Abstract
To learn about the cellular processes involved in Mg2+ homeostasis and the mechanisms allowing cells to cope with low Mg2+ availability, we performed RNA expression-profiling experiments and followed changes in gene activity upon Mg2+ depletion on a genome-wide scale. A striking portion of genes up-regulated under Mg2+ depletion are also induced by high Ca2+ and/or alkalinization. Among the genes significantly up-regulated by Mg2+ starvation, Ca2+ stress, and alkalinization are ENA1 (encoding a P-type ATPase sodium pump) and PHO89 (encoding a sodium/phosphate cotransporter). We show that up-regulation of these genes is dependent on the calcineurin/Crz1p (calcineurin-responsive zinc finger protein) signaling pathway. Similarly to Ca2+ stress, Mg2+ starvation induces translocation of the transcription factor Crz1p from the cytoplasm into the nucleus. The up-regulation of ENA1 and PHO89 upon Mg2+ starvation depends on extracellular Ca2+. Using fluorescence resonance energy transfer microscopy, we demonstrate that removal of Mg2+ results in an immediate increase in free cytoplasmic Ca2+. This effect is dependent on external Ca2+. The results presented indicate that Mg2+ depletion in yeast cells leads to enhanced cellular Ca2+ concentrations, which activate the Crz1p/calcineurin pathway. We provide evidence that calcineurin/Crz1p signaling is crucial for yeast cells to cope with Mg2+ depletion stress.
Mg2+ is the most abundant divalent cation in cells, where the ion predominantly serves as a counterion for solutes, particularly ATP and other nucleotides, RNA and DNA. By binding to RNAs and many proteins, Mg2+ also contributes to establishing and maintaining physiological structures and acts as an important cofactor in catalytic processes. Mg2+ also stabilizes membranes and active conformations of macromolecules (reviewed in references 18, 37, and 38). Cellular Mg2+ concentrations are in the millimolar range (∼15 to 20 mM), some 3 orders of magnitude higher than those of Ca2+ (100 to 200 nM) (4, 5, 17, 20, 38). The vast majority of Mg2+ is bound to ligands, leaving a small fraction of up to 5% in a free ionized state (38, 40). Cellular Mg2+ homeostasis involves systems facilitating influx and others that mediate extrusion of the ion. Mg2+ influx is an electrogenic process driven by the inside negative membrane potential and mediated by channels in the plasma membrane, either by TRPM6 and TRPM7 proteins in mammals (42, 43) or by members of the heterogeneous CorA/Mrs2/Alr1 protein family in prokaryotes, organelles, lower eukaryotes, and plants (15, 17, 23, 24, 53, 54). These high-affinity Mg2+ uptake systems allow cells to grow even in the presence of very low external Mg2+ concentrations. In mutants lacking these systems, cells survive only when provided with high external Mg2+ concentrations. Extrusion of Mg2+ occurs against the electrochemical gradient and is mediated by exchange against Na+, H+, or other ions, making use of their inside-directed gradients to drive the process (10, 40).
Although Ca2+ concentrations are several orders of magnitude lower than those of Mg2+, the two ions appear to affect each other in a mostly antiparallel fashion. In yeast, vacuolar Ca2+ accumulation is blocked by increased Mg2+ in the medium, and alr1Δ mutants having lower Mg2+ exhibit elevated Ca2+ (5, 17). In pancreatic acinar cells, an increase in intracellular Mg2+ results in a decrease of Ca2+ influx, whereas intracellular Ca2+ mobilization is associated with a reduction in Mg2+ (31). Moreover, extracellular Mg2+ is known to regulate K+ and Ca2+ channels in the plasma membrane (6, 29, 44). Intracellular Mg2+ concentrations in mammalian cells have been reported to change in response to hormonal stimuli, albeit much more slowly than do Ca2+ concentrations (10, 31, 40). In some cases, these mutual modulations may simply reflect a replacement of one divalent cation by the other, but Mg2+ effects on Ca2+ signaling have frequently been observed (31).
Mg2+ starvation of rats has been reported to elicit significant up-regulation of expression of genes involved in oxygen stress in thymocytes (35). These effects result from long-lasting Mg2+ starvation conditions (2 days) and may include immediate responses of cells to Mg2+ withdrawal as well as secondary effects reflecting induction of stress phenomena.
In an attempt to understand the direct effects of Mg2+ starvation, we followed a whole-genome approach in Saccharomyces cerevisiae. We set out to analyze short-term responses to Mg2+ withdrawal in yeast cells by transcriptomal analysis. A relatively confined set (<2%) of the total of 6,300 genes responded with a significant, at least twofold, increase in transcript levels. Most of them were found to be similarly up-regulated by other treatments that elicited a Ca2+ peak. In fact, we observed an increase of cytoplasmic Ca2+ immediately after cells were transferred to low-Mg2+ medium, and up-regulation of the calcineurin/Crz1p (calcineurin-responsive zinc finger protein) signaling pathway.
MATERIALS AND METHODS
Yeast strains, plasmids, and media.
S. cerevisiae strains used in this study are Y00000 (BY4741), Y05353 (BY4741; crz1::kanMX4) and Y05040 (BY4741; cnb1::kanMX4) from the EUROSCARF collection (http://web.uni-frankfurt.de/fb15/mikro/euroscarf/). The presence of the deletions was confirmed by qualitative PCR using two specific primer pairs for wild-type and deletion mutant strains (data not shown). Wild-type strain JS034-4C is described by Stadler and Schweyen (47). The Crz1-green fluorescent protein (GFP) fusion construct (pRSP97) is described by Polizotto and Cyert (36). Synthetic medium was prepared according to Sherman (46): for standard medium, 1 mM MgCl2 was added, and for Mg2+ free medium, the MgCl2 was omitted. EGTA (10 mM, pH 8.0) was added where indicated. Medium containing high Ca2+ was buffered with 50 mM MES (morpholineethanesulfonic acid [pH 6.0]).
Mg2+ starvation and RNA isolation.
For each experiment, two identical yeast cultures were grown in synthetic medium containing 1 mM MgCl2 to an optical density at 600 nm (OD600) of 0.5. The cultures were centrifuged, washed twice with prewarmed synthetic medium containing either 1 mM MgCl2 (+Mg2+) or no MgCl2 (−Mg2+), and resuspended in the same medium. For time course experiments, aliquots were removed at the indicated time points; for all other experiments, cells were harvested 70 min after the first wash. For the FK506 experiment, FK506 (Fujisawa GmbH, Munich) in dimethyl sulfoxide was added to a final concentration of 1 μg/ml (1.25 μM) 10 min before centrifugation and for all subsequent steps. Total RNA was isolated using the hot acidic phenol method (1). For microarray experiments, three chloroform extractions were performed instead of one.
DNA microarray analyses.
