. Mol. Med. 4:519-528. [DOI] [PubMed] [Google Scholar]
- 34.Matsuoka, M., K. Tani, and S. Asano. 1998. Interferon-alpha-induced G1 phase arrest through up-regulated expression of CDK inhibitors, p19Ink4D and p21Cip1 in mouse macrophages. Oncogene 16:2075-2086. [DOI] [PubMed] [Google Scholar]
- 35.Nardelli, D. T., J. P. Cloute, K. H. Luk, J. Torrealba, T. F. Warner, S. M. Callister, and R. F. Schell. 2005. CD4+ CD25+ T cells prevent arthritis associated with Borrelia vaccination and infection. Clin. Diagn. Lab. Immunol. 12:786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nardelli, D. T., M. A. Burchill, D. M. England, J. Torrealba, S. M. Callister, and R. F. Schell. 2004. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged gamma interferon-deficient mice treated with anti-interleukin-17 antibody. Clin. Diagn. Lab. Immunol. 11:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nardelli, D. T., T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-CD25 antibody treatment of mice vaccinated and challenged with Borrelia spp. does not exacerbate arthritis but inhibits borreliacidal antibody production. Clin. Vaccine Immunol. 13:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Farrell, A. M., D. A. Parry, F. Zindy, M. F. Roussel, E. Lees, K. W. Moore, and A. L. Mui. 2000. Stat3-dependent induction of p19INK4D by IL-10 contributes to inhibition of macrophage proliferation. J. Immunol. 164:4607-4615. [DOI] [PubMed] [Google Scholar]
- 39.Paul, W. E. 1993. Fundamental immunology, 3rd ed. Raven Press, New York, NY.
- 40.Potter, M. R., S. R. Rittling, D. T. Denhardt, R. J. Roper, J. H. Weis, C. Teuscher, and J. J. Weis. 2002. Role of osteopontin in murine Lyme arthritis and host defense against Borrelia burgdorferi. Infect. Immun. 70:1372-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakaguchi, S., N. Sakaguchi, M. Asano, M. Itoh, and M. Toda. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chain (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155:1151-1164. [PubMed] [Google Scholar]
- 42.Salazar, C. A., M. Rothemich, E. E. Drouin, L. Glickstein, and A. C. Steere. 2005. Human Lyme arthritis and the immunoglobulin G antibody response to the 37-kilodalton arthritis-related protein of Borrelia burgdorferi. Infect. Immun. 73:2951-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz, J. L., R. F. Schell, A. Hejka, D. M. England, and L. Konick. 1988. Induction of Lyme arthritis in LSH hamsters. Infect. Immun. 56:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoppmeyer, K., P. S. Norris, and M. Haas. 1999. Inhibition of T-cell acute lymphoblastic leukemia proliferation in vivo by re-expression of the p16INK4a tumor suppressor gene. Neoplasia 1:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steel, R. G. D., and J. H. Torrie. 1960. Principles and procedures of statistics with special reference to the biological sciences. McGraw-Hill Book Co., New York, NY.
- 46.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 47.Tavera-Mendoza, L. E., T. T. Wang, and J. H. White. 2006. p19INK4D and cell death. Cell Cycle 5:596-598. [DOI] [PubMed] [Google Scholar]
- 48.Tschan, M. P., U. R. Peters, J. F. Cajot, D. C. Betticher, M. F. Fey, and A. Tobler. 1999. The cyclin-dependent kinase inhibitors p18INK4c and p19INK4d are highly expressed in CD34+ progenitor and acute myeloid leukaemic cells but not in normal differentiated myeloid cells. Br. J. Haematol. 106:644-651. [DOI] [PubMed] [Google Scholar]
- 49.Williams, B. R., and Z. Zhu. 2006. Intrabody-based approaches to cancer therapy: status and prospects. Curr. Med. Chem. 13:1473-1480. [DOI] [PubMed] [Google Scholar]
- 50.Yokota, T., Y. Matsuzaki, K. Miyazawa, F. Zindy, M. F. Roussel, and T. Sakai. 2004. Histone deacetylase inhibitors activate INK4d gene through Sp1 site in its promoter. Oncogene 23:5340-5349. [DOI] [PubMed] [Google Scholar]
- 51.Yssel, H., M. C. Shanafelt, C. Soderberg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zelenay, S., T. Lopes-Carvalho, I. Caramalho, M. F. Moraes-Fontes, M. Rebelo, and J. Demengeot. 2005. Foxp3+ CD25− CD4 T cells constitute a reservoir of committed regulatory cells that regain CD25 expression upon homeostatic expansion. Proc. Natl. Acad. Sci. USA 102:4091-4096. [DOI] [PMC free article] [PubMed] [Google Scholar]