Abstract
The residual serological reactivity observed in patients cured of visceral leishmaniasis (VL) represents the major factor underlying the low efficiency of most anti-Leishmania serological approaches to assess posttherapeutic cure in VL. Herein, we have described a detuned flow cytometry-based methodology to detect anti-live (FC-ALPA-immunoglobulin G [IgG]) and anti-fixed (FC-AFPA-IgG) L. chagasi promastigote IgG, along the titration curve (1:2,000 to 1:128,000), as a tool to assess late (12 months after treatment [12 mAT]) and early (2 and 6 mAT) posttherapeutic cure of pediatric American visceral leishmaniasis. Reactivities were reported as the percentage of positive fluorescent parasite (PPFP), using a PPFP of 50% as a cutoff to segregate positive and negative results. Our data demonstrated that both FC-ALPA-IgG at 1:4,000 and FC-ALPA-IgG at 1:32,000 are useful for late cure assessment in VL, with 100% specificity and outstanding likelihood ratio indices. Cure assessment at 6 mAT also showed promising performance indices, identifying 81% and 71.4% of the treated patients with negative results. However, new interpretation parameters were necessary to monitor cure at 2 mAT. We then introduced the differential PPFP (ΔPPFP) of 25% as a new cutoff for early cure assessment at specific serum dilutions to analyze IgG reactivity by FC-ALPA-IgG and FC-AFPA-IgG. Our data demonstrated that at 2 mAT, ΔPPFP was >25% in 60% and 57.1% of treated patients, whereas at 6 mAT, a ΔPPFP of >25% was observed in 100% and 95.2% of samples assayed by FC-ALPA-IgG and FC-AFPA-IgG, respectively. Together, our findings showed the potential of both FC-ALPA-IgG and FC-AFPA-IgG regarding their applicability to detect differential serological reactivity and further contribution to posttherapeutic cure assessment in VL.
Visceral leishmaniasis (VL) is a systemic infection caused by an intracellular protozoan belonging to the Leishmania donovani complex: Leishmania (Leishmania) donovani, L. (L.) infantum, and L. (L.) chagasi (7). It is endemic in 62 countries, with a total of 200 million people at risk and an estimated 500,000 new cases each year worldwide (3). American visceral leishmaniasis, caused by L. chagasi, is a major health problem in many parts of Brazil. The disease is clinically characterized by fever, gradual weight loss, splenomegaly, hypergammaglobulinemia, and pancytopenia and is usually fatal without specific chemotherapy (13).
Following chemotherapy, the criterion adopted for effective VL treatment is based on clinical improvement besides negative results in parasitological methods (10). Although positive parasitological tests indicate the persistence of parasites and represent a definitive marker of ongoing infection and treatment failure, these invasive methods have low sensitivity even before therapeutic intervention (18, 19). Thus, one of the major challenges has been the establishment of laboratory tools to assess treatment effectiveness, considering that conventional approaches usually remain positive after chemotherapy and therefore fail when used as a cure criterion.
Although the high levels of Leishmania-specific antibodies have been successfully used for specific VL diagnosis, the residual serological reactivity observed in patients cured of VL represents the major factor underlying the low efficiency of most anti-Leishmania serological approaches to assess posttherapeutic cure in VL. Recently, several detuned (less-sensitive) methodologies have been proposed to resolve major restrictions regarding the use of conventional serological approaches. In this context, we have previously developed a flow cytometry-based methodology to detect anti-live trypomastigote antibodies as a reliable serological approach to monitor posttherapeutical cure in human Chagas' disease (9). The detection of anti-live trypomastigote antibodies by flow cytometry has been comparable to those by complement-mediated lysis, showing it to be a new alternative method to identify antimembrane antibodies. We have demonstrated that although conventional serology (indirect immunofluorescence and enzyme-linked immunosorbent assay) remained positive after treatment, the antimembrane-specific antibodies detected by flow cytometry were present only during active disease and not detected after successful chemotherapy. More recently, Rocha et al. (15, 16), using a similar flow cytometry-based methodology, also reported the clinical value of anti-live L. braziliensis antibodies for the detection of active cutaneous leishmaniasis.
Herein, we have assayed the performance of detuned anti-live and anti-fixed L. chagasi IgG detected by flow cytometry as a tool to monitor postchemotherapy cure of pediatric American visceral leishmaniasis. Our findings showed the potential of both FC-ALPA-IgG and FC-AFPA-IgG regarding their applicability to detect differential serological reactivity and further contribute to posttherapeutic cure assessment in VL.
MATERIALS AND METHODS
Patients.
As part of major studies focusing on the epidemiology and chemotherapy in VL, sera were obtained from 21 patients with amastigote-positive bone marrow aspirates and stored at −20°C, aiming to perform a retrospective serological investigation to provide an alternative serological approach to assess posttherapeutical cure in VL. The patients included in this study ranged from 6 months to 10 years old. Patients were enrolled at University Hospital in Montes Claros, Minas Gerais, Brazil. For each patient, a medical history was obtained and a complete physical examination performed. Treatment of patients was carried out with 1.0 mg/kg of body weight/day of amphotericin B during 14 days, and no parasites were detected in bone marrow aspirate collected after the end of treatment. They were followed for 12 months and considered cured during this period. For posttreatment evaluations, the blood samples were drawn before and 2, 6, and 12 months following the beginning of treatment. The serum samples were inactivated by heating for 30 min at 56°C and kept at −20°C until use. The inactivated sera were diluted in 0.15 M phosphate-buffered saline (PBS), pH 7.2, containing 10% heat-inactivated fetal bovine serum (PBS-10% FBS) and used to evaluate the presence of anti-L.chagasi antibodies by flow cytometry.
