Abstract
We isolated Enterococcus faecalis from the body fluids of dead larvae of the greater wax moth, Galleria mellonella. Extracellular gelatinase (GelE) and serine protease (SprE), both of which are considered putative virulence factors of E. faecalis, were purified from the culture supernatant of E. faecalis. In an attempt to elucidate their virulence mechanisms, purified GelE and SprE were injected into hemolymph of G. mellonella and evaluated with regard to their effects on the immune system of insect hemolymph. As a result, it was determined that E. faecalis GelE degraded an inducible antimicrobial peptide (Gm cecropin) which is known to perform a critical role in host defense during the early phase of microbial infection. The results obtained from the G. mellonella-E. faecalis infection model compelled us to assess the virulence activity of GelE against the complement system in human serum. E. faecalis GelE hydrolyzed C3a and also mediated the degradation of the alpha chain of C3b, thereby inhibiting opsonization and the formation of the membrane attack complex resultant from the activation of the complement cascade triggered by C3 activation. In contrast, E. faecalis SprE exhibited no virulence effect against the immune system of insect hemolymph or human serum tested in this study.
A host of studies have previously been conducted in an attempt to gain insight into the microbial virulence factors involved in the processes of human infectious diseases. Recently, four simple invertebrates—Caenorhabditis elegans (45); the fruit fly, Drosophila melanogaster (12); and two lepidopteran insects, Bombyx mori (25) and Galleria mellonella (4, 38)—have attracted interest due to their potential as good model systems for the screening of virulence factors of pathogenic microbes or for elucidating their actions in the host. Galleria mellonella is readily bred in the laboratory, and larvae in the last stage are an appropriate size for injecting samples into hemolymph, which tends to be difficult with C. elegans and D. melanogaster. It has also been reported that there is a positive correlation between the virulence of Pseudomonas aeruginosa in mice and G. mellonella models (21). Accordingly, the model of G. mellonella killing by microbial pathogens may generate information necessary for our understanding of the processes inherent to human infection (21).
Over the last 2 decades, enterococci have been recognized as being among the most common hospital-acquired pathogens causing a wide variety of diseases in humans. This class of pathogens can infect the bloodstream (3), urinary tract (15), endocardium (34), and biliary tract as well as burn wounds (23) and periodontal tissues (26). In addition, as enterococci are frequently isolated from polymicrobial flora (8, 37), the enterococci have been theorized to play a role in bacterial synergy (32, 33). Despite increasing recognition of the clinical importance of enterococcal infections, the pathogenic mechanisms utilized by this class of pathogens remain unclear. Among diverse enterococcal species, Enterococcus faecalis is known to be most responsible for enterococcal infections (23). E. faecalis has been reported to generate a variety of factors important for virulence, including aggregation substance (14), sex pheromones (42), cytolysin (5), hyaluronidase (26), MSCRAMM protein (40), and extracellular proteases (gelatinase and serine protease) (11), although the virulence mechanisms inherent to these factors also remain largely unknown. Gelatinase (GelE), which is the major interest of this study, was proposed to have an important role in biofilm formation (7, 17) and translocation of bacteria across intestinal cell layers (52). However, there are also still debates on the exact mechanism for the virulence activity of GelE.
Recently, we have isolated E. faecalis from the body fluids of dead G. mellonella larvae and have purified a GelE protein as an insecticidal toxin from E. faecalis culture media. The purified GelE protein was determined to be 99% identical to previously known E. faecalis GelE with regard to its amino acid sequence. Some bacterial proteases, such as elastases of Pseudomonas aeruginosa, have been reported to exert their virulence effects via altering host immune response (2, 41). We have thus performed a series of experiments to elucidate the relationship between E. faecalis GelE and the host immune system. We first evaluated the insecticidal activity of the purified GelE protein against G. mellonella larvae and also investigated the effect of GelE on the defense system of the hemolymph. The results from other previous works about P. aeruginosa elastase and the results from our work performed with a G. mellonella infection model led us to postulate that GelE may perform a critical role in the pathogenesis of enterococcal infection in human blood. Accordingly, we attempted to determine whether or not E. faecalis GelE affects the complement system and inhibits the immune reactions resultant from complement activation. To the best of our knowledge, this is the first paper to identify and describe the virulence mechanism of E. faecalis GelE in human serum.
