Abstract
Previous studies with group C meningococcal polysaccharide-tetanus toxoid (GCMP-TT) conjugates had suggested that the GCMP O-acetyl group masked the protective epitope for group C meningococci through steric hindrance or altered conformations. For this report, we confirmed this phenomenon and performed comparative studies with group Y meningococcal polysaccharide (GYMP)-TT to determine whether it might extend to other serogroups. The de-O-acetylated (dOA) polysaccharides (PSs) resulted in higher serum bactericidal activities (SBA) towards the O-acetylated (OA) meningococcal strains from the respective serogroups. High-resolution H-nuclear magnetic resonance spectroscopy at 500 MHz and competitive inhibition serum bactericidal assays were used to characterize the nature of the protective epitope. In head-to-head comparisons with OA PSs as SBA inhibitors, the dOA PSs provided 10 to 1,000 times better inhibition for GCMP in human and mouse antisera and 6 to 13 times better inhibition for GYMP in mouse antisera, using OA strains in all assays. In addition, the SBA for OA strains was highly correlated with dOA PS-specific immunoglobulin G (r = 0.72 to 0.98) for both GCMP and GYMP. The results suggest that there may be a generalized role for the O-acetyl group to provide an epitope of misdirected immunogenicity for meningococcal PS capsules, enabling escape from immune surveillance. In addition to greater chemical consistency, the dOA forms of GCMP and GYMP conjugate vaccines endow greater immunologic competence to the PSs, rendering them capable of eliciting higher levels of functional antibodies toward the protective epitopes.
Meningococcal disease continues to be of significant global concern, with an estimated 1.2 million cases and 135,000 deaths annually (52). In the United States, at the start of this century, there were 1,400 to 2,800 cases of meningococcal disease annually, with a mortality of 10 to 14% and severe sequelae in 11 to 19% of survivors (6). The causative agent, Neisseria meningitidis, is known to encompass at least 13 serogroups, based on the organism's polysaccharide (PS) capsule (44), although only 5 groups (A, B, C, W135, and Y) appear to be responsible for nearly all disease (24). The dominant serogroups in the United States are B, C, and Y, where each is responsible for about a third of the cases (6). Group B causes >50% of meningococcal disease in infants <1 year old (43), while groups C, Y, and W135 are responsible for >75% of cases for those >11 years old (6, 43).
Capsule-based immunizations with the T-cell-dependent meningococcal PS conjugate vaccines have been shown to be capable of not only eliminating disease in target populations (2, 35, 38) but also of reducing carriage (28), thereby reducing transmission and resulting in herd immunity (2, 39). Meningococcal group C conjugate vaccines were licensed in the United Kingdom without efficacy trials by relying on the demonstration of safety and immunogenicity in terms of serum bactericidal activity (SBA) (1, 7, 35, 38), which was previously established as a surrogate measurement of protective immunity or a predictor for protection (17); it has been recommended by the World Health Organization for evaluation of vaccine immune responses (54) and validated as a serologic correlate of protection using postlicensure efficacy estimates (1). More recently, the same correlate was applied in the licensure of a tetravalent (A, C, Y, and W135) conjugate vaccine in the United States (6).
Group C meningococcal polysaccharide (GCMP) is a homopolymer of α-(2,9)-linked sialic acids, which are O-acetylated (OA) at position C-8 or C-7 (4), and the full H-nuclear magnetic resonance (H-NMR) assignment with detailed O acetylation (OAc) pattern of the PS used in vaccine production has been reported (25). Group Y meningococcal polysaccharide (GYMP) is a heteropolymer consisting of repeating disaccharides of α-(2,4)-linked sialic acids attached to the 6 position of glucose residues: →6)Glcp-(α1→4)-NeupAc-(α2→. The sialic acids on the purified PS are OA at position C-9 or C-7 in various amounts depending on the strain (25). The O-acetyl moiety is likely present at C-8 on GCMP and C-7 on GYMP at the surface of the organisms. After purification of the PS, however, these O-acetyl groups eventually migrate to the neighboring hydroxyl (i.e., C-7 on GCMP and C-9 on GYMP) upon storage in solution (25).
Clinical trials of T-cell-independent GCMP vaccines in OA and de-O-acetylated (dOA) forms, conducted in the 1970s and 1980s, concluded that the OAc was not required for the immunogenicity of the vaccine (16, 37, 47), and it was suggested that the dOA form of the PS be used as a replacement for the OA vaccine for all age groups (47). We later confirmed the clinical GCMP OAc results using tetanus toxoid (TT) conjugates of GCMP in preclinical mouse studies, comparing varying amounts of OAc with the dOA form and demonstrating that the immunogenicity was inversely correlated to the level of OAc (32). In addition, the SBA was shown to be correlated with dOA PS-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) titers (32). The dOA conjugate was then evaluated in a series of clinical trials in the United Kingdom, where it was shown to be well tolerated and highly immunogenic in adults, children, and infants (7, 8, 10, 40-42, 45, 46). As a result, this meningococcal C conjugate vaccine was licensed as the only dOA conjugate (NeisVac-C; Baxter Healthcare Corporation) in 32 countries worldwide (37a), with no documented vaccine failures during the early years of the campaign.
In this report, a series of spectroscopic and serologic studies were conducted in an effort to explore and characterize the nature of the capsular PS protective epitope for groups C and Y, particularly with respect to the OAc. High-resolution H-NMR spectroscopy at 500 MHz was used to define the OAc status of the purified meningococcal PSs for use as inhibitors in competitive inhibition bactericidal assays.
(Portions of this work were presented at the conference Physico-Chemical Procedures for the Characterization of Vaccines at Les Pensières, Veyrier-du-Lac, France, 1 to 3 December 1999.)
MATERIALS AND METHODS
Strains.
The N. meningitidis strains C11 (also known as 60E), S1975, S225, S3536, S3790, and Slaterus were provided by Carl Frasch, Bacterial Polysaccharides Laboratory, U.S. Food and Drug Administration. PSs for conjugation were derived from the C11 strain for group C and from Slaterus for group Y. Strains for SBA assays were C11 for group C and S3790 for group Y.