Yeast cDNA arrays were obtained from the Ontario Cancer Institute Microarray Centre. Reverse transcription, probe cleanup, and microarray hybridization were performed according to the manufacturer's protocol. Two individual experiments including dye swap were performed. Microarrays were read using an axon GenePix 4000B laser scanner (Axon Instruments) and analyzed with the GenePix Pro 3.0 software. The Saccharomyces Genome Database (http://www.yeastgenome.org/) was used to extract the information on the genes regulated by Mg2+ starvation. The geneXplorer 2.0: Megayeast site from Stanford University (http://genome-www.stanford.edu/cgi-bin/yeast_stress/gx?n = megayeast&rx = 5&ry) was used to search for genes induced under general stress conditions.
Northern blot experiments.
Twenty-five-microgram samples of total RNA were subjected to gel electrophoresis and blotted to nitrocellulose membranes (27). 32P-labeled probes for hybridization were generated by either random primed labeling (Roche) from PCR-synthesized DNAs or hot PCR on genomic DNA using the following oligonucleotide primers: ENA1F (5′-TTATCGCGGTCAATGTGCTC), ENA1R (5′-ATCAAACTCACGTTGCCCTC), PHO89F (5′-TGCTTTACTGCTGGTTGGTG), and PHO89R (5′-AGCGTTGGCAACGTCATTAG). For quantification of RNA levels, the blots were rehybridized to an actin probe generated using the primers ACT1F (5′-ACCAAGAGAGGTATCTTGACTTTACG) and ACT1R (5′-GACATCGACATCACACTTCATGATGG). Documentation and analyses of the Northern blots were performed using the Amersham Biosciences Typhoon 8600 phosphorimaging system and the Molecular Dynamics Image Quant software.
Fluorescence microscopy.
For determination of the subcellular location of Crz1p, plasmid pRSP97 (a GFP-CRZ1 fusion construct) (36) was transformed into BY4741. The transformants were grown in synthetic complete medium lacking uracil (SC −Ura [containing 1 mM MgCl2 and 10 mg/liter methionine]) to an OD600 of 1 to 1.5. One-milliliter aliquots were spun in a tabletop centrifuge (30 s at 7,000 rpm), washed twice in SC −Ura medium (prewarmed to 30°C, with 1 mM Mg2+, 0 mM Mg2+, or 200 mM CaCl2, respectively), resuspended in the same medium, and incubated for 10 min at 30°C. Prior to microscopy, the cells were briefly spun in a Qualtron microcentrifuge and resuspended in a small volume of the supernatant.
Determination of cytoplasmic Ca2+ concentrations.
To express YC2-12 in yeast, a 2.5-kb BamHI-XhoI fragment from YC2.12 in pCS2 (30) was cloned into the yeast expression plasmid pVT-U (51). The resulting plasmid (pGW845) was transformed into BY4741. Transformants were grown in selective medium containing 1 mM Mg2+ to an OD600 of 0.5 to 1.5 and concentrated to 1/100 volume in a Qualtron microcentrifuge. Cytosolic Ca2+ concentration was measured as previously described (14, 26). Briefly, yeast cells stably expressing the sensor in the cytosol were immobilized on glass coverslips with concanavalin A (Sigma-Aldrich) and placed into an experimental chamber that allowed continuous perfusion and fast buffer switch. The microscope consists of a Nikon inverted microscope (Eclipse 300TE, Nikon, Vienna, Austria) equipped with CFI Plan Fluor ×40 oil immersion objective (NA 1.3; Nikon, Vienna, Austria), an epifluorescence system (150 W XBO; Optiquip, Highland Mills, NY), and a liquid-cooled charge-coupled device camera (−30°C; Quantix KAF 1400G2, Roper Scientific, Acton, MA). All devices were controlled by Metafluor 4.0 (Visitron Systems, Puchheim, Germany). To monitor the cytosolic free Ca2+ concentration, the cells were illuminated at 440 nm (Cameleon; 440AF21; Omega Optical, Brattleboro, VT). An optical beam splitter (Dual-View Micro-Imager; Optical Insights, Visitron Systems) was used in order to allow simultaneous emission rationing at 480 nm (480AF30; Omega Optical) and 535 nm (535AF26 with dichroic 455DRVP; Omega Optical).
Microarray data accession number.
Microarray data from this study are available at the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo) under accession no. GSE6687.
RESULTS
Genome-wide analysis of gene expression in response to Mg2+ starvation.
To determine how cells cope with deprivation of the essential metal ion Mg2+ and to learn about the cellular processes involved in Mg2+ transport, we performed whole-genome microarray experiments. Cells were grown in synthetic medium containing standard concentrations of Mg2+ (1 mM MgCl2) and then shifted to nominally Mg2+-free medium or to fresh medium containing standard concentrations of Mg2+. The expression profile of cells shifted to Mg2+-free medium was compared to that of cells grown in 1 mM Mg2+. Ninety minutes after the shift from 1 mM to nominally Mg2+-free medium, we found genes belonging to particular functional clusters to be up-regulated (Table 1). Among the genes most significantly induced were several genes encoding proteins involved in Na+, phosphate, and energy homeostasis: i.e., Na+ pump P-type ATPase genes and their stabilizing factors (ENA1, ENA2, ENA5, and STF2 and its homologue, YLR327c) and PHO89, required for sodium-dependent phosphate uptake. In contrast, genes encoding acid phosphatases (PHO3, PHO11, and PHO12) were found to be down-regulated. Other genes found to be up-regulated upon Mg2+ deprivation are involved in cytoskeleton organization (ABP1, MTI1, RVS167, SAC6, and SRV2) and membrane synthesis (ARE2, ERG26, and PLB3). The proteins encoded by these genes might stabilize the cytoskeleton and membranes to compensate for the lack of Mg2+, as Mg2+ is known to be crucial in stabilizing the cell shape and for membrane integrity. Moreover, typical stress response genes were induced as well as genes for carbohydrate and amino acid metabolism, vacuolar protein degradation, and a large set of genes with known or unknown function, which do not form obvious functional clusters (see Table 1 for details).
TABLE 1.
