Abstract
Because of the occurrence of genotype 3 hepatitis E virus (HEV) in regions of low endemicity, it is important to validate the currently used serological assays for diagnosing infections with viruses belonging to this lineage, since these assays only use antigens derived from genotype 1 and 2 viruses. We evaluated the Genelabs enzyme-linked immunosorbent assay (ELISA) and the RecomBlot from Mikrogen for the detection of HEV-specific immunoglobulin M (IgM) and IgG under conditions of low endemicity. We compared test results of 16 patients with locally acquired genotype 3 HEV, 8 genotype 1 patients, 167 healthy controls from the general population, and 101 cases with hepatitis due to other viral causes. The measured specificities of the ELISA (98%) and the RecomBlot (97%) were comparable to those given by the manufacturer for IgM but were significantly lower for IgG (93% by ELISA and 66% by immunoblotting, versus reported values of 98% for ELISA and 95% for blotting). Antibody levels detected following infections with genotype 3 were lower than those following genotype 1 infections except for those measured in the IgM ELISA. Reactivity to the four antigens used in the immunoblot assay were analyzed and showed differences in the IgM immunoblot reactions between genotype 1 patients and genotype 3 patients. The ORF3 antigen was the most specific antigen. The specificity could be improved by a combined testing regimen with confirmation by immunoblotting of all positive ELISA results and by raising the cutoff of the IgG immunoblot assay without loss of sensitivity. We conclude that a combination of ELISA and immunoblotting is needed for acceptable specificity and sensitivity of HEV assays under conditions of low endemicity.
Hepatitis E is an acute self-limiting disease that is common in Asia and Africa and considered emerging in many industrialized countries. After the discovery of the hepatitis E virus (HEV) by electron microscopy in 1983 and its subsequent characterization (2), HEV infections have been reported over a wide geographic area in Asia, Africa, the Middle East, and Central America (1). The lineages that are endemic in these regions differ antigenically and genetically and have been labeled Birma-like (genotype 1), Mexico-like (genotype 2), and China-like (genotype 4). In addition, besides travel-associated HEV infections, several reports have now shown indigenous circulation of a fourth lineage of HEV (genotype 3), both in humans and in swine in regions of the world previously considered free from HEV (3, 8, 10, 13-17).
HEV infections are recognized in The Netherlands as an imported disease related to travel to regions of endemicity but also as a result of indigenous transmission of HEV. In the travel-related cases, genotype 1 is frequently detected, whereas most locally acquired HEV cases are caused by genotype 3 viruses. In view of the increasing awareness of the occurrence of HEV in regions with a temperate climate, it is important to validate the currently used serological assays for diagnosis of infections with viruses belonging to the newly described lineage. Because viremia is thought to be limited to the acute phase of illness, the diagnosis of HEV infection is mainly dependent on serology, especially in cases involving late sample collection (9). Both HEV-specific immunoglobulin M (IgM) and IgG are generally detectable at the onset of symptoms. The titers of IgM decline rapidly during early convalescence but can be detected in some patients for 5 to 6 months (7). IgG can be detected in most patients for at least 1 year after acute infection.
Currently available commercial serological assays are based on genotype 1 and 2 antigens and might have lower sensitivity for detection of infections with the genotype 3 strains (4, 6, 18). In addition, the positive predictive value is lower in areas of low endemicity (like The Netherlands) than in the countries of where it is endemic, due to the low overall seroprevalence. These differences in test performance might lead to a different interpretation of the test results compared to the countries were HEV is highly endemic.
We evaluated the use of two commercially available serological assays for the detection of HEV-specific IgM and IgG under nonendemic conditions. We compared the test performance in samples from locally acquired genotype 3 HEV infections to results in travel-related cases (genotype 1 infections) and calculated the sensitivity and specificity of HEV serology in a region of low endemicity.
MATERIALS AND METHODS
Clinical samples.
The specificity of HEV serology was calculated by using serum samples collected from the general population of The Netherlands (n = 167). These sera were obtained from a cross-sectional epidemiological survey and were age matched to a set of samples from hepatitis patients that were submitted for HEV antibody testing.
The sensitivity of the HEV serology was determined using a panel of sera collected from 24 HEV patients with a positive PCR result either in their serum or stool. The PCR-positive HEV patients were derived from the screening of 1,027 unexplained hepatitis cases (8). Eight patients were infected with a genotype 1 strain, and 16 patients were infected with a genotype 3 strain with homology between 91% and 97% to virus strains detected in pigs from The Netherlands in 1999 (14). All patients had clinical symptoms of acute hepatitis and/or elevated liver enzymes. The average age in the patient group was 49 years (range, 26 to 85 years). Follow-up samples from these patients were used to further analyze the differences in responses between genotype 1 (two samples) and genotype 3 (nine samples).