Capsular PSs.
The group C and Y PSs were kindly provided by Baxter Vaccines (Beltsville, MD), according to their manufacturing protocols, using microfiltration and ultrafiltration procedures.
De-O acetylation of PSs.
The PSs were partially or completely de-O-acetylated by treatment with dilute base. Dialysis and lyophilization yielded PSs in which the extent and position of O acetylation were determined by high-resolution H-NMR spectroscopy at 500 MHz on a Bruker spectrometer.
Preparation of conjugates.
Native and base-treated GCMPs were depolymerized and activated by oxidation with NaIO4, which cleaves the C-8-C-7 sialic acid bond. Native and base-treated GYMPs were depolymerized with mild acid and activated using periodate oxidation of the sialic acid side chain (C-9-C-8 bond). Coupling of the PS fragments to TT (Statens Serum Institut, Copenhagen, Denmark) for the conjugate vaccines or human serum albumin (HSA) for ELISA coating antigens was carried out using reductive amination (23).
Preclinical studies and serologic assays.
The GCMP-TT and GYMP-TT conjugate bulks in saline containing 0.01% thimerosal were adsorbed on aluminum hydroxide. Each dose of the preclinical vaccine formulation contained 2 μg of conjugated PS. All vaccine formulations were tested in groups of 10 female Swiss Webster mice (Harlan Sprague Dawley), 4 to 6 weeks old, housed at Washington Biotechnology, Inc. (Baltimore, MD), under the direction of Sean O'Neill. Immunizations were performed on days 0, 28, and 42. Sera from mice were collected on days 0, 28, 38, and 52 and stored frozen until they were pooled and analyzed once for SBA and PS-specific ELISA IgG titer determinations, using complete lines with linear regions that contained several points (instead of using single points) for each serum (14). Mouse SBA and IgG titers were all standardized using in-house reference sera (three different sera for SBA and one serum for IgG) as controls to cover the range of the assays (14). The CDC 1992 reference serum (21) was used to standardize human IgG concentrations (in μg/ml).
Animal facilities were accredited by the American Association for Accreditation of Laboratory Animal Care, licensed by the state of Maryland, and registered with the U.S. Department of Agriculture and FDA. The animal maintenance, immunization, and blood sampling were in accordance with the applicable portions of the Animal Welfare Act and the Department of Health and Human Services Guide for the Care and Use of Laboratory Animals.
For group Y sera, the antibody-dependent complement-mediated SBA was determined as previously described (14). For group C sera, however, the SBA was assessed by a turbidimetric protocol (32) which was a modification of the previous method, where bacterial growth after complement-mediated killing is measured by turbidity instead of colony counts; this was used for all SBA-based potency measurements for the licensure of NeisVac-C. Sample sera were titrated in duplicate on 96-well microtiter plates. After heat inactivation, serial dilutions of the sample pools and reference sera were made on the plate using Gey's balanced salt solution (GIBCO BRL, Gaithersburg, MD). Baby rabbit complement (Pel-Freeze, Brown Deer, WI) and approximately 2 × 104 CFU/ml bacteria (Neisseria meningitidis strain C11 for serogroup C or strain 3790 for serogroup Y) were added to give a final reaction ratio of 1:1:2 (bacteria/complement/sera). This was incubated at 37°C and 5% CO2 for 1 hour with agitation (14). After the bactericidal reaction, for the group Y bacteria we followed the previous protocol for plating and counting (14), while for the group C bacteria we followed the alternative turbidimetric procedure (32). For group C, the growth of surviving bacteria was measured by diluting the 100-μl reaction mixture with 100 μl Mueller-Hinton broth (Difco), transferring a 75-μl aliquot of that mixture into 125 μl of Mueller-Hinton broth in a separate microtiter plate, and incubating for 12 h at 37°C and 5% CO2 with agitation. The absorbance at 650 nm was measured and the percentage growth plotted. SBA titers were obtained as the reciprocal of the serum dilution at 50% killing and were standardized for variations in complement activity by the use of a range of three reference sera performed concurrently (14).
The inhibition assays, modified from a previous method (14), were performed by preincubating the test sera, at fourfold the concentration normally achieving 100% killing of bacteria (to account for assay variability, making sure that essentially complete killing was achieved without inhibitor), with an equal volume of inhibitor for 1 h at 37°C and 5% CO2. The inhibitor was diluted to provide final concentrations in a 2-fold series for GCMP from 10 μg/ml to 19 pg/ml and in a 10-fold series for GYMP from 250 μg/ml to 0.25 pg/ml. Following this preincubation, the assays were completed as described above.
Clinical sera.
Human dOA GCMP-TT conjugate vaccine sera were obtained from the various clinical trials in the United Kingdom that have been reported previously (40, 42). Each 0.5-ml dose of the meningococcal C polysaccharide conjugate vaccine (NeisVac-C) provided by Baxter contained 10 μg of de-O-acetylated GCMP, 15 to 20 μg tetanus toxoid, and 0.5 mg aluminum hydroxide adjuvant. Human GCMP vaccine sera, from United Kingdom adults immunized with ACVax (GlaxoSmithKline), were provided by Keith Cartwright, Public Health Laboratory Service, United Kingdom The geometric means (GM) were calculated with 95% confidence intervals (CI) and compared by a two-tailed t test.
RESULTS
Preparation of GCMP and GYMP with various degrees of OAc.
The OAc patterns for GCMP and GYMP, as measured by H-NMR at 500 MHz, are summarized in Table 1. The OAc of GYMP, at C-7 or C-9, represented the status of the material upon extraction and purification from different strains, whereas the OAc of GCMP, at C-7 or C-8, represented the status after extraction from a single strain but processed in different ways. The three different C/C11 samples from Table 1 were obtained as follows: C/C111 represented freshly isolated material which contained mostly C-8 OAc, C/C112 represented material that was stored for approximately 6 months at 4°C with the OAc shifted to C-7, and C/C113 represented dOA GCMP obtained by base treatment. These high-molecular-mass samples (>100,000 Da) of OA and dOA PSs were used for the competitive inhibition bactericidal studies of this report.