Approvals for testing were obtained through the Ethical Committee of Universidade Estadual de Montes Claros. Informed consent was obtained from parents or legal guardians of minors.
Parasites.
L. chagasi promastigotes (MHOM/BR/74/PP75) were cultivated at 25°C in liver infusion tryptose. After serial passages in vitro, the parasites were harvested at the stationary growth phase and centrifuged at low speed (100 × g for 10 min at 25°C) to remove cell debris and erythrocytes. Prior to recovering the parasite suspension, the supernatant was left to rest for 10 min at room temperature. The supernatant containing most of the parasites was centrifuged at 1,000 × g for 10 min at 4°C. The pellet was washed twice in PBS supplemented with PBS-10% FBS. For the use of fixed promastigotes, the parasites were immediately resuspended in an equal volume of PBS and fluorescence-activated cell sorter (FACS) fix solution (0.33 M of paraformaldehyde, 0.048 M of sodium cacodylate, and 0.11 M of sodium chloride, pH 7.2; Sigma Chemical Corp., St. Louis, MO), washed in PBS, and stored at 4°C until use. The suspension of parasites was adjusted to 5 × 106/ml, and live or fixed promastigotes were used separately in immunofluorescence assays.
Immunofluorescence by flow cytometry.
Considering the relevance of the antigen source during measurements of IgG reactivity, two distinct antigenic preparations were assayed: live, membrane-intact promastigotes (providing the selective recognition of parasite surface antigens for the IgG reactivity [FC-ALPA-IgG]) and paraformaldehyde-fixed promastigotes of L. chagasi (providing the recognition of the complex mixture of membrane and intracellular antigens for the IgG reactivity [FC-AFPA-IgG]). Moreover, considering the applications of detuned serological approaches (assay of highly diluted samples), a broad range of serum dilutions (1:2,000 to 1:128,000) was tested for each sample.
The immunofluorescence assays were performed as described by Rocha et al. (15, 16), modified as follows. Briefly, 250,000 parasites/well were incubated at 37°C for 30 min in the presence of different dilutions of serum samples. After incubation with sera, the parasites were washed twice with 150 μl of PBS-10% FBS (at 1,000 × g for 10 min at 4°C) and reincubated at 37°C for 30 min in the dark, in the presence of fluorescein isothiocyanate (FITC)-conjugated anti-human IgG antibody (Sigma Chemical Corp., St. Louis, MO) diluted 1:1,200 in PBS-10% FBS for live parasites and 1:16,000 for fixed parasites. After a second wash procedure, the FITC-labeled parasites were fixed for 30 min with a FACS fix solution before analysis in the cytometer. Each assay included an internal control of nonspecific binding in which parasites, not exposed to human serum, were incubated with FITC-conjugated anti-human IgG. The optimum working concentrations of sera and anti-human antibody conjugates were determined with the checkerboard titration method.
FACSort data acquisition, storage, and analysis.
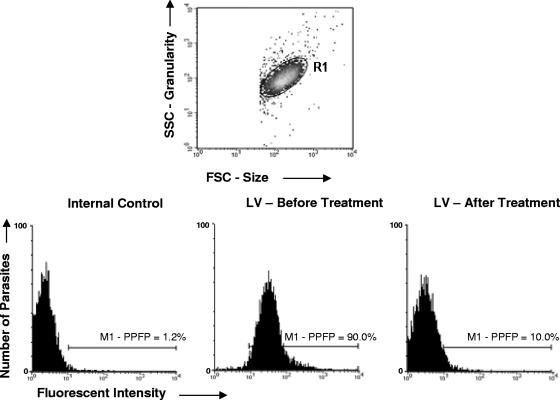
Flow cytometric measurements were performed on a Becton Dickinson FACSort interfaced to an Apple Quadra FACStation. The CellQuest software package was used in both data storage and analysis. Stained parasites were run in the cytometer, and 5,000 events per sample were acquired. Promastigotes were identified based on their specific forward (FSC) and side (SSC) light-scattering properties (FSC and SSC in log scale mode with gains of E00 and 300, respectively, and threshold set at 400 for the FSC channel). The relative FITC fluorescence intensity was quantified after adjustments of gains for FL1/FITC (FL1 in log scale mode with a gain of 630). The flow cytometer adjustments were maintained in the same way for the data acquisition of FC-ALPA-IgG and FC-AFPA-IgG. Data storage was performed in list mode format. Data analysis was performed by first selecting the promastigotes on FSC × SSC dot plots. Live and fixed parasites were found to assume similar and characteristic FSC × SSC dot plot distributions (Fig. 1, top panel). After the selection of the parasite population, the relative FITC fluorescence intensity was quantified for each test sample dilution by first establishing a maximum value of reactivity to the internal control for the second step reagent (FITC-conjugated anti-human IgG), where a marker (M1) was set up on the histogram representation of the FITC-conjugated internal control (Fig. 1, left bottom histogram). This marker was maintained to determine the reactivity in all data analyses performed on that experimental batch. The reactivity of IgG for each sample was reported as the percentage of positive fluorescent parasites (PPFP), which represent the frequency of parasite shift toward higher fluorescence intensity, across the M1 (Fig. 1, middle and right bottom histogram, respectively).
