Abstract
Moraxella catarrhalis causes acute otitis media in children and lower respiratory tract infections in adults and elderly. In children the presence of antibodies against the highly conserved outer membrane protein CD correlates with protection against infection, suggesting that this protein may be useful as a vaccine antigen. However, native CD is difficult to purify, and it is still unclear if recombinant CD (rCD) is a valid alternative. We performed a side-by-side comparison of the immunogenicities and efficacies of vaccine formulations containing native CD and rCD with adamantylamide dipeptide as the mucosal adjuvant. Intranasal vaccination of mice stimulated the production of high CD-specific antibody titers in sera and of secretory immunoglobulin A in mucosal lavages, which cross-recognized both antigens. While vaccination with native CD increased the number of interleukin-2 (IL-2)- and gamma interferon-producing cells, rCD mainly stimulated IL-4-secreting cells. Nevertheless, efficient bacterial clearance was observed in the lungs of challenged mice receiving native CD and in the lungs of challenged mice receiving rCD (96% and 99%, respectively). Thus, rCD is a promising candidate for incorporation in vaccine formulations for use against M. catarrhalis.
In the last two decades, Moraxella catarrhalis has emerged as an important mucosal pathogen (35). In children, it is one of the etiological agents of sinusitis, bronchitis, pneumonia, and acute otitis media (18, 23). In our hospital, between 1994 and 2001 the main bacterial etiological agents isolated from middle ear fluids of children were Haemophilus influenzae (45%) and Streptococcus pneumoniae (39%); the percentages of these organisms remained almost constant during this period, whereas the incidence of M. catarrhalis increased from 4 to 11%. In adults, M. catarrhalis is one of the etiological agents of recurrent infections, particularly in patients with chronic obstructive pulmonary disease, and is responsible for approximately 30% of the new cases (37). The clinical management of patients infected with M. catarrhalis also is a problem, since high costs are associated with established therapies and there is global emergence of antibiotic-resistant strains (35). Therefore, a vaccine able to block bacterial infection at the mucosal level would be an invaluable tool.
There are eight major outer membrane proteins of M. catarrhalis, and proteins C and D are two different stable forms of the same protein (11, 35). A single gene codes for a protein with a predicted molecular mass of 46 kDa, which migrates as a doublet during sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) at approximately 60 kDa (2, 28). CD is an integral porin-like protein that plays a role in bacterial attachment to host cell-associated mucins, promoting bacterial ascension through the Eustachian tube (15, 20, 21). The CD protein is considered a promising vaccine candidate because it is surface exposed and highly conserved (17, 30). The presence of exposed epitopes on its surface suggests that antibodies could bind CD on the intact bacteria (24, 30). The importance of antibody responses directed against CD is further supported by clinical evidence. In fact, patients who recover from an M. catarrhalis infection with high levels of antibodies against CD are less susceptible to reinfection than patients with low levels of antibodies or no antibodies against CD (22, 26).
Thus, the potential of CD as a candidate vaccine antigen has been explored in the past. Purified CD protein induced antibodies in guinea pigs and mice that not only bound to intact M. catarrhalis but also exhibited in vitro bactericidal activity against the pathogen (41). However, the fastidious growth properties of M. catarrhalis and the relatively poor expression of this protein make large-scale production of native CD (nCD) particularly difficult. Therefore, production of a recombinant CD protein (rCD) is the only valid alternative for mass vaccine production. In this context, a previous report suggested that rCD might be a potentially useful candidate antigen (20, 27). However, side-by-side comparisons between the recombinant and native antigens were not performed, which made it extremely difficult to assess whether rCD is indeed a valid alternative. In addition, this study was performed by injecting the rCD emulsified with incomplete Freund's adjuvant into Peyer's patches (27), thereby making it more difficult to predict responses to standard vaccination schedules in humans.
It was demonstrated previously that intranasally administered antigens trigger better immune responses in the respiratory tract and in the middle ear than antigens administered orally or parenterally trigger (16). Thus, it seems particularly attractive to assess the potential of a CD-based formulation administered by the intranasal route, using a mucosal challenge model with bacterial clearance as the read-out. Unfortunately, the use of this route generally induces relatively poor immune responses, with the exception of naturally acquired infections. However, this can be overcome by use of mucosal adjuvants.
We previously demonstrated that the mucosal adjuvant adamantylamide dipeptide (AdDP) (3, 5) enhances the immune responses against the outer membrane protein of H. influenzae P6 when it is coadministered by the intranasal route. This coadministration led to elicitation of a protective response against pulmonary or middle ear challenge with virulent bacteria (5). Thus, in the present work we performed a side-by-side comparison of the immunogenicities and efficacies of vaccine formulations containing nCD and rCD with AdDP as the mucosal adjuvant. The results obtained demonstrated that a candidate vaccine based on rCD and AdDP stimulates an immune response able to promote efficient bacterial clearance after pulmonary challenge of mice with a virulent M. catarrhalis strain.
MATERIALS AND METHODS
Animals.
BALB/c mice (ages, 8 to 12 weeks) were purchased from Gador Laboratories (Buenos Aires, Argentina) and Harlan-Winkelmann GmbH (Borchen, Germany) and were maintained under standard conditions. All experiments were approved by the local authorities.
Cell cultures.
Spleen cells were grown in RPMI 1640 supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, 50 μg/ml of streptomycin, 5 × 10−5 M 2-mercaptoethanol, and 1 mM l-glutamine (Gibco BRL, Karlsruhe, Germany).