Gene expression upon magnesium starvation
| Name and function | Identification no. | Induction or reduction | Coregulationa |
|---|---|---|---|
| Induction | |||
| Na, P, and energy homeostasis | |||
| ENA1 | YDR040C | 4.1 ± 1.5 | C A |
| ENA2 | YDR039C | 3.5 ± 1.3 | C |
| ENA5 | YDR038C | 3.1 ± 0.7 | C A |
| PMC1 | YGL006W | 5.5 ± 0.5 | C |
| PHO89 | YBR296C | 7.9 ± 2.5 | C A S |
| STF2 | YGR008C | 4.9 ± 2.0 | S |
| C metabolism | |||
| ARA1 | YBR149W | 2.9 ± 0.6 | |
| ATH1 | YPR026W | 2.7 ± 0.4 | S |
| GLK1 | YCL040W | 4.6 ± 1.2 | S |
| GPD1 | YDL022W | 3.2 ± 1.2 | S |
| MDH2 | YOL126C | 2.9 ± 0.6 | |
| TPS2 | YDR074W | 3.4 ± 0.7 | S |
| TSL1 | YML100W | 8.2 ± 3.1 | S |
| Amino acid metabolism | |||
| APE2 | YKL158W | 2.6 ± 0.3 | |
| ARO9 | YHR137W | 3.9 ± 1.5 | S |
| BNA2 | YJR078W | 3.9 ± 1.5 | C |
| GTT1 | YIR038C | 4.9 ± 1.9 | S |
| MET14 | YKL001C | 3.8 ± 1.7 | C A |
| MET3 | YJR010W | 3.9 ± 1.6 | C |
| Vacuolar protein degradation | |||
| ATG19 | YOL082W | 2.9 ± 0.5 | C S |
| PBI2 | YNL015W | 4.0 ± 1.7 | S |
| PEP12 | YOR036W | 4.5 ± 0.8 | C S |
| PEP4 | YPL154C | 4.5 ± 1.7 | A S |
| PRB1 | YEL060C | 9.3 ± 4.0 | C N S |
| Cytoskeleton organization | |||
| ABP1 | YCR088W | 3.1 ± 0.8 | |
| MTI1 | YJL020C | 3.5 ± 1.3 | |
| Membrane synthesis | |||
| ERG26 | YGL001C | 3.1 ± 0.9 | C |
| OSH6 | YKR003W | 3.0 ± 0.8 | |
| PLB3 | YOL011W | 3.6 ± 1.4 | N |
| SLI1 | YGR212W | 3.5 ± 1.2 | C S |
| Transporters | |||
| ENB1 | YOL158C | 2.9 ± 0.8 | C |
| MEP1 | YGR121C | 3.7 ± 0.6 | C N S |
| SUL1 | YBR294W | 2.8 ± 0.4 | |
| Stress response | |||
| DDR2 | YOL052C-A | 4.0 ± 1.9 | |
| DDR48 | YMR173W | 4.8 ± 1.6 | A S |
| GRX1 | YCL035C | 2.8 ± 0.8 | |
| HSP104 | YLL026W | 4.5 ± 1.3 | S |
| HSP42 | YDR171W | 4.0 ± 1.0 | S |
| SGE1 | YPR198W | 4.4 ± 1.7 | C |
| Others of known function | |||
| AKL1 | YBR059C | 2.2 ± 0.2 | |
| ATG5 | YPL149W | 3.8 ± 1.3 | C |
| CPS1 | YJL172W | 3.5 ± 1.2 | C N S |
| DCS1 | YLR270W | 2.7 ± 0.6 | S |
| DCS2 | YOR173W | 3.0 ± 0.5 | S |
| DIA1 | YMR316W | 9.3 ± 3.5 | C N S |
| ECM13 | YBL043W | 2.7 ± 0.2 | |
| ETR1 | YBR026C | 2.5 ± 0.2 | |
| FMS1 | YMR020W | 2.6 ± 0.5 | C |
| GPI18 | YBR004C | 3.2 ± 0.7 | C A |
| HAL5 | YJL165C | 3.3 ± 0.7 | N a |
| MRP8 | YKL142W | 3.4 ± 1.1 | S |
| PNC1 | YGL037C | 4.6 ± 1.2 | S |
| PTP2 | YOR208W | 2.9 ± 0.8 | |
| RCR1 | YBR005W | 6.3 ± 1.8 | C N S |
| REX3 | YLR107W | 3.7 ± 1.3 | |
| RPN7 | YPR108W | 2.8 ± 0.8 | |
| SOL4 | YGR248W | 2.7 ± 0.4 | S |
| TIS11 | YLR136C | 5.8 ± 2.0 | C A S |
| URA1 | YKL216W | 3.7 ± 1.3 | S |
| WTM1 | YOR230W | 4.2 ± 1.4 | a S |
| YPK1 | YKL126W | 3.4 ± 1.1 | C |
| YDL124W | 7.4 ± 2.9 | S | |
| Others of unknown function | |||
| FMP12 | YHL021C | 4.2 ± 1.3 | A a S |
| FMP46 | YKR049C | 2.6 ± 0.5 | |
| HOR7 | YMR251W-A | 7.4 ± 3.0 | A S |
| HUA1 | YGR268C | 4.1 ± 1.5 | C S |
| IML2 | YJL082W | 2.7 ± 0.4 | |
| MSC1 | YML128C | 2.6 ± 0.3 | A |
| ORM2 | YLR350W | 3.6 ± 1.5 | C |
| PIN3 | YPR154W | 4.1 ± 1.4 | S |
| PRM8 | YGL053W | 2.8 ± 0.7 | C |
| RTA1 | YGR213C | 6.6 ± 2.0 | C A |
| SRF4 | YDL023C | 2.9 ± 0.5 | |
| UBX6 | YJL048C | 2.7 ± 0.6 | |
| UIP3 | YAR027W | 3.3 ± 0.5 | C S |
| YSW1 | YBR148W | 2.9 ± 0.6 | |
| ZSP1 | YBR287W | 4.9 ± 2.0 | C A S |
| YLR327C | 5.1 ± 2.4 | C A S | |
| YDL010W | 3.2 ± 0.7 | C | |
| YDR391C | 4.3 ± 1.5 | S | |
| YEL074W | 4.9 ± 1.3 | ||
| YHR087W | 6.2 ± 2.9 | A | |
| YHR097C | 6.0 ± 0.6 | C N S | |
| YJL171C | 5.3 ± 2.3 | C N A S | |
| YKL151C | 3.1 ± 0.7 | S | |
| YLR414C | 11.0 ± 4.5 | C N S | |
| YNL208W | 7.7 ± 2.7 | C A S | |
| YOR220W | 16.3 ± 1.5 | C N S | |
| YOR289W | 2.9 ± 0.7 | S | |
| YOR385W | 3.1 ± 0.8 | C N S | |
| YCL042W | 4.5 ± 0.3 | S | |
| YDL011C | 2.7 ± 0.3 | ||
| YGL165C | 2.5 ± 0.2 | C N | |
| YGR110W | 2.6 ± 0.4 | C | |
| YIL055C | 3.1 ± 0.5 | ||
| YJL015C | 4.2 ± 0.4 | ||
| YJL016W | 4.3 ± 0.9 | C | |
| YJL144W | 3.6 ± 1.4 | S | |
| YJL152W | 3.0 ± 0.9 | ||
| YLR162W | 3.6 ± 0.5 | ||
| YLR194C | 11.5 ± 3.0 | C N | |
| YMR007W | 4.2 ± 1.0 | C | |
| YMR102C | 2.6 ± 0.4 | ||
| YMR304C-A | 3.3 ± 1.0 | C A s | |
| YNL092W | 4.3 ± 1.3 | C | |
| YNL115C | 3.9 ± 1.5 | ||
| YNL134C | 3.5 ± 0.8 | A S | |
| YOL048C | 2.9 ± 0.8 | ||
| YOR152C | 2.3 ± 0.2 | ||
| YPR197C | 3.0 ± 0.5 | ||
| Reduction | |||
| P homeostasis | |||
| PHO3 | YBR092C | −3.2 ± 1.2 | |
| PHO5 | YBR093C | −2.9 ± 0.9 | s |
| PHO12 | YHR215W | −3.4 ± 1.4 | A s |
| Others | |||
| YHB1 | YGR234W | −4.5 ± 1.4 | |
| YLR413W | −2.8 ± 0.7 | s |
A striking number of genes up-regulated upon Mg2+ starvation are also up-regulated under one ore more of the following conditions: Ca2+ stress, Na+ stress (57), or alkalization of the growth medium (45, 52) (see Table 1). In particular, of the 112 genes significantly up-regulated (± standard deviation of ≥2) 42 (38%) are known to be up-regulated by Ca2+ and 13 (12%) by high Na+, of which 11 are also induced by Ca2+, giving a total of 44 out of 112 genes (39%) up-regulated by temporal Mg2+ deprivation and also by short-term Ca2+ and/or Na+ stress. Furthermore, many of the genes induced/repressed by Mg2+ starvation seem to respond to general stress conditions as they are similarly regulated under various conditions of stress (16) (Table 1).