FIG. 1.
Representative flow cytometry charts used to determine the anti-L. chagasi FC-ALPA-IgG and FC-AFPA-IgG reactivities in serum samples. Promastigotes were first selected on FSC × SSC dot plots. Live and fixed parasites were found to assume similar and characteristic FSC × SSC dot plot distributions (top panel). The relative FL1/FITC fluorescence intensity was quantified by first establishing a maximum value of reactivity to the internal control (FITC-conjugated anti-human IgG), setting up a marker, M1 (left bottom histogram). This marker was maintained to determine the reactivity in all data analyses performed on the experimental batch. The reactivity of IgG for serum samples from VL patients before and after treatment was reported as the PPFP, which represents the frequency of parasite shift toward higher fluorescence intensity, across the M1 (middle and right bottom histograms, respectively).
Statistical analysis.
The receiver operating characteristic curve (ROC curve) was used to select the cutoff value to discriminate between positive and negative results of FC-ALPA-IgG and FC-AFPA-IgG (5). The test's global accuracy was also evaluated by taking the area under the ROC curve (AUC) as proposed by Swets (20).
The tests' performance levels were assessed by determining a range of indices expressed in percentages, based on the following formulas: (i) sensitivity = [true positives/(true positives + false negatives)] × 100; (ii) specificity = [true negatives/(true negatives + false positives)] × 100; (iii) positive predictive value (PPV) = [true positives/(true positives + false positives)] × 100; (iv) negative predictive value (NPV) = [true negatives/(true negatives + false negatives)] × 100.
The tests' performance levels were also investigated at distinct ranges of PPFP values by the determination of likelihood ratios (LR), as follows: positive LR = sensitivity/(1 - specificity); negative LR = (1 - sensitivity)/specificity.
RESULTS
FC-ALPA-IgG and FC-AFPA-IgG as laboratory tools for late cure assessment in VL.
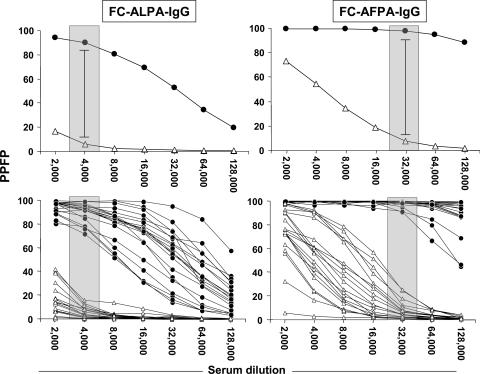
Initially, we characterized the profile of anti-L. chagasi IgG reactivity for live and fixed antigen preparations, aiming to outline the anti-Leishmania IgG titration curve and identify the serum dilutions able to better discriminate the reactivity of pooled samples collected before (BT) and 12 months after treatment (12 mAT). Data analysis demonstrated that FC-ALPA-IgG and FC-AFPA-IgG titration curves have distinct patterns of reactivity (PPFP values) for both pooled samples, depending on the antigen preparation used (Fig. 2, top panels). Higher values of PPFP were observed when fixed parasites were used as targets (Fig. 2, right top panel). However, despite the intrinsic profile, both FC-ALPA-IgG and FC-AFPA-IgG reactivities were able to discriminate higher IgG reactivities of BT samples compared to 12 mAT (Fig. 2, top panels).
FIG. 2.
Anti-L. chagasi FC-ALPA-IgG (left panels) and FC-AFPA-IgG (right panels) reactivities in serum samples from VL patients BT (•) and 12 mAT (▵). The results are expressed as mean PPFP (top graphs) and individual PPFP values (bottom graphs) from 1:2,000 to 1:128,000 serum dilutions. The patterned rectangles represent the selected serum dilutions of the higher segregation range between BT and 12 mAT (1:4,000 for FC-ALPA-IgG and 1:32,000 for FC-AFPA-IgG).
Additionally, the analysis of FC-ALPA-IgG and FC-AFPA-IgG titration curves allowed identification of the specific serum sample dilutions (1:4,000 for FC-ALPA-IgG and 1:32,000 for FC-AFPA-IgG) that better segregate the PPFP values of BT and 12 mAT (Fig. 2, top panels). Analysis of anti-Leishmania IgG reactivity using individual samples confirmed the higher segregation between BT and 12 mAT at selected serum dilutions (Fig. 2, bottom panels).
Together, these findings suggested that FC-ALPA-IgG and FC-AFPA-IgG are promising laboratory tools for late (12 mAT) cure assessment in VL.
The applicability of FC-ALPA-IgG and FC-AFPA-IgG for late cure assessment in VL is confirmed by outstanding performance indices.