MATERIALS AND METHODS
Bacterial cultures and insect rearing.
During rearing of G. mellonella, we found some larvae that appeared to be dead by bacterial infection. A bacterial strain was isolated from the larval cadavers of G. mellonella. In order to identify the bacteria, we conducted an analysis of the 16S rRNA sequence. As a result, the isolate was determined to be closest to the E. faecalis AY395018 strain found in the gypsy moth, Lymantria dispar, and is henceforth referred to as “E. faecalis GM” in this paper. Stocks were maintained on petri dishes containing 1% Bacto agar (Difco) in tryptic soy broth (TSB; Difco). The cultures were incubated for 20 h in TSB at 37°C. G. mellonella larvae were reared on an artificial diet (13). Last-instar larvae were used in this study, each having a body weight in excess of 120 mg.
Infection and survival experiments.
Enterococcus faecalis ATCC 51299, which was a human isolate, was selected as a control strain in this experiment. Galleria mellonella larvae were injected with washed mid-logarithmic-phase E. faecalis GM or E. faecalis ATCC 51299 (5 × 105 cells per larva) in 5 μl of autoclaved 10 mM sodium phosphate (NaP) buffer at a pH of 7.4. The test group used for infection by each of the bacterial strains consisted of 100 insects, and insect mortality was monitored at 6-h intervals for the first 24 h and then at 4-h intervals. In addition, 5 μl of a concentrate of the E. faecalis GM or ATCC 51299 culture supernatant (CS) containing 20 μg of proteins was injected into the hemocoel of each of 10 larvae. The CS of each E. faecalis strain was concentrated via ultrafiltration, using a 10-kDa cutoff membrane (Vivaflow 200, no. 05VF20045; Vivascience Ltd., United Kingdom) and adjusted to the same concentration. Alternatively, the concentrated CS of E. faecalis GM was heated for 5 min at 75°C, and the same amount of heat-treated sample was injected into each insect. As the injection of the concentrated CS of E. faecalis GM into the G. mellonella larvae resulted in rapid death of insects, the mortality of the insects was assessed at 5-min intervals.
Purification of GelE from concentrated CS of E. faecalis GM.
The culture of E. faecalis GM was centrifuged for 30 min at 10,000 × g. A 3-liter sample of cell-free CS was concentrated to ∼20 ml as described above. The concentrated CS was loaded onto a Sephadex G-100 column (2.5 by 70 cm) equilibrated with NaP buffer. Fractions were eluted at a flow rate of 6 ml/h and collected at intervals of 20 min. Every second fraction was assessed with regard to its insecticidal activity, in accordance with the following procedure. First, 50 μl of fraction was dried with a vacuum centrifugation system (Centra evaporator; Bioneer, Daejon, Korea) and resuspended in 6 μl of distilled water. Then, 5 μl was removed and injected into a G. mellonella larva. Five larvae were used for each test fraction. Fractions resulting in 100% insect mortality after overnight rearing were pooled and concentrated to ∼10 ml by ultrafiltration. The sample was then applied to a DEAE anion exchange column (SOURCE 15Q; Amersham Biosciences, Uppsala, Sweden). After sample injection, the column was washed with NaP buffer containing 50 mM NaCl. Then, the NaCl concentration was increased in a linear fashion up to 500 mM for 50 min, and the eluted fractions were collected in peak mode. Each fraction of two peaks was then concentrated with a Vivaspin concentrator equipped with a 10-kDa cutoff membrane and subjected to tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). To test the insecticidal activity of each of the purified proteins, the 5-μl samples, harboring various amounts of protein, were injected into G. mellonella larvae, after which insect mortality was monitored at the predetermined postinjection time. Ten larvae were used for each of the test groups. The N-terminal amino acid sequences of two purified proteins were determined via gas phase Edman degradation at the Korea Basic Science Institute. As described in Results, it was determined that the protein in the first peak was E. faecalis GelE, and the second peak was identified as a serine protease (SprE). In order to generate antibodies against GelE, the purified GelE was heat inactivated for 5 min at 75°C, as the injection of fresh GelE resulted in severe damage to the rabbit. According to conventional procedure, antiserum was taken 1 week after the booster injection of heat-inactivated antigen and employed in the immunoblot analysis (50). A 1,000-fold-diluted antiserum was used in this experiment.