Bacterial strains and growth conditions.
Pathogenic strains of M. catarrhalis were isolated from the middle ears of children with long-term otitis media with effusion at the Ricardo Gutiérrez Children's Hospital. The strains were maintained in pure skim milk, as well as in brain-heart infusion (BHI) broth containing 50% (vol/vol) glycerol, at −80°C until they were used. One strain (ARG2003-1) was selected for CD purification, cloning, and challenge studies based on its clearance rates in lungs of BALB/c mice. M. catarrhalis was streaked onto BHI agar or was grown in BHI broth and then was incubated overnight (ON) at 37°C in the presence of 5% CO2. Escherichia coli strains XL1-Blue and BL21(DE3) were used for cloning and expression of recombinant protein. Luria-Bertani broth or agar (20) was used for cultivation of E. coli strains.
Adjuvant.
AdDP was synthesized by Bachem (Bubendorf, Switzerland) according to good manufacturing practice guidelines, as described elsewhere (3).
SDS-PAGE and Western blot analysis.
SDS-PAGE was performed by using the methods of Laemmli (19). Proteins present on the gels were detected by using Coomassie blue. An immunoblot analysis was performed with mouse antiserum raised against nCD or rCD, as previously described (7).
Purification of native CD.
Native CD was purified by using a method described elsewhere (41), with minor modifications. In brief, M. catarrhalis was grown ON in BHI broth. Bacteria were harvested by centrifugation at 10,000 × g for 20 min at 4°C. The pellet was resuspended in 50 mM Tris-HCl (pH 8.0), and the cells were disrupted by sonication and centrifuged at 26,000 × g for 20 min at 4°C. The supernatant containing soluble bacterial proteins was discarded. The pellet was washed twice with buffer A (50 mM Tris-HCl [pH 8.0] containing 0.5% Triton X-100 and 10 mM EDTA) and twice with 50 mM Tris-HCl (pH 8.0) containing 0.1% SDS. The SDS-insoluble pellet was then resuspended in buffer A. Urea was added to a final concentration of 6 M, and the mixture was heated at 60°C for 30 min. Under these conditions nCD was solubilized and remained soluble after ON dialysis at 4°C against 50 mM Tris-HCl (pH 8.0). The nCD protein was found to be >95% pure as judged by SDS-PAGE and scanning densitometry, according to an analysis performed using the multi-Analyst software (Bio-Rad Laboratories, Inc). The protein concentration was determined by using a protein assay kit (Bio-Rad Laboratories) according to the manufacturer's instructions.
Construction and purification of rCD protein.
Restriction and modification enzymes were purchased from New England Biolabs. DNA manipulations were performed as described by Sambrook and Russell (29), and PCR amplification was performed by using a PCR reagent kit (Perkin-Elmer) according to the manufacturer's instructions. A DNA fragment encoding the mature CD protein was amplified from the chromosomal DNA of the M. catarrhalis isolate by PCR. A primer complementary to the 5′ end of the CD gene, 5′-GCGGGATCCGGTGTGACAGTCAGCCCACTACTA-3′, was designed to contain a BamHI site (underlined). The reverse primer, 5′-CGCGTCGACTTGAACAATCATATCTTTGGTTTG-3′, was designed to contain the 3′ end of the coding sequence and a SalI site (underlined). The resulting PCR product was ligated into the vector pCR2.1-TOPO (TOPO TA Cloning; Invitrogen) and then transformed into E. coli strain XL1-Blue. The fragment encompassing the CD gene was digested with BamHI and SalI and subsequently cloned into the expression vector pET23a(+) (Novagen) to generate plasmid pET23-CD, which was then transformed into E. coli strain BL21(DE3). The sequence of the insert was verified by DNA sequence analysis. The amino acid sequence exhibited 99% identity with the sequences of two previously reported CD proteins (15, 25). At the nucleotide level the sequence of our strain (GenBank accession number EF093799) differed at nine and seven nucleotides from the previously published sequences (GenBank accession numbers AY493741 and L10755, respectively). CD expression was verified by SDS-PAGE analysis and Coomassie blue staining before and after induction of the T7 promoter with isopropyl-β-d-thiogalactopyranoside (IPTG). Overexpression and purification of the His-tagged CD protein were performed under denaturing conditions by using QIAGEN protocols. The identity of the purified rCD was verified by Western blot analysis using mouse antisera raised against nCD. The purity and protein concentration were determined as described above for the nCD protein.
Immunization schedules and sample collection.
On days 0, 7, and 21, groups of 5 to 10 mice were vaccinated by intranasal inoculation (10 μl/nostril) with either nCD or rCD (40 μg/dose) and AdDP (200 μg/dose) as a mucosal adjuvant diluted in sterile phosphate-buffered saline (PBS). Control groups received either AdDP alone or PBS. No endotoxin activity was detected (<1 ng/ml) using a HEK-Blue lipopolysaccharide detection kit (InvivoGen). On day 31, serum samples were collected from blood from the tail vein and stored at −20°C until they were used. Then mice were sacrificed to obtain bronchoalveolar lavage (BAL), nasal lavage (NAL), and middle ear lavage (MEL) samples. NAL samples were obtained by gently flushing the nasal cavities from the posterior opening of the nose with 100 μl of PBS with 40 μM phenylmethylsulfonyl fluoride (PMSF) in each cavity after the mandible was removed. BAL samples were obtained by irrigation with 400 μl of PBS with 40 μM PMSF, using a blunted needle inserted into the trachea after a tracheotomy. MEL samples were obtained by irrigation of each tympanic cavity twice with 50 μl of PBS with 40 μM PMSF. The recovered washes were centrifuged at 10,000 × g for 5 min to remove debris, and supernatants were collected and stored at −20°C until they were used. Spleens were removed and pooled for analysis.