We constructed a ROC curve in order to determine the value of PPFP that was indicated as an edge to segregate individual samples and confine BT and 12 mAT within regions of distinct PPFP categories. Data from the ROC curve indicated that in both cases (FC-ALPA-IgG and FC-AFPA-IgG), a PPFP of 50% was the best cutoff edge to discriminate the PPFP values from BT and 12 mAT and categorize the IgG reactivity as negative (PPFP ≤ 50%) or positive (PPFP > 50%). Moreover, the AUC indicated that FC-ALPA-IgG and FC-AFPA-IgG displayed a perfect global accuracy (AUC, 1), following the classification proposed by Swets (20). Our findings demonstrated that both antigenic preparations were able to segregate all BT samples into a section of high PPFP values, whereas all 12 mAT samples were confined within a region of PPFP of ≤50% (Fig. 3, top panels). Specifically, at 12 mAT, the interpretation criteria applied to posttherapy seronegativity by FC-ALPA-IgG included values lower than or equal to 50% reactivity at a serum dilution of 1:4,000, whereas seronegativity by FC-AFPA-IgG included values lower than or equal to 50% reactivity at a serum dilution of 1:32,000. Further analysis confirmed the usefulness of FC-ALPA-IgG and FC-AFPA-IgG through outstanding performance indices, with 100% sensitivity and specificity besides outstanding predictive values (PPV and NPV of 100%) and accuracy (100%). Likelihood ratios further support the applicability of both methodologies for late cure assessment at 12 mAT after chemotherapy of VL patients (Table 1).
FIG. 3.
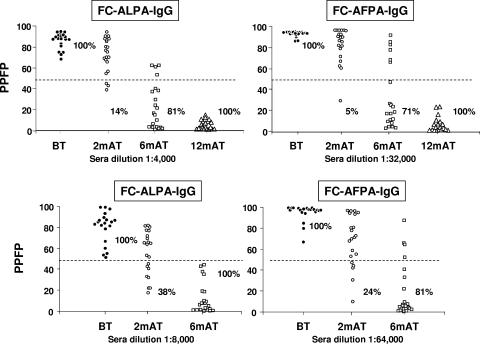
Anti-L. chagasi FC-ALPA-IgG (left panel) and FC-AFPA-IgG (right panel) reactivities of individual serum samples from VL patients BT (•), 2 mAT (○), 6 mAT (□), and 12 mAT (▵). The results are expressed as PPFP for individual samples, at serum dilutions of 1:4,000 for FC-ALPA-IgG and 1:32,000 for FC-AFPA-IgG (top panels). New interpretation parameters to monitor IgG reactivities early after chemotherapy are expressed as PPFP for individual samples, at serum dilutions of 1:8,000 for FC-ALPA-IgG and 1:64,000 for FC-AFPA-IgG (bottom panels). The dotted line represents the cutoff between negative and positive results. Specificity and sensitivity indices are provided on the graphs.
TABLE 1.
Performance indices of FC-ALPA-IgG and FC-AFPA-IgG at three different times after chemotherapy for VLa
| IgG and sample time | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | LR for PPFP of: |
|
|---|---|---|---|---|---|---|---|
| >50% | ≤50% | ||||||
| FC-ALPA-IgG (1:4,000) | |||||||
| 2 mAT | 100 (85-100) | 14 (4.7-33) | 53 (38-67) | 100 (44-100) | 86 (71-94) | 1.17 | 0.00* |
| 6 mAT | 100 (85-100) | 81 (60-92) | 84 (65-94) | 100 (81-100) | 100 (91-100) | 5.25 | 0.00* |
| 12 mAT | 100 (85-100) | 100 (85-100) | 100 (85-100) | 100 (85-100) | 100 (91-100) | >10* | 0.00* |
| FC-AFPA-IgG (1:32,000) | |||||||
| 2 mAT | 100 (85-100) | 5 (0.8-23) | 51 (36-67) | 100 (20-100) | 77 (61-86) | 1.05 | 0.00* |
| 6 mAT | 100 (85-100) | 71 (50-86) | 78 (60-90) | 100 (80-100) | 99 (90-100) | 3.50 | 0.00* |
| 12 mAT | 100 (85-100) | 100 (85-100) | 100 (85-100) | 100 (85-100) | 100 (91-100) | >10* | 0.00* |
Performance indices are expressed as percentages (with 95% confidence intervals in parentheses), except for LR values, which are expressed as probability ratios.
, LR value with high clinical significance.
Early cure assessment throughout FC-ALPA-IgG and FC-AFPA-IgG shows promising performance indices at 6 mAT.