TABLE 1.
OAc patterns of capsular PSs
| Serogroup/strain | % OAc
|
|||
|---|---|---|---|---|
| Total | C-7 | C-9 | C-8 | |
| Y/S1975 | <5 | <5 | <5 | NAa |
| Y/S225 | <5 | <5 | <5 | NA |
| Y/S3536 | 52 | 46 | 5 | NA |
| Y/S3790 | 90 | 80 | 10 | NA |
| Y/Slaterus | 93 | 32 | 61 | NA |
| C/C111b | 95 | 5 | NA | 90 |
| C/C112b | 75 | 65 | NA | 10 |
| C/C113b | <5 | <5 | NA | <5 |
NA, not applicable.
Serogroup C data are from reference 32.
Effect of OAc on the immunogenicity of GYMP-TT conjugates.
The physicochemical properties and immunogenicity in mice of tetanus toxoid conjugates prepared with oxidized GYMPs that are OA (>70% OAc) or dOA (<5% OAc) are summarized in Table 2. The dOA conjugate consistently generated higher PS-specific IgG titers when using dOA GYMP-HSA as the ELISA coat antigen as well as a higher SBA towards the OA S3790 strain after two and three injections; differences were approximately twofold at best.
TABLE 2.
Physicochemical properties and mouse immunogenicity of GYMP-TT vaccines
| Vaccine | % OAc | PS avg mol mass (kDa) | PS/protein ratio | PS-specific IgG titera (SBA titerb) after:
|
||
|---|---|---|---|---|---|---|
| 1 injection | 2 injections | 3 injections | ||||
| OA conjugate | >70 | 15 | 0.262 | 347,400 (36,600) | 395,100 (33,800) | 541,400 (38,950) |
| dOA conjugate | <5 | 12.5 | 0.214 | 500,900 (33,220) | 930,500 (60,250) | 1,038,200 (53,060) |
IgG was determined by ELISA with dOA-GYMP-HSA as coat antigen. Preimmune IgG titers were all <50.
Preimmune SBA titers were all <66. The SBA assay was conducted with OA strain 3790.
Competitive inhibition of SBA with GCMP inhibitors containing various OAc patterns.
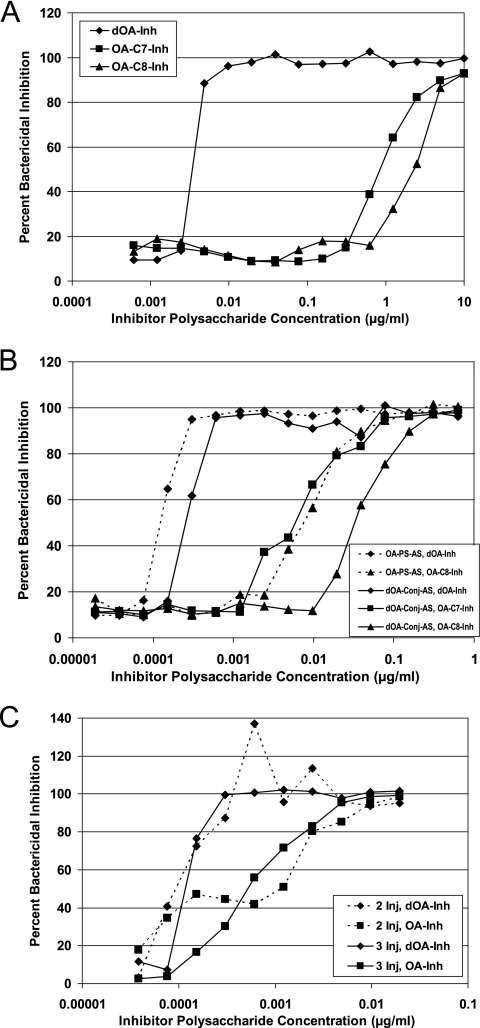
A competitive inhibition bactericidal assay was performed to assess the importance of the O-acetyl groups in the binding of functional (killing) mouse and human GCMP-specific antibodies to live meningococci, using the OA C11 strain (Fig. 1). GCMPs were used as inhibitors but with different states of OAc: OA at C-7, OA at C-8, and dOA (Table 1). The inhibition assay was performed with mouse sera after three subcutaneous (s.c.) injections (Fig. 1A), adult human sera after one intramuscular (i.m.) injection (Fig. 1B), and infant human sera after two or three i.m. injections (Fig. 1C). The dOA material was the superior inhibitor in all cases, regardless of the host and the OAc status of the vaccine antigen.
FIG. 1.
Competitive inhibition of SBA with GCMP inhibitors containing various OAc patterns, including dOA, OA at C-7, OA at C-8, and a mixture of C-7 and C-8 OAc (where no C number is given). Inh, inhibitor; AS, antisera; Conj, conjugate; Inj, injections. (A) Inhibition of SBA for anti-dOA-Conj (GCMP-TT) mouse sera. (B) Inhibition of SBA for anti-OA-PS (GCMP) and anti-dOA-Conj (GCMP-TT) adult human sera. (C) Inhibition of SBA for anti-dOA-Conj (GCMP-TT) infant human sera. (Adapted from reference 32 with permission of the publisher.)
For the mouse sera raised against the dOA GCMP-TT conjugate, the dOA GCMP inhibited the SBA for the OA strain 100 to 1,000 times better than the OA GCMP (Fig. 1A). When comparing the two different OA inhibitors, however, the C-7 OAc provided slightly better inhibition than the C-8 OAc (Fig. 1A).
