Abstract
We measured rubella virus immunoglobulin G (IgG) and IgM levels, as well as IgG avidity indexes, in serum samples taken before or after 6 months either after infection or after vaccination. The results obtained indicate that humoral immune responses are different after primary infection and after vaccination. This may have important consequences on the serological diagnosis of rubella virus infection.
Infection with rubella virus was endemic worldwide until the advent of rubella immunization in the early 1980s. Most vaccines used currently contain the RA 27/3 strain, which induces long-lasting immunity, as an antibody response is detectable for >20 years in 95% of patients (6). Because rubella vaccination coverage is not sufficient throughout the world, rubella cases are still reported. Clinical diagnosis of rubella is difficult and unreliable, as rubella virus infection can be asymptomatic in up to 50% of infected patients. Thus, laboratory confirmation is essential and relies on specific knowledge of rubella virus antibody kinetics (7, 10). In developed countries, rubella virus antibody testing is often recommended for all pregnant women who have not had a previous positive rubella virus antibody test. The aim of this screening is to identify women who are susceptible to rubella during pregnancy and for whom vaccination is advised in the immediate postpartum period and to prevent congenital rubella syndrome. This screening relies on serum testing for rubella virus-specific immunoglobulin G (RV-IgG). Tests for rubella virus-specific IgM (RV-IgM) are not indicated unless there is a history of rash or exposure to a rubella-like rash, but many gynecologists request IgM antibody testing because they are afraid to miss acute infection in early pregnancy. This practice should be discouraged, as the detection of RV-IgM is considered by some biologists as always indicative of a recent rubella virus infection. This misinterpretation has important implications in the management of pregnancy (11). In countries where vaccination is recommended to women of child-bearing age, a positive RV-IgM result is mostly due to vaccination or to nonspecific stimulation of the immune system rather than to primary rubella virus infection (1, 13, 14). Unnecessary tests for RV-IgM may thus lead to problems in interpretation because the positive predictive value of RV-IgM is poor, especially in countries where disease incidence is low. If RV-IgM is detected, it is therefore imperative to confirm a primary infection by using alternative tests. Among these, RV-IgG avidity may be helpful in distinguishing primary rubella virus infection from other situations where RV-IgM is detected (4, 5).
In this study, RV-IgG levels as well as RV-IgM levels and RV-IgG avidity kinetics after primary infection and after vaccination were analyzed in order to help biologists and clinicians in the interpretation of rubella serology.
All serum samples were collected from pregnant women and referred to our laboratory for rubella virus antibody screening or for additional testing when RV-IgM was detected.
RV-IgM was measured using the bioMérieux Vidas Rubella IgM enzyme immunoassay (cutoff index, 1.2) (bioMérieux, Marcy l'Etoile, France). RV-IgG was measured using the Beckman Access IgG immunoassay (cutoff, 15 IU/ml) (Beckman Coulter). The tests used for RV-IgG and RV-IgM determinations were performed according to the specifications of each manufacturer. RV-IgM and RV-IgG tests were carried out with serum samples collected <6 months either after the onset of infection (n = 33) or after vaccination (n = 33). The RV-IgG avidity index was measured in serum samples taken <6 months either after the onset of infection (n = 38) or after vaccination (n = 38) and in serum samples taken >6 months and up to 30 years either after the onset of infection (n = 25) or after vaccination (n = 25).
The RV-IgG avidity index was measured using our in-house urea wash method. This method is based on adding 6 M urea as a denaturing agent to the washing buffer to prevent the binding of low-avidity antibody to the antigen. Results are expressed as ratios of absorbance values for single serum dilutions with and without denaturing agent. An avidity index of <30% is considered very low, one between 30% and 70% is considered low, one between 70% and 90% is considered moderate, and one of >90% is considered high.
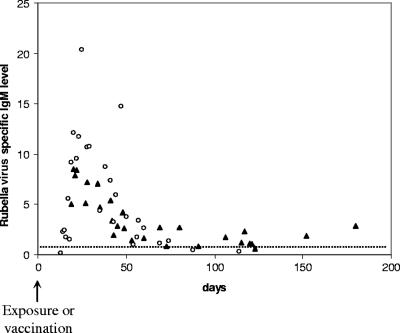
RV-IgM and RV-IgG levels and RV-IgG avidity indexes for samples taken <6 months either after primary rubella virus infection or after rubella vaccination.
Detection of RV-IgM in a serum sample collected 3 to 6 days after the onset of rash is the method of choice for diagnosis of acute rubella. It has been well described that RV-IgM is always detected for up to 8 weeks after natural infection by using the most sensitive techniques but is usually no longer detected after 12 to 14 weeks (2, 8, 15). Low concentrations of RV-IgM can be detected for much longer after vaccination (3, 9, 12) and may be difficult to distinguish from RV-IgM detected in primary rubella virus infection (5, 13, 14). Our results confirmed that after naturally acquired rubella, RV-IgM usually peaked within a few days after the onset of infection and then sharply decreased (levels divided by ∼2 every 3 weeks) (Fig. 1). In contrast, after vaccination, RV-IgM peaked at a lower level and was always detected for at least 3 months after vaccination (Fig. 1). After 3 months and for up to 6 months, RV-IgM was still detected at low levels in 7/8 cases.