We have further focused on the applicability of FC-ALPA-IgG and FC-AFPA-IgG as a tool for early cure assessment to be applied at 2 mAT and 6 mAT. For this purpose, we have initially used the same interpretation criteria proposed for late cure assessment at 12 mAT. Our findings demonstrated that both methodologies displayed good performance at 6 mAT, being able to identify 17/21 and 15/21 of the treated patients with negative results (PPFP ≤ 50%). Low performance indices were observed for both methodologies applied at 2 mAT, with 3 and 1 samples out of 21 displaying negative IgG reactivity (PPFP ≤ 50%) in FC-ALPA-IgG and FC-AFPA-IgG, respectively (Fig. 3, top panels). Performance indices demonstrated higher sensitivity and specificity of FC-ALPA-IgG and FC-AFPA-IgG at 6 mAT (sensitivity, 100% for both; specificity, 81% and 71%, respectively) compared to 2 mAT (sensitivity, 100% for both; specificity, 14% and 5%, respectively). Moreover, higher predictive values were observed at 6 mAT (PPV, 84% and 78%; NPV, 100% and 100%, respectively) compared to 2 mAT (PPV, 53% and 51%; NPV, 100% and 100%, respectively). Accuracy showed values of 100% and 99% at 6 mAT and 86% and 77% at 2 mAT. Likelihood ratios further support the clinical values of negative results derived from both methodologies. However, positive likelihood ratios did not support the clinical value of those methodologies for early cure assessment at 2 mAT or 6 mAT when using the same interpretation criteria used at 12 mAT (Table 1).
Establishing new interpretation parameters to monitor FC-ALPA-IgG and FC-AFPA-IgG reactivity early after chemotherapy.
The low performance of FC-ALPA-IgG and FC-AFPA-IgG for cure assessment at 2 mAT and 6 mAT led to the change of the interpretation parameters proposed for 12 mAT (Fig. 3, bottom panels). The analysis of anti-Leishmania IgG titration curves at different times after treatment, including BT, 2 mAT, and 6 mAT, allowed us to identify the serum dilutions 1:8,000 for FC-ALPA-IgG and 1:64,000 for FC-AFPA-IgG. Specifically, the new interpretation criteria postulate that seronegativity by FC-ALPA-IgG includes values lower than or equal to 50% reactivity at a serum dilution of 1:8,000, whereas that by FC-AFPA-IgG comprises values lower than or equal to 50% reactivity at a serum dilution of 1:64,000. These serum dilutions assured the seropositivity of all samples BT and the identification of a higher frequency of seronegativity at 2 mAT and 6 mAT. Further analysis of individual data sets of PPFP values using the selected serum dilutions demonstrated a gain on the FC-ALPA-IgG and FC-AFPA-IgG performance in identifying seronegativity at 2 mAT and 6 mAT (Fig. 3, bottom panels). The range of PPFP values detected by FC-ALPA-IgG demonstrated that 8/21 and 21/21 of the patients displayed negative results at 2 mAT and 6 mAT (Fig. 3, left bottom panel). These indices are higher in comparison to the 3/21 and 17/21 previously identified by the criterion proposed for late cure assessment (Fig. 3, left top panel). The range of PPFP values detected by FC-AFPA-IgG demonstrated that 5/21 and 17/21 of the patients displayed negative results at 2 mAT and 6 mAT (Fig. 3, right bottom panel). These indices are higher in comparison to the 1/21 and 15/21 previously identified by the criterion proposed for late cure assessment (Fig. 3, right top panel). Performance indices demonstrated that changes in the interpretation criteria for FC-ALPA-IgG and FC-AFPA-IgG applied to early cure assessment led to higher specificity at 6 mAT (specificity, 100% and 81%, respectively) as well as at 2 mAT (specificity, 38% and 24%, respectively). Moreover, higher positive predictive values were observed at 6 mAT (PPV, 100% and 84%, respectively) as well as at 2 mAT (PPV, 61% and 57%, respectively). Accuracy analysis demonstrated a slight increase for the FC-AFPA-IgG applied at 2 mAT. Comparative analysis of the specificity for FC-ALPA-IgG and FC-AFPA-IgG using the new interpretation parameters demonstrated a gain of cure assessment at 2 mAT and 6 mAT with values from 5.0% to 24% and 71.0% to 81.0%, respectively (Fig. 3 and Table 2). Analysis of likelihood ratio values further confirmed the gain of the new proposed interpretation criteria, mainly for FC-ALPA-IgG, highlighting the values of both LR of >50% and LR of ≤50% to define the clinical status of a given patient at 6 mAT. On the other hand, our data demonstrated that both methodologies do not result in an outstanding significant clinical value when applied at 2 mAT (Table 2).
TABLE 2.
Performance indices of FC-ALPA-IgG and FC-AFPA-IgG for early cure assessment after chemotherapy in VLa
| IgG and sample time | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | LR for PPFP of: |
|
|---|---|---|---|---|---|---|---|
| >50% | ≤50% | ||||||
| FC-ALPA-IgG (1:8,000) | |||||||
| 2 mAT | 100 (85-100) | 38 (21-60) | 61 (45-76) | 100 (67-100) | 86 (72-95) | 1.62 | 0.00* |
| 6 mAT | 100 (85-100) | 100 (85-100) | 100 (85-100) | 100 (85-100) | 100 (91-100) | >10* | 0.00* |
| FC-AFPA-IgG (1:64,000) | |||||||
| 2 mAT | 100 (85-100) | 24 (11-45) | 57 (41-71) | 100 (57-100) | 82 (67-92) | 1.31 | 0.00* |
| 6 mAT | 100 (85-100) | 81 (62-93) | 84 (65-94) | 100 (82-100) | 99 (89-99) | 5.25 | 0.00* |
Performance indices are expressed as percentages (with 95% confidence intervals in parentheses), except for LR values, which are expressed as probability ratios.