For the adult human sera, the dOA GCMP inhibited approximately 100-fold better than the C-8 OA GCMP, regardless of the OAc status (OA GCMP versus dOA GCMP-TT) of the vaccine material used to elicit the bactericidal antibodies (Fig. 1B). The C-7 OA GCMP again inhibited better (nearly 10-fold) than the C-8 OA GCMP with the dOA GCMP-TT antisera and was even stronger than what was observed for the mouse (Fig. 1B). Nonetheless, the dOA GCMP was greater than 10-fold stronger than the C-7 OA GCMP as an inhibitor (Fig. 1B).
For the infant human sera raised against the dOA GCMP-TT conjugate (at 4 and 5 months of age), the dOA GCMP was approximately 10-fold better at inhibiting, regardless of the number of injections used to raise the sera (Fig. 1C). For this particular study, however, the OA GCMP inhibitor contained an even mixture of C-7 and C-8 OA GCMP.
Overall, the slopes of the inhibition lines were quite similar (Fig. 1A and B), except for the infants (Fig. 1C), where there might be a steeper slope for the dOA inhibitor compared with the OA material. In this case, the steeper slope might indicate higher avidity for the dOA GCMP with the infant sera.
Competitive inhibition of SBA with GYMP inhibitors containing various OAc patterns.
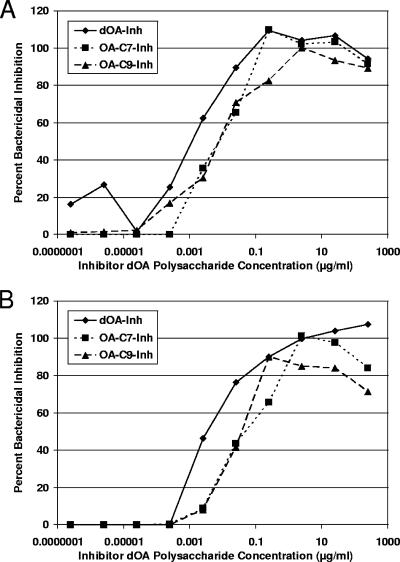
A competitive inhibition bactericidal assay was also performed to assess the importance of the O-acetyl groups in the binding of functional (killing) mouse GYMP-specific antibodies to live meningococci, using the OA S3790 strain (Fig. 2). GYMPs were used as inhibitors but with different states of OAc: OA at C-7, OA at C-9, and dOA (Table 1). The inhibition assay was performed with mouse sera raised against dOA GYMP-TT (Fig. 2A) and OA GYMP-TT (Fig. 2B) after three s.c. injections. The dOA GYMP inhibitor was consistently better (6- to 13-fold) than the OA GYMP inhibitor, regardless of the OAc status of the vaccine material, but the difference was not as dramatic as what was observed for the GCMP inhibitors.
FIG. 2.
Competitive inhibition of SBA with GYMP inhibitors containing various OAc patterns, including dOA, OA at C-7, and OA at C-9. Inh, inhibitor. (A) Inhibition of SBA for anti-dOA-GYMP-TT mouse sera. (B) Inhibition of SBA for anti-OA-GYMP-TT mouse sera.
Correlation of SBA with ELISA IgG using dOA PS-HSA coat antigens.
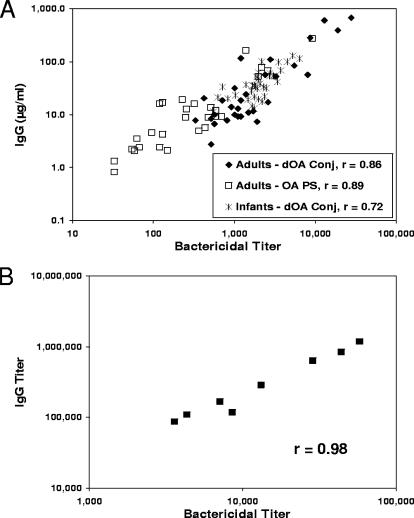
Strong correlations have been observed between IgG and SBA when using dOA PS-HSA conjugates as ELISA coat antigens (Fig. 3). Figure 3A shows the GCMP scatter plots for IgG versus SBA, using adult human sera (n = 30) to dOA GCMP-TT after one i.m. injection (r = 0.86), adult human sera (n = 28) to OA GCMP after one i.m. injection (r = 0.89), and infant human sera (n = 36) to dOA GCMP-TT after three i.m. injections (r = 0.72). Similarly, Fig. 3B shows the scatter plot for mouse sera (n = 8) raised against dOA GYMP-TT after three s.c. injections (r = 0.98). All SBA data were generated with OA strains. All IgG data were generated with dOA ELISA coat antigens.
FIG. 3.
Correlation of SBA with ELISA IgG using dOA PS-HSA coat antigens. (A) GCMP scatter plots for IgG versus SBA, using adult human antisera to dOA GCMP-TT, adult human antisera to OA GCMP, and infant human antisera to dOA GCMP-TT. Conj, conjugate. (B) GYMP scatter plots for IgG versus SBA for mouse antisera to dOA GYMP-TT. (Panel A adapted from reference 32 with permission of the publisher.)
It is noteworthy that all the GCMP human data lie on the same line, regardless of age, OAc, or conjugation status of the vaccine antigen. The infant SBA titer (GM, 1,998; CI, 1,663 to 2,400) and IgG in μg/ml (GM, 36.8; CI, 29.8 to 45.4) scatter plot overlapped the adult SBA titer (GM, 1,730; CI, 1,128 to 2,652) and IgG in μg/ml (GM, 27.0; CI, 15.8 to 46.2) scatter plot for the dOA conjugate; however, the OA GCMP vaccine showed a significantly reduced SBA titer (GM, 281; CI, 162 to 486) and IgG levels in μg/ml (GM, 9.32; CI, 5.33 to 16.28) compared with the dOA GCMP conjugate vaccine in adults (P < 0.0001 for SBA and P = 0.0067 for IgG). It should also be noted that ELISA IgM analyzed from the same group C human sera, using the same dOA GCMP-HSA coat antigens, showed little or no correlation with SBA (data not shown), yielding r values that ranged from 0.10 to 0.33.
