Abstract
The enzyme-linked immunospot (ELISPOT) assay is a powerful tool for measuring antigen-specific cellular immune responses. The ability to use frozen peripheral blood mononuclear cells (PBMC) facilitates testing samples in multicenter clinical trials; however, unreliable ELISPOT responses may result if samples are not handled properly. Exposure of frozen PBMC to suboptimal storage temperature (−20°C) or repeated cycling between more optimal storage temperatures (less than −130°C and −70°C) reduced the quality of frozen PBMC, as assessed by cell viability and functional ELISPOT response measures. Cell viability as assessed by trypan blue dye exclusion was reduced, and the percentage of apoptotic cells, as determined by the Guava Nexin assay, was significantly increased after these events. The functional gamma interferon ELISPOT responses to phytohemagglutinin (PHA) mitogen, a CD4 T-cell-specific antigen (varicella-zoster virus), and a CD8 T-cell-specific antigen (pool containing known cytomegalovirus, Epstein-Barr virus, and influenza virus peptides) were all significantly reduced after suboptimal storage events. However, for a given suboptimal storage event, the magnitude of the reduction varied between individuals and even among aliquots within an individual bleed, indicating the need for sample-specific acceptance criteria (AC). The percent viable or percent apoptotic cells after thaw, as well as the functional ELISPOT response to PHA, were all effective when applied with limits as AC for separating samples damaged during storage from valid control samples. Although all three AC measures could be effectively applied, the apoptosis AC limit applied was best for separating samples that could respond to antigenic stimulation from samples that could not effectively respond.
Cell-mediated immune responses can be assessed by using a variety of methods, including proliferation assays, cytotoxic-T-lymphocyte assays, tetramer staining, intracellular cytokine staining, and the cytokine enzyme-linked immunospot (ELISPOT) assay (2, 3, 9, 10, 12-14, 16, 20-22, 27, 28). The advantages of the ELISPOT assay are that it is relatively simple to perform, does not require large numbers of cells, can be performed with frozen cell samples, and is sensitive (4, 13, 27). The gamma interferon (IFN-γ) ELISPOT assay has been applied as an effective method for monitoring cellular immune responses in vaccine clinical trials (10, 12, 14, 19, 26, 30). Assay of frozen peripheral blood mononuclear cells (PBMC) allows for high-throughput testing of samples obtained in multicenter clinical trials. Use of frozen cells reduces variability in the assessment of immunologic responses by allowing for the testing of multiple bleeds from a subject within the same assay run.
The quality of a PBMC sample affects response detection in the IFN-γ ELISPOT assay. Multiple factors can influence the quality of PBMC preparations from the time of blood collection through the point of sample testing. PBMC frozen on the date of blood draw have been shown to perform as well as fresh PBMC in the ELISPOT assay (17, 21, 27). However, PBMC isolated from blood that was stored overnight prior to processing exhibited decreased ELISPOT responses compared to PBMC processed on the same day as blood collection (27). Numerous studies have focused on the impact of freezing or thawing procedures on PBMC quality for cell-based assay applications (1, 5, 8, 9, 15, 25, 32). Criteria for the evaluation of PBMC quality upon thaw have been reported in the past. Many have focused on assessing cell viability by trypan blue dye exclusion or the functional ability of cells to respond to stimulation with mitogen (4, 5, 8, 9, 15, 32). Other groups have utilized the response to control antigens as quality control measures (6, 7, 21, 23, 32), but this may restrict to evaluation of either a CD4 or CD8 T-cell-specific response and only be applicable to the subset of subjects who have a measurable response to those specific antigens.
There is a lack of data investigating storage or handling events that may impact functional antigen-specific responses detected in the IFN-γ ELISPOT assay. In order to better understand the impact of factors such as storage, handling, and shipping conditions on sample quality, multiple vials of cells from single donor bleeds were subjected to suboptimal storage and handling within a controlled setting, and the effects on both CD4 and CD8 T-cell-specific response detection in the IFN-γ ELISPOT assay were assessed. IFN-γ ELISPOT responses to UV-treated varicella-zoster virus (VZV), a known CD4 T-cell-specific antigen (27), and to a peptide pool of known CD8 T-cell epitopes for cytomegalovirus, Epstein-Barr virus, and influenza (group A) virus (7) were evaluated.
The studies reported here describe cell health measures (trypan blue viability and apoptosis) and nonspecific functional responses (ELISPOT response to phytohemagglutinin [PHA]) compared to antigen-specific ELISPOT responses obtained with samples exposed to suboptimal storage. Further, we describe the application of specific limits for these measures as acceptance criteria (AC) to identify samples “damaged” and rendered incapable of providing an accurate antigen-specific ELISPOT response for the subject. These studies show that maintaining proper temperature during storage and minimizing the exposure to changes in storage temperature are critical for optimal ELISPOT response detection. We further demonstrate that specific AC limits can be applied to separate optimal, “valid” samples from samples impacted by suboptimal storage, “invalid” samples, to improve the quality of data analyses after ELISPOT testing with frozen PBMC samples.
MATERIALS AND METHODS
Temperature measurements.
Temperatures within a shipping container containing dry ice were obtained by using a TempTale dry ice probe measuring device (Sensitech, Beverly, MA). A box containing 90 frozen vials of freezing media (90% fetal bovine serum-10% dimethyl sulfoxide) was placed into a Styrofoam shipping container with 1 in. of dry ice at the bottom. Temperature probes were placed at the bottom of the dry ice and in the middle of the sample box. The shipping container was then filled with dry ice, and temperature probes were placed at the top surface of the dry ice and in the air space above the dry ice. The shipping container was sealed and stored at room temperature over the time course of data recording. The temperature data was downloaded from the TempTale probes using software provided by the manufacturer.
Sample collection.
Blood or leukapheresis product was collected either locally (blood from consenting subjects at Merck Health Services or product provided by Biological Specialty Corp., Comar, PA) or at multiple sites in a clinical study. The clinical protocol was institutional review board approved, and all subjects consented. Blood or leukapheresis were processed on the date of collection for PBMC isolation and freezing.
PBMC preparation.
PBMC were isolated from whole blood or leukapheresis products. Briefly, diluted sample was layered onto Histopaque-1077 (Sigma, St. Louis, MO) and centrifuged at 400 × g. The buffy layer was removed, and PBMC were isolated, frozen, and thawed as previously described (27). In accordance with our validated ELISPOT assay testing format, PBMC were not cultured (incubation over period of time at 37°C) prior to the evaluation of cell health measures (trypan blue dye exclusion, apoptosis) or performing the ELISPOT assay testing.
Sample storage and temperature changes.
Frozen PBMC aliquots were stored in the vapor phase within a liquid nitrogen freezer (LN2, less than −130°C). Samples from clinical sites were shipped encased in dry ice overnight to the Merck central laboratory, where they were directly transferred into LN2 freezer storage until they were thawed for evaluation and assay testing. Aliquots of frozen PBMC were transferred from LN2 freezer storage, on dry ice, to either −70°C or −20°C freezers for storage experiments. For cycling experiments, the samples were transferred, on dry ice, between −70°C and LN2 freezers. One cycle represents the transfer from LN2 to −70°C and back to LN2. Samples indicated as exposed to multiple cycling events were stored at −70°C for periods from 24 to 72 h for each cycle before transfer back to LN2 storage. Samples described as exposed to a single storage cycle event were transferred into −70°C for a period of 5 weeks. All samples were placed back into LN2 freezer storage until ELISPOT testing was performed. Control samples were stored continuously in the LN2 freezer. Each vial was tested independently in the ELISPOT assay.