ENA1 and PHO89 transcripts are rapidly induced upon Mg2+ starvation.
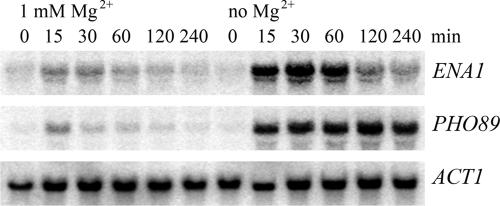
To confirm that induction of transcripts is due to depletion of Mg2+ and to investigate the kinetics of transcriptional induction by Mg2+ starvation, we performed Northern blot analyses of two genes highly induced in the microarray experiment, ENA1, which encodes a P-type ATPase (19), and PHO89, a gene required for phosphate uptake (28). For this experiment, two cultures were grown under identical conditions. One culture was washed and resuspended in medium lacking Mg2+ as described in Materials and Methods, and the other was mock treated with medium containing 1 mM Mg2+. As shown in Fig. 1, both ENA1 and PHO89 were indeed highly induced within 15 min of Mg2+ starvation (lanes 8 to 12), whereas only a very small transient increase of ENA1 and PHO89 transcript levels can be observed in cultures treated the same way with the regular growth medium (1 mM Mg2+). This small response in the control samples is probably due to the treatment of the cells (centrifugation and supply of fresh medium). While PHO89 mRNA steady-state levels remained high for at least 4 h after Mg2+ depletion, ENA1 induction appeared more temporal as the gene expression was again down-regulated after 2 h. Thus, at least for certain genes, this induction by Mg2+ depletion appears transient.
FIG. 1.
ENA1 and PHO89 transcripts are induced upon Mg2+ starvation. Strain JS034-4C was grown in synthetic medium containing 1 mM Mg2+ to an OD600 of 0.5, washed twice with SD medium containing either 1 mM Mg2+ or lacking Mg2+, and then incubated in the same medium. Samples were drawn at the indicated time points, and total RNA was prepared. Twenty-five micrograms of total RNA was loaded per lane, and Northern blot analysis was performed using radiolabeled probes specific for ENA1, PHO89, and ACT1 as a loading control.
Induction of ENA1 and PHO89 transcription by low Mg2+ is mediated by the transcription factor Crz1p and requires calcineurin signaling.
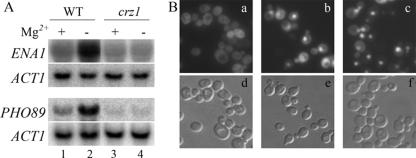
Many of the genes induced by Mg2+ starvation have previously been shown to be under the control of the calcineurin-dependent transcription factor Crz1p: i.e., the calcineurin/Crz1p-dependent signaling pathway (9, 57). Thus, we investigated whether transcriptional induction upon Mg2+ starvation is dependent on the transcription factor Crz1p. For this purpose, Mg2+ starvation and subsequent Northern blot analysis were performed on wild-type and crz1Δ strains. As seen in the Northern blot (Fig. 2A, lanes 1 and 2), both the ENA1 and PHO89 transcripts were increased by Mg2+ starvation. No such induction was observed in the crz1Δ cells (lanes 3 and 4). Moreover, steady state levels of ENA1 and PHO89 were slightly lower in crz1Δ cells grown in regular medium compared to the isogenic wild type (compare lanes 1 and 3).
FIG. 2.
The transcription factor Crz1p is required for the induction of ENA1 and PHO89 and translocated to the nucleus upon Mg2+ starvation. (A) Levels of expression of ENA1 and PHO89 in wild-type (WT [BY4741]) (lanes 1 and 2) and crz1Δ (lanes 3 and 4) cells were compared under standard conditions (1 mM Mg2+, lanes 1 and 3) or 70 min of Mg2+ starvation (lanes 2 and 4). Northern blot analysis is shown. (B) Strain BY4741-CRZ1-GFP was grown at 28°C to log-phase in SD −Ura medium containing 1 mM MgCl2 (a and d) and then washed and incubated for 10 min at 28°C in SD −Ura medium containing either no MgCl2 (b and e) or 1 mM MgCl2-200 mM CaCl2-50 mM MES (pH 6) (c and f) before cells were harvested for microscopy and analyzed.
The activity of Crz1p is regulated by the Ca2+/calmodulin-dependent protein phosphatase calcineurin. Dephosphorylation of the transcription factor Crz1p results in its translocation to the nucleus, where it becomes active (36, 49). To determine whether Mg2+ starvation also promotes nuclear localization of Crz1p, we followed the localization of GFP-tagged Crz1p (36) under standard and Mg2+ starvation conditions, respectively. GFP-Crz1p is located in the cytoplasm under normal growth conditions (Fig. 2B, a and d). Yet, when the cells were shifted to Mg2+-free medium, nuclear accumulation of GFP-tagged Crz1p was observed within 10 min (Fig. 2B, b and e). The same was true when cells were challenged with 200 mM Ca2+ (Fig. 2B, c and f), indicating that under both circumstances Crz1p is targeted to the nucleus to induce its target genes. However, it has to be noted that more GFP-Crz1p stays in the cytoplasm upon Mg2+ depletion than under Ca2+ stress. This observation is reminiscent of Crz1p nuclear translocation upon Na+ (800 mM) or mild Ca2+ (≤150 mM) stress, where only partial translocation to the nucleus had been seen (49). When cells were mock treated (centrifuged and washed) with fresh medium containing 1 mM MgCl2, GFP-Crz1p stayed in the cytoplasm (data not shown). Neither GFP alone, nor an Msn2-GFP fusion protein was translocated to the nucleus upon Mg2+ depletion (data not shown). Msn2, a transcriptional activator related to Msn4p, is activated under stress conditions, resulting in its translocation from the cytoplasm to the nucleus, where it binds DNA at stress response elements of responsive genes, inducing gene expression (http://www.yeastgenome.org).