DISCUSSION
We had previously concluded, from the H-NMR spectra of purified GCMP, that both the C-7 OA and dOA forms had similar conformations around their glycosidic (C-2 to C-9) bonds, which were significantly different from that of the C-8 OA analogue that is likely present on the surface of the organism (32). We have now concluded, from the H-NMR data for GYMP from other investigators (25), that the C-9 OA repeating unit and its dOA counterpart have similar conformations around their glycosidic linkages (i.e., sialic acid and glucose). In addition, the C-7 OA analogue of GYMP, as likely present on the surface of the organism, seems to display significant conformational differences around its sialic acid linkages but not around the glucose bond, which does not seem to be affected by the OAc status of the sialic acid. We further propose that the differences in conformation around the sialic acid bond between the C-7 and C-9 OA forms could be explained by the fact that the O-acetyl located at C-7, in addition to the linkage at C-4, influences the rotation of the sialic acid glycosidic bonds, whereas the O-acetyl at C-9, being away from these two centers, has no influence on the rotation (torsional angle), which therefore allows the conformational identity between C-9 OA and dOA forms of GYMP.
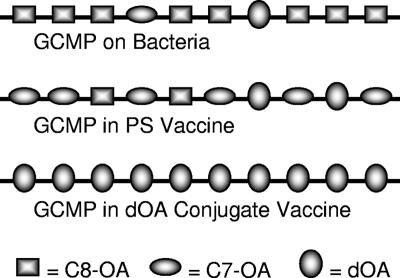
The dOA GYMP-TT conjugate was consistently more immunogenic (not statistically significant by this design) in mice than its OA counterpart, for both ELISA IgG and SBA, in a manner similar to what was previously observed for GCMP in mice (32) and in agreement with earlier human GCMP data (16, 37, 47). Although the ELISA result might be interpreted as an assay-based enhancement toward the dOA epitope, which used only the dOA material to coat the plates, the measurement of SBA with an OA strain eliminates any dOA assay bias. As illustrated in Fig. 4, the OA strains would still have some dOA targets to allow effective killing by antibodies raised against dOA vaccines. Likewise, OA vaccines would have some dOA epitopes to elicit antibodies to dOA targets, but they would not be as efficient in doing so. While this might appear to be a compelling argument for the significance of dOA epitopes, conclusive evidence would be best provided by competitive inhibition bactericidal assays.
FIG. 4.
Schematic representation of OAc patterns on GCMP from different sources.
The 10- to 1,000-fold increase in inhibition of SBA against the OA strain for various GCMP vaccine-induced antisera, using dOA GCMP as the inhibitor, appears to grossly approximate a mathematical relationship derived from the ratio of dOA to OA material. For example, there should be greater than 10-fold fewer dOA groups on the OA material, as crudely approximated in Fig. 4; therefore, greater than 10-fold more OA material is required to achieve the same level of inhibition as with the dOA material. The conformational differences observed between C-7 OA and C-8 OA PSs may also help to explain the reason for the differences in their bactericidal inhibition, with a greater difference observed in human antisera, i.e., since the C-7 OA conformation resembles that of the dOA PS (32), the C-7 OA showed greater inhibition than the C-8 OA PS. Nonetheless, the dOA material was the best inhibitor by at least an order of magnitude, even if the antibody-eliciting material was OA and the strain used in the bactericidal assay was OA, thereby confirming the dOA region or conformation as the target of protective antibodies. Since the slopes of the inhibition lines were very similar, it would appear that the inhibition differences reflect a difference in the density of the protective epitope (14). The infant sera, however, might indicate that there is an additional avidity factor in favor of the dOA inhibitor, since the OA inhibitor slopes were generally not as steep.
For group Y meningococci, inhibition differences between the dOA and OA material were not as dramatic as those observed for group C, but there was nonetheless a difference of about 1 log. The dOA GYMP competitively inhibited the SBA of both dOA and OA GYMP-TT conjugate vaccine-induced mouse antibodies directed against the OA strain at concentrations that were 6- to 13-fold lower than that required for the OA GYMP. The smaller differences might be partly due to the difficulties of obtaining H-NMR analyses that are precisely timed with the use of the material in the bactericidal assay, particularly considering the structural differences between GCMP and GYMP, i.e., it is quite possible that the O-acetyl groups for GYMP had migrated sooner than anticipated to the C-9 position, which would be conformationally more related to the dOA form, thereby accounting for the smaller inhibition differences than what was observed for GCMP.
The group C SBA correlated strongly with dOA-GCMP-specific IgG as measured by ELISA using the dOA GCMP-HSA conjugate as the coat antigen, regardless of the nature of the vaccine (i.e., OAc and conjugation status) or age group of the individuals (i.e., adults versus infants). Even though only OA bacteria were used for the bactericidal assay, the SBA was strongly correlated to dOA-GCMP-specific IgG in the same way for all sera, providing further evidence for the specificity of the protective epitope. As with group C, the group Y SBA also correlated strongly with dOA-GYMP-specific IgG as measured by ELISA using the dOA GYMP-HSA conjugate as the coat antigen.
The consistent serologic scatter plots suggest that an IgG correlate to the SBA “gold standard” serologic correlate of protection (1, 7) would be more reliable if one were to use dOA material as the ELISA coat antigen. When the O-acetyl groups were completely eliminated from the coat antigen, qualitative differences reported by others (46, 53) were not observed. In addition, antibody avidity was no longer an issue, contrary to what others have reported using OA material (18), i.e., all points from all sera fell on the same line with the same linear correlation. The only exception ever observed was for infant sera after a single injection of dOA GCMP-TT at 2 months of age, where there was a small shift off the line, indicating a possible difference in avidity (data not shown). By the second injection, however, given at 3 months, the response appeared identical to adults.
Since there are always some dOA epitopes on the OA bacteria, as well as the extracted OA PS (Fig. 4) (4, 25), the presentation of OA PS to the immune system during vaccination should still allow a protective response to the minimally available dOA protective epitope. As demonstrated in the United Kingdom, efficacious vaccine formulations have been accomplished by different manufacturers (2, 35, 39), regardless of the presence of OAc as a chemical mechanism of disguise.
