Abstract
The role intestinal epithelial cells play in the pathogenesis of amebic colitis is poorly understood. Herein, we demonstrate that secreted and soluble ameba (Entamoeba histolytica) proteins (SAP) induce expression of the chemoattractant monocyte chemotactic protein (MCP) in the colonic epithelial cell lines Caco-2, T84, and LS174T. MCP-1 mRNA induction was both dose and time dependent, with peak induction occurring at 8 h and with 100 μg/ml of SAP. Significant increase in MCP-1 protein expression was observed after 12 h. SAP failed to activate any of the mitogen-activated protein kinase pathways or IκB kinase activity. Moreover, inhibiting the classical pathway of NF-κB activation did not affect SAP-induced MCP-1 expression. Instead, we find that SAP-induced MCP-1 expression is dependent on posttranslational modification of the NFκB p65 subunit. SAP induced phosphorylation of p65 and enhanced NF-κB transcriptional activity, which are phosphatidylinositol 3-kinase (PI3 kinase) dependent. Treatment with PI3 kinase inhibitor LY290004 significantly abrogated the activation of Akt, p65, and MCP-1 mRNA induction. We conclude that colonic epithelial cells play a role in the initiation of inflammation by secreting chemokines in response to soluble ameba components.
Entamoeba histolytica is an enteric protozoan parasite that is responsible for the disease amebiasis in humans. The disease affects 50 million people globally and is the fourth leading parasitic cause of death (14). Host inflammatory responses are thought to play an important role in the pathogenesis of intestinal amebiasis. However, the roles of intestinal epithelial cells (IEC) and mediators of colonic inflammation have not been fully elucidated.
Invasive amebiasis is characterized by infiltration of immune cells such as leukocytes and lymphocytes (5). It has not been well established if this cellular infiltration is a cause or consequence of inflammation. The parasite has previously been shown to elicit interleukin-8 (IL-8) production from colonic epithelial cells (4, 15). IL-8 is a potent chemoattractant primarily for neutrophils, and its secretion alone does not explain the homing of other immune cells such as monocytes and lymphocytes seen in the amebic lesions (5). Monocyte chemotactic protein 1 (MCP-1) belongs to a group of C-C or β-chemokines, is produced by a variety of cells, including IEC, and is potently chemotactic for monocytes, lymphocytes, and basophils (10). Recently, amebic infection in a human intestinal xenograft model has been shown to increase a number of genes in the epithelial cells, including the MCP-1 gene (16). However, the mechanism of induction is not known and also it is not clear if ameba components themselves can directly induce this chemokine.
The transcription factor NF-κB regulates a number of genes involved in immune response and inflammation. It is composed of two subunits, most commonly of p65 and P50. Only p65 has the transactivating domains and hence is critical for NF-κB activity. NF-κB is activated by different pathways, including classical, alternate, atypical, and p105 pathways (12). It is increasingly being recognized that posttranslational modifications of the NF-κB p65 subunit are equally important in the transactivating function of the transcription factor (3). The two important posttranslational events that determine the strength, duration, and efficacy of NF-κB function are phosphorylation and acetylation. Importantly, this p65 phosphorylation can lead to transcription of a set of NF-κB target genes independent of IκB kinase (IKK) degradation. IKK, Akt, NIK, and casein kinase II have been implicated in the phosphorylation of cysteine residues at different positions of the p65 subunit (3, 12). In this study, we show that soluble ameba proteins (SAP) can induce MCP-1 from IEC by a unique pathway involving phosphatidylinositol 3-kinase (PI3 kinase) and NF-κB p65.
MATERIALS AND METHODS
Cells, reagents, and ameba components.
Human adenocarcinomal cell lines Caco-2, T84, and LS174T from ATCC were grown to confluent monolayers in minimum essential medium with 5% serum and penicillin and streptomycin for 5 to 7 days. Antibodies against MCP-1, total p65, and phospho-p65 are from Santa Cruz Biotech. Antibodies against IκB, phospho-IκB, total Akt, and phospho-Akt and LY290004 are from Cell Signaling. PD98089 and SB203580 are from Calbiochem. All other reagents are from Sigma. SAP were prepared by three cycles of freeze-thaw lysis of log-phase E. histolytica virulent strain HM1:IMSS and quantified by bicinchoninic acid assay (Pierce) as described previously (15). For transwell studies, ameba trophozoites were added to Corning transwell inserts with a pore diameter of 0.6 μM, with Caco-2 cells in the bottom well.