Sera from patients infected with hepatitis A (n = 23; a kind gift from S. Bruisten, GGD Amsterdam), B (n = 20), and C (n = 58) viruses were analyzed to investigate possible cross-reactivity with these other known common causes of acute viral hepatitis. All control hepatitis patients were serologically confirmed as having acute infections and, in the case of the hepatitis A patients, this conclusion was also confirmed by a positive PCR result.
IgG/IgM HEV ELISA (Genelabs Diagnostics).
The HEV-specific IgG and IgM enzyme-linked immunosorbent assays (ELISAs) were performed according to the manufacturer's instructions (Genelabs Diagnostics Inc., California). The ELISA is based on recombinant proteins from the ORF2 gene, which encodes the major capsid protein, and the ORF3 gene, which encodes a short protein of unknown function from genotype 1 and 2 HEV strains expressed in Escherichia coli (Genelabs) (4, 6, 18).
Positive and negative control samples provided with the kit were included in each run. In addition, an internal low-positive IgG or IgM sample was tested to control for intra-assay variation. Cutoff values were calculated as 0.500 (for IgG) or 0.400 (for IgM) plus the mean absorbance of the nonreactive controls. Ratios of ≥1 (optical density of the test sample divided by the cutoff) were considered positive. The specificities of the HEV-specific ELISAs conducted with healthy blood donors, as indicated by the manufacturer, were 97% (n = 1,260) and 98% (n = 917) for IgM and IgG, respectively, in the regions where HEV is not endemic and 96% and 85% in the regions where it is endemic. The sensitivity of the IgM ELISA is time dependent and was reported as 93% (n = 150) if the patient was tested within 14 days after the onset of disease. The sensitivities were determined by the manufacturer in sera from HEV outbreaks and were 95% (n = 150) and 97% (n = 103) for IgM and IgG, respectively.
IgG/IgM HEV RecomBlot (Mikrogen).
The RecomBlot uses recombinant antigens that are separated by gel electrophoresis and transferred to nitrocellulose membrane (Western blot assay). Antigens on the immunoblot are the N-terminal part of the capsid antigen (glutathione S-transferase fusion protein O2N; 50 kDa), the C-terminal part of the capsid antigen in three fragments (triple band; O2C; 38 to 41 kDa), the middle part of the capsid antigen (O2M; 28 kDa), and the ORF3 protein (O3; 15 kDa) of genotypes 1 and 2. The HEV immunoblot assay was performed according to the manufacturer's instructions. Prior to testing for HEV-specific IgM antibodies, sera were depleted of IgG antibodies with Gullsorb (Gull Laboratories) to prevent possible interisotype competition and false-positive results caused by rheumatoid factors. An internal low-positive IgM control sample was also included during each run. All four bands on the immunoblot were scored individually on intensity (scores of 0 to 3), and the scores were summarized to a maximal score of 12. A specimen was considered positive for anti-HEV IgM or IgG when the total score of the test was higher than 5 or 3, respectively. Samples scoring exactly 5 (IgM) or 3 (IgG) were considered intermediate.
Reported sensitivity of the HEV recombinant blot was 85.7% and 97.5% for IgM and IgG, respectively, as determined in acute genotype 1 HEV patients from Madras. Specificity was reported as 100% and 85% in regions where it is not endemic for IgM and IgG, respectively, as reported by the manufacturer. In regions where it is endemic, specificity calculated in healthy controls was 98.5% for IgM and only 56% for IgG detection as a diagnostic test for recent infection, indicating high exposure to HEV in the general population in these regions and reflecting past (asymptomatic) HEV infection. A random set of 65 specimens from the panel used for the ELISA was tested by immunoblotting.
Combined testing regimen of prescreening by ELISA and confirmation with immunoblotting.
For optimization of the HEV serology under circumstances of low endemicity, a combined testing regimen of screening by ELISA followed by confirmation by immunoblotting was investigated. With this approach all patient samples were first screened in both the IgM and IgG HEV ELISA. All IgM- and/or IgG-reactive samples were tested in both the IgG and IgM immunoblot assay. The results of the immunoblot assay are considered conclusive irrespective of previous IgG or IgM responses detected in the ELISA.
BioNumerics software and statistical analysis.