Detection of CD-specific antibodies by enzyme-linked immunosorbent assays.
CD-specific antibody titers in NAL, BAL, MEL, and serum samples were determined by enzyme-linked immunosorbent assays. In brief, 96-well Nunc-Immuno MaxiSorp assay plates (Nunc) were coated with 0.5 μg/well of nCD or rCD in coating buffer (carbonate [pH 9.4]) for 2 h at 37°C and then washed four times with PBS-0.05% Tween 20 (PBS-T). The plates were blocked with PBS-T containing 1% bovine serum albumin for 2 h at 37°C. Serial twofold dilutions of samples in PBS-T were added (50 μl/well), and the plates were incubated ON at 4°C. After four washes, antibody binding was revealed by use of either horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) or phosphatase alkaline-conjugated α-chain-specific rabbit anti-mouse IgA antibodies (ICN) as secondary antibodies. The plates were incubated for 2 h at 37°C, and after four washes, the reactions were visualized by use of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) in 0.1 M citrate-phosphate buffer (pH 4.35) containing 0.01% H2O2 or p-nitrophenyl phosphate in 10 mM diethanolamine (pH 9.5) containing 0.5 mM MgCl2. The absorbance was determined at a wavelength of 405 nm. Endpoint titers were expressed as the reciprocal log2 of the last dilution, which provided an optical density that was >0.1 U above the background value (preimmune sera).
Bactericidal assay.
The bactericidal capacity of the antibodies present in serum and BAL fluids from immunized mice was assessed as previously described (27). In brief, pooled sera and BAL samples were heat inactivated by incubation at 56°C for 30 min. Bacteria were grown in BHI broth at 37°C with shaking until the optical density at 600 nm was 0.2. Then the culture was diluted 1:4,000 in sterile PCGM (3.26 mM NaH2PO4, 12.8 mM NaHPO4, 125 mM NaCl, 1.25 mM CaCl2, 0.5 mM MgCl2, 1% gelatin), and 20 μl was mixed with 10 μl of diluted sera and 10 μl of PBS and incubated at room temperature for 30 min. Then 10 μl of complement, prepared by adsorbing normal human serum with protein G to remove IgG, was added, and the reaction mixture was incubated at 37°C for another 30 min. Finally, 200 μl of PCGM was added, and 50-μl reaction mixtures were plated onto agar plates in duplicate. The plates were incubated ON, and colonies were counted the next day. The bactericidal titer was defined as the highest dilution at which at least 50% killing was observed. Viable bacterial counts at the time that complement was added were determined for each experiment. Appropriate controls were included in the assay, and they included bacteria and complement in the absence of serum or BAL fluid to ensure that the complement source did not kill bacteria; bacteria plus either serum or BAL fluid in the absence of complement to ensure that the samples were not toxic to the bacteria; and bacteria and buffer alone to ensure the viability of bacteria.
Determination of IL-2-, IL-4-, and IFN-γ secreting cells.
The numbers of interleukin-2 (IL-2)-, IL-4-, and gamma interferon (IFN-γ)-secreting cells were determined by an enzyme-linked immunospot (ELISPOT) assay (Becton-Dickinson). Spleen cells were added at final concentrations of 5 × 105 and 1 × 106 cells/well and incubated in quadruplicate in the absence or presence of either nCD or rCD (10 μg/ml). To determine the number of CD8+ IFN-γ-secreting cells, CD4+ cells were depleted by using Dynabeads M-450 epoxy (Dynal Biotech) coated with anti-CD4 antibodies (clone L3T4; BD Pharmingen) according to the manufacturers' instructions (4). Spots were scanned with an ImmunoSpot series 3A analyzer and were counted using the ImmunoSpot v3.2 image analyzer software (C.T.L.).
Measurement of cellular proliferation.
Proliferation assays were performed in triplicate, as previously described (6). Briefly, spleen cells (5 × 105 cells/well) were incubated for 4 days in the presence of rCD. Eighteen hours before harvest, 1 μCi [3H]thymidine (Amersham International, Freiburg, Germany) was added to each well. Cells were harvested on paper filters (Filtermat A; Wallac, Freiburg, Germany) by using a cell harvester (Inotech, Wohlen, Switzerland), and the amount of incorporated [3H]thymidine was determined with a gamma scintillation counter (Wallac 1450; Micro-Trilux).
Bacterial challenge studies.
Bacteria were grown ON on BHI agar plates in the presence of 5% CO2, harvested, and then washed twice and resuspended in saline. Eight days after the last boost, mice were anesthetized by intraperitoneal injection of 100 μl of PBS containing 2.5 mg of ketamine, and a bolus inoculum containing 5 × 108 CFU of live bacteria in 50 μl of saline was introduced into the lungs via an intratracheal cannula. The concentration of the inoculum was estimated by determining the optical density at 600 nm and was confirmed by determining the number of CFU by plating serial dilutions on BHI agar plates. Mice were sacrificed 4 h after inoculation of the lungs, and the intact lungs were excised, placed in 200 μl of sterile saline, and homogenized (27). The efficiency of bacterial clearance was established by determining the number of viable bacteria present in lung homogenates by plating serial dilutions of the samples on BHI agar plates and incubating the plates at 37°C in the presence of 5% CO2 (5, 27).
