Abstract
Humans reliably produce high concentrations of borreliacidal OspC antibodies specific for the seven C-terminal amino acids shortly after infection with Borrelia burgdorferi. We show that dogs also produce OspC borreliacidal antibodies but that their frequencies, intensities, and antigenicities differ significantly. The findings therefore confirm a major difference between the borreliacidal antibody responses of humans and canines with Lyme disease.
Lyme disease is a tick-associated zoonosis caused by Borrelia burgdorferi sensu lato. The bacterium induces a variety of clinical manifestations in humans, including skin lesions, pericarditis, inflammation of the nervous system, and arthritis (14). An immunodominant antibody response during human illness involves the production of outer surface protein C (OspC) borreliacidal antibodies (9, 12, 13) that activate complement to kill the spirochete (10). High titers of borreliacidal OspC antibodies are reliably produced shortly after infection, and the response can be detected by a flow cytometric test (2, 4). This method of detection also requires live organisms, such as B. burgdorferi 50772 spirochetes, because these unique spirochetes lack the ability to produce OspA and OspB but do express OspC. This characteristic is essential since the expression of OspA or OspB prevents OspC antibodies from binding (4). Not surprisingly, the complexity of this test has contributed to decreased use despite the high sensitivity and specificity (2, 4). Researchers recently reported (12), however, that human OspC borreliacidal antibodies appear to be entirely specific for a conserved epitope within the C-terminal seven amino acids (C7). A peptide-based enzyme-linked immunosorbent assay (ELISA) could therefore likely accurately detect the response. In addition, the epitope could be a valuable constituent of a human Lyme disease vaccine.
B. burgdorferi spirochetes also cause similar clinical abnormalities in dogs. Canine Lyme disease manifests most often as subclinical polyarthritis and/or periarteritis (15) but can progress to renal disease (7), cardiac disorders (11), or arthritis (16). In addition, infected dogs produce borreliacidal antibodies that are also detected by using unique ospA- and ospB-defective spirochetes such as B. burgdorferi 50772 (3). We therefore determined whether B. burgdorferi 50772-specific borreliacidal antibodies in immune sera from dogs with Lyme disease were also induced by the C-terminal epitope of OspC.
Challenge and confirmation of infection.
Fifteen healthy 14-week-old laboratory-reared beagles were used. To ensure that the animals were not exposed to B. burgdorferi previously, serum samples were obtained prior to the study, and seronegativity was confirmed by using a B. burgdorferi 297 whole-cell ELISA (17). Ticks were also collected from a focus of endemicity near La Crosse, WI (8), and infection was confirmed by examining the midguts from 50 adult male ticks after staining with fluorescein isothiocyanate-labeled OspA monoclonal antibody H5332 (8). B. burgdorferi was detected in 15 (30%) ticks. Ten male and 10 female ticks were then selected randomly and placed into a rubber cup that was secured to the left dorsal-anterior region of each animal for 1 week. The dogs were isolated, fed commercial food and water ad libitum, and observed daily for lameness.
To confirm transmission of spirochetes by the tick challenge, 4-mm-punch skin biopsies were obtained from the tick bite sites at 34 and 90 days postchallenge, placed into separate tubes containing 9 ml of modified (1) Barbour-Stoenner-Kelly medium, incubated at 34°C, and examined weekly by dark-field microscopy for 4 weeks. B. burgdorferi was recovered from 12 (80%) and 11 (73%) dogs at 34 and 90 days postchallenge, respectively (Table 1). In addition, spirochetes were recovered from at least one skin biopsy from 14 (93%) dogs, and 4 (27%) dogs also developed lameness in one or more limbs that lasted for at least three observation periods.
TABLE 1.
Recovery of B. burgdorferi from skin biopsies and development of lameness in tick-challenged dogs
| Dog no. | Recovery of B. burgdorferi after:
|
Lamenessa | |
|---|---|---|---|
| 34 days | 90 days | ||
| 1 | + | + | − |
| 2 | + | − | + |
| 3b | − | − | − |
| 4 | + | + | + |
| 5 | + | + | − |
| 6 | − | + | − |
| 7 | + | − | − |
| 8 | − | + | − |
| 9 | + | + | − |
| 10 | + | + | + |
| 11 | + | + | − |
| 12 | + | + | + |
| 13 | + | + | − |
| 14 | + | + | − |
| 15 | + | − | − |
Development of lameness in one or more joints lasting three or more days.