Real-time PCR.
Total RNA was extracted with TRIzol reagent (Invitrogen) and quantified, and 5 μg of RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and oligo(dT) per the manufacturer's instructions. Two microliters of cDNA was used for real-time PCR.
Amplifications were carried out with a QIAGEN Quantitect SYBR green PCR kit at the following cycling conditions: 94°C hold for 10 min, followed by 40 cycles of denaturation at 94°C for 20 s, annealing at 60°C for 30 s, and extension at 72°C for 60 s using MCP-1 forward primer 5′GATCTCAGTGCAGAGGCTCG3′, MCP-1 reverse primer 5′TGCTTGTCCAGGTGGTCCAT3′, 18S forward primer 5′GGATGCGTGCATTTATCAGA3′, 18S reverse primer 5′GTTGATAGGGCAGACGTTCG3′, GAPDH forward primer 5′GAAGGTGAAGGT CGGAGT3′, and GAPDH reverse primer 5′GAAGATGGTGATGGGATTTC3′. Specificity of amplification was checked by melt curve analysis. MCP-1 expression was normalized against 18S rRNA for initial MCP-1 expression studies and with GAPDH for subsequent inhibition studies. Change (n-fold) over control levels was determined according to the comparative cycle threshold method as described by Bas et al. (1).
Western blotting.
Cytoplasmic or nuclear extracts were collected using an NE-PER (Pierce) kit per the instructions of the manufacturer. Protein concentrations were estimated by the micro-bicinchoninic acid method (Pierce). Equal amounts of the samples were then separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Bio-Rad). The membranes were blocked in 3.5% skim milk and Tris-buffered saline-Tween 20 (TBST) (20 nM Tris-HCl, pH 7.5, 500 mM NaCl, 0.1% Tween 20) at 4°C overnight, incubated with primary antibodies in skim milk-TBST at 4°C overnight, washed three times with TBST, and incubated with horseradish peroxidase-conjugated secondary antibody in skim milk-TBST overnight at 4°C. After three washes each in 20 mM Tris-HCL (pH 7.5)-500 mM NaCl-0.39. Tween 20 (TBS-3T) and TBST, the blot was developed with an enhanced chemiluminescence system (Amersham Pharmacia Biotech, Piscataway, NJ) according to the instructions of the manufacturer. Actin and histone were used as loading controls for cytoplasmic and nuclear extracts, respectively.
In vitro kinase assay.
Cells were incubated with cell lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 25 mM β-glycerophosphate, 2 mM EDTA, 2 mM pyrophosphate, 1 mM orthovanadate, 1% Triton X-100, 1 mM dithiothreitol, 1 mM NaF, and protease inhibitors) followed by the addition of IKK-α antibody. Following overnight end-to-end rotation of tubes at 4°C, immunoprecipitates were washed three times with lysis buffer and once with kinase buffer (20 mM Tris, pH 7.5, 1 mM MnCl2, 10 mM MgCl2, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 2 mM NaF, and 1 mM dithiothreitol). Immunoprecipitates were finally resuspended in 20.0 μl of kinase buffer containing 5μCi of [γ-32P]ATP and incubated at 30°C for 30 min. Glutathione S-transferase-IκB-α (Santa Cruz Biotech) (1 μg) was used as the substrate. The reaction was terminated by boiling the mixture with SDS sample buffer for 5 min. Finally, the protein was resolved by 10% SDS-PAGE, the gel was dried, and the radioactive bands were visualized by autoradiography. Cell lysates were also checked for IKK-α expression for normalization.
Luciferase reporter assay.
NF-κB transactivation was studied using luciferase reporter plasmids from Stratagene according to the manufacturer's instructions. Briefly, vector DNA (pNF-κB-Luc with or without pFC-MEKK positive-control plasmid) was allowed to form complexes with FuGene, which was used at a 0.003% (vol/vol) final concentration. Cells were washed once in serum-free medium, and the DNA-FuGene mixture containing the pNF-κB-Luc plasmid was added to 40 to 50% confluent Caco-2 cells. The transfection continued at 37°C for 6 h, after which the medium was changed to normal growth medium and the cells were allowed to grow for another 48 h. Transfected cells were treated with SAP for 6 h, luciferase was extracted with a luciferase assay system (Promega), and the emitted light was measured with a luminometer.