FIG. 1.
RV-IgM levels measured <6 months after natural infection (circles) and <6 months after vaccination (triangles). The cutoff is shown by a dotted line.
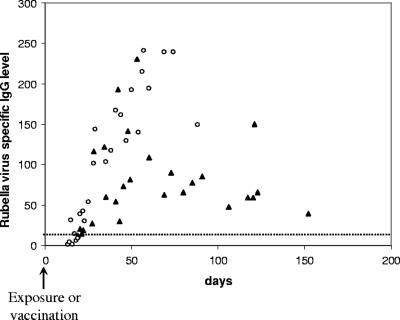
RV-IgG was generally detected within 2 to 3 weeks after the onset of infection. These results are in accordance with previous studies (2). We observed that within 2 months, the level of RV-IgG was usually higher after primary infection than after rubella vaccination and, in most cases, remained significantly higher (Fig. 2).
FIG. 2.
RV-IgG levels measured <6 months after natural infection (circles) and <6 months after vaccination (triangles). The cutoff is shown by a dotted line.
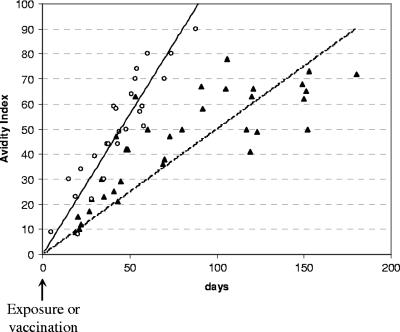
In patients with primary rubella virus infection, there was an avidity increase from very low indexes (<30%) to moderate indexes (70 to 90%) within 2 months after infection: in 9/26 cases, avidity indexes were very low, in 15/26 cases they were low, and in 2/26 cases they were moderate. Between 2 and 3 months after infection, most avidity indexes were moderate (7/9 were moderate and 2/9 were high). High-avidity indexes (>90%) were constantly observed after three or more months of infection (3/3). The overall analysis indicated a very quick maturation of RV-IgG after naturally acquired rubella virus infection (Fig. 3). After rubella vaccination, avidity indexes increased much more slowly. Indeed, within 2 months after vaccination, avidity indexes ranged from very low (11/16) to low (5/16). For periods of >2 months, avidity indexes remained low (13/22) or moderate (9/22). High-avidity indexes were never observed after vaccination, regardless of the observation time (Fig. 3).
FIG. 3.
IgG avidity indexes measured <6 months after natural infection (circles and solid curve) and <6 months after vaccination (triangles and dotted curve).
Figure 4 illustrates differences in RV-IgM, RV-IgG, and RV-IgG avidity kinetics between two patients, namely, one after recent vaccination (Fig. 4A) and one after recent primary rubella virus infection (Fig. 4B). These two cases show that RV-IgM is detected longer after vaccination than after natural infection. Additionally, RV-IgG levels are higher after natural infection. As for the RV-IgG avidity index, it rapidly reaches a higher level after natural infection than after vaccination, where it stabilizes at a low index.
FIG. 4.
RV-IgM (▴) and RV-IgG (•) levels and RV-IgG avidity indexes (□). Cutoffs are indicated for RV-IgM (dashed-dotted line) and RV-IgG (dashed line). (A) Kinetics for recent vaccination; (B) kinetics for recent primary rubella virus infection.
RV-IgG avidity indexes for samples taken >6 months either after primary rubella virus infection or after rubella vaccination.
Our study shows that 6 months or more after primary infection, all patients had IgG avidity indexes of >85%, and most patients (21/25) had indexes of >90% (Fig. 5). Remarkably, after rubella vaccination, IgG avidity stabilizes at a lower level than that after primary infection, and many years later, it usually remains low (6/25) or moderate (19/25). For vaccinated patients, reinfection cannot be excluded and may explain why, in some cases, the avidity index observed a long time after vaccination is similar to the avidity index observed after primary infection.
FIG. 5.
IgG avidity indexes measured >6 months after vaccination (triangles) and >6 months after natural infection (circles).
Conclusions.
To reduce the incidence of congenital rubella syndrome, routine prenatal screening and postpartum vaccination for seronegative women are recommended. Regarding consequences of positive serological results in the management of pregnancy, these must be interpreted with caution for pregnant women. Indeed, misinterpretation of results can lead to anxiety for pregnant women and sometimes to useless termination of pregnancy. This is why interpretation of positive results should be based on as much information as possible concerning clinical signs, contact with rash, previous immunization, and antibody screening.