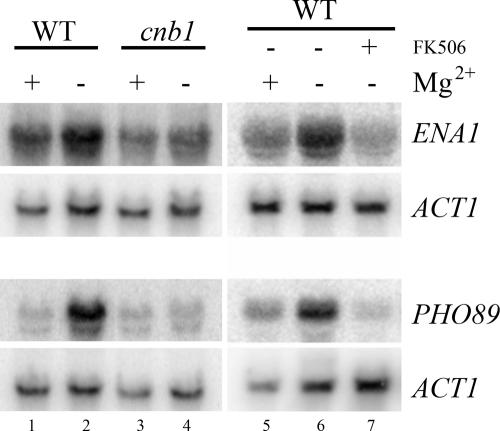
To ascertain whether transcriptional induction of ENA1 and PHO89 is also dependent on calcineurin, the phosphatase responsible for dephosphorylation of Crz1p, we performed Mg2+ starvation experiments in the calcineurin mutant (cnb1Δ) and in wild-type cells in the presence of the calcineurin inhibitor FK506. As shown in Fig. 3, transcriptional activation of ENA1 and PHO89 was indeed dependent on calcineurin function: while the wild-type cells starved for Mg2+ showed normal induction of both transcripts (Fig. 3, lanes 1, 2, 5, and 6), cnb1Δ cells (Fig. 3, lanes 3 and 4) or cells pretreated with FK506 did not induce the two transcripts upon Mg2+ depletion (Fig. 3, lane 7). Treatment of the cells with only dimethyl sulfoxide (the solvent for FK506) did not abolish the transcriptional response to Mg2+ depletion (data not shown). Similarly, addition of FK506 to the control experiment (1 mM Mg2+) did not affect expression (data not shown). Taken together, our results show that removal of Mg2+ from the growth medium results in activation of the calcineurin/Crz1p signaling pathway.
FIG. 3.
The transcriptional response to Mg2+ starvation is dependent on calcineurin. Expression of ENA1 and PHO89 upon Mg2+ starvation (70 min) was followed in cnb1Δ mutant cells and in wild-type (WT) cells in the presence or absence of the calcineurin inhibitor FK506 (1.25 μM), which was added to the culture 10 min prior to the washes. Northern blot analysis is shown.
Cells lacking calcineurin/Crz1p signaling are sensitive to low Mg2+.
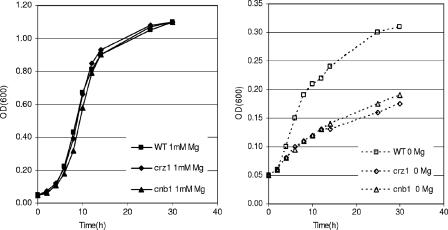
We have shown that yeast cells react to Mg2+ deprivation by activating a number of genes via the calcineurin/Crz1p pathway. To investigate whether this response is necessary for cells to cope with low-Mg2+ stress, we analyzed the growth on Mg2+-depleted medium of mutants defective for calcineurin signaling. As shown in Fig. 4 (left panel), growth of cells lacking either Crz1p or Cnb1p (calcineurin B), the regulatory subunit of calcineurin, is indistinguishable from that of wild-type cells on synthetic medium containing standard magnesium concentrations. In contrast, when Mg2+ is omitted from the medium crz1Δ or cnb1Δ mutants exhibit clearly reduced growth compared to the wild type (Fig. 4, right panel), indicating that the induction of the calcineurin pathway is important for cells to cope with Mg2+ depletion.
FIG. 4.
crz1Δ and cnb1Δ cells are sensitive to low Mg2+. BY4741 wild-type (WT) and crz1Δ and cnb1Δ mutant cells were cultured in synthetic SD medium containing 1 mM Mg2+ overnight, washed three times in distilled H2O, and then inoculated (OD600 of 0.05) into synthetic SD medium containing 1 mM Mg2+ (left panel) or no Mg2+ (right panel). Cells were incubated at 28°C with shaking, and growth was followed by measuring the OD600.
External Ca2+ is required for the induction of ENA1 and PHO89 in response to low Mg2+.
Next, we investigated the role of Ca2+ in the calcineurin/Crz1p dependent up-regulation of ENA1 and PHO89 upon Mg2+ depletion. While in animal cells, the endoplasmic reticulum is the major site of intracellular Ca2+ storage and release, this does not seem to be the case for yeast (50). The two major sources for calcium in yeast are the vacuole and the medium (12, 41). Therefore, we asked whether the source of the calcium signal is outside the cell. We compared the ENA1 and PHO89 induction from cells grown in medium containing standard concentrations of Ca2+ with those grown in medium containing the Ca2+ chelator EGTA (Fig. 5). When 10 mM EGTA was added to the medium before and after the shift to Mg2+-free medium, the induction was completely abolished (Fig. 5, lanes 3 and 4), as compared to standard Ca2+ conditions (Fig. 5, lanes 1 and 2). Thus, we conclude that external calcium contributes to the transcriptional induction by Mg2+ depletion and that external calcium can indeed become limiting for the response, as the signal is completely abolished when EGTA is added to the media.
FIG. 5.
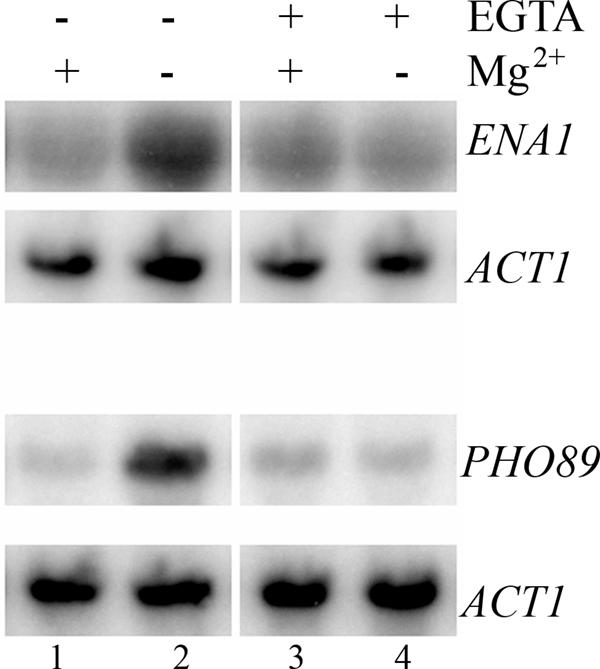
External Ca2+ is required for the induction of ENA1 and PHO89 upon Mg2+ starvation. Expression of ENA1 and PHO89 upon Mg2+ starvation (70 min) was followed in the presence or absence of the Ca2+ chelator EGTA (10 mM). Northern blot analysis is shown.
Cytoplasmic Ca2+ levels increase upon Mg2+ depletion with dependence on external Ca2+.