Statistical analysis.
Blots were scanned for densitometric units by use of ImageJ software. Statistical analysis to check significance was done with Student's t test using Prism software. Graphs plotted were from two to three independent experiments, and error bars in all graphs represent means ± standard deviations.
RESULTS
SAP induce MCP-1 mRNA and protein production in colonic epithelial cell lines.
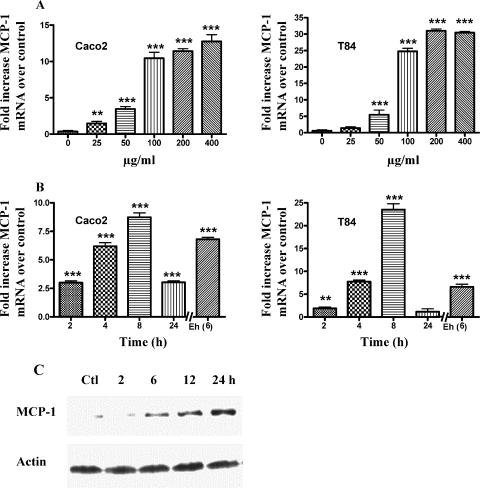
Human colonic epithelial cells Caco-2 and T84 were treated with different concentrations (0 to 400 μg/ml) of SAP for 8 h and checked for induction of MCP-1 mRNA expression by real-time PCR. As shown in Fig. 1A, SAP-induced MCP-1 mRNA in a concentration-dependent manner in both Caco-2 and T84 cells. However, at concentrations greater than 100 μg/ml the dose-dependent effect faded away (Fig. 1A). Based on these results, Caco-2 and T84 cells were treated with 100 μg/ml SAP for 0 to 24 h and checked for MCP-1 mRNA expression and protein production. As shown in Fig. 1B, SAP induced significant expression of MCP-1 mRNA as early as 2 h, reaching a peak at 6 to 8 h. We have also observed high levels of MCP-1 mRNA expression in LS174T colonic cells (data not shown). Interestingly, live ameba in transwell culture inserts also induced significant amounts of MCP-1 mRNA expression at 6 h in Caco-2 and T84 cells, clearly implicating that the putative effector component is soluble and is secreted from live ameba. Little MCP-1 protein expression was observed after 6 h in response to SAP but increased steadily thereafter, with significant expression seen after 12 h (Fig. 1C) in Caco-2 cells.
FIG. 1.
E. histolytica components induce MCP-1 mRNA and protein production from colonic epithelial cells. (A) Confluent Caco-2 and T84 cells were treated with different concentrations of SAP for 8 h. Total cellular RNA was extracted by the TRIzol method, and real-time PCR was performed as described in Materials and Methods. **, P < 0.01; ***, P < 0.001 (over untreated controls). (B) Epithelial cells were treated with 100 μl/mg of SAP for different time periods as indicated. Results in the last lane [Eh (6)] represent results from live E. histolytica amebas (2.5 × 106) in transwells incubated with colonic cell monolayers for 6 h. Data represent changes (n-fold) of mRNA expression over that for the control in experiments repeated thrice with similar results. **, P < 0.01; ***, P < 0.001 (over untreated controls). (C) Caco-2 cells were treated with 100 μl/mg of SAP for different time periods, and whole-cell lysates were subjected to SDS-PAGE and Western blot analysis with MCP-1 antibody and actin antibodies. Ctl, control.
Ameba-induced MCP-1 mRNA induction is independent of IKK activity and IκB-α degradation.
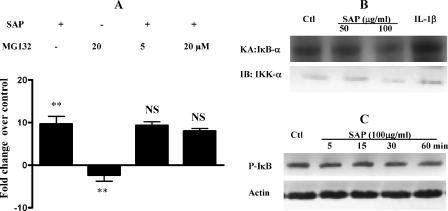
As MCP-1 expression is regulated by the transcription factor NF-κB, we checked its role in ameba-induced MCP-1 induction. Surprisingly, pretreating cells with the proteasome inhibitor MG132 failed to suppress SAP-induced MCP-1 production while significantly reducing basal MCP-1 mRNA expression (Fig. 2A). SAP treatment also failed to induce IKK activity (Fig. 2B) and to phosphorylate IκB (Fig. 2C).