Statistical analysis.
In the immunogenicity and protection studies the significance of differences between two groups was determined by Student's unpaired two-tailed t test with transformed data (log10 or log2), and the significance of differences among three or more groups was determined by a one-way analysis of variance with the Tukey-Kramer test as the multiple-comparison test. Viable bacterial counts were compared by using the nonparametric Mann-Whitney U test for two groups and the Krustal-Wallis test with Dunn's multiple-comparison test for three or more groups. For parametric or log-transformed data, the results were expressed as means ± standard errors of the means, whereas for nonparametric data the results were expressed as medians and ranges. Differences were considered significant at a P value of <0.05.
RESULTS
Characterization of the rCD protein.
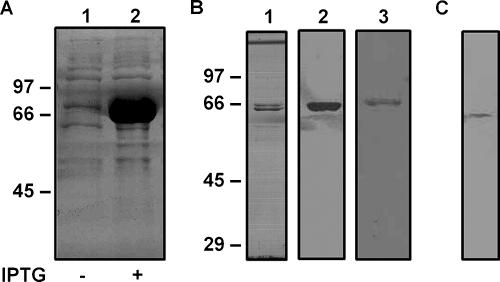
The rCD protein was evaluated by SDS-PAGE and densitometry, as well as by Western blot analysis using serum samples obtained from mice vaccinated with nCD or rCD (Fig. 1A and B). These approaches allowed us to determine the purity and identity of the rCD preparation used in the vaccination studies. The results obtained demonstrated that rCD could be efficiently purified by Ni2+-agarose affinity chromatography (>95% purity) and that this recombinant protein is specifically recognized by antibodies raised against both nCD and rCD. In addition, total proteins of M. catarrhalis were analyzed by Western blot analysis using an antiserum raised against rCD, thereby confirming the cross-reactivity of the two proteins (Fig. 1C). This finding was further supported by the results of competitive binding inhibition assays, which showed that nCD specifically binds to antibodies raised against rCD, blocking their capacity to bind to immobilized rCD protein (data not shown).
FIG. 1.
Characterization of nCD and rCD proteins. (A) Total proteins from E. coli BL21(pET23-CD) were analyzed for rCD expression on a 10% SDS-PAGE gel with Coomassie blue stain. Lane 1, E. coli BL21(pET23-CD) before induction; lane 2, E. coli BL21(pET23-CD) after induction. (B) rCD from cell extract was purified by Ni2+-agarose chromatography, separated by 10% SDS-PAGE, and visualized by Coomassie blue staining (lane 1) and Western blotting using mouse antisera raised against rCD (lane 2) and nCD (lane 3). (C) Total proteins from M. catarrhalis were analyzed by Western blotting using a mouse antiserum raised against rCD. The positions of the molecular mass standards (in kDa) are indicated on the left.
Intranasal immunization with nCD or rCD coadministered with AdDP elicits humoral responses in both serum and mucosal secretions.
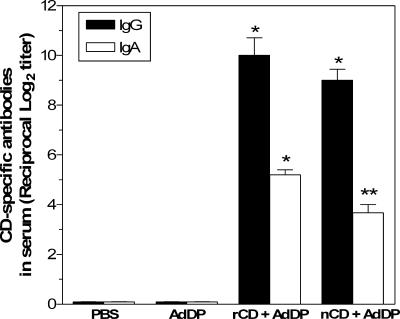
High titers of CD-specific antibodies were observed after intranasal immunization with either nCD or rCD coadministered with AdDP (Fig. 2). There were no statistically significant differences between the endpoint titers observed for the two groups, thereby confirming that under our experimental conditions rCD is as immunogenic as nCD. On the other hand, no significant differences in the IgG titers were observed when sera from rCD- or nCD-vaccinated animals were tested using plates coated with either nCD or rCD (data not shown). This further confirmed that rCD-specific antibodies are able to recognize nCD. The levels of CD-specific serum IgA were slightly higher in animals vaccinated with rCD than in animals that received nCD (Fig. 2); however, the differences were not statistically significant (P > 0.05). No CD-reacting antibodies were observed in control animals.
FIG. 2.
CD-specific serum antibodies after intranasal immunization with nCD or rCD using AdDP as the mucosal adjuvant. The data are presented as the reciprocal log2 mean endpoint titers, and the error bars indicate standard errors of the means. Differences were statistically significant at P values of <0.0001 (one asterisk) and <0.0005 (two asterisks) for comparisons with the control groups.
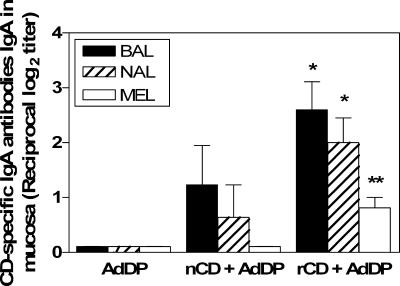
To evaluate the capacities of the different formulations to elicit a mucosal immune response in vaccinated mice, the presence of CD-specific secretory IgA (sIgA) in mucosal lavages was analyzed (Fig. 3). Significant increases in the levels of CD-specific sIgA were observed in NAL, BAL, and MEL samples from mice vaccinated with rCD. In contrast, a weaker CD-specific sIgA response in NAL and BAL samples was observed in mice vaccinated with nCD than in mice vaccinated with rCD, and anti-CD sIgA was not detected in MEL samples from these mice.