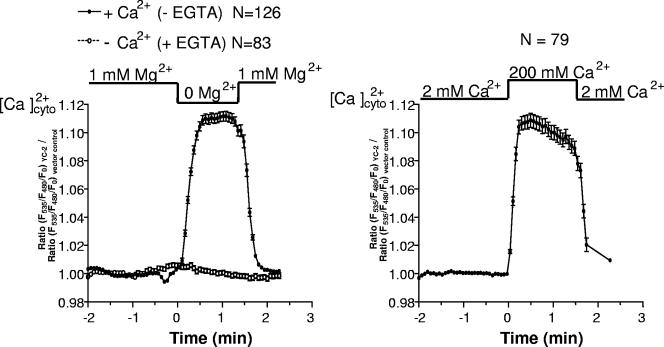
Since removal of Mg2+ from the medium resulted in activation of the calcineurin/Crz1p pathway and was dependent on external Ca2+, we wanted to know if this was caused by an increase in cytosolic Ca2+ concentration upon Mg2+ removal. A number of external stimuli, such as Ca2+ or Na+ stress, alkalinization of the growth medium, alpha factor, and hyper- and hypo-osmotic stress have so far been shown to trigger a rise in cytoplasmic Ca2+ (3, 11, 32, 52, 57). To determine cytoplasmic Ca2+ concentrations, we expressed the cytosol-targeted Ca2+-sensing protein YC2-12 (30) in yeast under the strong constitutive ADH1 promoter. This sensor is a member of the “cameleon family” of Ca2+ indicators and consists of tandem fusions of a cyan-emitting fluorescent protein, calmodulin, the calmodulin-binding peptide M13, and a yellow-emitting fluorescent protein. Upon binding of Ca2+ to calmodulin, this protein binds to the M13 domain, resulting in an increase in the fluorescence resonance energy transfer between the flanking fluorophores. To show that the sensor is working in yeast, we challenged cells with 200 mM external Ca2+. This treatment resulted in a cytoplasmic Ca2+ peak (Fig. 6, right panel). Next, we investigated cytosolic Ca2+ upon Mg2+ depletion. As shown in Fig. 6 (left panel), removal of extracellular Mg2+ resulted in a rapid increase of the cytosolic Ca2+ concentration in the presence of 2 mM external Ca2+. This signal was reversible and normalized upon readdition of Mg2+ into the external solution. In contrast, in the absence of extracellular Ca2+ (i.e., Ca2+-free buffer containing 10 mM EGTA), no cytosolic Ca2+ elevation was obtained in response to Mg2+ removal. These data indicate that the cytoplasmic elevation of Ca2+ in response to the removal of external Mg2+ essentially depends on the presence of extracellular Ca2+, strongly indicating that an influx of Ca2+ is occurring under these conditions. The cytoplasmic Ca2+ elevation upon removal of extracellular Mg2+ closely matched the peak obtained in response to 200 mM external Ca2+.
FIG. 6.
Cytoplasmic Ca2+ levels increase upon Mg2+ depletion in dependence of external Ca2+. Cytosolic free Ca2+ concentrations were analyzed upon Mg2+ depletion (left panel) or upon addition of 200 mM Ca2+ (right panel) using fluorescence resonance energy transfer microscopy.
DISCUSSION
Interactions of Ca2+ and Mg2+.
Cellular Ca2+ and Mg2+ levels appear linked in many circumstances, such that high Mg2+ results in low Ca2+ and vice versa. Thus, internal Mg2+ or Ca2+ concentrations can be reciprocally modulated by altering medium concentrations of Mg2+ or Ca2+, by hormone stimulation, or by mutations of ion transporters (5, 17, 31). There are Ca2+-dependent Mg2+ transporters, possibly antiporters, present in hepatocytes (13, 39), as well as a Ca2+/Mg2+ exchanger in the apical rat liver plasma membrane (7). Also, Mg2+ acts at an extracellular site on L-type Ca2+ channels to regulate Ca2+ influx (2); the same is found on T-type Ca2+ channels (44). There is evidence that this modulatory effect of Mg2+ involves the EF-hand motif of the COOH-terminus of Ca2+ channels (6). In the present study, we found that the transcriptomal response of Mg2+ depletion is very similar to the Ca2+ stress response: i.e., yeast cells respond to Mg2+ withdrawal from growth medium with an immediate upshift of intracellular Ca2+ concentrations; activation of the transcription factor Crz1p via calcineurin, a Crz1p phosphatase; and the upregulation of a gene set known to be under the control of the calcineurin/Crz1p pathway. Mg2+ depletion thus elicits a response which is widely similar to that of Ca2+ stress, high Na+, or alkalinization.
Induction of the calcineurin/Crz1p pathway by low-Mg2+ stress.
This study commenced with the observation that short-term Mg2+ depletion in the yeast S. cerevisiae induces a number of genes known to be calcineurin/Crz1p regulated (45, 52, 57). This induction is immediate and transient. Crz1p is the vital transcription factor for Ca2+ signaling in yeast. Crz1p contains a zinc finger motif for DNA binding and binds specifically to the calcineurin-dependent response element, a 24-bp DNA sequence both necessary and sufficient for Ca2+-induced, calcineurin-dependent gene expression (48). The conserved Ca2+/calmodulin-regulated protein phosphatase calcineurin dephosphorylates and thereby activates Crz1p (49). Calcineurin is inhibited by the immunosuppressive drugs FK506 and cyclosporine and is essential for the antigen-dependent activation of T lymphocytes in higher eukaryotes (8, 25, 34). When dephosphorylated by calcineurin, Crz1p translocates from the cytoplasm to the nucleus, where it binds its target DNA in order to activate downstream genes (49; reviewed in reference 9). Similarly, we found that Crz1p shifted to the nucleus also upon Mg2+ depletion, as in the case of Ca2+ or Na+ stress (57), and Crz1p target genes were upregulated. Since this effect was missing in the calcineurin mutant and was inhibited by the calcineurin inhibitor FK506, dephosphorylation of Crz1p by calcineurin is a prerequisite. Taken together, short-term Mg2+ depletion results in the induction of the calcineurin/Crz1p pathway and requires both calcineurin and Crz1p function. This response is necessary for yeast cells to function properly under conditions of Mg2+ depletion, as calcineurin/Crz1p pathway mutants displayed reduced growth in Mg2+-depleted medium. The parallel induction of Crz1p by Mg2+ shortage and Ca2+ stress is reminiscent of the regulation of the transcription factor Aft1p, which, upon iron shortage as well as Co2+ stress, moves to the nucleus and induces its target genes (47, 55). In both instances, shortage of the more abundant ions (iron/Mg2+) mimics the effects induced by imbalances in the respective ions at lower physiological concentrations (Co2+/Ca2+). Also in both cases, these distinct responses confer resistance to the stress induced by metal ion shortage, or overexposure, respectively.
Mg2+ depletion induces rapid Ca2+ influx.
Depletion of external Mg2+ concentrations has no obvious short-term effect on intracellular Mg2+ concentrations (4, 17). Accordingly, the signal for switching on the calcineurin/Crz1 pathway is likely to arise from effects of low Mg2+ at the cell surface and to be mediated through the cell membrane. K+ and Ca2+ channels have been reported to open when Mg2+ falls below threshold concentrations (2, 33, 44, 56), and an effect of Mg2+ withdrawal on the opening of Ca2+ channels and influx of Ca2+ thus appears to be likely. In fact, we have demonstrated here that external Ca2+ is essential for the induction of Crz1p target genes by Mg2+ depletion. Moreover, upon Mg2+ depletion external Ca2+ is rapidly internalized, leading to high cytoplasmic Ca2+ similar to that observed in the presence of high Ca2+ concentrations in the medium.