ELISA and immunoblot results were entered into BioNumerics 4.00 from Applied Maths to compare and analyze the differences between patients and control groups. A chi-square analysis was performed to determine the significance of differences in seroprevalence between two groups. P values of <0.01 were considered significant.
RESULTS
Specificity of the HEV-specific IgM and IgG ELISA.
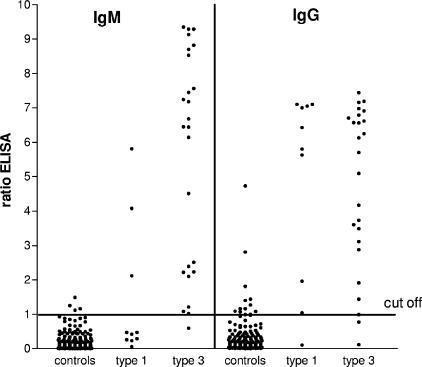
In total, 4 of the 167 controls (2.4%) had a positive IgM response in the ELISA and 12 persons (7.2%) had detectable IgG (Table 1). The ratios (optical density/cutoff) were low (<2) with two exceptions for IgG (2.8 and 4.7) (Fig. 1). In total two controls (1.2%) had both an IgM and IgG positive result in the ELISA, but both the signals were low (ratio < 1.50) and could not be confirmed by immunoblot analysis. The calculated specificity of the ELISA was 98% and 93% for IgM and IgG, respectively (Table 2).
TABLE 1.
Serological IgM and IgG results in the general population and in patients acutely infected with HEV genotype 1 and genotype 3
| Group and test | No. of controls or patients | No. (%) of samples positive for:
|
||
|---|---|---|---|---|
| IgM | IgG | Both IgM and IgG | ||
| Controls | ||||
| ELISA | 167 | 4 (2.4) | 12 (7.2) | 2 (1.2) |
| Blotting | 65 | 2 (3.1) | 22 (33.8) | 2 (3.1) |
| Combineda | 167 | 1 (0.5) | 6 (3.6) | 1 (0.5) |
| Genotype 1 patients | ||||
| ELISA | 8 | 3 (38) | 8 (100) | 3 (38) |
| Blotting | 8 | 8 (100) | 8 (100) | 8 (100) |
| Combineda | 8 | 8 (100) | 8 (100) | 8 (100) |
| Genotype 3 patients | ||||
| ELISA | 16 | 16 (100) | 14 (88) | 14 (88) |
| Blotting | 16 | 14 (88) | 16 (100) | 14 (88) |
| Combineda | 16 | 14 (88) | 16 (100) | 14 (88) |
Combined results are from initial screening of samples by ELISA with only positive results confirmed by immunoblotting.
FIG. 1.
HEV-specific IgM and IgG antibodies measured by ELISA in the general population and in genotype 1- and genotype 3-infected HEV patients. Serum samples were from controls (n = 167) from the general population of The Netherlands, 8 genotype 1-infected patients (including 2 follow-up samples), and 16 genotype 3-infected patients (including 9 follow-up samples).
TABLE 2.
Reported and observed sensitivities and specificities of the HEV ELISA and immunoblot assayc
| Ig and assay | Sensitivity (%)
|
Specificity (%)
|
||
|---|---|---|---|---|
| Reported (n) | Observed (type 1/type 3) | Reported (n) | Observed (n) | |
| IgM | ||||
| ELISA | 95 (150) | 38/100b | 97 (1,260) | 98 (167) |
| Blotting | 86 (40) | 100/88 | 99.5a (197) | 97 (65) |
| Combination | 100/88 | 99 (167) | ||
| IgG | ||||
| ELISA | 97 (103) | 100/88 | 98 (917) | 93 (167) |
| Blotting | 98 (40) | 100/100 | 85.3a (197) | 66 (65)b |
| Combination | 100/100 | 96 (167) | ||
Data from regions where HEV is not endemic.
Values in boldface are significantly different from the reported values.
n, number of samples tested.
Specificity of HEV-specific IgM and IgG immunoblot assay.