IFN-γ ELISPOT assay.
The IFN-γ ELISPOT assay was performed as previously described (27). Briefly, polyvinylidene difluoride 96-well plates (Millipore, Bedford, MA) were coated with anti-IFN-γ antibody (Endogen, Rockford, IL). A total of 5 × 105 PBMC were stimulated with antigen for 16 to 20 h. UV-treated VZV antigens, from clarified cell culture supernatants of VZV-infected MRC-5 cells, were used at a 1:80 final dilution in the assay as a known CD4 T-cell-specific antigen. The control antigen for VZV was prepared from uninfected MRC-5 cells and used at a 1:160 final dilution in the assay to normalize MRC-5 antigen content across the MRC-5 control and VZV antigen preparations tested. A pool containing 23 major histocompatibility complex class I-restricted peptides to cytomegalovirus, Epstein-Barr virus, and influenza virus (group A) prepared as previously described (7) was used at 2 μg/ml for each peptide, as a known CD8 T-cell-specific antigen (termed the EPI peptide pool in this report). Dimethyl sulfoxide was used as the negative control for the peptide pool (at same concentration present in EPI peptide pool antigen). PHA (Sigma) was used at 5 μg/ml as a positive control mitogen. After incubation with antigen, the plates were washed, and a biotinylated anti-IFN-γ second antibody (Endogen) was added. After overnight incubation at 4°C, the plates were washed, treated with streptavidin-alkaline phosphatase (Pierce, Rockford, IL) and washed again. Spots were developed with NBT/BCIP substrate (Pierce), and the plates were allowed to dry overnight. Spots were enumerated by using an ImmunoSpot image analyzer system (Cellular Technologies Limited, Cleveland, OH).
Viability.
Percent viability was determined by trypan blue dye exclusion. Cell suspension was mixed with 0.04% trypan blue dye, and live and dead cells were scored by counting them on a hemacytometer (Hycor, Garden Grove, CA).
Apoptosis assay.
Apoptosis was assessed by using the Guava Nexin kit and the Guava PCA system (Guava Technologies, Hayward, CA). The Guava Nexin assay utilizes two stains (annexin V and 7-amino actinomycin D [7-AAD]) to quantitate the percentage of apoptotic cells. Changes in cell membrane structure occur early in apoptosis. Namely, phosphatidylserine (PS) is translocated from the inner layer of the lipid bilayer to the outer layer (29). The exposure of PS on the cell surface is the basis of the Guava Nexin assay. Annexin V is a phospholipid-binding protein that binds PS on the cell surface, and 7-AAD is a cell impermeant dye that is excluded from live cells but taken up by late-stage apoptotic cells as the membrane becomes porous. Cells that stain positive for both dyes are in the later stages of apoptosis, prior to cell death. The Nexin assay was performed according to the manufacturer's protocol with the following exception. Once a uniform cell suspension was prepared, as described above, cells were not washed to remove culture media by centrifugation at 300 to 400 × g for 10 min at 2 to 8°C; instead, 8 μl of the cell suspension containing 8 × 104 PBMC was added directly to the Guava Nexin reaction. Cell samples were then stained with annexin V and 7-AAD for 20 min on ice. Nexin buffer was added to stop the staining reaction, and data were acquired on the Guava PCA system immediately. Cells were gated based on forward scatter (size), and results are reported as the percentage of gated cells that are positive for both annexin V and 7-AAD.
Statistics.
To assess the impact of suboptimal storage procedures on the VZV ELISPOT response, the PHA ELISPOT response, the EPI peptide pool ELISPOT response, cell viability as measured by trypan blue, and the apoptotic cell percentage as measured by the Nexin assay, a model, containing main effects for sample and storage condition, and the interaction term was applied to the natural log-transformed (ln) ELISPOT responses and logit transformed percentages. The model was fit using the GLM procedure in SAS. Least-squares (LS) means were obtained for each storage condition. Pairwise differences in LS means between the optimal LN2 storage condition and each of the suboptimal conditions and 95% confidence intervals (95% CI) on the differences were determined by exponentiating the ln-transformed ELISPOT differences and ln-transformed CI. Odds ratios between the LS mean logit-transformed percentages from samples stored in the optimal LN2 freezer storage condition and those stored in each of the suboptimal conditions and 95% CI on the odds ratios were determined by back-transforming the differences between the logit-transformed percentages and logit-transformed CI.
The 3σ lower bounds were determined for ELISPOT response to PHA, the percent viability by trypan blue, and the percent apoptosis by Nexin assay using 130 PBMC samples obtained in a clinical study with the PBMC isolated and frozen on the date of blood draw. Samples were stored frozen in optimal LN2 freezer storage with a single shipment in dry ice packaging to a central lab for assay testing. The lower bound for the ELISPOT response to PHA was determined by using the natural log-transformed PHA response results and the lower bound for trypan blue dye exclusion viability and percent apoptosis were determined by using the logit (pi/[1 − pi])-transformed viability and apoptosis percentages (values greater than 97% were assigned a value of 97%, and values less than 3% were assigned a value of 3%).
RESULTS
Temperatures in shipping container.
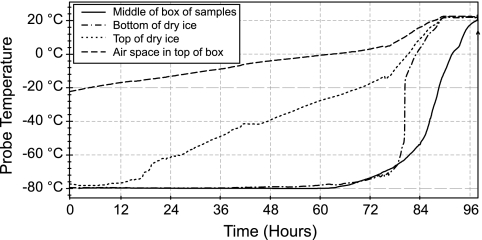
Frozen PBMC samples could be exposed to changing temperature during shipment (e.g., from clinical sites to central laboratory for assay testing). A TempTale device was used to measure the temperature within a dry ice shipping container. Four temperature probes were placed throughout a shipping container packed with dry ice and a box of frozen sample vials. As shown in Fig. 1, the temperature at the bottom of the dry ice and within the middle of the sample box remained below −70°C for up to 72 h. Importantly, the temperature measured at the top surface of the dry ice began to rise at about 12 h and reached −60°C by 24 h. The temperature measured within the air space above the dry ice level rose to −20°C within only a few hours. These data demonstrate that the packaging of frozen samples for shipping directly influences the storage environment to which they become exposed. If samples are not fully encased within the dry ice, they may become exposed to temperatures from −60°C up to −20°C or warmer over the period of sample shipment.
FIG. 1.
Temperatures within dry ice shipping container, measured with a TempTale probe reader system.
Impact of −20°C temperature exposure during storage.