FIG. 3.
CD-specific secretory IgA antibodies in BAL, NAL, and MEL samples obtained from mice after intranasal immunization with nCD or rCD using AdDP as the adjuvant. The data are presented as the reciprocal log2 mean endpoint titers, and the error bars indicate standard errors of the means. Differences were statistically significant at P values of <0.005 (one asterisk) and <0.01 (two asterisks) for comparisons with the control group.
The quality of the antibodies stimulated after vaccination was assessed by evaluating the bactericidal activities of sera and BAL fluids. Interestingly, the serum and BAL samples from mice immunized with rCD showed bactericidal activity up to dilutions of 1:64, and 1:4, respectively, whereas no activity was detected when samples from nCD-vaccinated mice were tested (Table 1). The preimmune sera from vaccinated and control mice showed no bactericidal activity.
TABLE 1.
Bactericidal activities of sera and BAL fluids of mice immunized with nCD or rCD using AdDP as a mucosal adjuvant
| Vaccination group | Sample | Bactericidal titera
|
|
|---|---|---|---|
| Preimmune | Postimmune | ||
| PBS | Sera | <2 | <2 |
| BAL | <2 | <2 | |
| nCD + AdDP | Sera | <2 | <2 |
| BAL | NDb | <2 | |
| rCD + AdDP | Sera | <2 | 64 |
| BAL | ND | 4 | |
Bactericidal titers are expressed as the reciprocal of the highest dilution of either serum or BAL fluid capable of killing at least 50% of the bacteria compared with controls. The results were obtained from duplicate bacterial plates, and no significant variations between the two plates were found.
ND, not determined.
Cellular responses stimulated after intranasal vaccination with nCD and rCD.
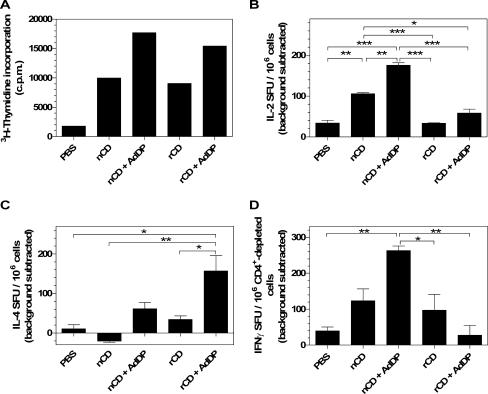
The cellular immune responses induced by vaccination were first evaluated by assessing the proliferative capacity of spleen cells after in vitro restimulation with either nCD or rCD. Similar proliferative responses were observed in animals immunized with nCD or rCD alone (Fig. 4A). Coadministration of nCD or rCD protein with AdDP resulted in induction of a stronger proliferative response (Fig. 4A). No statistically significant differences were observed in mice vaccinated with nCD or rCD. We also analyzed the number of IL-2-secreting cells present in spleens of immunized mice by the ELISPOT assay. The number of cells was increased in mice that received either nCD or rCD, and the responses were stronger in the mice vaccinated with AdDP as the adjuvant (Fig. 4B). Interestingly, the responses were significantly stronger (P < 0.001) in animals that were vaccinated with nCD alone or with nCD plus AdDP than in animals that received rCD or rCD plus AdDP (Fig. 4B).
FIG. 4.
Cellular immune responses in mice after vaccination with either nCD or rCD using AdDP as the adjuvant. (A) Proliferation was assessed after 4 days of in vitro restimulation of spleen cells in the presence of the CD protein by measuring [3H]thymidine incorporation (cpm). The data are mean numbers of cpm for triplicate samples after subtraction of background values for nonstimulated cells. (B and C) Total splenocytes were incubated for 40 h in the presence of either nCD or rCD, and the numbers of IL-2-producing cells (B) and IL-4-producing cells (C) were determined by ELISPOT assays. (D) CD4+-depleted splenocytes were incubated for 16 h in the presence of either nCD or rCD, and the number of IFN-γ-producing cells was determined by an ELISPOT assay. The data in panels B, C, and D are presented as the numbers of spot-forming units (SFU)/106 cells in stimulated samples after subtraction of the background value for nonstimulated cells. The error bars indicate standard errors of the means. Differences between the experimental groups were statistically significant at P values of <0.05 (one asterisk), <0.01 (two asterisks), and <0.001 (three asterisks).
We then analyzed the number of IL-4-secreting cells present in spleens from vaccinated mice after in vitro restimulation with either nCD or rCD. The number of IL-4-secreting cells was not increased in animals immunized with nCD, whereas a slight increase was observed when cells from rCD-vaccinated mice were tested (Fig. 4C). On the other hand, the presence of AdDP in the formulation resulted in an increased number of IL-4-secreting cells, and the response was significantly stronger in mice that received rCD (P < 0.05) (Fig. 4C). These results are in agreement with those obtained for IL-2-secreting cells (Fig. 4B).
Finally, we determined the number of IFN-γ-producing cells in vaccinated animals. As shown in Fig. 4D, the number of CD8+ IFN-γ-secreting cells was greater in mice vaccinated with nCD protein plus AdDP than in the PBS control group (P < 0.01) and in mice that received rCD (P < 0.05) or nCD alone. Interestingly, coadministration of AdDP with rCD resulted in a reduction in the number of IFN-γ-secreting cells compared to the number in animals that received rCD alone (Fig. 4D).