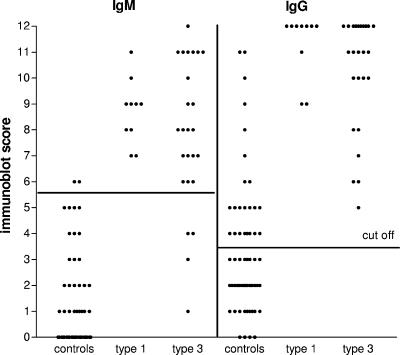
A total of 65 serum samples from the general population of The Netherlands were tested to estimate the specificity of HEV-specific IgM and IgG immunoblotting in The Netherlands. Two persons (3.1%) tested positive for IgM, both with a score of 6, just above the cutoff level of 5 (Table 1). The calculated specificity of the IgM immunoblot assay was 97% (Table 2; Fig. 2). Both of the control patients that tested positive for IgM also had IgG antibodies (scores of 5 and 11). Twenty-two controls (34%) tested positive for IgG in the immunoblot assay (n = 22), leading to a specificity of only 66%. The specificity of the IgG immunoblot assay could be increased to 79% or 90% if the cutoff level were increased from 3 to 4 or 5, respectively, without affecting the sensitivity in both the genotype 1- and 3-infected patient groups.
FIG. 2.
HEV-specific IgM and IgG antibodies measured by immunoblot assay in the general population and in genotype 1- and genotype 3-infected HEV patients. Serum samples were from controls (n = 65) from the general population of The Netherlands, 8 genotype 1-infected patients (including 2 follow-up samples), and 16 genotype 3-infected patients (including 9 follow-up samples).
Specificity of a combined testing regimen of prescreening by ELISA and confirmation with immunoblotting.
With the combined testing regimen applied to the 167 control samples, only one positive IgM immunoblot result was obtained. The sample tested originally IgM negative but IgG positive in the ELISA (Table 1). None of the four originally solitary IgM responses in the ELISA could be confirmed by the IgM immunoblot assay. Of the 12 IgG-positive ELISA results, only half could be confirmed by immunoblotting (3.6%), of which 1 person also had detectable IgM antibodies (scores of 6 for IgM and 11 for IgG). Thus, the combination of the ELISA and immunoblot assay led to a specificity of 99% and 96% for IgM and IgG serology, respectively, under nonendemic circumstances.
Sensitivity of the HEV-specific IgM and IgG ELISA in genotype 1- and genotype 3-infected HEV patients.
The sensitivities of the IgM and IgG HEV-specific ELISAs were determined in acute-phase sera from PCR-positive HEV patients. Sera from 8 patients who were infected with genotype 1 HEV and 16 patients with a genotype 3 infection were analyzed (Table 1). Five (63%) of the HEV genotype 1-infected patients tested negative in the IgM ELISA, while all genotype 3-infected patients (n = 16) were positive for IgM in the ELISA (Table 1). For IgG, all 8 genotype 1 patients were IgG positive, whereas 88% (14/16) of genotype 3-infected patients were positive for HEV-specific IgG. Therefore, the calculated sensitivities of the IgM ELISA were 38% in genotype 1-infected and 100% in genotype 3-infected patients; the calculated sensitivities of the IgG ELISA were 100% in genotype 1-infected and 88% in genotype 3-infected patients.
Sensitivities of the HEV-specific IgM and IgG immunoblot assays in genotype 1-infected and genotype 3-infected HEV patients.
All genotype 1-infected patients tested positive for both IgM and IgG in the immunoblot assays (sensitivity, 100% and 100%) (Table 1; Fig. 2). Two genotype 3-infected patients tested negative for IgM, but all patients in this group were IgG positive, leading to sensitivities of 88% and 100% for IgM and IgG, respectively, for genotype 3 infections. One patient in the genotype 3-infected group had a low IgG response of 5, whereas all other signals were higher. In general, lower immunoblot assay scores were detected more often in the genotype 3-infected patients than in the genotype 1-infected group for both the IgM and IgG responses, but these differences were not significant (Fig. 2).
Sensitivity of the combined testing regimen of prescreening by ELISA and confirmation with immunoblotting.
The sensitivity of IgM detection in a combined schedule was high for genotype 1 patients (100%), but two patients were missed in the genotype 3 patient group, leading to a sensitivity of 88%. With the combined approach, the sensitivity was 100% for IgG for both the genotype 1 and genotype 3 patients (Table 1).
Analysis of the reactivity against the O2N, O2M, O2C, and O3 antigens used in the immunoblot assay.
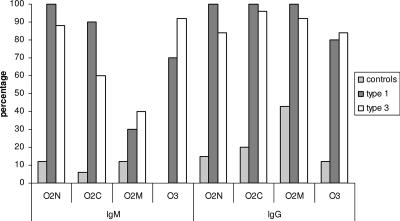
The reactivity of each serum sample to individual bands in the immunoblot assay (O2N, O2M, O2C, and O3) was scored separately and compared between controls and the genotype 1- and 3-infected patients (Fig. 3). If only immunoblotting scores of individual bands of 2 to 3 were considered reactive, the antigens O2N, O2C, and O2M reacted with 12, 6, and 12% of control sera (n = 65) for IgM, respectively, and no reactivity to O3 was observed in the IgM blot in the control group. For IgG, the antigens O2N, O2C, O2M, and O3 reacted with 15, 20, 43, and 12% of the control sera (scores of 2 or 3), respectively. In HEV-infected patients, the O2M antigen had a low sensitivity, between 30 and 40%, in the IgM assay for both genotype 1- and 3-infected patients (Fig. 3).