FIG. 2.
Ameba components do not activate IKK activity. (A) Caco-2 cells were treated or not (−) with MG132 for 2 h followed by SAP for 2 h. RNA was extracted and real-time PCR for MCP-1 was done as described in the text. Experiments were repeated three times. **, P < 0.01. NS, change not significant compared to results of treatment with SAP alone. (B) Caco-2 cells were treated with different concentrations of SAP for 30 min, cell lysates were immunoprecipitated using IKK-α antibody, and an in vitro kinase assay was done using glutathione S-transferase-IκB-α as the substrate. Equal quantities (20 μg) of cellular proteins were subjected to Western blotting to check the IKK-α levels. KA, kinase assay; IB, immunoblotting; Ctl, control. A representative blot from two experiments is shown. (C) Cells were treated with 100 μg/ml of SAP for different time periods and cell lysates subjected to SDS-PAGE and Western blotting with antibodies against phosphorylated IκB (P-IκB). The blot was stripped and reprobed with actin antibodies. A blot from one of three independent experiments is shown.
Ameba components induce NF-κB transcriptional activity and p65 phosphorylation.
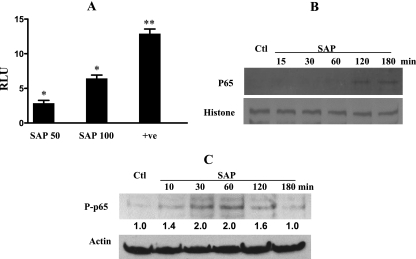
Despite the lack of IκB degradation, ameba protein treatment was found to result in around a sixfold increase of NF-κB transcriptional activity (Fig. 3A). To decipher the mechanism underlying the apparently contradicting observations of a lack of IKK activation but increased NF-κB transactivation, we focused on the dynamics of the p65 subunit. First, we checked its nuclear translocation, but we did not observe significant accumulation until after 2 h (Fig. 3B). To check if ameba components activate NF-κB by posttranslational modification, we checked the phosphorylation status of the p65 subunit. Indeed, SAP was found to increase the levels of phosphorylated p65 following 30 min of treatment (Fig. 3C).
FIG. 3.
SAP induces NF-κB transactivation and p65 phosphorylation. (A) Caco-2 cells were transfected with pNF-κB-Luc plasmid with or without the positive control (p-MEKK), treated with different concentrations of SAP for 6 h, and assayed for luciferase activity as described in the text. Results are from three independent studies. *, P < 0.05; **, P < 0.01; RLU, relative luciferase units compared to values for the untreated cells; +ve, positive control. (B) Cells were treated with 100 μg/ml of SAP for different time periods, nuclear extracts were prepared as described in the text, and 10 μg/ml of protein was separated by SDS-PAGE and checked for p65 nuclear translocation. The blot was stripped and reprobed with histone antibody for normalization. A blot from one of three experiments is shown. Ctl, control. (C) Whole-cell lysates from cells treated as described above were subjected to Western blotting and probed with antibody specific to phosphorylated p65 (P-p65) and then with antibody against actin. Representative blots from two experiments, in which P-p65 levels are normalized to actin following densitometric scanning of blots, are shown. Expression in control cells was assigned an arbitrary value of 1, and expressions relative to this value are shown for treated cells.
E. histolytica activates Akt.
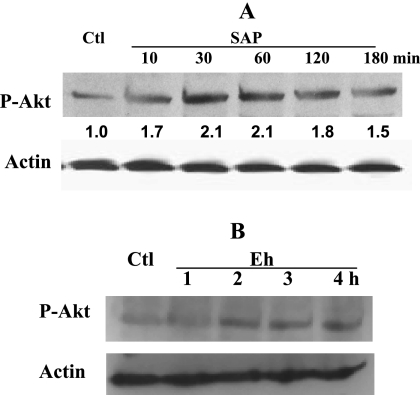
As Akt is one of the proposed upstream kinases implicated in p65 phosphorylation (9), its activation was checked with an antibody that specifically recognizes phosphorylated Akt. As shown in Fig. 4A, SAP treatment rapidly increased phosphorylated Akt levels as early as 15 min and this activation continued for 2 h. Moreover, secreted products from live E. histolytica trophozoites separated by a semipermeable membrane also activated Akt after 2 h of treatment of epithelial cells (Fig. 4B).