The evidence that the dOA epitope is the primary target for bactericidal antibodies has provided the likely explanation for why the dOA GCMP conjugate vaccine has been shown to have “superior immunogenicity” in toddlers (41), as well as the capacity to reduce the number of doses for a primary series in infants (8, 45). It would appear unlikely that the difference in carrier proteins contributed significantly to the difference in PS-specific immunogenicity, since the IgG was so strongly correlated to the SBA, which was completely and preferentially inhibited by the dOA PS.
The presence of the O-acetyl group in capsular PSs has been widely observed and characterized from other pathogens, including meningococcal groups A (22), H (33), I (30), K (31, 51), W135 (22), and 29E (5), Escherichia coli K1 (36), Staphylococcus aureus serotypes 5 and 8 (13), Salmonella enterica serovar Typhi Vi (49), pneumococci type 9V (29), and group B streptococcal serotypes Ia, Ib, II, III, V, and VI (26). The role of acetylation, however, is still being debated. For example, the immunodominance of the O-acetyl epitope observed for meningococcal A (3), E. coli K1 (36), and S. enterica serovar Typhi Vi (49) has been interpreted by some as evidence for a protective target (3). These studies, however, did not directly demonstrate the specificity of bactericidal or functionally protective antibodies; they only provided immunogenicity data and ELISA inhibition results, which are greatly affected by the chemistry of the processed antigens. On the other hand, the pneumococcal study showed that the O-acetyl group was not required to elicit opsonic antibodies (29), and the staphylococcal study showed that the dOA PS-specific antibodies were opsonophagocytic for OA strains (13).
Preliminary studies in mice were initiated for OAc on group W135, and no significant difference was observed for SBA inhibition with OA versus dOA material (data not shown); however, OAc also did not appear to serve any immunologic purpose. This might be expected since most of the group W135 strains are dOA (27). A recent study by Giardina et al. also showed little or no difference in SBA for human sera tested against OA+ versus OA- strains (15); these sera were obtained from individuals immunized with a tetravalent PS vaccine containing groups A, C, Y, and W135 PSs. The SBA for one serum, however, could only be inhibited by the OA- PS and not the OA+ PS, even while using the OA+ target strain (15), thus supporting the dOA epitope as the protective target for group W135.
In contrast to pneumococci, which globally produce at least 90 different capsular PSs (20), there are only 13 meningococcal capsular PS-based serogroups (44), with only 5 causing nearly all disease worldwide (24) and only 2 or 3 dominating in a given geographical location (24, 44). Under these circumstances, serotype/serogroup replacement, as observed in response to pneumococcal conjugate vaccines, with respect to invasive disease (11) and carriage (34) may not have evolved for meningococci to circumvent the host defense, given OAc as an alternative mechanism. In fact, meningococcal serogroup replacement has never been clearly documented for PS-based vaccines. For example, the broad 1999-2000 introduction of meningococcal C conjugate vaccines in the United Kingdom was an acknowledged success (2, 35, 38) and has not resulted in replacement with a B serogroup (2, 12, 35, 50), even though there is an identified genetic mechanism for capsule switching (48). In addition, no serogroup replacement was observed in the U.S. military after 34 years of PS vaccine use (9).
OAc may have evolved to also allow for a less contentious coexistence, as would be necessary for these obligate commensal bacteria that only occasionally cause disease (19). It is therefore interesting that, unlike pneumococcal capsular serotypes, OAc has been identified for the majority of meningococcal capsular serogroups. The O-acetyl moiety appears to be used for masking the protective dOA epitope of the capsular polysaccharide, either by steric hindrance or conformational change. Alternatively, or additionally, the O-acetyl group might provide an immunodominant epitope to act as a decoy, distracting the immune system from the more appropriate (and possibly immunorecessive) target and resulting in less functionally active antibodies to help enable a carriage state in the host.
In conclusion, the competitive bactericidal inhibition data, using NMR-defined polysaccharide inhibitors, provided strong evidence that the protective epitopes for both GCMP and GYMP are contained on the bacteria in the dOA form. We speculate that the role of the O-acetyl group for the PS on the organism is to form an epitope of misdirected immunogenicity, masking the susceptible target and thus escaping immune surveillance. In addition to enabling greater chemical consistency through the elimination of an intrinsic isomeric microheterogeneity, the dOA form of the vaccine PSs may provide better protection against meningococcal disease than the OA form.
Acknowledgments
We thank Diane Hebblewaite, Katherine Nauman, and June Saunders for technical assistance on serologic assays.