FIG. 3.
Distribution of the incidence of strongly reactive HEV-specific bands in an immunoblot assay in the general population and in HEV genotype 1- and genotype 3-infected patients. Total number of serum samples tested: 65 controls, 10 genotype 1, and 25 genotype 3. Only immunoblot scores of individual bands of 2 to 3 were considered reactive, following the manufacturer's instructions.
The correlations between the IgM and IgG ELISA results and individual reactivities to the separate antigens in the immunoblot assay were calculated using test results from both HEV patients and controls (n = 100). The highest correlation was observed for the O3 antigen for both IgM (r = 0.75) and IgG (r = 0.79) results. A low reactivity to the O3 antigen in the IgM and IgG immunoblot assay was also associated with negative results in the ELISA. The lowest correlation was detected for the O2M antigen, with r values of only 0.28 and 0.46 for the IgM and IgG assays, respectively. The other two antigens had correlations of r = 0.66 and r = 0.71 for O2N and O2C for IgG, respectively, and r = 0.57 and r = 0.49 for IgM, respectively.
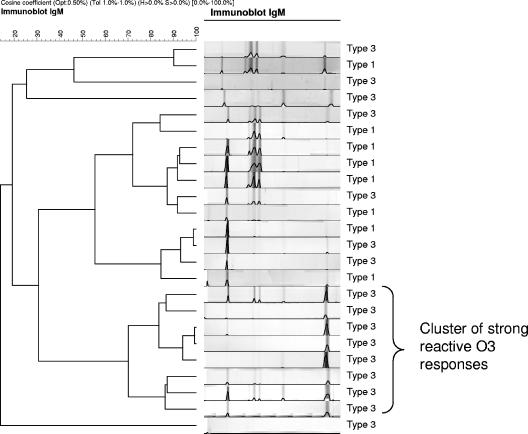
Comparison on the basis of intensity of the IgM response to the four antigens with the BioNumerics software showed that eight type 3 cases (50%) had a high O3 response in combination with reactivity with the other three antigens. This pattern was distinct from those observed in genotype 1 patients (n = 8) and the other genotype 3 patients (n = 8). The genotype 1 HEV-infected patients also showed in general a very strong O2N response compared to genotype 3 patients (Fig. 4). A similar comparison of the IgG response based on the intensity of the four antigens showed no differences between genotype 1- and genotype 3-infected patients. Most patients were reactive to all four antigens used in the assay.
FIG. 4.
Distribution of reactive HEV-specific bands in the IgM immunoblot assay of HEV genotype 1- and genotype 3-infected patients. Acute-phase serum samples from 8 genotype 1 and 16 genotype 3 patients were analyzed.
Cross-reactivity of HEV serology in HAV-, HBV-, and HCV-infected patients.
Sera from confirmed HAV, HBV, and HCV patients were analyzed in the HEV IgM and IgG ELISAs and confirmatory immunoblot assay to detect possible cross-reactivity (Table 3). A relatively high proportion of HBV-infected (20%) and HCV-infected (9%) patients tested positive for IgG antibodies after immunoblot assay confirmation, but the results were not significantly different from those in the control group. Increasing the cutoff level for the IgG immunoblot assay to 5 decreased the number of reactive HBV samples to 10% and HCV samples to 5%.
TABLE 3.
Cross-reactivity in sera from patients with HAV, HBV, or HCV infection in the HEV IgM and IgG ELISAs and after immunoblotting confirmationa
| Test and infection | Frequency (%) of cross-reactivity with HEV Ig
|
||
|---|---|---|---|
| Only IgM | Only IgGb | IgM and IgGb | |
| ELISA | |||
| HAV | 2/23 (9) | 1/23 (4) | 1/23 (4) |
| HBV | 1/20 (5) | 3/20 (15) | 0/20 (0) |
| HCV | 6/58 (10) | 15/58 (26)* | 7/58 (12)* |
| Combined regimen | |||
| HAV | 0/23 (0) | 0/23 (0) | 0/23 (0) |
| HBV | 0/20 (0) | 4/20 (20) | 0/23 (0) |
| HCV | 3/58 (5) | 5/58 (9) | 0/58 (0) |
In total, 23, 20, and 58 HAV, HBV, and HCV confirmed cases, respectively, were analyzed for cross-reactivity in the HEV serology according to the combined testing regimen. All patients were first screened with both the IgM and IgG HEV ELISAs. All IgM- and/or IgG-reactive samples were then tested in both the IgG and IgM immunoblot assays. The results of the immunoblot assay were considered conclusive irrespective of previous IgG or IgM responses detected in the ELISA.