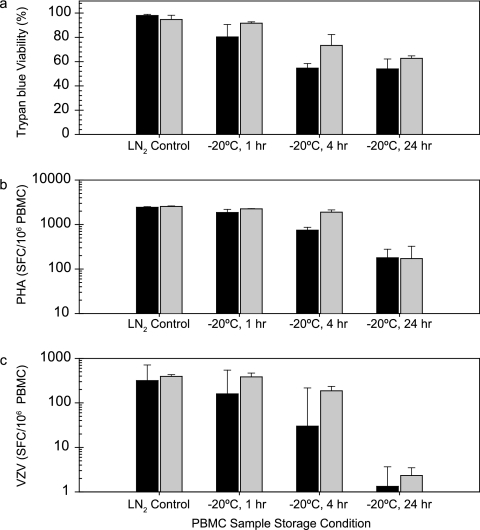
Since the temperature above the dry ice level within a shipping container can rise to −20°C, it is important to understand the potential impact on functional assessment of ELISPOT responses with samples exposed to those conditions. Frozen samples, maintained in vapor-phase LN2 freezer storage, were exposed to various lengths of storage in a −20°C freezer to simulate possible exposure during shipment. Initially, samples were transferred to −20°C storage for periods from 24 h to 3 weeks. After 24 h of storage at −20°C, IFN-γ ELISPOT responses to VZV antigen (CD4 T-cell antigen) were undetectable, and responses to PHA (mitogen) were sixfold lower than control samples stored continuously in a LN2 freezer (data not shown). Next, exposure to −20°C storage for periods from one to 24 h were evaluated, conditions that more closely reflect what may be experienced during actual periods of sample shipment. Figure 2 shows the percent viability of the cells after thaw (Fig. 2a) and the average ELISPOT response to PHA mitogen (Fig. 2b) and VZV antigen (Fig. 2c) for three frozen vials of PBMC from each of two donors. These data are representative of the observations made for PBMC from multiple donors. Control samples were stored continuously in a LN2 freezer. After 1 h of −20°C storage, the ELISPOT response to VZV antigen for donor M4866 was reduced approximately twofold from the LN2 freezer stored control, while the response for donor M5020 was unaffected. However, after 4 h of −20°C storage the ELISPOT response for donor M5020 PBMC to VZV antigen was reduced approximately twofold compared to LN2 freezer controls. After 24 h of −20°C storage the ELISPOT response to VZV antigen was essentially undetectable with PBMC from either donor. The ELISPOT responses to PHA were not affected after 1 h of −20°C storage but were reduced to very low levels after 24 h of storage. The percent viability (by trypan blue exclusion) was also reduced, but not as dramatically as the reductions observed in ELISPOT responses to VZV and PHA.
FIG. 2.
Effect of short-term −20°C temperature storage on PBMC viability and IFN-γ ELISPOT responses. Samples were transferred from LN2 freezer into −20°C freezer for the times indicated and then returned to the LN2 freezer until assay testing. Averages and standard deviations for three vials tested per condition are shown for the percent viability determined by trypan blue dye exclusion (a), PHA ELISPOT response (b), and VZV ELISPOT response (c). Bars: ▪, donor M4866; □, donor M5020.
Impact of cycling the temperature of storage.
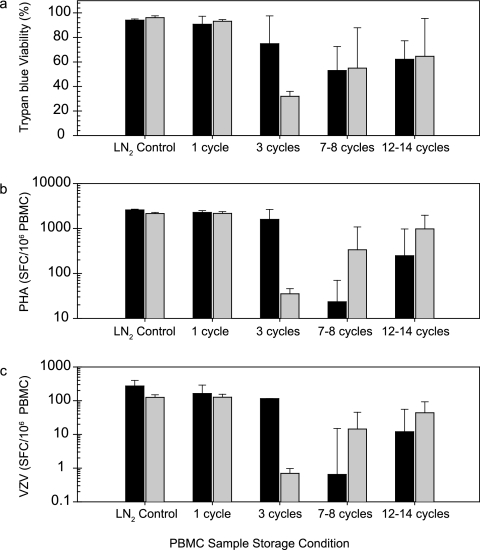
Samples can potentially be moved between LN2 freezer and dry ice storage several times over the course of frozen storage, such as transfers between clinical processing sites and central testing labs, and during handling in the laboratory prior to assay testing. The effect of cycling the storage temperature between conditions of −70°C and LN2 freezers on PBMC sample viability and IFN-γ ELISPOT responses was assessed. Samples were cycled between an LN2 freezer and a −70°C freezer from 1 to 14 temperature cycles over a period from 1 to 5 weeks. Figure 3 shows the percent viability of PBMC after thaw (Fig. 3a), along with the average ELISPOT responses to PHA mitogen (Fig. 3b) and VZV antigen (Fig. 3c), for five vials per treatment group from each of two donors. As observed with the exposure to −20°C storage, the results after cycling the storage temperature of the frozen aliquots were variable between donors. In addition, there was variability of impact from the storage temperature cycling on individual aliquots within an individual donor bleed sample, as demonstrated by the large error bars. There was a complete loss of detectable ELISPOT response to VZV antigen with all aliquots of PBMC from donor M1481 tested after three temperature cycles (Fig. 3c). However, some aliquots from this donor, though not all, were still able to provide VZV antigen-specific response after 12 to 14 storage temperature cycles. The reduction in VZV-specific (CD4) response for individual PBMC vials from the same donor was neither consistent nor predictable with the storage temperature cycling. A similar trend was observed for ELISPOT responses to PHA mitogen with temperature-cycled PBMC aliquots (Fig. 3b). The percent viability upon thaw by trypan blue exclusion (Fig. 3a) was also affected, with a trend toward overall reduction similar to that observed for ELISPOT response to VZV and PHA, but was not as dramatically reduced as the functional responses measured by ELISPOT.
FIG. 3.
Effect of cycling the temperature of storage on PBMC viability and IFN-γ ELISPOT responses. One cycle represents transfer from an LN2 freezer storage to −70°C freezer storage and back to LN2 freezer storage. After the indicated number of storage cycles, samples were stored in an LN2 freezer until assay testing. Averages and standard deviations of five vials tested per condition are shown for the percent viability by trypan blue dye exclusion (a), PHA ELISPOT response (b), and VZV ELISPOT response (c). Bars: ▪, donor M4866; □, donor M1481.
AC measures.
The data presented have demonstrated that exposure to different temperature conditions during storage or handling can affect the ELISPOT response detected. Although there is evidence of a decline in response due to the storage conditions evaluated, the effect was variable both among individuals and among aliquots within the same individual bleed. This indicates the need for sample-specific criteria to identify when a sample has been compromised during handling, storage, and/or shipment. A large number of aliquots of PBMC (100 vials) were collected and frozen from a single bleed for two individual donors and the aliquots stored in a LN2 freezer (less than −130°C). We removed one set of aliquots for each donor (40 vials) and exposed them to −20°C storage for a period of 8 h, followed by transfer back to LN2 storage. Another set of aliquots for each donor (40 vials) were subjected to three cycles of storage between LN2 (less than −130°C) and −70°C over a 1-week period. Each individual PBMC vial was then thawed and assessed for viability by trypan blue dye exclusion, determining the percent apoptosis in the Nexin assay, and evaluating the functional responses to CD4 (VZV) and CD8 (EPI peptide pool) T-cell-specific antigens and PHA mitogen in the IFN-γ ELISPOT assay.
The changes in viability, apoptosis, and ELISPOT responses after these frozen storage changes were analyzed compared to controls (20 vials) continuously stored in the LN2 freezer (less than −130°C). The analysis was performed on data for individual donor and with both donors combined. The statistical results comparing treatments were consistent (the same P value was obtained) whether analyzed by individual donor or by the donors combined. The data for the combined donor analysis are provided in Tables 1 and 2.