, LR value with high clinical significance.
Introducing ΔPPFP as a new strategy applied to early cure assessment in VL.
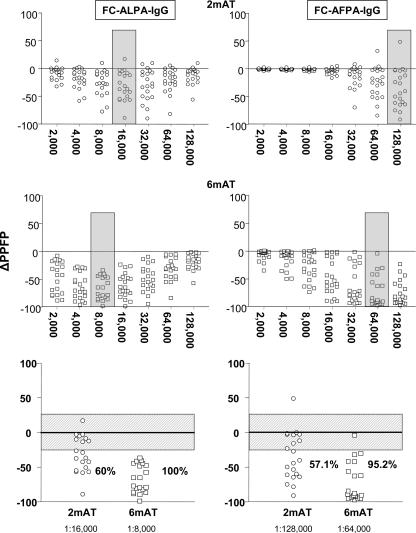
The analysis of differential reactivity detected by paired samples (delta reactivity [Δ]) is a promising strategy in clinical laboratory investigations. We have further focused on the anti-Leishmania IgG reactivity using the ΔPPFP, defined as the difference between PPFP after treatment and PPFP before treatment [ΔPPFP = PPFP (2 mAT) - PPFP (BT), or ΔPPFP = PPFP (6 mAT) - PPFP (BT)]. Initially, we evaluated the ΔPPFP values throughout the FC-ALPA-IgG and FC-AFPA-IgG titration curves, aiming to identify the serum dilutions that yielded the higher differential reactivities. Our findings confirmed the sera dilution 1:8,000 and 1:16,000 as the better choices to analyze ΔPPFP at 6 mAT for FC-ALPA-IgG and FC-AFPA-IgG, respectively (Fig. 4, middle panels). However, we have found out that at 2 mAT the serum dilutions of 1:64,000 for FC-ALPA-IgG and 1:128,000 for FC-AFPA-IgG yielded better differential reactivities (Fig. 4, top panels). Once we defined the specific serum dilutions for ΔPPFP analysis at 2 mAT and 6 mAT, we evaluated the ability of this parameter to detect differential seroreactivity early following therapeutic intervention in VL. Data analysis was performed after the establishment of a gray zone (Fig. 4, bottom panels) corresponding to the first quartile of the ΔPPFP range (cutoff edge of 25%, considering PPFP values from 0 to 100%). We found that this gray zone strategy would give further strength to data interpretation, since it would avoid interference regarding the possible intrinsic flow cytometric measurement variability. Using this interpretation criterion we observed that at 2 mAT, 12 out of 20 (60%) and 12 out of 21 (57.1%) individuals displayed a drop in PPFP reactivity detected by FC-ALPA-IgG and FC-AFPA-IgG, respectively (Fig. 4, bottom panels). Moreover, 20/20 (100%) and 18/21 (95.2%) of the treated patients showed, at 6 mAT, a decrease in anti-Leishmania IgG reactivity as assayed with FC-ALPA-IgG and FC-AFPA-IgG, respectively (Fig. 4, bottom panels). Together, our findings suggest the use of ΔPPFP as a novel strategy to be applied to early cure assessment in VL at 2 mAT and 6 mAT.
FIG. 4.
Differential anti-L. chagasi IgG reactivities of paired samples (ΔPPFP) detected by FC-ALPA-IgG (left panels) and FC-AFPA-IgG (right panels) and defined as the variation between PPFP at 2 mAT (○) and 6 mAT (□) and PPFP values BT (•). The results are expressed as ΔPPFP for each pair of samples. The rectangle identifies the serum dilution that yielded the higher differential reactivity at 2 mAT (1:16,000 for FC-ALPA-IgG and 1:128,000 for FC-AFPA-IgG) and at 6 mAT (1:8,000 for FC-ALPA-IgG and 1:64,000 for FC-AFPA-IgG). Data analysis was performed after the establishment of a gray zone (the patterned rectangle in the bottom panels) corresponding to the first quartile of the ΔPPFP range (cutoff of 25%).
DISCUSSION
In clinical practice, following chemotherapy, the criterion adopted for effective VL treatment is based on clinical improvement and a negative result by parasitological methods (10). Even though the parasitological methods, such as direct microscopic demonstration and culture isolation of Leishmania parasites from aspirates, have high specificity, their use in clinical laboratories has some limitations, mainly due to the low sensitivity of these procedures (19), which are also invasive, time-consuming, and require experienced personnel.
Moreover, despite being noninvasive, most types of serological tests that evaluate the presence of specific antibodies remain positive for long periods after cure. For this reason, there is a need for the development of new tests which could minimize the occurrence of positive serology after parasite elimination. In this context, many studies have proposed different antigen preparations and methodologies and evaluated their performance as laboratory tools to monitor postchemotherapy effectiveness in VL (1, 2, 6, 12, 14, 17).