*, significantly different (P < 0.01) from the control group (results are shown in Table 1).
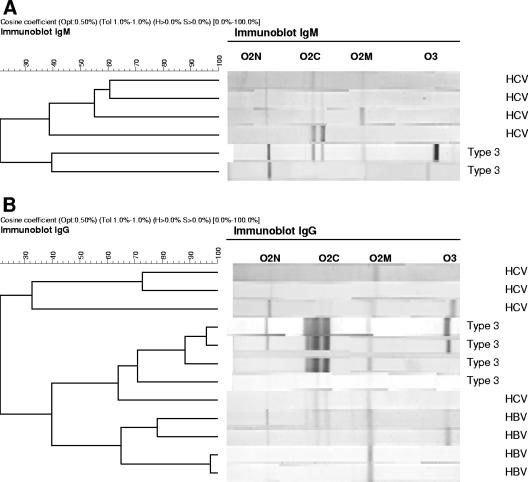
Comparison of the IgG response based on the detected intensity to the four antigens in the immunoblot assay revealed distinct patterns between HEV patients and the HAV, HBV, and HCV patients, with two exceptions. These two were HBV patients, and they clustered with the HEV patients with reactivity to all four antigens (total scores of 9 and 10), probably reflecting past infection (Fig. 5). All three IgM-positive HCV patients (scores of 7, 8, and 9) had no O3 band, in contrast with the majority (83%) of confirmed HEV cases (Fig. 5). All HEV-reactive specimens tested negative by PCR.
FIG. 5.
Distribution of reactive HEV-specific bands in the IgM and IgG immunoblot assay of HBV- and HCV-infected patients. (A) Detected IgM responses in serum samples from reactive HCV patiens (n = 4) and HEV patients (n = 2). (B) Detected IgG responses in serum samples from reactive HBV (n = 4) and HCV (n = 4) patients and HEV patients (n = 4).
DISCUSSION
HEV is a cause of unexplained hepatitis in The Netherlands, and it is therefore recommend that HEV infection should be investigated in all patients with unexplained acute hepatitis, despite their travel history (8, 15). Better awareness among physicians about the possibility of locally acquired HEV infections in industrialized countries has led to an increased rate of detection of genotype 3 infections. For the physician in attendance and the patient himself, the knowledge of which genotype is responsible for the infection is not of real clinical importance, since the diagnosis and the management remain the same. However, there are some indications that genotype 3 infections usually do cause a milder disease compared to genotype 1 (11). In addition, determination of the genotype can be important in epidemiological studies and helpful in revealing the possible (local) sources of HEV infection and other risk factors.
Several commercial serological assays are on the market, but the antigens used are derived from genotype 1 and 2 viruses, and experience with these assays and their test performance comes mostly from regions of high endemicity. Because the diagnosis of HEV infection is mainly dependent on serological testing, the currently used diagnostic tests were evaluated for use under circumstances with low-level genotype 3 HEV circulation.
Our data show that the observed specificity of the serological assays under these conditions was good for IgM but less optimal for IgG. The observed specificities for the two IgG assays, ELISA (93%) and immunoblot (66%), were both significantly lower than those provided by the manufacturer (98% for the ELISA and 85.3% for the immunoblot assay). Seroprevalence for HEV-specific antibodies is known to be age dependent in regions of endemicity (5). Therefore, the lower specificity could be caused by the fact that our control group was age matched to the general group of samples from patients with suspected HEV that were sent into our laboratory. This leads to a selection of an older population than the healthy young blood donors that were used as control groups by the manufacturer.