TABLE 1.
Effects of suboptimal storage temperature and temperature cycling on IFN-γ ELISPOT responses during storagea
| Storage condition and stimulating agent | Avg no. of SFC/106 PBMC | Fold difference | 95% CI | P |
|---|---|---|---|---|
| Controlb | ||||
| VZV antigen | 351 | |||
| EPI peptide pool | 302 | |||
| PHA mitogen | 2,492 | |||
| Suboptimal storage tempc | ||||
| VZV antigen | 7 | 49.3 | 31.4-77.4 | <0.001 |
| EPI peptide pool | 11 | 26.4 | 15.8-44.2 | <0.001 |
| PHA mitogen | 112 | 22.3 | 12.5-39.9 | <0.001 |
| Storage temp cyclingd | ||||
| VZV antigen | 3 | 108.6 | 69.2-170.3 | <0.0001 |
| EPI peptide pool | 22 | 14.0 | 8.4-23.4 | <0.0001 |
| PHA mitogen | 408 | 6.1 | 3.4-10.9 | <0.0001 |
A total of 100 frozen aliquots of PBMC from each of two donors (M3143 and M5020) were tested. Results were combined across both donor PBMC samples. The analysis for individual donors was consistent with the pooled analysis. One assay run testing aliquots for donor M3143 was lost; so, for this donor, the results for analysis were reduced to 15 aliquots for the control and 34 aliquots each for suboptimal storage temperature and temperature cycling during storage.
A total of 20 aliquots from each donor were maintained in LN2 storage.
A total of 40 aliquots from each donor were subjected to an 8-h period of storage at −20°C and then returned to LN2 storage.
A total of 40 aliquots from each donor were subjected to three cycles of storage change between LN2 to −70°C and back to LN2.
TABLE 2.
Effects of suboptimal storage temperature and temperature cycling on cell health during storagea
| Storage condition and cell health indicator | Avg no. of cells (%)b | Odds ratio | 95% CI | P |
|---|---|---|---|---|
| Controlc | ||||
| Viability | 97.5 | |||
| Apoptosis | 7.1 | |||
| Suboptimal storage tempd | ||||
| Viability | 63.2 | 23.0 | 18.5-28.7 | <0.001 |
| Apoptosis | 55.8 | 0.06 | 12.5-39.9 | <0.001 |
| Storage temp cyclinge | ||||
| Viability | 76.5 | 12.2 | 9.8-15.1 | <0.0001 |
| Apoptosis | 41.7 | 0.11 | 0.09-0.12 | <0.0001 |
A total of 100 frozen aliquots of PBMC from each of two donors (M3143 and M5020) were tested. Results were combined across both donor PBMC samples. The analysis for individual donors was consistent with the pooled analysis. One assay run testing aliquots for donor M3143 was lost; so, for this donor, the results for analysis were reduced to 15 aliquots for the control and 34 aliquots each for suboptimal storage temperature and temperature cycling during storage.
By definition, the number of detectable living cells indicates viability and the number of nonliving cells indicates apoptosis.
A total of 20 aliquots from each donor were maintained in LN2 storage.
A total of 40 aliquots from each donor were subjected to an 8-h period of storage at −20°C and then returned to LN2 storage.
A total of 40 aliquots from each donor were subjected to three cycles of storage change between LN2 to −70°C and back to LN2.
On average, the exposure of frozen PBMC samples to 8 h of −20°C storage resulted in an ∼49-fold reduction in ELISPOT response to VZV (CD4) antigen and an ∼26-fold reduction in ELISPOT response to the EPI peptide pool (CD8) antigen. Responses to nonspecific mitogen stimulation were also reduced, with an ∼22-fold reduction in ELISPOT response to PHA. All of these decreases in functional ELISPOT response were significant (P < 0.001). Exposure to −20°C during frozen storage also impacted the measures of cell health status after thaw and subsequent assay. On average, the odds of a cell being viable by trypan blue dye exclusion were ∼23-fold higher in samples continuously stored in LN2 than in samples exposed to −20°C storage for 8 h. The odds of a cell undergoing apoptosis (as measured by the Nexin assay) were ∼16-fold higher within a sample with exposure to −20°C storage temperature for 8 h than in samples continuously stored at LN2.
Exposure of frozen PBMC samples to three cycles of storage between LN2 (less than −130°C) and −70°C, resulted in an average reductions of ∼100-fold in ELISPOT response to VZV (CD4) antigen and ∼14-fold in ELISPOT response to the EPI peptide pool (CD8) antigen. Responses to nonspecific mitogen stimulation were also reduced, with a mean ∼6-fold mean reduction in ELISPOT response to PHA. All of these decreases in functional ELISPOT response were significant (P < 0.0001). Exposure to temperature cycling during frozen storage also impacted measures of cell health after thaw. On average, the odds of a cell being viable by trypan blue dye exclusion was ∼12-fold higher in samples continuously stored in LN2 than in samples cycled between LN2 storage (less than −130°C) and −70°C. The odds of a cell undergoing apoptosis (as measured by the Nexin assay) were ∼9-fold higher within a sample cycled between LN2 storage and −70°C than samples continuously stored at LN2.
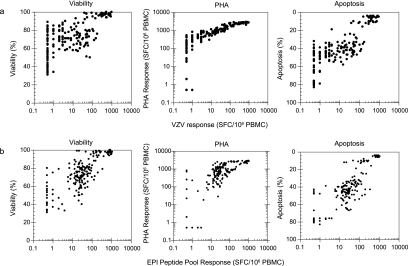
The data for all samples tested from each donor were further evaluated for a comparison of viability, ELISPOT response to PHA mitogen, and percent apoptosis with the antigen-specific ELISPOT responses obtained, irregardless of the storage exposure (Fig. 4). The measures of cell health were plotted against either the CD4 (Fig. 4a) or CD8 (Fig. 4b) antigen-specific ELISPOT responses. The plots indicate that all three measures, whether associated with the measures of cell health status (percent viability or apoptosis) or a functional response capability (ELISPOT response to PHA mitogen), positively correlated with response to both the CD4- and CD8-specific antigens. Given the positive associations observed between these measures and ELISPOT response, none of the measures could be excluded from the list of potential candidates upon which AC could be based in order to identify when a sample has been compromised during handling, storage, and/or shipment.
FIG. 4.
Plots of viability, IFN-γ ELISPOT response to PHA, and percent apoptosis with antigen-specific IFN-γ ELISPOT responses compared to antigen-specific IFN-γ ELISPOT response. (a) AC correlation with VZV antigen response (CD4-specific); (b) AC correlation with EPI peptide pool response (CD8-specific).
These three AC indicators were further evaluated using a large number (n = 130) of frozen PBMC samples collected during the conduct of a clinical study, stored under optimal (LN2 freezer storage) conditions according to specific handling standard operating procedures, and exposed to a single storage temperature cycling event during shipment on dry ice from the clinical sites to a central testing lab, and all assay testing was performed in a standardized, validated IFN-γ ELISPOT assay. The 3σ lower bounds for these samples were ≥89% for percent viability, ≥1,025 SFC/106 PBMC for IFN-γ ELISPOT response to PHA, and <18% for the percent apoptosis by Nexin assay after thaw. Determination of these lower bounds, together with the positive correlation observed between the AC measures and CD4 and CD8 antigen-specific responses in the single donor aliquot study, support the selection of AC limits from actual sample collection in an applied setting, as opposed to limits derived strictly from laboratory controlled studies. Controlled laboratory studies have the potential to produce samples of better quality than may be encountered in an applied setting.