We also thank Baxter Healthcare Corporation for the early support of this work after acquisition of North American Vaccine, Inc., where the studies were originally conceived.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Andrews, N., R. Borrow, and E. Miller. 2003. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin. Diagn. Lab. Immunol. 10:780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Berry, D. S., F. Lynn, C. H. Lee, C. E. Frasch, and M. C. Bash. 2002. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect. Immun. 70:3707-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharjee, A. K., H. J. Jennings, C. P. Kenny, A. Martin, and I. C. Smith. 1975. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J. Biol. Chem. 250:1926-1932. [PubMed] [Google Scholar]
- 5.Bhattacharjee, A. K., H. J. Jennings, and C. P. Kenny. 1978. Structural elucidation of the 3-deoxy-D-manno-octulosonic acid containing meningococcal 29-e capsular polysaccharide antigen using carbon-13 nuclear magnetic resonance. Biochemistry 17:645-651. [DOI] [PubMed] [Google Scholar]
- 6.Bilukha, O. O., and N. Rosenstein. 2005. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb. Mortal. Wkly. Rep. Recomm. Rep. 54(RR07):1-21. [PubMed] [Google Scholar]
- 7.Borrow, R., N. Andrews, D. Goldblatt, and E. Miller. 2001. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect. Immun. 69:1568-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, R., D. Goldblatt, A. Finn, J. Southern, L. Ashton, N. Andrews, G. Lal, C. Riley, R. Rahim, K. Cartwright, G. Allan, and E. Miller. 2003. Immunogenicity of, and immunologic memory to, a reduced primary schedule of meningococcal C-tetanus toxoid conjugate vaccine in infants in the United kingdom. Infect. Immun. 71:5549-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brundage, J. F., M. A. Ryan, B. H. Feighner, and F. J. Erdtmann. 2002. Meningococcal disease among United States military service members in relation to routine uses of vaccines with different serogroup-specific components, 1964-1998. Clin. Infect. Dis. 35:1376-1381. [DOI] [PubMed] [Google Scholar]
- 10.Burrage, M., A. Robinson, R. Borrow, N. Andrews, J. Southern, J. Findlow, S. Martin, C. Thornton, D. Goldblatt, M. Corbel, D. Sesardic, K. Cartwight, P. Richmond, and E. Miller. 2002. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect. Immun. 70:4946-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2005. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease-United States, 1998-2003. Morb. Mortal. Wkly. Rep. 54:893-897. [PubMed] [Google Scholar]
- 12.Diggle, M. A., and S. C. Clarke. 2005. Increased genetic diversity of Neisseria meningitidis isolates after the introduction of meningococcal serogroup C polysaccharide conjugate vaccines. J. Clin. Microbiol. 43:4649-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fattom, A. I., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusco, P. C., F. Michon, J. Y. Tai, and M. S. Blake. 1997. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and nonhuman primates. J. Infect. Dis. 175:364-372. [DOI] [PubMed] [Google Scholar]
- 15.Giardina, P. C., E. Longworth, R. E. Evans-Johnson, M. L. Bessette, H. Zhang, R. Borrow, D. Madore, and P. Fernsten. 2005. Analysis of human serum immunoglobulin G against O-acetyl-positive and O-acetyl-negative serogroup W135 meningococcal capsular polysaccharide. Clin. Diagn. Lab. Immunol. 12:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glode, M. P., E. B. Lewin, A. Sutton, C. T. Le, E. C. Gotschlich, and J. B. Robbins. 1979. Comparative immunogenicity of vaccines prepared from capsular polysaccharides of group C Neisseria meningitidis O-acetyl-positive and O-acetyl-negative variants and Escherichia coli K92 in adult volunteers. J. Infect. Dis. 139:52-59. [DOI] [PubMed] [Google Scholar]
- 17.Goldschneider, I., E. C. Gotschlich, and M. S. Artenstein. 1969. Human immunity to the meningococcus. I. The role of humoral antibodies. J. Exp. Med. 129:1307-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison, L. H. 2006. Prospects for vaccine prevention of meningococcal infection. Clin. Microbiol. Rev. 19:142-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holder, P. K., S. E. Maslanka, L. B. Pais, J. Dykes, B. D. Plikaytis, and G. M. Carlone. 1995. Assignment of Neisseria meningitidis serogroup A and C class-specific anticapsular antibody concentrations to the new standard reference serum CDC1992. Clin. Diagn. Lab. Immunol. 2:132-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jennings, H. J., A. K. Bhattacharjee, D. R. Bundle, C. P. Kenny, A. Martin, and I. C. Smith. 1977. Structures of the capsular polysaccharides of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J. Infect. Dis. 136(Suppl.):S78-S83. [DOI] [PubMed] [Google Scholar]
- 23.Jennings, H. J., and C. Lugowski. 1981. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J. Immunol. 127:1011-1018. [PubMed] [Google Scholar]
- 24.Jódar, L., I. M. Feavers, D. Salisbury, and D. M. Granoff. 2002. Development of vaccines against meningococcal disease. Lancet 359:1499-1508. [DOI] [PubMed] [Google Scholar]
- 25.Lemercinier, X., and C. Jones. 1996. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides from Neisseria meningitidis used in vaccine production. Carbohydr. Res. 296:83-96. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, A. L., V. Nizet, and A. Varki. 2004. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc. Natl. Acad. Sci. USA 101:11123-11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longworth, E., P. Fernsten, T. L. Mininni, U. Vogel, H. Claus, S. Gray, E. Kaczmarski, and R. Borrow. 2002. O-acetylation status of the capsular polysaccharides of serogroup Y and W135 meningococci isolated in the UK. FEMS Immunol. Med. Microbiol. 32:119-123. [DOI] [PubMed] [Google Scholar]
- 28.Maiden, M. C., J. M. Stuart, and The UK Meningococcal Carriage Group. 2002. Carriage of serogroup C meningococci 1 year after meningococcal C conjugate polysaccharide vaccination. Lancet 359:1829-1831. [DOI] [PubMed] [Google Scholar]
- 29.McNeely, T. B., J. M. Staub, C. M. Rusk, M. J. Blum, and J. J. Donnelly. 1998. Antibody responses to capsular polysaccharide backbone and O-acetate side groups of Streptococcus pneumoniae type 9V in humans and rhesus macaques. Infect. Immun. 66:3705-3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michon, F., J. R. Brisson, R. Roy, F. E. Ashton, and H. J. Jennings. 1985. Structural determination of the capsular polysaccharide of Neisseria meningitidis group I: a two-dimensional NMR analysis. Biochemistry 24:5592-5598. [DOI] [PubMed] [Google Scholar]