Data for dog 3 were removed from the subsequent table and Fig. 1.
Antibody responses after infection.
Blood samples were obtained 90 days after the tick challenge and examined by Western blotting and a borreliacidal antibody test. Western blotting was performed by using B. burgdorferi 297 and standard techniques. Briefly, the spirochetes were boiled in treatment buffer, and 150 μg of protein was loaded onto a 10 to 20% linear gradient polyacrylamide gel and electrophoresed. The proteins were then transferred to a polyvinylidene difluoride membrane, cut into strips, blocked with 1% bovine serum albumin in phosphate-buffered saline (PBS, pH 7.2)-0.1% Tween 20, and incubated sequentially at room temperature with dog serum diluted 1:100 and horseradish peroxidase-labeled anti-dog immunoglobulin G. Reactions were detected by development with the TMB Membrane peroxidase substrate system (Kirkegaard & Perry Laboratories, Gaithersburg, MD). Serum from a healthy dog was used as a negative control.
Borreliacidal antibodies were detected by flow cytometry as described previously (4, 5) by combining 5 × 104 B. burgdorferi 50772 spirochetes with serum and complement (guinea pig serum [50% hemolytic complement, ≥200 units/ml]) and incubating the suspension at 35°C. Following incubation, a 100-μl aliquot of the assay suspension was combined with PBS and acridine orange, and the spirochetes were analyzed for killing by using a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). A ≥13% increase in fluorescence intensity compared to a normal serum control was considered positive (2). In addition, the assays were performed in duplicate, and the presence of blebbed nonmotile B. burgdorferi organisms was confirmed by microscopy.
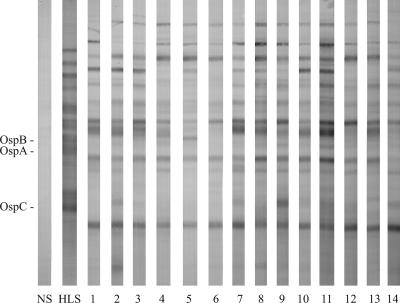
The animal with negative biopsy results (dog 3) was also seronegative by Western blotting and a whole-cell ELISA (data not shown), so the dog had likely not become infected and the serum was not evaluated further. In contrast, the 14 dogs with positive skin biopsies also produced immunoglobulin G antibodies against numerous B. burgdorferi proteins (Fig. 1). The specificities of the dog antibody responses were similar, but they differed significantly from the responses typically detected during human infection (6). As an example, the human Lyme disease serum control, collected from a patient who became infected in the same region as that where the ticks were collected, contained OspA, OspB, and OspC antibodies. In contrast, the Western blots of the immune dog sera did not detect OspA or OspB antibodies, and only rarely (dog 9) were antibodies specific for OspC detected. In addition, the immune dog sera typically contained high levels of antibodies specific for an uncharacterized, approximately 20-kDa protein that was not detected in the human serum. Additional studies to more completely characterize these differences should prove valuable for future efforts to develop effective canine Lyme disease tests or vaccines.
FIG. 1.
Western blots of normal dog serum (NS), typical human Lyme disease serum (HLS), and immune sera (lanes 1 to 14) from dogs infected with Borrelia burgdorferi by tick challenge.
In this study, however, our efforts were focused on determining whether the OspC borreliacidal antibody response was also immunodominant during canine illness. We therefore quantitated the levels of OspC and C7 antibodies by using OspC and C7 ELISAs (17), respectively, and compared the responses to the titers of borreliacidal antibodies. Antibodies specific for C7 were not detected (data not shown). OspC antibodies were detected in 12 (86%) immune sera (Table 2), but as suggested by the Western blots, the levels were significantly lower than those detected routinely during human infection (13). In addition, nine (64%) sera contained borreliacidal antibodies at titers (titer range, 1:640 to 1:20,480) seen commonly during human illness (4, 9, 12, 13), but one OspC-positive serum did not contain borreliacidal activity and two OspC-negative sera contained borreliacidal antibodies. The results therefore confirmed previous findings (3) that high concentrations of B. burgdorferi 50772-specific borreliacidal antibodies were produced during canine Lyme disease, but in contrast to the case for human illness, the response did not correlate closely with the detection of OspC antibodies.