High intracellular Ca2+ may also be helpful for the release of internal Mg2+ stores, perhaps maintaining the availability of free Mg2+ upon medium Mg2+ depletion.
Ca2+/calcineurin signaling is conserved from yeast to mammals. Activation of Crz1p by calcineurin is reminiscent of the regulation of the mammalian NFAT1 to -4 transcription factor proteins by Ca2+/calcineurin-dependent signaling. Similar to the situation in yeast, a rise in intracellular Ca2+ activates calcineurin in mammals, which in turn dephosphorylates all four NFAT proteins, leading to their rapid nuclear import (8, 22). Depending upon which binding partners are involved Ca2+/calcineurin-NFAT-mediated signaling pathways regulate gene expression either positively or negatively. Binding partners can be AP-1 (composed of Fos and Jun proteins), MEF2, GATA proteins, and histone deacetylases (21). Especially with respect to diseases associated with altered serum Mg2+ levels (hypomagnesemia and hypermagnesemia), it will be interesting to learn whether changes in the serum Mg2+ availability also influence Ca2+ signaling in higher eukaryotes.
Acknowledgments
We thank M. Iliev for technical support and D. F. Steele, C. Schüller, and G. Brunt for many helpful suggestions. We are grateful to Fujisawa GmbH (Munich) for providing FK506.
This work was supported by the Austrian Science Fund (FWF).
Footnotes
Published ahead of print on 2 March 2007.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology, p. 13.12.1-13.12.5. J. Wiley and Sons, New York, NY.
- 2.Bara, M., and A. Guit-Bara. 2001. Magnesium regulation of Ca2+ channels in smooth muscle and endothelial cells of human allantochorial placental vessels. Magnes. Res. 14:11-18. [PubMed] [Google Scholar]
- 3.Batiza, A. F., T. Schulz, and P. H. Masson. 1996. Yeast respond to hypotonic shock with a calcium pulse. J. Biol. Chem. 271:23357-23362. [DOI] [PubMed] [Google Scholar]
- 4.Beeler, T., K. Bruce, and T. Dunn. 1997. Regulation of cellular Mg2+ by Saccharomyces cerevisiae. Biochim. Biophys. Acta 1323:310-318. [DOI] [PubMed] [Google Scholar]
- 5.Beeler, T., K. Gable, C. Zhao, and T. Dunn. 1994. A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae. J. Biol. Chem. 269:7279-7284. [PubMed] [Google Scholar]
- 6.Brunet, S., T. Scheuer, R. Klevit, and W. A. Catterall. 2005. Modulation of CaV1.2 channels by Mg2+ acting at an EF-hand motif in the COOH-terminal domain. J. Gen. Physiol. 126:311-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cefaratti, C., A. Romani, and A. Scarpa. 2000. Differential localization and operation of distinct Mg2+ transporters in apical and basolateral sides of rat liver plasma membrane. J. Biol. Chem. 275:3772-3880. [DOI] [PubMed] [Google Scholar]
- 8.Clipstone, N. A., and G. R. Crabtree. 1992. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature 357:695-697. [DOI] [PubMed] [Google Scholar]
- 9.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 311:1143-1150. [DOI] [PubMed] [Google Scholar]
- 10.Dai, L. J., G. Ritchie, D. Kerstan, H. S. Kang, D. E. Cole, and G. A. Quamme. 2001. Magnesium transport in the renal distal convoluted tubule. Physiol. Rev. 81:51-84. [DOI] [PubMed] [Google Scholar]
- 11.Denis, V., and M. S. Cyert. 2002. Internal Ca2+ release in yeast is triggered by hypertonic shock and mediated by a TRP channel homologue. J. Cell Biol. 156:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn, T., K. Gable, and T. Beeler. 1994. Regulation of cellular Ca2+ by yeast vacuoles. J. Biol. Chem. 269:7273-7278. [PubMed] [Google Scholar]
- 13.Fagan, T., and A. Romani. 2000. Activation of Na+- and Ca2+-dependent Mg2+ extrusion by alpha(1)- and beta-adrenergic agonists in rat liver cells. Am. J. Physiol. 279:G943-G950. [DOI] [PubMed] [Google Scholar]
- 14.Frieden, M., R. Malli, M. Samardzija, N. Demaurex, and W. F. Graier. 2002. Subplasmalemmal endoplasmic reticulum controls K(Ca) channel activity upon stimulation with a moderate histamine concentration in a human umbilical vein endothelial cell line. J. Physiol. 540:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gardner, R. C. 2003. Genes for magnesium transport. Curr. Opin. Plant Biol. 6:263-267. [DOI] [PubMed] [Google Scholar]
- 16.Gasch, A. P., P. T. Spellman, C. M. Kao, O. Carmel-Harel, M. B. Eisen, G. Storz, D. Botstein, and P. O. Brown. 2000. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 11:4241-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graschopf, A., J. A. Stadler, M. K. Hoellerer, S. Eder, M. Sieghardt, S. D. Kohlwein, and R. J. Schweyen. 2001. The yeast plasma membrane protein Alr1 controls Mg2+ homeostasis and is subject to Mg2+-dependent control of its synthesis and degradation. J. Biol. Chem. 276:16216-16222. [DOI] [PubMed] [Google Scholar]
- 18.Günther, T. 1993. Mechanisms and regulation of Mg2+ efflux and Mg2+ influx. Miner. Electrolyte Metab. 19:259-265. [PubMed] [Google Scholar]
- 19.Haro, R., B. Garciadeblas, and A. Rodriguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 20.Iida, H., Y. Yagawa, and Y. Anraku. 1990. Essential role for induced Ca2+ influx followed by [Ca2+]i rise in maintaining viability of yeast cells late in the mating pheromone response pathway. A study of [Ca2+]i in single Saccharomyces cerevisiae cells with imaging of fura-2. J. Biol. Chem. 285:13391-13399. [PubMed] [Google Scholar]
- 21.Im, S. H., and A. Rao. 2004. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol. Cells 18:1-9. [PubMed] [Google Scholar]
- 22.Klee, C. B., H. Ren, and X. Wang. 1998. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J. Biol. Chem. 273:13367-13370. [DOI] [PubMed] [Google Scholar]
- 23.Knoop, V., M. Groth-Malonek, M. Gebert, K. Eifler, and K. Weyand. 2005. Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genomics 274:205-216. [DOI] [PubMed] [Google Scholar]
- 24.Kolisek, M., G. Zsurka, J. Samaj, J. Weghuber, R. J. Schweyen, and M. Schweigel. 2003. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J. 22:1235-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, J., J. J. D. Farmer, W. L. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-CsA and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 26.Malli, R., M. Frieden, M. Trenker, and W. F. Graier. 2005. The role of mitochondria for Ca2+ refilling of the ER. J. Biol. Chem. 280:12114-12122. [DOI] [PubMed] [Google Scholar]