Application of AC limits to frozen PBMC samples.
Consistent with data from the previous temperature treatment studies, exposure of samples to suboptimal temperature or temperature cycling can reduce both antigen-specific and mitogen ELISPOT responses, reduce viability, and increase the percentage of cells undergoing apoptosis. We sought to identify whether assigned AC limits applied to the testing of frozen PBMC samples was effective for separating samples that produce lower antigen-specific ELISPOT responses after suboptimal storage events from optimally stored, control samples. Trypan blue dye viability of ≥89% after thaw, a response to PHA in the ELISPOT assay of ≥1,000 spot-forming cells (SFC) per million PBMC, and a Nexin assay apoptosis limit of <18% after thaw of PBMC were applied as AC. These AC limits were applied to the data obtained with the 100 PBMC aliquots, from the two donors (M3143 and M5020), that had been subjected to the three storage conditions described (LN2 control, storage temperature cycling [between −130°C and −70°C], 8 h at −20°C).
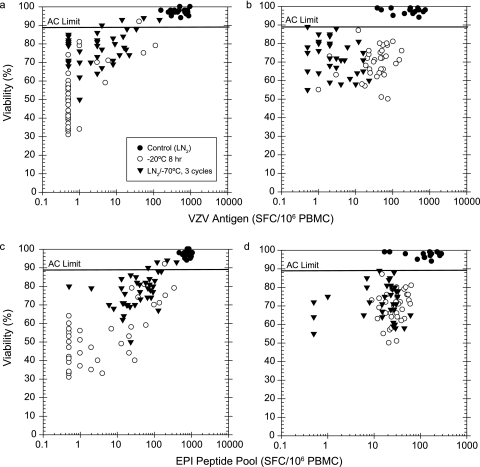
Plots of the percent viable cells (by trypan blue dye exclusion) versus the IFN-γ ELISPOT antigen-specific response to VZV (CD4) and peptide pool (CD8) for individual PBMC aliquots are shown in Fig. 5. Application of ≥89% viable by trypan blue dye exclusion as an AC limit for a valid result effectively discriminated control LN2-stored samples from the majority of samples impacted by exposure to suboptimal storage events. All of the optimal, LN2-stored samples had trypan blue viability of ≥89%, and 94 and 98% of the M3143 and M5020 aliquots, respectively, that had either been cycled between LN2 storage and −70°C or stored at −20°C for 8 h (suboptimal storage events) had viability results of <89%.
FIG. 5.
Application of trypan blue dye viability AC, with an AC limit of ≥89% viable, to assay testing with frozen PBMC after optimal LN2 storage, 8 h exposure to −20°C storage, and storage temperature cycling. (a) Donor M3143 response to VZV antigen; (b) donor M5020 response to VZV antigen; (c) donor M3143 response to EPI peptide pool; (d) donor M5020 response to EPI peptide pool.
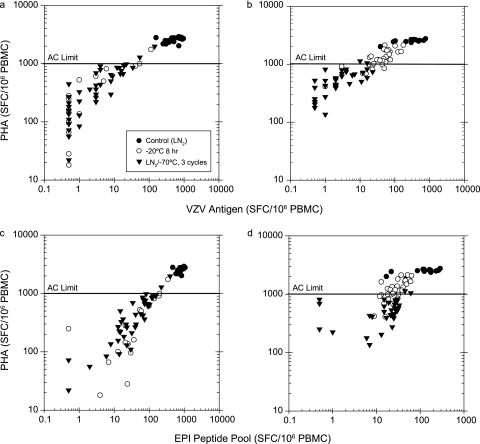
Plots of ELISPOT response to PHA (mitogen) versus the antigen-specific response to VZV (CD4) and the EPI peptide pool (CD8) for individual PBMC aliquots are shown in Fig. 6. Application of an ELISPOT response to PHA of ≥1,000 SFC/106 PBMC as an AC for a valid result effectively separated the optimal LN2-stored samples from a majority of samples exposed to suboptimal storage conditions. All of the optimal, LN2-stored samples had ELISPOT responses to PHA of ≥1,000 SFC/106 PBMC, while 3 and 68% of the M3143 and M5020 aliquots, respectively, stored at −20°C for 8 h had ELISPOT responses to PHA of ≥1,000 SFC/106 PBMC. Only 5 and 6% of the M3143 and M5020 aliquots, respectively, cycled between LN2 and −70°C storage conditions had an ELISPOT response to PHA of ≥1,000 SFC/106 PBMC.
FIG. 6.
Application of IFN-γ ELISPOT response to PHA AC, with an AC limit of ≥1,000 SFC, to assay testing with frozen PBMC after optimal LN2 storage, 8 h exposure to −20°C storage, and storage temperature cycling. (a) Donor M3143 response to VZV antigen; (b) donor M5020 response to VZV antigen; (c) donor M3143 response to EPI peptide pool; (d) donor M5020 response to EPI peptide pool.
The PHA AC effectively discriminated the samples most damaged after storage events, the samples with the greatest degree of reduction in antigen-specific ELISPOT response observed. Although performing acceptably overall as an AC, the PHA of ≥1,000 SFC cutoff had a more modest effect in selecting some of the samples impacted less severely by the suboptimal storage events (those with more modest reduction in antigen-specific response). This was particularly evident with donor M5020 samples, where suboptimal storage at −20°C for 8 h reduced to a lesser degree the response to both the CD4 and CD8 antigens, as well as to PHA. In addition, application of a specific PHA limit as an AC is dependent upon experience with the assay within a particular lab and the imaging system used for enumeration of spot counts. The ImmunoSpot system within our lab was qualified for performance of spot counting in the range required for this AC cutoff with a precision estimate (%RSD) of 18% for spot counting across the dynamic range from 11 up to 1,000 spots per well (or 22 to 2,000 SFC per million PBMC) (data not shown).
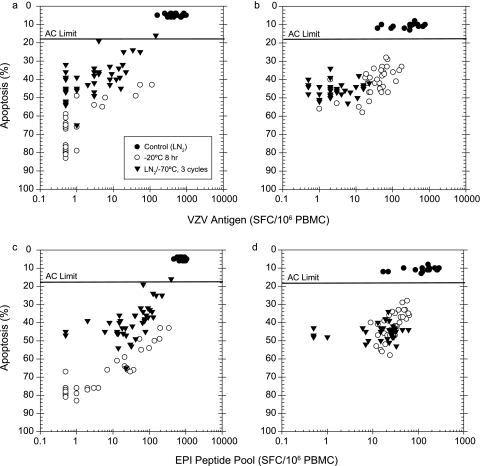
Plots of the percent apoptotic cells (by Nexin assay) versus IFN-γ ELISPOT antigen-specific response to VZV (CD4) and peptide pool (CD8) for individual PBMC aliquots are shown in Fig. 7. Application of a Nexin assay apoptosis result of <18% as an AC for a valid result effectively discriminated the control LN2-stored samples from the majority of samples impacted by exposure to suboptimal storage events. All of the optimal LN2-stored samples had Nexin assay apoptosis results of <18, and 99% of the M3143 and M5020 aliquots exposed to suboptimal storage (either cycled LN2 and −70°C storage or storage at −20°C for 8 h) had Nexin assay apoptosis results of ≥18%.