Serological results are highly dependent on the patient tested and the technique used. However, it has previously been published that humoral immune responses are different after rubella vaccination and after primary rubella virus infection, regardless of the technique used (1, 12). In our study, we demonstrated that analysis of RV-IgM levels in consecutive samples can help to distinguish primary infection from vaccination, since the kinetics of RV-IgM are very different depending on whether it appears postvaccination or post-primary infection. However, to confirm such an important conclusion, RV-IgM-positive test results for pregnant women should always be completed using an alternative method, such as RV-IgG avidity testing. We noticed that maturation of RV-IgG is slower after vaccination than after naturally acquired infection. Moreover, we showed that the RV-IgG avidity index stabilizes at moderate levels after vaccination and at high levels after primary infection.
We also demonstrated or confirmed that IgG avidity and IgG antibody levels, important parameters of the quality of the humoral immune response, are lower after vaccination than after primary rubella virus infection. Yet because it is well known that the rubella vaccine is very efficient, we may suppose that the induced cellular immune response plays a major role in the protection observed. This hypothesis deserves further study.
It is still necessary to include rubella virus antibody screening in antenatal care, even in countries with well-established vaccination programs. Most diagnosis problems can now be solved with improved and close analysis of serological results, as described here.
Acknowledgments
We thank Richard Keros for revision of the English used in this report.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Al-Nakib, W., J. M. Best, and J. E. Banatvala. 1975. Rubella-specific serum and nasopharyngeal immunoglobulin responses following naturally acquired and vaccine-induced infection. Prolonged persistence of virus-specific IgM. Lancet i:182-185. [DOI] [PubMed] [Google Scholar]
- 2.Banatvala, J. E., and D. W. G. Brown. 2004. Rubella. Lancet 363:1127-1137. [DOI] [PubMed] [Google Scholar]
- 3.Banatvala, J. E., et al. 1985. Persistence of rubella antibodies after vaccination: detection after experimental challenge. Rev. Infect. Dis. 7:S86-S90. [DOI] [PubMed] [Google Scholar]
- 4.Best, J. M., et al. 2002. Interpretation of rubella serology in pregnancy—pitfalls and problems. BMJ 325:147-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böttiger, B., and I. P. Jensen. 1997. Maturation of rubella IgG avidity over time after acute rubella infection. Clin. Diagn. Virol. 8:105-111. [DOI] [PubMed] [Google Scholar]
- 6.Enders, G., and U. Nickerl. 1988. Rubella vaccination: antibody persistence for 14-17 years and immune status of women without and with a history of vaccination. Immun. Infekt. 16:58-64. [PubMed] [Google Scholar]
- 7.Grangeot-Keros, L. 2003. Techniques biologiques du diagnostic anténatal des infections virales et de la toxoplasmose. Rev. Fr. Lab. 353:29-32. [Google Scholar]
- 8.Grangeot-Keros, L., et al. 1988. Prenatal and postnatal production of IgM and IgA antibodies to rubella virus studied by antibody capture immunoassay. J. Infect. Dis. 158:138-143. [DOI] [PubMed] [Google Scholar]
- 9.Grangeot-Keros, L., and G. Enders. 1997. Evaluation of a new enzyme immunoassay based on recombinant rubella virus-like particles for detection of immunoglobulin M antibodies to rubella virus. J. Clin. Microbiol. 35:398-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingrand, D. 2003. Diagnostic anténatal des infections rubéoliques. Rev. Fr. Lab. 353:41-45. [Google Scholar]
- 11.Morgan-Capner, P., N. S. Crowcroft, and PHLS Joint Working Party of the Advisory Committees of Virology and Vaccines and Immunisation. 2002. Guidelines on the management of, and exposure to, rash illness in pregnancy (including consideration of relevant antibody screening programmes in pregnancy). Commun. Dis. Public Health 5:59-71. [PubMed] [Google Scholar]
- 12.O'Shea, S., et al. 1985. Development and persistence of class-specific antibodies in the serum and nasopharyngeal washings of rubella vaccinees. J. Infect. Dis. 151:89-98. [DOI] [PubMed] [Google Scholar]
- 13.Thomas, H., et al. 1992. Persistent rubella-specific IgM reactivity in the absence of recent primary rubella and rubella reinfection. J. Med. Virol. 36:188-192. [DOI] [PubMed] [Google Scholar]
- 14.Thomas, H., et al. 1999. Simultaneous IgM reactivity by EIA against more than one virus in measles, parvovirus B19 and rubella infection. J. Clin. Virol. 14:107-118. [DOI] [PubMed] [Google Scholar]
- 15.Thomas, H. I. J., et al. 1992. Persistence of specific IgM and low avidity specific IgG1 following primary rubella. J. Virol. Methods 39:149-155. [DOI] [PubMed] [Google Scholar]