Reduction of antibacterial activity of the insect hemolymph and degradation of Gm cecropin by GelE.
The G. mellonella larvae were inoculated with live Escherichia coli K112 (5 × 103 cells/larva) as described by Kim et al. (27). After 24 h of further rearing, the hemolymph was collected and centrifuged in order to remove hemocytes and cell debris. Cell-free hemolymph (150 μl) was added to 150 μl of NaP buffer containing 5 μg of purified GelE or SprE or no proteases and incubated for 15 min at room temperature. Each sample was then mixed with an equal volume of 10% acetic acid. Each mixture was vigorously stirred overnight at room temperature and centrifuged for 30 min at 10,000 × g. The acid extract obtained as a supernatant was dried completely via vacuum centrifugation and resuspended in 0.01% acetic acid to a final concentration of 5 mg/ml. Samples were assessed for antibacterial activity against E. coli in a colony count assay, as described by Kim et al. (27). The experiments were repeated three times, and the mean values were used to construct a graph. In addition, the proteolytic activities of GelE and SprE against a Gm cecropin, namely, a cecropin-like antimicrobial peptide previously purified from G. mellonella hemolymph (27), was evaluated via tricine SDS-PAGE and high-performance liquid chromatography (HPLC). Synthetic Gm cecropin (20 μg) was incubated for 30 min with 2 μg of GelE or SprE in NaP buffer at room temperature. The mixtures were then subjected to tricine SDS-PAGE and C18 reverse-phase HPLC. The fractions were then eluted in a continuous 1.0%/min linear gradient of acetonitrile in 0.1% trifluoroacetic acid. Peptide fragments generated via GelE proteolysis were collected through the HPLC column and tested for their antibacterial properties against E. coli in radial diffusion assays (29).
Serum bactericidal and hemolytic assays.
Fresh normal human serum (NHS) samples were collected from three healthy volunteers. NHS (15 μl) was mixed with 4 μg of GelE, SprE, heat-inactivated GelE (hGelE), or no protein in 30 μl of NaP buffer containing 170 mM NaCl and then incubated for 1 h at room temperature. Five microliters of washed mid-logarithmic-phase E. faecalis GM solution (4 × 104 CFU/ml) was then added to the mixture. After one additional hour of incubation at 37°C in a rotary incubator, 20 μl of the mixture was plated on tryptic soy agar. The resultant colonies were then counted after overnight incubation at 37°C. For controls, NHS and GelE were heat inactivated at 56°C for 30 min and at 75°C for 5 min, respectively. Data were expressed as the percent survival in each sample compared to that in heat-inactivated NHS (hNHS) [(number of CFU in sample/number of CFU in hNHS) × 100]. The hemolytic activity of NHS against rabbit erythrocytes was also determined using a modified version of the procedure described in the work of Jiang et al. (24). In brief, 20 μl of NHS was mixed with 70 μl of phosphate-buffered saline (PBS) containing 2 μg of GelE, SprE, or hGelE or no protein and incubated for 1 h at 37°C. Rabbit erythrocytes (106 cells) in 10 μl of PBS containing 1% bovine serum albumin were added to the mixture and incubated for an additional hour at 37°C. After 3 min of centrifugation at 10,000 × g, the optical density of the supernatant was measured at 405 nm. Twenty percent (vol/vol) hNHS in PBS was employed as a negative control sample. The percent lysis of rabbit erythrocytes in each sample was calculated according to the following equation: percent lysis = (A405 of sample − A405 of PBS control)/(A405 of NHS sample − A405 of PBS control) × 100. In addition, the inhibitory effect of GelE on the hemolytic activity of NHS was evaluated in a dose-dependent manner. In this assay, GelE in 90 μl of the incubation mixture (20 μl NHS plus 70 μl PBS) was twofold serially diluted in a concentration range from 0.05 to 0.4 μM. All experiments were repeated at least three times, and the mean values were used in the construction of graphs.
Detection of complement factor C3 deposition on the bacterial surface.