Intranasal immunization with nCD or rCD enhances the pulmonary clearance of M. catarrhalis.
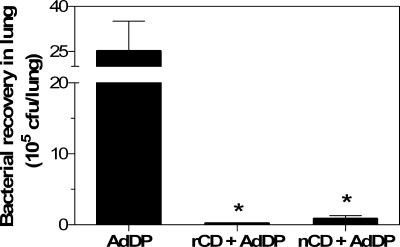
The comparative efficacies of nCD- and rCD-based vaccine formulations were evaluated by studying the pulmonary clearance of a clinical isolate of M. catarrhalis after challenge. Mice immunized with either nCD or rCD protein-based vaccine showed more efficient bacterial clearance in the lungs (P < 0.03) than control mice showed (8.8 × 104 and 1.8 × 104 CFU/lung, respectively, compared with 2.6 × 106 CFU/lung) (Fig. 5). In fact, 96% and 99% reductions in the number of viable bacteria were observed in nCD- and rCD-vaccinated mice, respectively. These results indicate that nCD and rCD can stimulate similar levels of bacterial clearance (P > 0.05).
FIG. 5.
M. catarrhalis recovery from lung homogenates obtained from mice intranasally vaccinated with either nCD or rCD coadministered with AdDP. Immunized mice were challenged by the intratracheal route with 5 × 108 CFU of M. catarrhalis. Bacterial clearance was assessed after 4 h by plating serial dilutions of the lung homogenates onto BHI agar plates. The data are presented as the mean numbers of CFU/lung; the error bars indicate standard errors of the means. An asterisk indicates that the difference was statistically significant at a P value of <0.03 for a comparison with the AdDP control group.
DISCUSSION
There is a clear need for a vaccine able to prevent diseases caused by M. catarrhalis. Like most human pathogens, M. catarrhalis gains entrance to the body via the mucosal surface. Thus, it would be desirable to stimulate a local immune response at the portal of entry, since sIgA plays a major role in the defense against mucosal pathogens (38). In this context, mucosal vaccination can lead to stimulation of humoral and cellular immune responses at both the mucosal and systemic levels. However, despite the concept of a common mucosal immune system, whereby immune cells activated at one site disseminate to remote mucosal tissues, there is a significant degree of compartmentalization. This constrains the choice of vaccination route for inducing effective immune responses at the specific effector sites (14, 16). Intranasal vaccination seems to be particularly attractive for stimulating responses in the respiratory tract. However, administration of soluble antigens by this route generally does not induce effective immune responses. This problem can be overcome by using mucosal adjuvants (10, 31, 39, 40). However, there are safety concerns associated with the use of some of these molecules in humans (e.g., toxicity and retrograde homing to neural tissues) (34).
The use of vaccines against M. catarrhalis based on a native antigen, such as the proposed outer membrane protein CD is, unfortunately, not suitable for large-scale production under good manufacturing practice conditions. However, a previous study showed that injection into Peyer's patches of rCD emulsified in Freund's adjuvant enhances pulmonary clearance of M. catarrhalis in a mouse model (27). rCD can be purified by using a simple and cost-effective method. Nevertheless, selection of a recombinant immunogen as a vaccine component is based not only on the ability to purify large amounts of antigen to homogeneity but also on demonstration that the recombinant protein retains the immunological properties of the native protein. In this context, no comparative studies to test nCD and rCD had been performed.
In the present study, we performed a side-by-side evaluation of the immunogenicities and efficacies of nasal vaccine formulations based on nCD and rCD. Our results show that intranasal immunization with CD using a mucosal adjuvant with an adequate safety profile, such as AdDP, induces an efficient CD-specific sIgA response in the middle ear and lungs, as well as strong humoral and cellular systemic responses. Immunoblot assays also showed that both rCD and nCD are cross-recognized by antibodies raised after vaccination with either nCD or rCD. Vaccination with rCD and AdDP resulted in stimulation of IL-4-secreting cells that was stronger than the stimulation in animals immunized with nCD plus AdDP. On the other hand, vaccination with nCD and AdDP resulted in a significant increase in the number of IL-2- and IFN-γ-producing cells. Thus, use of rCD seems to result in stronger Th2 polarization, which is in agreement with the improved sIgA responses observed in mucosal territories.
Although M. catarrhalis is a pathogen that is restricted to humans, the murine pulmonary clearance model is widely accepted as a cost-efficient screening tool for new vaccine candidates (9, 12, 13, 27, 32, 36). Thus, we used this model to evaluate the efficacy of the CD-specific responses stimulated after intranasal immunization with rCD or nCD plus AdDP. Our results demonstrated that there was enhanced bacterial clearance in the lungs of both nCD- and rCD-vaccinated mice challenged with M. catarrhalis. There were no statistically significant differences in bacterial clearance between animals immunized with nCD and animals immunized with rCD. Thus, it seems that enhanced bacterial clearance correlates with the presence of CD-specific IgG in serum from vaccinated mice. However, no bactericidal activity was observed when sera from mice immunized with nCD were tested. This suggests that effector mechanisms other than bactericidal antibodies are responsible for the clearance observed in nCD-vaccinated mice. In this context, it is important to highlight the fact that efficient production of IFN-γ was observed only in nCD-vaccinated animals. These findings are not unusual in vaccinology, since different effector mechanisms seem to be stimulated by different licensed vaccines against whooping cough (1, 8, 33).