To date, although these serological approaches distinguish the mean serological reactivities between groups of patients before and after treatment, at clinical laboratories the analysis of a given serological data set must be considered at an individual level, in order to release a conclusive report. Usually, the establishment of a cutoff for serological reactivity needs to be applied to segregate positive and negative samples. Therefore, the development of detuned serological approaches, based on high serum dilutions, with high sensitivity to VL diagnosis and outstanding specificity to assess posttherapeutic cure in VL, at an individual level, represents a relevant challenge.
Herein, we have established a new flow cytometry-based methodology and compared the performance of anti-live (FC-ALPA-IgG) and anti-fixed (FC-AFPA-IgG) L. chagasi IgG as tools for cure assessment in VL at 12 months after treatment. Outstanding performance indices suggested the applicability of both FC-ALPA-IgG and FC-AFPA-IgG to discriminate negative and positive results, as alternative tools for cure assessment in VL.
Despite our serological approach applied at 12 mAT corroborating the posttherapeutic cure assessment, the recovery of healthy clinical status at 12 mAT is by itself considered a definitive cure criterion. Indeed, in Brazil there is a general consensus that treated VL patients are only considered cured if they remain free of signs or symptoms up to 12 months after treatment (10). Therefore, the major current challenge in the field of cure assessment in VL is the establishment of alternative laboratory methods that contribute to the prediction of treatment effectiveness early after therapeutic intervention. We assayed FC-ALPA-IgG and FC-AFPA-IgG to assess cure at 2 and 6 months after treatment. Although our findings demonstrated promising results at 6 months, the performance indices at 2 months failed to demonstrate differential reactivity, independent of the use of live or fixed L. chagasi promastigotes. The low performance of FC-ALPA-IgG and FC-AFPA-IgG for cure assessment at 2 mAT led to the change of the interpretation parameters, assuming a higher serum dilution as a rational alternative. However, in spite of the increment in performance observed for samples collected at 6 mAT, analysis at 2 mAT still remains without clinical value. It has been proposed in all forms of leishmaniasis, including VL, that a sterile cure does not occur and it indicates the need for a definition of cure by the use of alternative serological interpretation. Successful therapy could possibly be monitored by the decline of anti-Leishmania antibody levels. There is a consensus in clinical laboratory immunology that although seronegativity after effective cure is a late phenomenon, this process is often absent after clinical and parasitological cures of several human pathological conditions (2, 4, 8, 11, 14, 21). Considering such a statement, the analysis of shift in seroreactivity toward lower levels instead of seronegativity has been used to assess posttherapeutic cure in a wide range of human diseases diagnosed serologically. Advances in laboratory immunology, bringing high sensitivity to detection of IgG reactivities, require new perspectives for data interpretation in these cases. In this context, the analysis of differential anti-L. chagasi IgG reactivity of paired samples (ΔPPFP) detected by FC-ALPA-IgG and FC-AFPA-IgG, defined as the variation between IgG reactivity after and before treatment, appeared to be a new strategy proposed for early cure assessment in VL.
The overall comparative analysis highlighted that FC-ALPA-IgG presents a better performance than FC-AFPA-IgG. The striking advantage of using live parasites as the antigenic source for antibody detection is that only epitopes on the outside membrane are available for IgG binding. This property of live parasites avoids the binding of IgG to intracellular components that can be responsible for cross-reactivity. However, considering the laboriousness of working with live parasites, we are currently evaluating the usefulness of IgG subclasses for anti-fixed promastigote antibodies to overcome the mentioned lower overall performance of FC-AFPA-IgG. We expect this new approach will improve the performance of FC-AFPA-IgG as it is applied for early cure assessment in VL.
In conclusion, our data demonstrated the potential of flow cytometry as a tool for noninvasive assessment of the success of visceral leishmaniasis treatment. The high performance level of the flow cytometry fluorescence is due to photomultiplier detectors coupled with the possibility of assaying higher serum dilutions than those usually tested by conventional methodologies, and these are probably the major features responsible for the higher performance level of the proposed methodology. Moreover, the adjustable capacity of flow cytometry, herein to count 5,000 parasites per assay, improved the test confidence of the proposed variable, the PPFP. Since several clinical laboratories have considered the acquisition of a flow cytometer, in the near future it will be feasible to implement this new test in the serological routine. It is important to mention that since all patients enrolled in our investigation were successfully treated and none of them relapsed, a definitive conclusion about the utility of the antibodies detected by flow cytometry to predict posttherapeutic cure or relapse still remains to be elucidated.