FIG. 4.
E. histolytica activates Akt. (A) Caco-2 cells were treated with 100 μg/ml of SAP for different times, and cell lysates were checked for phosphorylation of Akt by Western blotting. Blots were reprobed with actin antibody for normalization. Blots from one of three studies are shown, and values are relative densitometric units of phosphorylated Akt (P-Akt) following normalization to actin. (B) E. histolytica (Eh) trophozoites (106) were added to the transwell culture with confluent Caco-2 cells at the bottom as described in Materials and Methods, and cell lysates were extracted at different times and checked for phosphorylated Akt as described above. Actin expression was analyzed to check equal loading. Each experiment was repeated twice. Ctl, control.
SAP-induced p65 activation is PI3 kinase but not MAP kinase dependent.
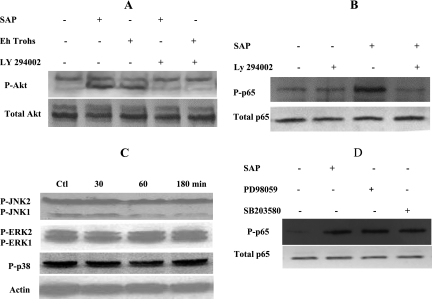
As Akt is a substrate for PI3 kinase, we checked the role of this kinase in SAP-induced Akt and p65 phosphorylation. As expected, inhibition of PI3 kinase with LY294002 inhibited SAP-induced Akt phosphorylation (Fig. 5A) but interestingly also abrogated ameba-induced p65 phosphorylation (Fig. 5B). To study the role of mitogen-activated protein (MAP) kinases that are purported to function downstream of PI3 kinase/Akt, we checked their activation by ameba components (Fig. 5C), but we failed to see activation of extracellular signal-regulated kinase (ERK), Jun N-terminal protein kinase, or p38 MAP kinase. Consistently, treatment of colonic cells with inhibitors of ERK and p38 MAP kinase failed to suppress SAP-induced p65 phosphorylation (Fig. 5D).
FIG. 5.
SAP-induced p65 phosphorylation is dependent on PI3 kinase but is independent of MAP kinases. (A) Caco-2 cells were pretreated with PI3 kinase inhibitor LY294002 for 1 h prior to SAP or E. histolytica trophozoite (Eh Trohs) (in transwell) exposure for 2 h, and whole-cell lysates were checked for phosphorylated Akt (P-Akt) levels in relation to total Akt expression. (B) Lyates from cells pretreated or not (−) with LY294002 followed by SAP for 30 min were subjected to SDS-PAGE and Western blotting using antibodies against phosphorylated p65 (P-p65) and total p65. (C) Caco-2 cells were exposed to SAP for different time periods, and cell lysates were checked for activation of MAP kinases by use of phosphospecific antibodies against Jun N-terminal protein kinase, ERK, and p38 MAP kinase. Actin expression was checked for normalization. Ctl, control. (D) Cells were pretreated with 50 μM of PD98059 (ERK inhibitor) or SB203580 (p38 inhibitor) for 2 h before incubation with SAP for 30 min, and whole-cell lysates were checked for phosphorylation of p65. The blot was stripped and reprobed with antibody against total p65. For all, representative blots from two or three independent experiments are shown.
PI3 kinase inhibition suppresses SAP-induced MCP-1 mRNA expression.
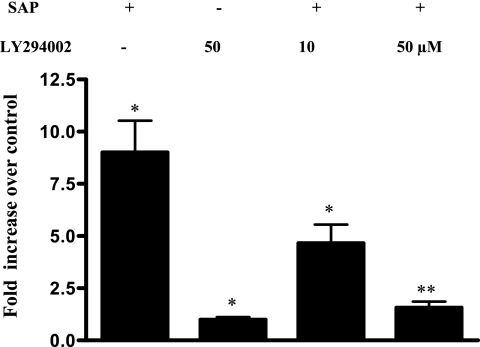
Finally, we checked if PI3 kinase inhibition can suppress MCP-1 mRNA induction by ameba components. As shown in Fig. 6, LY294002 significantly inhibited SAP-induced MCP-1 mRNA expression even at the low concentration of 10 μM and completely abrogated SAP-induced MCP-1 mRNA expression at a high concentration of 50 μM.