Experiments were conducted in accordance with the method described previously in the work of Ren et al. (39). In brief, 25 μl of NHS was mixed with 42 μl of PBS containing 2 μg of GelE or SprE and incubated for 1 h in a rotary incubator at 37°C. The washed mid-logarithmic-phase E. faecalis GM solution (2 × 107 CFU/ml) (183 μl) was added to the sample and incubated for 10 additional minutes at 37°C. After three washings with PBS, the bacteria were resuspended in 30 μl of reducing SDS sample buffer (10 μl of 0.5 M Tris-HCl [pH 6.8] containing 10% SDS plus 20 μl of 60 mM dithiothreitol) and boiled for 5 min. The boiled sample was then centrifuged for 5 min at 3,000 rpm, and 10 μl of the supernatant was electrophoresed on an 8% SDS-PAGE gel. The gel was then transferred onto nitrocellulose membranes in Tris-glycine buffer (25 mM Tris, 192 mM glycine, 20% methanol, pH 8.3) for immunoblot analysis, which was conducted using a commercially available antibody (C7761; Sigma) against human complement component 3 (C3), in accordance with the methods of Towbin et al. (50). For a positive or negative control sample, 25 μl of NHS or hNHS, respectively, was incubated in 42 μl of PBS containing no proteases for 1 h, and each sample was subjected to the same procedure for immunoblot analysis as described above.
Proteolysis of human C3, C3b, and C3a by GelE.
One microliter of NHS was added to 10 μl of NaP buffer containing 1 μg of GelE or SprE and then incubated for 20 min at room temperature. The sample was dried completely via vacuum centrifugation and resuspended in 50 μl of SDS-PAGE sample buffer. Five microliters was removed and electrophoresed on an 8% SDS-PAGE gel. In addition, 10 μl of log-phase E. faecalis GM solution (4 × 104 CFU/ml in PBS) was added to 190 μl TSB containing 60 μl of NHS. After incubation at 37°C for a predetermined time, the mixture was centrifuged for 10 min at 10,000 × g. Ten microliters of supernatant was removed and dried via vacuum centrifugation. It was then resuspended in 150 μl of SDS-PAGE sample buffer, and a 5-μl sample was subjected to SDS-PAGE. Two duplicate SDS-PAGE gels were employed for immunoblot analysis, which was conducted with an antibody against human C3. In order to determine the proteolytic activity of GelE against purified C3 components, 2 μg of commercially available C3 (C2910; Sigma), C3a (224881; Calbiochem, Germany), or C3b (204860; Calbiochem, Germany) was mixed with 10 μl of NaP buffer containing 1 μg of GelE. After 20 min of incubation at 37°C, the mixtures were subjected to 8% SDS-PAGE or 16.5% tricine SDS-PAGE.
Nucleotide sequence accession numbers.
The full 16S rRNA sequence of E. faecalis GM was deposited in GenBank under accession no. EF120452. The nucleotide sequence of gelE from E. faecalis GM has been registered in GenBank under accession no. EF105504.
RESULTS
Insecticidal activity of E. faecalis GM and CS of E. faecalis GM.
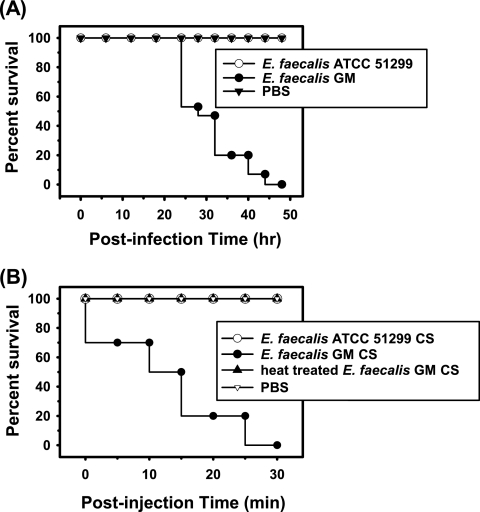
Galleria mellonella larvae were artificially infected via injection of E. faecalis GM into the hemocoel. Insect mortality was then evaluated at 6-hour intervals (Fig. 1A). No dead insects were observed until 24 h postinfection. About half of the test insects were killed at 28 h after infection, and all of the insects were dead 48 h later. In contrast, injection of E. faecalis ATCC 51299 resulted in no detectable insecticidal effects throughout the entirety of the incubation period. In order to determine whether secretions from E. faecalis GM exerted insecticidal effects, 5 μl of concentrated CS was injected into each of 10 larvae. As a result, all of the insect larvae turned black within 5 min, and all were dead 30 min later (Fig. 1B). However, neither the heat-treated CS of E. faecalis GM nor the CS of E. faecalis ATCC 51299 evidenced any insecticidal effects. According to these results, E. faecalis GM was determined to secrete a heat-labile agent with insecticidal activity.