Acknowledgments
We are thankful to Fabíola Karla Correa Ribeiro for the English and for grammatical review of the manuscript.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq, Brazil, grant 479957/01-0.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Anam, K., F. Afrin, D. Banerjee, N. Pramanik, S. K. Guha, R. P. Goswami, S. K. Saha, and N. Ali. 1999. Differential decline in Leishmania membrane antigen-specific immunoglobulin G (IgG), IgM, IgE, and IgG subclass antibodies in Indian kala-azar patients after chemotherapy. Infect. Immun. 67:6663-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Matta, V. L., S. Hoshino-Shimizu, R. Dietze, and C. E. Corbett. 2000. Detection of specific antibody isotypes and subtypes before and after treatment of American visceral leishmaniasis. J. Clin. Lab. Anal. 14:5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desjeux, P. 1996. Leishmaniasis—public health aspects and control. Clin. Dermatol. 14:417-423. [DOI] [PubMed] [Google Scholar]
- 4.Galvão, L. M., R. M. Nunes, J. R. Cancado, Z. Brener, and A. U. Krettli. 1993. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans. R. Soc. Trop. Med. Hyg. 87:220-223. [DOI] [PubMed] [Google Scholar]
- 5.Greiner, M., D. Pfeiffer, and R. D. Smith. 2000. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prev. Vet. Med. 45:23-41. [DOI] [PubMed] [Google Scholar]
- 6.Kumar, R., K. Pai, K. Pathak, and S. Sundar. 2001. Enzyme-linked immunosorbent assay for recombinant K39 antigen in diagnosis and prognosis of Indian visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 8:1220-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lainson, R., and J. J. Shaw. 1987. Evolution, classification and geographical distribution, p. 1-120. In W. Peters and R. Killick-Kendric (ed.), The leishmaniases in biology and medicine. Academic Press, London, England.
- 8.Malafiej, E., and E. Spiewak. 2001. The significance of the level of antibodies in the evaluation of the effects of treatment of toxocariasis. Wiad. Parazytol. 47:805-810. [PubMed] [Google Scholar]
- 9.Martins-Filho, O. A., M. E. S. Pereira, J. F. Carvalho, J. R. Cançado, and Z. Brener. 1995. Flow cytometry, a new approach to detect anti-live trypomastigote antibodies and monitor the efficacy of specific treatment in human Chagas' disease. Clin. Lab. Diagn. Immunol. 2:569-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ministério da Saúde, Brasil. 2003. Manual de vigilância e controle de leishmaniose visceral, secretaria de vigilância em saúde. Ministério da Saúde, Brasília, Brazil.
- 11.Mitre, E., and T. B. Nutman. 2006. IgE memory: persistence of antigen-specific IgE responses years after treatment of human filarial infections. J. Allergy Clin. Immunol. 117:939-945. [DOI] [PubMed] [Google Scholar]
- 12.Passos, S., L. P. Carvalho, G. Orge, S. M. Jeronimo, G. Bezerra, M. Soto, C. Alonso, and E. M. Carvalho. 2005. Recombinant Leishmania antigens for serodiagnosis of visceral leishmaniaiais. Clin. Diagn. Lab. Immunol. 12:1164-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson, R. D., and S. A. de Queiroz. 1996. Clinical spectrum of leishmaniasis. Clin. Infect. Dis. 22:1-13. [DOI] [PubMed] [Google Scholar]
- 14.Ravindran, R., K. Anam, B. C. Bairagi, B. Saha, N. Pramanik, S. K. Guha, R. P. Goswami, D. Banerjee, and N. Ali. 2004. Characterization of immunoglobulin G and its subclass response to Indian kala-azar infection before and after chemotherapy. Infect. Immun. 72:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocha, R. D. R., C. M. F. Gontijo, S. M. Elói-Santos, A. T. Carvalho, R. Corrêa-Oliveira, M. J. Marques, O. Genaro, W. Mayrink, and O. A. Martins-Filho. 2002. Anti-live Leishmania (Viannia) braziliensis promastigote antibodies, detected by flow cytometry, to identify active infection in American cutaneous leishmaniasis. Rev. Soc. Bras. Med. Trop. 35:551-552. [DOI] [PubMed] [Google Scholar]
- 16.Rocha, R. D. R., C. M. F. Gontijo, S. M. Elói-Santos, A. T. Carvalho, R. Corrêa-Oliveira, T. C. Ferrari, M. J. Marques, O. Genaro, W. Mayrink, and A. O. Martins-Filho. 2006. Clinical value of anti-live Leishmania (Viannia) braziliensis immunoglobulin G subclasses, detected by flow cytometry, for diagnosing active localized cutaneous leishmaniasis. Trop. Med. Int. Health 11:156-166. [DOI] [PubMed] [Google Scholar]
- 17.Santarém, N., A. Tomás, A. Ouaissi, J. Tavares, N. Ferreira, A. Manso, L. Campino, J. M. Correia, and A. Codeiro-da-Silva. 2005. Antibodies against a Leishmania infantum peroxiredoxin as a possible marker for diagnosis of visceral leishmaniasis and for monitoring the efficacy of treatment. Immunol. Lett. 101:18-23. [DOI] [PubMed] [Google Scholar]
- 18.Sundar, S., K. Kumar, V. P. Singh, and T. M. Mahopatra. 1991. Diagnostic lag period in kala-azar: test for early diagnosis needed. J. Assoc. Physicians India 39:651. [PubMed] [Google Scholar]
- 19.Sundar, S., and M. Rai. 2002. Laboratory diagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 9:951-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swets, J. A. 1998. Measuring the accuracy of diagnostic systems. Science 240:1285-1293. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka, J., T. Ohkusa, T. Yokoyama, T. Matsuhisa, T. Kawai, H. Hashimoto, T. Tomita, K. Hori, K. Nakajima, T. Matsumoto, and H. Miwa. 2006. Host serological response to Helicobacter pylori after successful eradication: long-term follow-up in patients with cured and persistent infection. Aliment. Pharmacol. Ther. 24:239-248.17209872 [Google Scholar]