FIG. 6.
SAP-induced MCP-1 induction is PI3 kinase dependent. Caco-2 cells were pretreated or not (−) with different concentrations of LY294002 for 1 h followed by SAP treatment for 2 h. RNA was extracted and real-time PCR done as described in the text. The experiment was repeated three times, and increase (n-fold) of MCP-1 over the level for untreated cells is depicted in the graph (*, P < 0.01 [over control]; **, P < 0.05 [over that of SAP alone]).
DISCUSSION
Herein, we report that soluble ameba components induce MCP-1, a potent macrophage chemoattractant, in IEC via alternate activation of the NF-κB pathway. While previous studies reported induction of IL-8, a neutrophil chemoattractant, by the parasite (4, 15), the role of these immune cells in ameba killing is doubtful as observed in vitro, wherein highly virulent strains of ameba could resist neutrophils at a high ratio of 3,000 neutrophils per ameba (5). More so, the neutrophils may contribute to host tissue damage. As invasion progresses, the ulcers extend deep into the submucosa, and during this late invasive stage, macrophages and lymphocytes are seen (5). Despite their presence in the lesions, the efficacy of monocytes and macrophages in killing trophozoites is not very clear but is probably related to their state of activation. Moreover, the ameba has been shown to secrete a monocyte locomotion inhibition factor, which has been implicated in the absence of macrophages in severe amebic lesions (8). Apparently, whether macrophages home in and kill trophozoites depends on several factors, such as the balance among epithelial MCP-1 production, parasite release of inhibitory factors, and release of other activating factors, like tumor necrosis factor alpha and gamma interferon. Nonetheless, monocytes and macrophages are present in amebic lesions and might play a role both in the destruction of host tissue and also in the killing of trophozoites. As MCP-1 is also able to activate cytotoxic T cells (7) and these cells have been found to kill ameba trophozoites (13), this observation is of great importance to pathogenesis.
NF-κB is an important transcription factor that is usually activated by a classical pathway involving IκB degradation and increased NF-κB nuclear translocation or a noncanonical pathway involving p65 phosphorylation. While IKK has been shown to be involved in both pathways, it is mandatory for the former but dispensable for the latter. The p65 subunit is phosphorylated at four serine residues by several kinases, and this posttranslational modification is either supplementary to classical IKK-dependent NF-κB activation or required for the transcription of certain NF-κB-dependent genes (12). Some reports suggest that phosphorylation of p65 enhances nuclear transport through an unknown mechanism but most probably via decreased affinity to IκB-α (2); this could be the reason for the delayed increase of nuclear expression of p65 (Fig. 3B) in response to ameba components and the delayed peak induction of MCP-1 mRNA at 8 h. Nonetheless, rapid induction at 2 h without the apparent increase of p65 is most probably related to the phospho-p65-mediated gene transcription seen in the luciferase reporter studies. MCP-1 is constitutively regulated by the classical NF-κB pathway, as is observed by its inhibition by the proteasome inhibitor MG132 in cells not treated with SAP. It could reasonably be argued that NF-κB-dependent genes are under the regulation of redundant pathways and could be modulated in a stimulus-dependent manner. We previously observed that prolonged treatment of macrophage-primed IEC with ameba proteins inhibits NF-κB activation via a negative regulation of IKK activity by stress proteins (6).
It is not clear if PI3 kinase or Akt directly phosphorylates the p65 subunit in IEC in response to ameba components. IKK-α/β and P38, which have previously been shown to mediate p65 phosphorylation (9, 11), were not involved in this case (Fig. 2 and 5D), and the role of other kinases, such as Bruton's tyrosine kinase or IKK-i/IKK-ɛ, needs to be explored. In summary, we demonstrate a novel signaling pathway in colonic epithelial cells for activation of MCP-1 by the gut pathogen E. histolytica and hypothesize that this could have an impact on the outcome of infection.