Assay specificity could be increased without loss of sensitivity by using a combined testing regimen, with the ELISA as a screening assay followed by confirmatory testing in an immunoblot assay. The combined testing regimen seems crucial to limit the risk of misdiagnosing hepatitis patients. We detected only one IgM- and IgG-positive person in the control group (immunoblot score of 2 for IgM and 11 for IgG), but a possible HEV infection could not be confirmed with a positive PCR result. A negative PCR result cannot exclude an infection, and the serologic response in this control does indicate recent contact with HEV. Since this concerns a healthy control, this could reflect an asymptomatic infection, but cross-reactivity cannot be ruled out; however, since all four HEV antigens were clearly reactive on the immunoblot, this is not a very likely explanation. A large proportion of the HCV patients (73%) had a strong IgG response to the O2M antigen. O2M was also found to have the lowest correlation between immunoblotting and ELISA results. Further research is needed to see if these findings are caused by cross-reacting antigens of HCV or if a previous (subclinical) infection with HEV does occur more often in HCV-infected patients.
An unexpected result was the low sensitivity (37%) of the IgM ELISA in the group of genotype 1-infected patients instead of the hypothesized lower sensitivity in the genotype 3 patients caused by the use of nonhomologous antigens. This low sensitivity of the IgM ELISA was correlated to the absence of a response to the O3 antigen detected in the immunoblot assay. The O3 antigen was found to be the most specific of all antigens used, and the analysis of follow-up serum samples from HEV patients by immunoblotting revealed that the response to the O3 antigen is usually the last to develop. Therefore, samples collected in the acute phase of illness may test negative. Similarly, the difference in immunoblot patterns between genotype 3- and genotype 1-infected patients observed here is intriguing, but as dates of onset of illness for the HEV cases in this study were not available, follow-up of a better-defined patient group is needed. The findings imply that samples positive for only IgG may reflect past but also recent infection. The level of IgG may be helpful, as almost all PCR-confirmed cases had high levels of IgG (immunoblot scores of ≥6).
The lower responses on average in genotype 3 patients compared to genotype 1 cases had little influence on the sensitivity, because the signals were in most cases well above the cutoff levels. For this reason, it is unlikely that many HEV patients acutely infected with genotype 3 are missed, although the timing of sampling may be important. The use of heterologous antigens does most likely contribute to lower reactivity in the group of genotype 3 patients, but other factors might also have had an influence. So far, most infections with genotype 3 strains have resulted in a rather mild course of illness, in elderly persons with a high prevalence of underlying illness, suggesting that genotype 3 strains are not as virulent and possibly are less immunogenic than the other genotypes (11).
We have no indication that acute HEV patients are missed on a large scale because of the use of possible inadequate antigens in the diagnostic assays. These findings are consistent with other reports where genotype 1 antigens are found to react equally well with serum samples from both primates and patients infected with the four known genotypes of HEV (12, 19). Although in general lower reactivity in HEV genotype 3-infected patients was seen compared to genotype 1 infections, we conclude that the currently used assays are acceptable for testing suspected genotype 1 and 3 HEV infections in patients in settings of low endemicity. However, we recommend a combined testing regimen with confirmation by immunoblotting of positive ELISA results for optimal specificity and sensitivity.
Acknowledgments
We thank Annika Haagsman, Bas van de Veer, and Marjan Kuijer for their technical assistance, René Benne and Peter Schneeberger for the collection of sera from HEV patients, and Sylvia Bruisten and Alex Koek from the GGD Amsterdam for collection of sera from the HAV-infected patients.
Part of this study was financially supported by EU grant 502571 “Enteric Virus Emergence, New Tools (EVENT).”