- 27.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 28.Martinez. P., and B. L. Persson. 1998. Identification, cloning and characterization of a derepressible Na+-coupled phosphate transporter in Saccharomyces cerevisiae. Mol. Gen. Genet. 258:628-638. [DOI] [PubMed] [Google Scholar]
- 29.Michailova, A., J. Saucerman, M. E. Belik, and A. D. McCulloch. 2005. Modeling regulation of cardiac KATP and L-type Ca2+ currents by ATP, ADP, and Mg2+. Biophys. J. 88:2234-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyawaki, A., J. Llopis, R. Heim, J. M. McCaffery, J. A. Adams, M. Ikura, and R. Y. Tsien. 1997. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388:882-887. [DOI] [PubMed] [Google Scholar]
- 31.Mooren, F. C., S. Turi, D. Gunzel, W. R. Schlue, W. Domschke, J. Singh, and M. M. Lerch. 2001. Calcium-magnesium interactions in pancreatic acinar cells. FASEB J. 15:659-672. [DOI] [PubMed] [Google Scholar]
- 32.Muller, E. M., N. A. Mackin, S. E. Erdman, and K. W. Cunningham. 2003. Fig 1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 278:38461-38469. [DOI] [PubMed] [Google Scholar]
- 33.Nilius, B., and T. Voets. 2004. Diversity of TRP channel activation. Novartis Found. Symp. 258:140-149. [PubMed] [Google Scholar]
- 34.O'Keefe, S. J., J. Tamura, R. L. Kincaid, M. J. Tocci, and E. A. O'Neill. 1992. FK506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature 357:692-694. [DOI] [PubMed] [Google Scholar]
- 35.Petrault, I., W. Zimowska, J. Mathieu, D. Bayle, E. Rock, A. Favier, Y. Rayssiguier, and A. Mazur. 2002. Changes in gene expression in rat thymocytes identified by cDNA array support the occurrence of oxidative stress in early magnesium deficiency. Biochim. Biophys. Acta 1586:92-98. [DOI] [PubMed] [Google Scholar]
- 36.Polizotto, R., and M. S. Cyert. 2001. Calcineurin-dependent nuclear import of the transcription factor Crz1p requires Nmd5p. J. Cell Biol. 154:951-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romani, A. 2007. Regulation of magnesium homeostasis and transport in mammalian cells. Arch. Biochem. Biophys. 458:90-102. (First published 7 August 2006; doi: 10.1016/j.abb.2006.07.012.) [DOI] [PubMed] [Google Scholar]
- 38.Romani, A., and A. Scarpa. 1992. Regulation of cell magnesium. Arch. Biochem. Biophys. 298:1-12. [DOI] [PubMed] [Google Scholar]
- 39.Romani, A., C. Marfella, and A. Scarpa. 1993. Hormonal stimulation of Mg2+ uptake in hepatocytes. Regulation by plasma membrane and intracellular organelles. J. Biol. Chem. 268:15489-15495. [PubMed] [Google Scholar]
- 40.Romani, A. M., and A. Scarpa. 2000. Regulation of cellular magnesium. Front. Biosci. 5:D720-D734. [DOI] [PubMed] [Google Scholar]
- 41.Rudolph, H. K., A. Antebi, G. R. Fink, C. M. Buckley, T. E. Dorman, J. Levitre, L. S. Davidow, J. Mao, and D. T. Moir. 1989. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell 58:133-145. [DOI] [PubMed] [Google Scholar]
- 42.Schlingmann, K. P., and T. Gudermann. 2005. A critical role of TRPM channel-kinase for human magnesium transport. J. Physiol. 566:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz, C., A. L. Perraud, C. O. Johnson, K. Inabe, M. K. Smith, R. Penner, T. Kurosaki, A. Fleig, and A. M. Scharenberg. 2003. Regulation of vertebrate cellular Mg2+ homeostasis by TRPM7. Cell 114:191-200. [DOI] [PubMed] [Google Scholar]
- 44.Serrano, J., S. R. Dashti, E. Perez-Reyes, and S. W. Jones. 2000. Mg2+ block unmasks Ca2+/Ba2+ selectivity of alpha1G T-type calcium channels. Biophys. J. 79:3052-3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Arino. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319-1333. [DOI] [PubMed] [Google Scholar]
- 46.Sherman, F. 1991. Methods Enzymol. 194:3-21. [DOI] [PubMed] [Google Scholar]
- 47.Stadler, J. A., and R. J. Schweyen. 2002. The yeast iron regulon is induced upon cobalt stress and crucial for cobalt tolerance. J. Biol. Chem. 277:39649-39654. [DOI] [PubMed] [Google Scholar]
- 48.Stathopoulos, A. M., and M. S. Cyert. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11:3432-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stathopoulos-Gerontides, A., J. Guo, and M. S. Cyert. 1999. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes Dev. 13:798-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strayle, J., T. Pozzan, and H. K. Rudolph. 1999. Steady-state free Ca2+ in the yeast endoplasmic reticulum reaches only 10 μM and is mainly controlled by the secretory pathway pump pmr1. EMBO J. 18:4733-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vernet, T., D. Dignard, and D. Y. Thomas. 1987. A family of yeast expression vectors containing the phage f1 intergenic region. Gene 52:225-233. [DOI] [PubMed] [Google Scholar]
- 52.Viladevall, L., R. Serrano, A. Ruiz, G. Domenech, J. Giraldo, A. Barcelo, and J. Arino. 2004. Characterization of the calcium-mediated response to alkaline stress in Saccharomyces cerevisiae. J. Biol. Chem. 279:43614-43624. [DOI] [PubMed] [Google Scholar]
- 53.Wachek, M., M. C. Aichinger, J. A. Stadler, R. J. Schweyen, and A. Graschopf. 2006. Oligomerization of the Mg2+-transport proteins Alr1p and Alr2p in yeast plasma membrane. FEBS J. 273:4236-4249. [DOI] [PubMed] [Google Scholar]
- 54.Weghuber, J., F. Dieterich, E. M. Froschauer, S. Svidovà, and R. J. Schweyen. 2006. Mutational analysis of functional domains in Mrs2p, the mitochondrial Mg2+ channel protein of Saccharomyces cerevisiae. FEBS J. 273:1198-1209. [DOI] [PubMed] [Google Scholar]
- 55.Yamaguchi-Iwai, Y., R. Ueta, A. Fukunaka, and R. Sasaki. 2002. Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J. Biol. Chem. 277:18914-18918. [DOI] [PubMed] [Google Scholar]
- 56.Yamaoka, K., and M. Kameyama. 2003. Regulation of L-type Ca2+ channels in the heart: overview of recent advances. Mol. Cell Biochem. 253:3-13. [DOI] [PubMed] [Google Scholar]
- 57.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 277:31079-31088. [DOI] [PubMed] [Google Scholar]