TABLE 2.
ELISA titers and borreliacidal activities in immune sera before (untreated) and after removal of OspC or C7 antibodies
| Serum | OspC ELISA titera,c
|
Borreliacidal antibody titerb,c
|
|||
|---|---|---|---|---|---|
| Untreated | OspC removed | Untreated | OspC removed | C7 removed | |
| Human | 20,480 | ND | 10,240 | ND | ND |
| 1 | 160 | — | ND | — | — |
| 2 | 320 | ND | 5,120 | 160 | 5,120 |
| 3 | 80 | ND | 1,280 | 640 | 1,280 |
| 4 | 160 | ND | 2,560 | 640 | 5,120 |
| 5 | 640 | — | ND | — | — |
| 6 | 320 | — | ND | — | — |
| 7 | ND | — | ND | — | — |
| 8 | 160 | ND | 5,120 | 1,280 | 5,120 |
| 9 | 320 | ND | 640 | 640 | 1,280 |
| 10 | 640 | ND | 2,560 | 160 | 5,120 |
| 11 | 2,560 | ND | 20,480 | 1,280 | 20,480 |
| 12 | 160 | ND | 640 | 80 | 320 |
| 13 | 640 | ND | 5,120 | 640 | 5,120 |
| 14 | ND | — | ND | — | — |
Reciprocal of highest dilution with absorbance value >0.100 unit above normal serum control value.
Reciprocal of highest dilution with significant borreliacidal activity. Sera from each animal were evaluated concurrently to negate interassay variability.
ND, none detected; —, not tested.
Effects of removing OspC or C-terminal fragment (C7) antibodies on borreliacidal activity.
To confirm this significant divergence from the human serologic response, we used a procedure described previously (4, 12) to remove the OspC- or C7-specific antibodies and then determined the effects on the borreliacidal activity. Briefly, 1-ml volumes of Tetralink tetrameric avidin resin (Promega, Madison, WI) were loaded into separate 10- by 70-mm columns, and 0.5 mg of recombinant OspC or C7 was solubilized in PBS and passed over a column. A 1-ml volume of each immune serum diluted 10-fold with PBS was then passed over the column several times. The columns were regenerated between serum samples by using 0.1 M citric acid (pH 3), and a human serum control was passed over the columns at periodic intervals to confirm their ability to remove antibodies.
As reported previously (12), passage over the recombinant OspC or C7 column completely removed the antibodies and borreliacidal activity from the human Lyme disease serum control (Table 2). However, removing the C7 antibodies did not affect the borreliacidal activity in the dog sera. In contrast, removal of the OspC antibodies reduced the borreliacidal activity significantly (≥4-fold reduction) in seven (78%) dog sera, but considerable borreliacidal activity remained even after additional passages over a rejuvenated column. In addition, the absence of OspC antibodies failed to affect two of the borreliacidal antibody-positive sera. The findings therefore confirmed that some canines with Lyme disease produce significant levels of borreliacidal antibodies specific for OspC but that the response does not always predominate and the region responsible is distinct from the epitope recognized by human OspC borreliacidal antibodies.
Conclusions.