FIG. 7.
Application of percent apoptosis AC, with an AC limit of ≤18%, to assay testing with frozen PBMC after optimal LN2 storage, 8 h exposure to −20°C storage, and storage temperature cycling. (a) Donor M3143 response to VZV antigen response; (b) donor M5020 response to VZV antigen; (c) donor M3143 response to EPI peptide pool; (d) donor M5020 response to EPI peptide pool.
All three AC measures were effective for identifying samples with reduced capability to respond to antigen-specific stimulation compared to the LN2 stored controls for donor M3143. Application of the apoptosis and viability AC was more effective than the PHA functional response criteria for donor M5020 samples, where the response to PHA was more modestly reduced after some suboptimal storage events. A comparison of the geometric mean count (GMC) for antigen-specific responses using the three AC limits applied was performed (Table 3). The results show that apoptosis (Nexin assay) provided the best separation of samples relative to their ability to respond to antigen, as exemplified by the highest GMC obtained for valid samples and the largest difference between GMCs for valid versus invalid samples. There were mixed results with the other two criteria applied, with the PHA AC limit performing better than viability for donor M3143 samples and the viability limit performing better than PHA for donor M5020 samples.
TABLE 3.
GMCs for ELISPOT responses with trypan blue viability, PHA ELISPOT response, and apoptosis AC limits applieda
| AC measure | Donor M3143
|
Donor M5020
|
||||
|---|---|---|---|---|---|---|
| n | GMC
|
n | GMC
|
|||
| VZV | EPI | VZV | EPI | |||
| Trypan blue viability | ||||||
| <89% | 75 | 2.0 | 10.3 | 67 | 10.0 | 20.3 |
| ≥89% | 25 | 276.0 | 575.1 | 16 | 178.2 | 102.0 |
| PHA response | ||||||
| <1,000 | 77 | 2.1 | 10.8 | 43 | 3.8 | 14.5 |
| ≥1,000 | 23 | 394.8 | 693.2 | 40 | 89.9 | 55.6 |
| Apoptosis | ||||||
| >18% | 79 | 2.2 | 11.1 | 68 | 9.7 | 20.1 |
| ≤18% | 21 | 460.7 | 756.3 | 15 | 251.7 | 117.0 |
Results from 100 aliquots (donor M3143) or 83 aliquots (donor M5020) collected from a single donor bleed. Twenty aliquots optimally stored in an LN2 freezer (less than −130°C) and 34 to 40 aliquots for each donor were subjected to suboptimal storage by transfer to −20°C for 8 h or cycling between LN2 and −70°C storage (three cycles). n, number of aliquots.
We also looked at the effect on GMC with these AC limits applied to samples collected in a suboptimal manner (PBMC isolation and freezing after overnight storage of blood) during conduct of a clinical study. The data were collected from 138 samples, representing multiple bleeds for 46 individual subjects. Application of the AC limits to the VZV antigen-specific ELISPOT results for these samples produced the highest GMC for valid samples and the largest differential between valid and invalid sample GMC with the apoptosis criteria limit applied. The PHA AC limit ranked second in performance, based upon the same criteria, with viability ranking third with the lowest GMC for valid samples and smallest difference observed between valid and invalid sample GMC (unpublished results).
DISCUSSION
We have previously reported a validated ELISPOT assay for assessment of CD4 T-cell responses to VZV antigens incorporating optimized procedures for testing frozen PBMC that produces results consistent with those detected using freshly isolated PBMC (27). Other reports have also shown that the medium used for cell freezing, along with the specific protocols for freezing and thawing, can influence the ability to use frozen PBMC in lieu of freshly isolated cells (1, 5, 8, 24). It has also been reported that PBMC optimally stored over long periods (LN2, less than −130°C) can provide good recovery (cell number) and viability (15) and produce consistent IFN-γ ELISPOT responses over the storage period (27). However, there is little information available addressing factors during storage that may influence frozen PBMC sample quality relative to IFN-γ ELISPOT testing and the antigen-specific results obtained.
The data presented in this report support that the quality of sample storage and handling is critical for maintaining optimal integrity for data acquisition. Exposure of frozen PBMC samples to suboptimal storage (such as −20°C) for even brief periods or excessive cycling of samples between storage temperatures can lead to a reduction in sample quality and detected ELISPOT result. These events reduced the health of PBMC whether assessed by trypan blue dye exclusion viability or by quantifying the percentage of apoptotic cells with the Guava Nexin assay.
In addition, the suboptimal storage events reduced response detection in the ELISPOT assay after stimulation with mitogen and CD4 or CD8 T-cell-specific antigens. The reduction in ELISPOT response after suboptimal storage was neither predictable nor consistent for samples from a specific donor bleed. This can be a significant issue in clinical studies in which potential suboptimal storage events have occurred but may not have been documented for the samples. The lack of predictable reduction in antigen-specific response means there is an inability to apply a “correction factor” to the results obtained with these samples. Therefore, it is critical to identify compromised samples and remove them from any data analyses.
Common methods applied for assessing PBMC quality of frozen PBMC for cell based assays include trypan blue dye exclusion assessing viability of cells (15, 32) and assay response to either mitogen (13, 19, 26, 27) or a selected antigen (6, 7, 9, 17, 21, 23). In the studies presented here, the percent apoptotic cells for a sample provided the best indication whether a PBMC sample would retain functional antigen-specific ELISPOT response. Trypan blue viability had to be applied with a stringent cutoff (≥89% viable) to be effective for separating the optimal samples from those that could not respond effectively to antigen. Response to PHA was also effective but has potential, with a high limit applied, to mark as invalid samples from subjects who may not respond strongly to mitogen while their antigen-specific responses are unaffected. In the studies reported here, the percent apoptosis limit performed better as an acceptance criterion than either trypan blue viability or PHA response. Apoptosis provided the best differentiation between optimally and suboptimally stored samples, resulting in the highest GMC for valid samples and the largest differential between antigen-specific GMC for valid compared to nonvalid samples.
Previous studies have reported that freezing medium can affect the level of apoptosis (1) and that cryopreservation (LN2 storage) does not significantly affect the level of apoptosis (24) but that storage at higher temperatures (−30°C) could increase apoptosis and reduce trypan blue viability (11). The study by Fowke et al. did not report any difference in apoptosis level observed between sample storage at −70°C or −150°C. Consistent with their observations, we observed a reduction in trypan blue viability and an increase in apoptosis with samples exposed to a period of suboptimal −20°C storage. Further, we report that the impact could result with exposure as brief as 1 h and were strongly evident after just 4 to 8 h of storage in this temperature. Also consistent with their findings, we did not observe a reduction in trypan blue viability or an increase in apoptosis after storage at −70°C (data from one cycle into −70°C storage spanning a 5-week period) compared to liquid nitrogen stored (less than −135°C) samples. However, our studies provide additional information that repeated cycling of frozen PBMC samples between otherwise acceptable conditions can reduce trypan blue viability, increase the percentage of apoptotic cells, and result in the inability of PBMC samples to respond appropriately in a functional ELISPOT assay. The impact on functional response was observed for both CD4 and CD8 T-cell-specific antigens as well as after mitogen (PHA) stimulation.