- 31.Michon, F., J. R. Brisson, R. Roy, F. E. Ashton, and H. J. Jennings. 1985. Structural determination of the capsular polysaccharide of Neisseria meningitidis group K: a 2D-NMR analysis. Can. J. Chem. 63:2781-2786. [DOI] [PubMed] [Google Scholar]
- 32.Michon, F., C. H. Huang, E. K. Farley, L. Hronowski, J. Di, and P. C. Fusco. 2000. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev. Biol. (Basel) 103:151-160. [PubMed] [Google Scholar]
- 33.Michon, F., R. Roy, H. J. Jennings, and F. E. Ashton. 1984. Structural elucidation of the capsular polysaccharide of Neisseria meningitidis group H. Can. J. Chem. 62:1519-1524. [Google Scholar]
- 34.Millar, E. V., K. L. O'Brien, J. P. Watt, M. A. Bronsdon, J. Dallas, C. G. Whitney, R. Reid, and M. Santosham. 2006. Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin. Infect. Dis. 43:8-15. [DOI] [PubMed] [Google Scholar]
- 35.Miller, E., D. Salisbury, and M. Ramsay. 2001. Planning, registration, and implementation of an immunisation campaign against meningococcal serogroup C disease in the UK: a success story. Vaccine 20(Suppl. 1):S58-S67. [DOI] [PubMed] [Google Scholar]
- 36.Orskov, F., I. Orskov, A. Sutton, R. Schneerson, W. Lin, W. Egan, G. E. Hoff, and J. B. Robbins. 1979. Form variation in Escherichia coli K1: determined by O-acetylation of the capsular polysaccharide. J. Exp. Med. 149:669-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peltola, H., A. Safary, H. Kayhty, V. Karanko, and F. E. Andre. 1985. Evaluation of two tetravalent (ACYW135) meningococcal vaccines in infants and small children: a clinical study comparing immunogenicity of O-acetyl-negative and O-acetyl-positive group C polysaccharides. Pediatrics 76:91-96. [PubMed] [Google Scholar]
- 37a.Pollabauer, E. M., R. Petermann, and H. J. Ehrlich. 2005. Group C Meningococcal polysaccharide-tetanus toxoid conjugate vaccine: a meta-analysis of immunogenicity, safety and posology. Hum. Vaccines 1:131-139. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay, M. E., N. Andrews, E. B. Kaczmarski, and E. Miller. 2001. Efficacy of meningococcal serogroup C conjugate vaccine in teenagers and toddlers in England. Lancet 357:195-196. [DOI] [PubMed] [Google Scholar]
- 39.Ramsay, M. E., N. J. Andrews, C. L. Trotter, E. B. Kaczmarski, and E. Miller. 2003. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ 326:365-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richmond, P., R. Borrow, J. Findlow, S. Martin, C. Thornton, K. Cartwright, and E. Miller. 2001. Evaluation of de-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and de-O-acetylated serogroup C strains. Infect. Immun. 69:2378-2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richmond, P., R. Borrow, D. Goldblatt, J. Findlow, S. Martin, R. Morris, K. Cartwright, and E. Miller. 2001. Ability of 3 different meningococcal C conjugate vaccines to induce immunologic memory after a single dose in UK toddlers. J. Infect. Dis. 183:160-163. [DOI] [PubMed] [Google Scholar]
- 42.Richmond, P., D. Goldblatt, P. C. Fusco, J. D. Fusco, I. Heron, S. Clark, R. Borrow, and F. Michon. 1999. Safety and immunogenicity of a new Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine in healthy adults. Vaccine 18:641-646. [DOI] [PubMed] [Google Scholar]
- 43.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, L. Lefkowitz, M. L. Cartter, R. Danila, P. Cieslak, K. A. Shutt, T. Popovic, A. Schuchat, L. H. Harrison, and A. L. Reingold. 1999. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J. Infect. Dis. 180:1894-1901. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 45.Southern, J., A. Crowley-Luke, R. Borrow, N. Andrews, and E. Miller. 2006. Immunogenicity of one, two or three doses of a meningococcal C conjugate vaccine conjugated to tetanus toxoid, given as a three-dose primary vaccination course in UK infants at 2, 3 and 4 months of age with acellular pertussis-containing DTP/Hib vaccine. Vaccine 24:215-219. [DOI] [PubMed] [Google Scholar]
- 46.Southern, J., S. Deane, L. Ashton, R. Borrow, D. Goldblatt, N. Andrews, P. Balmer, R. Morris, J. S. Kroll, and E. Miller. 2004. Effects of prior polysaccharide vaccination on magnitude, duration, and quality of immune responses to and safety profile of a meningococcal serogroup C tetanus toxoid conjugate vaccination in adults. Clin. Diagn. Lab. Immunol. 11:1100-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinhoff, M. C., E. B. Lewin, E. C. Gotschlich, and J. B. Robbins. 1981. Group C Neisseria meningitidis variant polysaccharide vaccines in children. Infect. Immun. 34:144-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swartley, J. S., A. A. Marfin, S. Edupuganti, L. J. Liu, P. Cieslak, B. Perkins, J. D. Wenger, and D. S. Stephens. 1997. Capsule switching of Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 94:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szu, S. C., X. R. Li, A. L. Stone, and J. B. Robbins. 1991. Relation between structure and immunologic properties of the Vi capsular polysaccharide. Infect. Immun. 59:4555-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trotter, C. L., M. E. Ramsay, S. Gray, A. Fox, and E. Kaczmarski. 2006. No evidence for capsule replacement following mass immunization with meningococcal serogroup C conjugate vaccines in England and Wales. Lancet Infect. Dis. 6:616-617. [DOI] [PubMed] [Google Scholar]
- 51.Van der Kaaden, A., G. J. Gerwig, J. P. Kamerling, J. F. Vliegenthart, and R. H. Tiesjema. 1985. Structure of the capsular antigen of Neisseria meningitidis serogroup K. Eur. J. Biochem. 152:663-668. [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization. 2001. Epidemics of meningococcal disease. African meningitis belt, 2001. Wkly. Epidemiol. Rec. 76:282-288. [PubMed] [Google Scholar]
- 53.World Health Organization. 2004. Recommendations for the production and control of meningococcal group C conjugate vaccines. In WHO Expert Committee on Biological Standardization, 2001, Fifty-second report. Technical report series, no. 924, 2004, annex 2. World Health Organization, Geneva, Switzerland. [PubMed]
- 54.World Health Organization. 2004. Recommendations for the production and control of meningococcal group C conjugate vaccines. In WHO Expert Committee on Biological Standardization, 2003, Fifty-third report. Technical report series, no. 926, 2004, annex 3, addendum 2003, appendix 1. World Health Organization, Geneva, Switzerland.