FIG. 1.
Survival of insects upon injection with E. faecalis GM or CS. Kaplan-Meier survival plots of G. mellonella injected with E. faecalis GM or ATCC 51299 (A) and CS of each strain (B). For a control, 5 μl of PBS was injected into each of the test larvae.
Purification of GelE and SprE.
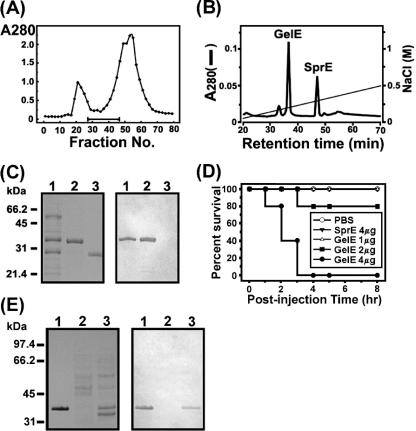
To purify an insecticidal protein from the concentrated CS of E. faecalis GM, we adopted a two-step procedure, consisting of a gel filtration chromatography step and an anion-exchange chromatography step. Figure 2A shows a profile of Sephadex G-100 gel filtration chromatography. Fractions of interest were subjected to anion-exchange HPLC as a final purification step, which yielded two peaks (Fig. 2B). The first and second peaks were eluted in the gradient at 0.17 M and 0.2 M NaCl, respectively. Each peak was verified to harbor a protein with a single band of 34 kDa or 26 kDa in tricine SDS-PAGE, thereby indicating that the proteins were purified to apparent homogeneity (Fig. 2C). Edman degradation showed that the N-terminal 30-amino-acid sequences of the first and second proteins matched exactly those of the processed, mature forms of the GelE and SprE proteins of E. faecalis, respectively. Furthermore, we determined the entire structure of the GelE gene (gelE) for the gelatinase from E. faecalis GM via PCR, which was conducted with several primers designed on the basis of the nucleotide sequence of E. faecalis gelE (GenBank accession no. D85393). As a result, gelE of E. faecalis GM shared 98.7% identity at the nucleotide level and 99% identity at the amino acid sequence level with gelE of E. faecalis V583 (data not shown). Therefore, we concluded that the purified protein in the first peak was an extracellular GelE protein of E. faecalis GM. Also, considering its N-terminal sequence, molecular mass, and electrophoretic mobility, the second protein was identified as an extracellular SprE protein. When the insecticidal activity of the purified GelE or SprE protein was tested against G. mellonella larvae, GelE evidenced lethal activity in a dose-dependent manner, but SprE manifested no such activity, even when 4 μg was injected (Fig. 2D). The results of our immunoblot analysis revealed that GelE was present in the CS of E. faecalis GM but not in the CS of E. faecalis ATCC 51299, thereby indicating that the control strain did not secrete detectable quantities of GelE (Fig. 2E).
FIG. 2.