In conclusion, our comparative study strongly supports the use of rCD as an antigen for mucosal vaccine formulations against M. catarrhalis, particularly in combination with AdDP as an adjuvant. Interestingly, we have recently shown that intranasal immunization with recombinant P6 protein and AdDP provides protection against otitis media and lung infection by nontypeable H. influenzae (5). These results suggest that it might be possible to use AdDP for a combined nasal vaccine aimed at protecting against respiratory infections caused by H. influenzae and M. catarrhalis.
Acknowledgments
We are indebted to M. Vazquez of the Bacteriology Laboratory of Ricardo Gutiérrez Children's Hospital for providing M. catarrhalis isolates. We thank K. Schulze for expert assistance.
This work was supported in part by Deutsche Forschungsgemeinschaft grant GU 482/2-3 to C.A.G.
Editor: D. L. Burns
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Ausiello, C. M., F. Urbani, A. La Sala, R. Lande, A. Piscitelli, and A. Cassone. 1997. Acellular vaccines induce cell-mediated immunity to Bordetella pertussis antigens in infants undergoing primary vaccination against pertussis. Dev. Biol. Stand. 89:315-320. [PubMed] [Google Scholar]
- 2.Bartos, L. C., and T. F. Murphy. 1988. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J. Infect. Dis. 158:761-765. [DOI] [PubMed] [Google Scholar]
- 3.Becker, P. D., R. S. Corral, C. A. Guzman, and S. Grinstein. 2001. Adamantylamide dipeptide as effective immunoadjuvant in rabbits and mice. Vaccine 19:4603-4609. [DOI] [PubMed] [Google Scholar]
- 4.Becker, P. D., S. Fiorentini, C. Link, G. Tosti, T. Ebensen, A. Caruso, and C. A. Guzman. 2006. The HIV-1 matrix protein p17 can be efficiently delivered by intranasal route in mice using the TLR 2/6 agonist MALP-2 as mucosal adjuvant. Vaccine 24:5269-5276. [DOI] [PubMed] [Google Scholar]
- 5.Bertot, G. M., P. D. Becker, C. A. Guzman, and S. Grinstein. 2004. Intranasal vaccination with recombinant P6 protein and adamantylamide dipeptide as mucosal adjuvant confers efficient protection against otitis media and lung infection by nontypeable Haemophilus influenzae. J. Infect. Dis. 189:1304-1312. [DOI] [PubMed] [Google Scholar]
- 6.Borsutzky, S., V. Fiorelli, T. Ebensen, A. Tripiciano, F. Rharbaoui, A. Scoglio, C. Link, F. Nappi, M. Morr, S. Butto, A. Cafaro, P. F. Muhlradt, B. Ensoli, and C. A. Guzman. 2003. Efficient mucosal delivery of the HIV-1 Tat protein using the synthetic lipopeptide MALP-2 as adjuvant. Eur. J. Immunol. 33:1548-1556. [DOI] [PubMed] [Google Scholar]
- 7.Burnette, W. N. 1981. “Western blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal. Biochem. 112:195-203. [DOI] [PubMed] [Google Scholar]
- 8.Canthaboo, C., L. Williams, D. K. Xing, and M. J. Corbel. 2000. Investigation of cellular and humoral immune responses to whole cell and acellular pertussis vaccines. Vaccine 19:637-643. [DOI] [PubMed] [Google Scholar]
- 9.Chen, D., J. C. McMichael, K. R. VanDerMeid, D. Hahn, T. Mininni, J. Cowell, and J. Eldridge. 1996. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect. Immun. 64:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliani, M. M., G. Del Giudice, V. Giannelli, G. Dougan, G. Douce, R. Rappuoli, and M. Pizza. 1998. Mucosal adjuvanticity and immunogenicity of LTR72, a novel mutant of Escherichia coli heat-labile enterotoxin with partial knockout of ADP-ribosyltransferase activity. J. Exp. Med. 187:1123-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldblatt, D., M. W. Turner, and R. J. Levinsky. 1990. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J. Infect. Dis. 162:1128-1135. [DOI] [PubMed] [Google Scholar]
- 12.Helminen, M. E., I. Maciver, J. L. Latimer, L. D. Cope, G. H. McCracken, Jr., and E. J. Hansen. 1993. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect. Immun. 61:2003-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helminen, M. E., I. Maciver, J. L. Latimer, J. Klesney-Tait, L. D. Cope, M. Paris, G. H. McCracken, Jr., and E. J. Hansen. 1994. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170:867-872. [DOI] [PubMed] [Google Scholar]
- 14.Heritage, P. L., B. J. Underdown, A. L. Arsenault, D. P. Snider, and M. R. McDermott. 1997. Comparison of murine nasal-associated lymphoid tissue and Peyer's patches. Am. J. Respir. Crit. Care Med. 156:1256-1262. [DOI] [PubMed] [Google Scholar]
- 15.Holm, M. M., S. L. Vanlerberg, I. M. Foley, D. D. Sledjeski, and E. R. Lafontaine. 2004. The Moraxella catarrhalis porin-like outer membrane protein CD is an adhesin for human lung cells. Infect. Immun. 72:1906-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren, J., and C. Czerkinsky. 2005. Mucosal immunity and vaccines. Nat. Med. 11:S45-S53. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao, C. B., S. Sethi, and T. F. Murphy. 1995. Outer membrane protein CD of Branhamella catarrhalis: sequence conservation in strains recovered from the human respiratory tract. Microb. Pathog. 19:215-225. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, M. A., W. L. Drew, and M. Roberts. 1981. Branhamella (Neisseria) catarrhalis—a lower respiratory tract pathogen? J. Clin. Microbiol. 13:1066-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.McMichael, J. C. 2000. Vaccines for Moraxella catarrhalis. Vaccine 19(Suppl. 1):S101-S107. [DOI] [PubMed] [Google Scholar]