The immunodominant borreliacidal antibody response during human infection with B. burgdorferi is reliably specific for the C-terminal seven amino acids of OspC (12). Dogs with Lyme disease may also produce OspC borreliacidal antibodies, but the response may not predominate and the antigenicities of the antibodies differ significantly. This finding has important implications for future efforts to develop canine Lyme disease tests and vaccines.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Callister, S. M., K. L. Case, W. A. Agger, R. F. Schell, R. C. Johnson, and J. L. E. Ellingson. 1990. Effects of bovine serum albumin on the ability of Barbour-Stoenner-Kelly medium to detect Borrelia burgdorferi. J. Clin. Microbiol. 28:363-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Callister, S. M., D. A. Jobe, W. A. Agger, R. F. Schell, T. J. Kowalski, S. D. Lovrich, and J. A. Marks. 2002. Ability of the borreliacidal antibody test to confirm Lyme disease in clinical practice. Clin. Diagn. Lab. Immunol. 9:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callister, S. M., D. A. Jobe, R. F. Schell, S. D. Lovrich, K. L. Onheiber, and J. B. Korshus. 2000. Detection of borreliacidal antibodies in dogs after challenge with Borrelia burgdorferi-infected Ixodes scapularis ticks. J. Clin. Microbiol. 38:3670-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callister, S. M., R. F. Schell, L. C. L. Lim, D. A. Jobe, K. L. Case, G. L. Bryant, and P. E. Molling. 1994. Detection of borreliacidal antibodies by flow cytometry: an accurate, highly specific serodiagnostic test for Lyme disease. Arch. Intern. Med. 154:1625-1632. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1995. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. Morb. Mortal. Wkly. Rep. 44:590-591. [PubMed] [Google Scholar]
- 7.Dambach, D. M., C. A. Smith, R. M. Lewis, and T. J. Van Winkle. 1997. Morphologic, immunohistochemical, and ultrastructural characterization of a distinctive renal lesion in dogs putatively associated with Borrelia burgdorferi infection: 49 cases (1987-1992). Vet. Pathol. 34:85-96. [DOI] [PubMed] [Google Scholar]
- 8.Jackson, C. A., S. D. Lovrich, W. A. Agger, and S. M. Callister. 2002. Reassessment of a Midwestern Lyme disease focus for Borrelia burgdorferi and the human granulocytic ehrlichiosis agent. J. Clin. Microbiol. 40:2070-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jobe, D. A., S. D. Lovrich, R. F. Schell, and S. M. Callister. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in patients with early Lyme disease. Clin. Diagn. Lab. Immunol. 10:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraiczy, P., K. P. Hunfeld, S. Peters, R. Wurzner, G. Acker, B. Wilske, and V. Brade. 2000. Borreliacidal activity of early Lyme disease sera against complement-resistant Borrelia afzelii FEM1 wild-type and an OspC-lacking FEM1 variant. J. Med. Microbiol. 29:917-928. [DOI] [PubMed] [Google Scholar]
- 11.Levy, S. A., and P. H. Duray. 1988. Complete heart block in a dog seropositive for Borrelia burgdorferi. Similarity to human carditis. J. Vet. Intern. Med. 2:138-144. [DOI] [PubMed] [Google Scholar]
- 12.Lovrich, S. D., D. A. Jobe, R. F. Schell, and S. M. Callister. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin. Diagn. Lab. Immunol. 12:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rousselle, J. C., S. M. Callister, R. F. Schell, S. D. Lovrich, D. A. Jobe, J. A. Marks, and C. A. Wieneke. 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J. Infect. Dis. 178:733-741. [DOI] [PubMed] [Google Scholar]
- 14.Steere, A. C. 1989. Lyme disease. N. Engl. J. Med. 321:586-596. [DOI] [PubMed] [Google Scholar]
- 15.Straubinger, R. K., A. F. Straubinger, B. A. Summers, and R. H. Jacobson. 2000. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J. Infect. Dis. 181:1069-1081. [DOI] [PubMed] [Google Scholar]
- 16.Summers, B. A., A. F. Straubinger, R. H. Jacobson, Y.-F. Chang, M. J. Appel, and R. K. Straubinger. 2005. Histopathological studies of experimental Lyme disease in the dog. J. Comp. Pathol. 133:1-13. [DOI] [PubMed] [Google Scholar]
- 17.Wieneke, C. A., S. D. Lovrich, S. M. Callister, D. A. Jobe, J. A. Marks, and R. F. Schell. 2000. Evaluation of whole-cell and OspC enzyme-linked immunosorbent assays for discrimination of early Lyme borreliosis from OspA vaccination. J. Clin. Microbiol. 38:313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]