Purification of GelE from concentrated CS of E. faecalis GM. (A) Profile of Sephadex G-100 gel filtration chromatography. The optical density of each fraction was measured at 280 nm. The bar represents the range of fractions evidencing 100% mortality in our “infection and survival” test. (B) Anion-exchange chromatography as a final step in the purification of GelE. Two peaks, corresponding to gelatinase and serine protease, were indicated by GelE and SprE, respectively. (C) Tricine SDS-PAGE and immunoblot analysis. Left panel, a tricine SDS-PAGE gel stained with Coomassie blue; right panel, immunoblot analysis conducted with antiserum against GelE. Lane 1, pooled fractions (no. 26 to 46) from gel filtration chromatography, as indicated by the horizontal bar in panel A; lane 2, purified GelE; lane 3, purified SprE. (D) Kaplan-Meier survival plots of G. mellonella injected with differing quantities of purified GelE, ranging from 1 to 4 μg per larva, or SprE. Also, 5 μl of PBS was injected into the larvae as a control. (E) Detection of GelE in the concentrated CS of E. faecalis GM. Left panel, a 10% SDS-PAGE gel stained with Coomassie blue; right panel, immunoblot analysis using antiserum against GelE as a probe. Lane 1, purified GelE; lane 2, CS of E. faecalis ATCC 51299; lane 3, CS of E. faecalis GM.
Proteolytic effect of GelE on the Gm cecropin of G. mellonella hemolymph.
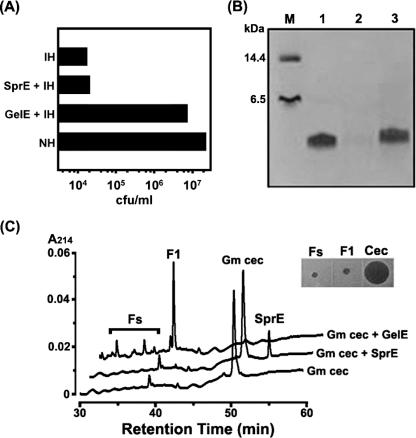
When the cell-free hemolymph of immunized G. mellonella larvae was incubated with GelE, its antibacterial activity was significantly attenuated (Fig. 3A). After 15 min of incubation, the activity was comparable to that of the hemolymph from naïve insects, thereby suggesting that the inducible antimicrobial activity of G. mellonella hemolymph was abolished during the incubation with GelE. Accordingly, we assessed the effects of GelE on an inducible antimicrobial peptide (Gm cecropin) from G. mellonella. Figure 3B shows that Gm cecropin was degraded by GelE but not by SprE. In addition, the HPLC chromatogram indicated that the digestion of Gm cecropin by GelE generated a discrete major peak (Fig. 3C, F1) as well as several minor peaks (Fig. 3C, Fs). When fractions of Fs and F1 were evaluated with regard to their antibacterial activities via radial diffusion assays, none of them was determined to retain antimicrobial activity (Fig. 3C, inset).
FIG. 3.
Effects of GelE on antibacterial activity of G. mellonella hemolymph. (A) As shown in the acid extract sample of the immunized hemolymph incubated with GelE (GelE + IH), its antibacterial activity was significantly attenuated and close to that of nonimmunized hemolymph (NH). Error bars were too small to express. (B) A Coomassie blue-stained 16.5% tricine SDS-PAGE gel containing purified Gm cecropin and Gm cecropin treated with GelE or SprE. Lane M, standard marker; lane 1, 2 μg of Gm cecropin; lane 2, 2 μg of Gm cecropin treated with GelE; lane 3, 2 μg of Gm cecropin treated with SprE. (C) HPLC chromatograms of the GelE-induced digestion of Gm cecropin. Inset, radial diffusion assay for antibacterial activities of Fs, F1, and intact Gm cecropin (Cec).
Effects of GelE on the bactericidal and hemolytic activities of human serum.