- 21.McMichael, J. C., and B. A. Green. 2003. Vaccines for Moraxella catarrhalis and non-typeable Haemophilus influenzae. Curr. Opin. Investig. Drugs 4:953-958. [PubMed] [Google Scholar]
- 22.Murphy, T. F., A. L. Brauer, C. Aebi, and S. Sethi. 2005. Identification of surface antigens of Moraxella catarrhalis as targets of human serum antibody responses in chronic obstructive pulmonary disease. Infect. Immun. 73:3471-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy, T. F., A. L. Brauer, B. J. Grant, and S. Sethi. 2005. Moraxella catarrhalis in chronic obstructive pulmonary disease: burden of disease and immune response. Am. J. Respir. Crit. Care Med. 172:195-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy, T. F., C. Kirkham, E. DeNardin, and S. Sethi. 1999. Analysis of antigenic structure and human immune response to outer membrane protein CD of Moraxella catarrhalis. Infect. Immun. 67:4578-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy, T. F., C. Kirkham, and A. J. Lesse. 1993. The major heat-modifiable outer membrane protein CD is highly conserved among strains of Branhamella catarrhalis. Mol. Microbiol. 10:87-97. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, T. F., C. Kirkham, D. F. Liu, and S. Sethi. 2003. Human immune response to outer membrane protein CD of Moraxella catarrhalis in adults with chronic obstructive pulmonary disease. Infect. Immun. 71:1288-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy, T. F., J. M. Kyd, A. John, C. Kirkham, and A. W. Cripps. 1998. Enhancement of pulmonary clearance of Moraxella (Branhamella) catarrhalis following immunization with outer membrane protein CD in a mouse model. J. Infect. Dis. 178:1667-1675. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, T. F., and M. R. Loeb. 1989. Isolation of the outer membrane of Branhamella catarrhalis. Microb. Pathog. 6:159-174. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 30.Sarwar, J., A. A. Campagnari, C. Kirkham, and T. F. Murphy. 1992. Characterization of an antigenically conserved heat-modifiable major outer membrane protein of Branhamella catarrhalis. Infect. Immun. 60:804-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staats, H. F., R. J. Jackson, M. Marinaro, I. Takahashi, H. Kiyono, and J. R. McGhee. 1994. Mucosal immunity to infection with implications for vaccine development. Curr. Opin. Immunol. 6:572-583. [DOI] [PubMed] [Google Scholar]
- 32.Unhanand, M., I. Maciver, O. Ramilo, O. Arencibia-Mireles, J. C. Argyle, G. H. McCracken, Jr., and E. J. Hansen. 1992. Pulmonary clearance of Moraxella catarrhalis in an animal model. J. Infect. Dis. 165:644-650. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg, B. M., S. David, H. Beekhuizen, F. R. Mooi, and R. van Furth. 2000. Protection and humoral immune responses against Bordetella pertussis infection in mice immunized with acellular or cellular pertussis immunogens. Vaccine 19:1118-1128. [DOI] [PubMed] [Google Scholar]
- 34.van Ginkel, F. W., R. J. Jackson, Y. Yuki, and J. R. McGhee. 2000. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J. Immunol. 165:4778-4782. [DOI] [PubMed] [Google Scholar]
- 35.Verduin, C. M., C. Hol, A. Fleer, H. van Dijk, and A. van Belkum. 2002. Moraxella catarrhalis: from emerging to established pathogen. Clin. Microbiol. Rev. 15:125-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verghese, A., E. Berro, J. Berro, and B. W. Franzus. 1990. Pulmonary clearance and phagocytic cell response in a murine model of Branhamella catarrhalis infection. J. Infect. Dis. 162:1189-1192. [DOI] [PubMed] [Google Scholar]
- 37.Verghese, A., D. Roberson, J. H. Kalbfleisch, and F. Sarubbi. 1990. Randomized comparative study of cefixime versus cephalexin in acute bacterial exacerbations of chronic bronchitis. Antimicrob. Agents Chemother. 34:1041-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams, R. C., and R. J. Gibbons. 1972. Inhibition of bacterial adherence by secretory immunoglobulin A: a mechanism of antigen disposal. Science 177:697-699. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto, S., H. Kiyono, M. Yamamoto, K. Imaoka, K. Fujihashi, F. W. Van Ginkel, M. Noda, Y. Takeda, and J. R. McGhee. 1997. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc. Natl. Acad. Sci. USA 94:5267-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto, S., Y. Takeda, M. Yamamoto, H. Kurazono, K. Imaoka, K. Fujihashi, M. Noda, H. Kiyono, and J. R. McGhee. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J. Exp. Med. 185:1203-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, Y. P., L. E. Myers, U. McGuinness, P. Chong, Y. Kwok, M. H. Klein, and R. E. Harkness. 1997. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol. Med. Microbiol. 17:187-199. [DOI] [PubMed] [Google Scholar]