Acknowledgments
This study was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research (CIHR). S.J.K. was the recipient of a McGill University graduate fellowship, and I.D. holds a Canadian Association of Gastroenterology-Axcan Pharma-CIHR Research and Fellowship Award.
We thank Elaine De Heuvel for excellent technical assistance.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Bas, A., G. Forsberg, S. Hammarström, and M.-L. Hammarström. 2004. Utility of the housekeeping genes 18srRNA, β-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand. J. Immunol. 59:566-573. [DOI] [PubMed] [Google Scholar]
- 2.Bohuslav, J., L.-F. Chen, H. Kwon, Y. Mu, and W. C. Greene. 2004. p53 induces NF-κB activation by an IκB kinase-independent mechanism involving phosphorylation of p65 by ribosomal S6 kinase 1. J. Biol. Chem. 279:26115-26125. [DOI] [PubMed] [Google Scholar]
- 3.Chen, L.-F., and W. C. Greene. 2004. Shaping the nuclear action of NF-κB. Nat. Rev. Mol. Cell Biol. 5:392-401. [DOI] [PubMed] [Google Scholar]
- 4.Eckmann, L., S. L. Reed, J. R. Smith, and M. F. Kagnoff. 1995. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J. Clin. Investig. 96:1269-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinosa-Cantellano, M., and A. Martinez-Palomo. 2000. Pathogenesis of intestinal amebiasis: from molecules to disease. Clin. Microbiol. Rev. 13:318-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammanadiminti, S. J., and K. Chadee. 2006. Suppression of NF-κB activation by Entamoeba histolytica in intestinal epithelial cells is mediated by heat shock protein 27. J. Biol. Chem. 281:26112-26120. [DOI] [PubMed] [Google Scholar]
- 7.Kim, J. J., L. K. Nottingham, J. I. Sin, A. Tsai, L. Morrison, J. Oh, K. Dang, Y. Hu, K. Kazahaya, M. Bennett, T. Dentchev, D. M. Wilson, A. A. Chalian, J. D. Boyer, M. G. Agadjanyan, and D. B. Weiner. 1998. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J. Clin. Investig. 102:1112-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kretschmer, R., M. L. Collado, M. G. Pacheco, M. C. Salinas, M. Lopez-Osuna, M. Lecuona, E. M. Castro, and J. Arellano. 1985. Inhibition of human monocyte locomotion by products of axenically grown Entamoeba histolytica. Parasite Immunol. 7:527-543. [DOI] [PubMed] [Google Scholar]
- 9.Madrid, L. V., M. W. Mayo, J. Y. Reuther, and A. S. Baldwin, Jr. 2001. Akt stimulates the transactivation potential of the RelA/p65 subunit of NF-κB through utilization of the IκB kinase and activation of the mitogen-activated protein kinase p38. J. Biol. Chem. 276:18934-18940. [DOI] [PubMed] [Google Scholar]
- 10.Murphy, P. M. 1994. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12:593-633. [DOI] [PubMed] [Google Scholar]
- 11.Sizemore, N., N. Lerner, N. Dombrowski, H. Sakurai, and G. R. Stark. 2002. Distinct roles of the IκB kinase α and β subunits in liberating nuclear factor κB (NF-κB) from IκB and in phosphorylating the p65 subunit of NF-κB. J. Biol. Chem. 277:3863-3869. [DOI] [PubMed] [Google Scholar]
- 12.Viatour, P., M.-P. Merville, V. Bours, and A. Chariot. 2005. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 30:43-51. [DOI] [PubMed] [Google Scholar]
- 13.Vohra, H., U. Kaur, A. K. Sharma, V. Bhalla, and D. Bhasin. 2003. Effective human defense against E. histolytica: high amoebicidal activity of lymphocytes and monocytes in amoebic liver abscess patients until 3 months follow-up. Parasitol. Int. 52:193-202. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. 1997. WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28-29 January 1997. Epidemiol. Bull. 18:13-14. [PubMed] [Google Scholar]
- 15.Yu, Y., and K. Chadee. 1997. Entamoeba histolytica stimulates interleukin 8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology 112:1536-1547. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Z., and S. L. Stanley, Jr. 2004. Stereotypic and specific elements of the human colonic response to Entamoeba histolytica and Shigella flexneri. Cell. Microbiol. 6:535-554. [DOI] [PubMed] [Google Scholar]