The second goal of these studies was to identify AC that could be applied during assay performance to distinguish valid from invalid sample results. Numerous studies to date have reported the viability of PBMC samples, with trypan blue dye exclusion and the response to mitogen being used as indicators of PBMC quality upon thaw (8, 15, 18, 23, 32). However, these measures have not been systematically evaluated with frozen PBMC samples for establishing AC and correlation with performance in ELISPOT assay testing. Our data support that viability by trypan blue dye exclusion can function as an AC, but these studies would support that the limit must be set relatively high (an AC limit of ≥89% viable in our testing format). The functional ELISPOT response to PHA mitogen was also effective for discriminating samples that could still provide an acceptable antigen-specific response from samples that had substantially reduced responses after storage or handling events. While the functional response to PHA (AC limit of ≥1,000 SFC) was effective, the limit applied is specific for the instrument and the settings used for plate well counting. The cutoff value was effective within our lab, and the PHA cutoff was repeatable on a qualified plate reader. However, this cutoff value must be determined for a particular assay protocol and within each lab performing testing using a set of samples designed for such an evaluation. The PHA AC limit will change depending upon cell number per assay well and the instrument performing the spot enumeration. Although this spot counting was within the dynamic performance range for our instrument, from analytical validation performed, this may be outside the effective performance range for other instruments. Apoptosis was the most effective as an AC (limit of <18%) in the studies reported and after assessment of clinical study samples (unpublished data). Measurement of apoptosis was performed by using a commercial assay kit on a specific instrument from the same vendor designed to support the assay. The apoptosis limit applied in our testing should be more consistent and applicable across laboratories, with a Guava PCA instrument, than a specific well spot count criteria assigned for the functional response to PHA.
ELISPOT assay testing is increasingly being used for numerous clinical programs including studies for human immunodeficiency virus, VZV, hepatitis C virus, malaria, other infectious diseases, and cancer targets (2, 10, 12, 14, 16, 19, 20, 22, 28, 31). The impact on effective evaluation of ELISPOT data derived from suboptimal samples that have been compromised during frozen storage or handling could be tremendous within these programs. Areas that can impact sample quality must be identified and procedural controls put in place to minimize any impact. In addition, there is a need for effectively identifying samples that have been compromised during the storage or handling process prior to assay testing. The data presented here support important areas for focus during execution of clinical trials with frozen PBMC and ELISPOT evaluation, along with AC that can effectively be applied to improve the data acquired and used for study analyses.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Abrahamsen, J. F., A. M. Bakken, and O. Bruserud. 2002. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion 42:1573-1580. [DOI] [PubMed] [Google Scholar]
- 2.Arlen, P., K.-Y. Tsang, J. L. Marshall, A. Chen, S. M. Steinberg, D. Poole, P. Horan Hand, J. Schlom, and J. M. Hamilton. 2000. The use of a rapid ELISPOT assay to analyze peptide-specific immune response in carcinoma patients to peptide versus recombinant poxvirus vaccines. Cancer Immunol. Immunother. 49:517-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanuma, H., M. Sharp, H. T. Maecker, V. C. Maino, and A. M. Arvin. 2000. Frequencies of memory T cells specific for varicella-zoster virus, herpes simplex virus, cytomegalovirus by intracellular detection of cytokine expression. J. Infect. Dis. 181:859-866. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, T., S. Stark, A. Grant, C. Hartnett, M. Tsang, and A. Kalyuzhny. 2002. A multidonor ELISPOT study of IL-1B, IL-2, IL-4, IL-6, IL-13, IFN-γ and TNF-α release by cryopreserved human peripheral blood mononuclear cells. J. Immunol. Methods 270:171-182. [DOI] [PubMed] [Google Scholar]
- 5.Baust, J. M., R. van Buskirk, and J. G. Baust. 2000. Cell viability improves following inhibition of cryopreservation-induced apoptosis. In Vitro Cell Dev. Dev. Biol. Animal 36:262-270. [DOI] [PubMed] [Google Scholar]
- 6.Currier, J., U. Visawapoka, S. Tovanabutra, C. Mason, D. Birx, F. McCutchan, and J. Cox. 2006. CTL epitope distribution patterns in the Gag and Nef proteins of HIV-1 from subtype A-infected subjects in Kenya: use of multiple peptide sets increases the detectable breadth of the CTL response. BMC Immunol. 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Currier, J. R., E. G. Kuta, E. Turk, L. B. Earhart, L. Loomis-Price, S. Janetzki, G. Ferrari, D. L. Birx, and J. H. Cox. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157-172. [DOI] [PubMed] [Google Scholar]
- 8.Disis, M. L., C. dela Rosa, V. Goodell, L.-Y. Kuan, J. C. C. Chang, K. Kuus-Reichel, T. M. Clay, H. K. Lyerly, S. Bhatia, S. Ghanekar, V. C. Maino, and H. T. Maecker. 2006. Maximizing the retention of antigen specific lymphocyte function after cryopreservation. J. Immunol. Methods 308:13-18. [DOI] [PubMed] [Google Scholar]
- 9.Doherty, T. M., A. Demissie, D. Menzies, P. Andersen, G. Rook, A. Zumla, and V. S. Group. 2005. Effect of sample handling on analysis of cytokine responses to Mycobacterium tuberculosis in clinical samples using ELISA, ELISPOT, and quantitative PCR. J. Immunol. Methods 298:129-141. [DOI] [PubMed] [Google Scholar]
- 10.Firbas, C., B. Jilma, E. Tauber, V. Buerger, S. Jelovcan, K. Lingnau, M. Buschle, J. Frisch, and C. S. Klade. 2006. Immunogenicity and safety of a novel therapeutic hepatitis C virus (HCV) peptide vaccine: a randomized, placebo controlled trial for dose optimization in 128 healthy subjects. Vaccine 24:4343-4353. [DOI] [PubMed] [Google Scholar]
- 11.Fowke, K. R., J. Behnke, C. Hanson, K. Shea, and L. M. Consentino. 2000. Apoptosis: a method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J. Immunol. Methods 244:139-144. [DOI] [PubMed] [Google Scholar]
- 12.Goepfert, P. A., H. Horton, M. J. McElrath, S. Gurunathan, G. Ferrari, G. D. Tomaras, D. C. Montefiori, M. Allen, Y.-L. Chiu, P. Spearman, J. D. Fuchs, B. A. Koblin, W. A. Blattner, S. Frey, M. C. Keefer, L. R. Baden, L. Corey, and N. H. V. T. Network. 2005. High-dose recombinant canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J. Infect. Dis. 192:1249-1259. [DOI] [PubMed] [Google Scholar]