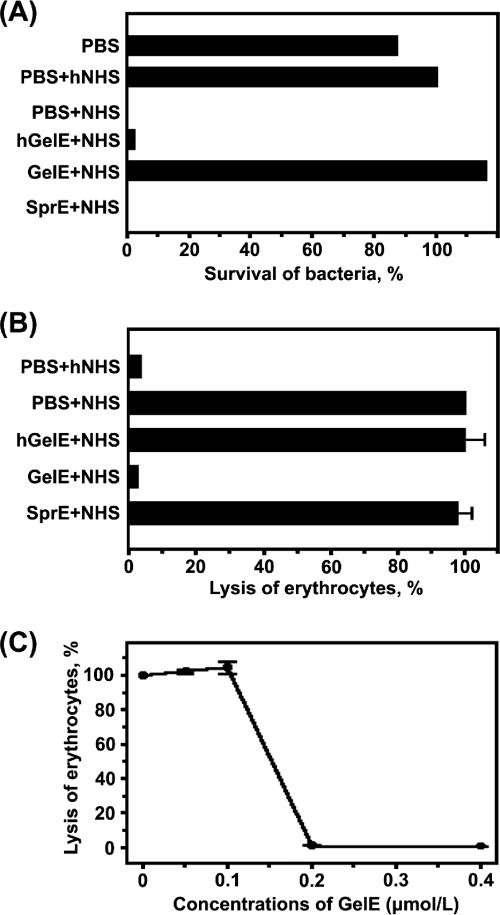
In order to determine whether GelE affects the bactericidal and hemolytic activities caused by the complement in human serum, we evaluated the bacterium-killing and hemolytic activities exhibited by NHS or GelE-treated NHS, using rabbit erythrocytes. As is shown in Fig. 4A, no viable colonies were detected in the sample that was plated on tryptic soy agar at 1 h after the incubation of 200 E. faecalis GM cells in 30% NHS. However, incubation with 30% NHS pretreated with GelE resulted in a bacterial survival rate of 100%. In contrast, SprE and hGelE did not affect bacterial killing by NHS, thereby indicating that the proteolytic activity of GelE was responsible for the abrogation of the bactericidal effects of NHS. In parallel, the complement-mediated lysis of rabbit erythrocytes was also inhibited by GelE, but neither SprE nor hGelE was shown to affect the hemolytic activity of NHS (Fig. 4B). In a dose-dependent experiment, it was determined that GelE, at concentrations in excess of 0.2 μmol/liter, exhibited >90% inhibitory activity against complement-mediated hemolysis (Fig. 4C).
FIG. 4.
Effect of GelE on complement activation in human seurm. (A) Role of GelE in complement-mediated killing of E. faecalis GM. The survival rate of E. faecalis GM in the PBS-hNHS sample was determined to be 100%, and the relative survival rate of each sample was measured. Error bars were too small to express. (B) Inhibitory effect of GelE on the complement-mediated lysis of rabbit erythrocytes. Lysis of erythrocytes in the PBS-NHS sample was determined to be 100%. (C) Dose-dependent effect of GelE on complement-mediated erythrocyte lysis. E. faecalis GM SprE was used as a negative control and had no effect on the complement-mediated lysis of erythrocytes (data not shown). Spontaneous erythrocyte lysis was <1% and was not represented.
Effect of GelE on cell surface deposition of C3.
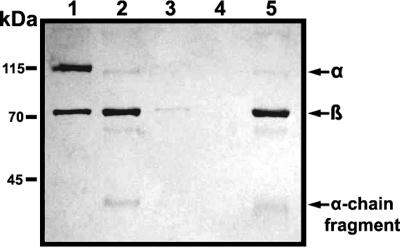
In order to examine in more detail the inhibitory effects of GelE on complement activity in NHS, we also attempted to ascertain, via immunoblot analysis, whether GelE affected the deposition of C3b on the cell surface upon the incubation of bacteria in NHS (Fig. 5). When bacteria were incubated with NHS or SprE-treated NHS, C3α, C3β, and the α-chain fragment were identified in the protein samples separated from the bacterial surface. In contrast, C3 components deposited on the bacterial surface were not detected after the incubation of bacteria with hNHS or GelE-treated NHS. From this result, it was suggested that GelE inhibited the complement-mediated opsonization of bacteria.
FIG. 5.
Effect of GelE on cell surface deposition of C3. The protein samples on E. faecalis GM were detected via immunoblot analysis. The nitrocellulose membrane was probed with anti-C3 antibody, which had been diluted to a concentration of 1:1,000 in antibody solution (1% skim milk in Tris-buffered saline containing 0.05% Tween 20). The secondary antibody used in this experiment was horseradish peroxidase-conjugated goat anti-rabbit antibody, as appropriate, at a dilution of 1:8,000. One microgram of purified C3 was employed as a control. Lane 1, purified C3; lane 2, NHS incubated with PBS; lane 3, hNHS incubated with PBS; lane 4, NHS incubated with GelE; lane 5, NHS incubated with SprE. Arrows indicate the α chain, β chain, and α-chain fragments of human C3.
Proteolytic effect of GelE on C3 in NHS and purified C3, C3a, and C3b.