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Aggarwal, R., and S. R. Naik. 1997. Epidemiology of hepatitis E: past, present and future. Trop. Gastroenterol. 18:49-56. [PubMed] [Google Scholar]
- 2.Balayan, M. S., A. G. Andjaparidze, S. S. Savinsaya, E. S. Ketiladze, D. M. Braginsky, A. P. Savinov, and V. F. Poleschuk. 1983. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology 20:23-31. [DOI] [PubMed] [Google Scholar]
- 3.Clemente-Casares, P., S. Pina, M. Buti, R. Jardi, M. Martin, S. Bofill-Mas, and R. Girones. 2003. Hepatitis E virus epidemiology in industrialized countries. Emerg. Infect. Dis. 9:448-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colac, D., D. Ogunc, F. Gunseren, S. Velipasaoglu, M. R. Aktekin, and M. Gultekin. 2002. Seroprevalence of antibodies to hepatitis A and E viruses in pediatric age groups in turkey. Acta Microbiol. Immunol. Hung. 49:93-97. [DOI] [PubMed] [Google Scholar]
- 5.Corwin, A. L., H. B. Khiem, E. T. Clayson, K. S. Pham, T. T. Vo, T. Y. Vu, T. T. Cao, D. Vaughn, J. Merven, T. L. Richie, M. P. Putri, J. He, R. Graham, F. S. Wignall, and K. C. Hyams. 1996. A waterborne outbreak of hepatitis E virus transmission in south-western Vietnam. Am. J. Trop. Med. Hyg. 54:559-562. [DOI] [PubMed] [Google Scholar]
- 6.Daniel, H. D., A. Warier, P. Abraham, and G. Sridharan. 2004. Age-wise exposure rates to hepatitis E virus in a southern Indian patient population without liver disease. Am. J. Trop. Med. Hyg. 71:675-678. [PubMed] [Google Scholar]
- 7.Favorov, M. O., H. A. Fields, M. A. Purdy, T. L. Yashina, A. G. Aleksandrov, M. J. Alter, D. M. Yarasheva, D. W. Bradley, and H. S. Margolis. 1992. Serologic identification of hepatitis E virus infection in epidemic and endemic settings. J. Med. Virol. 36:246-250. [DOI] [PubMed] [Google Scholar]
- 8.Herremans, M., H. Vennema, J. Bakker, B. van der Veer, E. Duizer, C. A. Benne, K. Waar, B. Hendrixks, P. Schneeberger, G. Blaauw, M. Kooiman, and M. P. G. Koopmans. 2007. Swine-like hepatitis E viruses are a cause of unexplained hepatitis in The Netherlands. J. Viral Hepatol. 14:140-146. [DOI] [PubMed] [Google Scholar]
- 9.Jameel, S. 1999. Molecular biology and pathogenesis of hepatitis E virus. Expert Rev. Mol. Med. 6:1-16. [DOI] [PubMed] [Google Scholar]
- 10.Mansuy, J. M., J. M. Peron, C. Bureau, L. Alric, J. P. Vinel, and J. Izopet. 2004. Immunologically silent autochthonous acute hepatitis E virus infection in France. J. Clin. Microbiol. 42:912-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizuo, H., Y. Yazaki, K. Sugawara, F. Tsuda, M. Takahashi, T. Nishizawa, and H. Okamoto. 2005. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J. Med. Virol. 76:341-349. [DOI] [PubMed] [Google Scholar]
- 12.Obriadina, A., J. H. Meng, T. Ulanova, K. Trinta, A. Burkov, H. A. Fields, and Y. E. Khudyakov. 2002. A new enzyme immunoassay for the detection of antibody to hepatitis E virus. J. Gastroenterol. Hepatol. 17:S360-S364. [DOI] [PubMed] [Google Scholar]
- 13.Schlauder, G. G., S. M. Desai, A. R. Zanetti, N. C. Tassopoulos, and I. K. Mushahwar. 1999. Novel hepatitis E virus (HEV) isolates from Europe: evidence for additional genotypes of HEV. J. Med. Virol. 57:243-251. [DOI] [PubMed] [Google Scholar]
- 14.Van der Poel, W. H. M., F. Verschoor, R. van der Heide, M. Kooreman, and A. M. de Roda Husman. 2001. Hepatitis E virus sequences in swine related to sequences in humans, The Netherlands. Emerg. Infect. Dis. 7:970-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waar, K., M. M. P. T. Herremans, H. Vennema, M. P. G. Koopmans, and C. A. Benne. 2004. Hepatitis E is a cause of unexplained hepatitis in The Netherlands. J. Clin. Virol. 33:145-149. [DOI] [PubMed] [Google Scholar]
- 16.Widdowson, M.-A., W. J. M. Jaspers, W. H. M. van der Poel, F. Verschoor, A. M. de Roda Husman, H. L. J. Winter, H. L. Zaaijer, and M. Koopmans. 2003. Cluster of cases of acute hepatitis associated with hepatitis E virus infection acquired in The Netherlands. Clin. Infect. Dis. 36:29-33. [DOI] [PubMed] [Google Scholar]
- 17.Zanetti, A. R., G. G. Schlauder, L. Romano, E. Tanzi, P. Fabris, G. J. Dawson, and I. K. Mushahwar. 1999. Identification of a novel variant of hepatitis E virus in Italy. J. Med. Virol. 57:356-360. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, J. Z., S. W. Im, S. H. Lau, T. N. Chau, S. T. Lai, S. P. Ng, M. Peiris, C. Tse, T. K. Ng, and M. H. Ng. 2002. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J. Med. Virol. 66:40-48. [DOI] [PubMed] [Google Scholar]
- 19.Zhou, Y. H., R. H. Purcell, and S. U. Emerson. 2003. An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotype 1, 2, 3, and 4. Vaccine 22:2578-2585. [DOI] [PubMed] [Google Scholar]