- 13.Kaufhold, R. M., J. A. Field, M. J. Caulfield, S. Wang, H. Joseph, M. A. Wooters, T. Green, H. F. Clark, D. Krah, and J. G. Smith. 2005. Memory T-cell response to rotavirus detected with a gamma interferon enzyme-linked immunospot assay. J. Virol. 79:5684-5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating, S. M., P. Bejon, T. Berthoud, J. M. Vuola, S. Todryk, D. P. Webster, S. J. Dunachie, V. S. Moorthy, S. J. McConkey, S. C. Gilbert, and A. V. S. Hill. 2005. Durable human memory T cells quantifiable by cultured enzyme-linked immunospot assays are induced by heterologous prime boost immunization and correlate with protection against malaria. J. Immunol. 175:5675-5680. [DOI] [PubMed] [Google Scholar]
- 15.Kleeberger, C. A., R. H. Lyles, J. B. Margolick, C. R. Rinaldo, J. P. Phair, and J. V. Giorgi. 1999. Viability and recovery of peripheral blood mononuclear cells cryopreserved for up to 12 years in a multicenter study. Clin. Diagn. Lab. Immunol. 6:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koibuchi, T., T. M. Allen, M. Lichterfeld, S. K. Mui, K. M. O'Sullivan, A. Trocha, S. A. Kalams, R. P. Johnson, and B. D. Walker. 2005. Limited sequence evolution within persistently targeted CD8 epitopes in chronic human immunodeficiency virus type 1 infection. J. Virol. 79:8171-8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreher, C. R., M. T. Dittrich, R. Guerkov, B. O. Boehm, and M. Tary-Lehmann. 2003. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J. Immunol. Methods 278:79-93. [DOI] [PubMed] [Google Scholar]
- 18.Kvarnstrom, M., M. C. Jenmalm, and C. Ekerfelt. 2004. Effect of cryopreservation on expression of Th1 and Th2 cytokines in blood mononuclear cells from patients with different cytokine profiles, analyzed with three common assays: an overall decrease of interleukin-4. Cryobiology 49:157-168. [DOI] [PubMed] [Google Scholar]
- 19.Levin, M. J., J. G. Smith, R. M. Kaufhold, D. Barber, A. R. Hayward, C. Y. Chan, I. S. F. Chan, D. J. J. Li, W. W. Wang, P. M. Keller, A. Shaw, J. L. Silber, K. Schlienger, I. Chalikonda, S. J. R. Vessey, and M. J. Caulfield. 2003. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-dose VZV vaccine. J. Infect. Dis. 188:1336-1344. [DOI] [PubMed] [Google Scholar]
- 20.Linette, G. P., D. Zhang, F. S. Hodi, E. P. Jonasch, S. Longerich, C. P. Stowell, I. J. Webb, H. Daley, R. J. Soiffer, A. M. Cheung, S. G. Eapen, S. V. Fee, K. M. Rubin, A. J. Sober, and F. G. Haluska. 2005. Immunization using autologous dendritic cells pulsed with the melanoma-associated antigen gp100-derived G280-9V peptide elicits CD8+ immunity. Clin. Cancer Res. 11:7692-7699. [DOI] [PubMed] [Google Scholar]
- 21.Maecker, H., J. Moon, S. Bhatia, S. Ghanekar, V. Maino, J. Payne, K. Kuus-Reichel, J. Chang, A. Summers, T. Clay, M. Morse, H. K. Lyerly, C. DeLaRosa, D. Ankerst, and M. Disis. 2005. Impact of cryopreservation on tetramer, cytokine flow cytometry, and ELISPOT. BMC Immunol. 6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mwau, M., I. Cebere, J. Sutton, P. Chikoti, N. Winstone, E. G.-T. Wee, T. Beattie, Y.-H. Chen, L. Dorrell, H. McShane, C. Schmidt, M. Brooks, S. Patel, J. Roberts, C. Conlon, S. L. Rowland-Jones, J. J. Bwayo, A. J. McMichael, and T. Hanke. 2004. A human immunodeficiency virus 1 (HIV-1) clade A vaccine in clinical trials: stimulation of HIV-specific T-cell responses by DNA and recombinant modified vaccinia virus Ankara (MVA) vaccines in humans. J. Gen. Virol. 85:911-919. [DOI] [PubMed] [Google Scholar]
- 23.Reimann, K. A., M. Chernoff, C. L. Wilkening, C. E. Nickerson, A. L. Landay, and T. A. I. A. T. Laboratories. 2000. Preservation of lymphocyte immunophenotype and proliferative response in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type-1-infected donors: implications for multicenter clinical trials. Clin. Diagn. Lab. Immunol. 7:352-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riccio, E. K. P., I. Neves, Jr., D. M. Banic, S. Corte-Real, M. das Gracas Alcerim, M. Morgado, C. T. Daniel-Ribeiro, and M. D. F. Ferreira-da-Cruz. 2002. Cryopreservation of peripheral blood mononuclear cells does not significantly affect the levels of spontaneous apoptosis after 24-h culture. Cryobiology 45:127-134. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar, S., V. Kalia, and R. C. Montelaro. 2003. Caspace-mediated apoptosis and cell death of rhesus macaque CD4+ T cells due to cryopreservation of peripheral blood mononuclear cells can be rescued by cytokine treatment after thawing. Cryobiology 47:44-58. [DOI] [PubMed] [Google Scholar]
- 26.Smith, J. G., M. Levin, R. Vessey, I. S. F. Chan, A. R. Hayward, X. Lui, R. M. Kaufhold, J. Clair, I. Chalikonda, C. Chan, M. Bernard, W. W. Wang, P. Keller, and M. J. Caulfield. 2003. Measurement of cell-mediated immunity with a varicella-zoster virus-specific interferon-gamma ELISPOT assay: responses in an elderly population receiving a booster immunization. J. Med. Virol. 70:S38-S41. [DOI] [PubMed] [Google Scholar]
- 27.Smith, J. G., X. Liu, R. M. Kaufhold, J. Clair, and M. J. Caulfield. 2001. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin. Diagn. Lab. Immunol. 8:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tassignon, J., W. Brny, S. Dahmani, L. Zhou, P. Stordeur, B. Byl, and D. De Groote. 2005. Monitoring of cellular responses after vaccination against tetanus toxoid: comparison of the measurement of IFN-γ production by ELISA, ELISPOT, flow cytometry and real-time PCR. J. Immunol. Methods 305:188-198. [DOI] [PubMed] [Google Scholar]
- 29.van Engeland, M., L. J. W. Nieland, F. C. S. Ramaekers, B. Schutte, and C. P. M. Reutelingsperger. 1998. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry 31:1-9. [DOI] [PubMed] [Google Scholar]
- 30.Wang, R., T. L. Richie, M. F. Baraceros, N. Rahardjo, T. Gay, J.-G. Banania, Y. Charoenvit, J. E. Epstein, T. Luke, D. A. Freilich, J. Norman, and S. L. Hoffman. 2005. Boosting of DNA vaccine-elicited gamma interferon responses in humans by exposure to malaria parasites. Infect. Immun. 73:2863-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg, A., C. Tierney, M. A. Kendall, R. J. Bosch, J. Patterson-Bartlett, A. Erice, M. S. Hirsch, B. Polsky, and A. C. T. G. Team. 2006. Cytomegalovirus-specific immunity and protection against viremia and disease in HIV-infected patients in the era of highly active antiretroviral therapy. J. Infect. Dis. 193:488-493. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg, A., L. Zhang, D. Brown, A. Erice, B. Polsky, M. S. Hirsch, S. Owens, and K. Lamb. 2000. Viability and functional activity of cryopreserved mononuclear cells. Clin. Diagn. Lab. Immunol. 7:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]