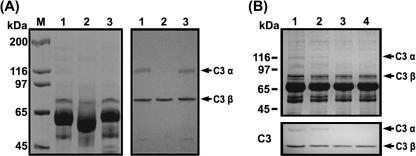
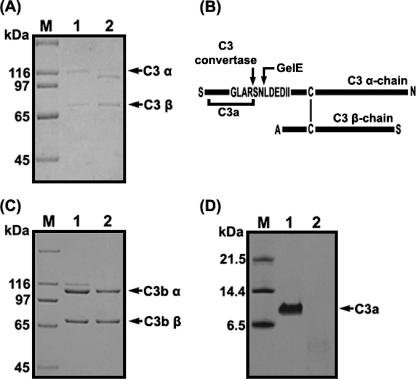
In order to characterize the proteolytic activity of GelE against C3 in human serum, NHS preincubated with GelE or SprE was subjected to SDS-PAGE, either stained with Coomassie blue or transferred onto nitrocellulose membranes, and then probed with anti-human C3 antibody (Fig. 6A). Whereas the immunoblots of NHS (Fig. 6A, lane 1) or SprE-treated NHS (Fig. 6A, lane 3) revealed intact α and β chains of C3 (115 and 75 kDa, respectively), the α-chain band disappeared from the gel upon the incubation of NHS with GelE (Fig. 6A, lane 2). In addition, we evaluated changes occurring in the C3 components of NHS incubated with E. faecalis GM (Fig. 6B). When E. faecalis GM (∼400 CFU) was incubated for 6 h with 30% (vol/vol) NHS in TSB, the α and β chains of C3 were found to have remained intact. In contrast, the α chain of C3 was not detected in the NHS samples incubated for 12 or 24 h. From Fig. 6A and B, it became evident that the α chain of C3 was destroyed via the action of GelE secreted from E. faecalis GM upon incubation for more than 12 h. Furthermore, in order to determine whether or not GelE directly hydrolyzed the components of C3, purified C3, C3a, or C3b was treated with GelE and subjected to SDS-PAGE. As a result, the band corresponding to the β chain of C3 remained unaltered, as was also observed in the case of the NHS sample incubated with GelE. However, the α-chain band was observed at a position lower than that of the intact C3 (Fig. 7A), which appeared to be equivalent to the position of the C3b α chain without C3a, which is an N-terminal 9-kDa component of the α chain of C3 (Fig. 7C). When the α chain generated from C3 treated with GelE was transferred to a polyvinylidene difluoride membrane and subjected to Edman degradation analysis, its N-terminal 10-amino-acid sequence was identified to be LDEDIIAEEN. Accordingly, it was concluded that GelE cleaved the peptide bond between Asn and Leu residues, which is close to the scissile bond of C3 convertase (Fig. 7B). In addition, C3a was determined to be further degraded by GelE, as it was not detected in the SDS-PAGE gel after incubation with GelE (Fig. 7D).
FIG. 6.
Degradation of complement factor C3 α chain by GelE, as determined via SDS-PAGE. (A) Proteolysis of the C3 α chain in NHS by GelE. Left panel, 8% SDS-PAGE gel conducted with NHS; right panel, immunoblot analysis. Lane M, molecular mass marker; lane 1, NHS incubated with PBS; lane 2, NHS incubated with GelE; lane 3, NHS incubated with SprE. Arrows indicate the intact α and β chains of C3 present in NHS. (B) Change in the C3 α chain in NHS by E. faecalis GM. Equal volumes of the mixtures were removed and subjected to SDS-PAGE and immunoblot analysis. Upper panel, an SDS-PAGE gel; lower panel, immunoblot analysis. Lane 1, NHS incubated with PBS; lane 2, NHS incubated with E. faecalis GM for 6 h; lane 3, NHS incubated with E. faecalis GM for 12 h; lane 4, NHS incubated with E. faecalis GM for 24 h.
FIG. 7.
Proteolysis of complement factor C3 and C3a by GelE. (A) SDS-PAGE performed with purified C3. Lane M, molecular mass marker; lane 1, C3 incubated with PBS; lane 2, C3 incubated with GelE. (B) Schematic view of C3 cleavage by GelE. The cleavage sites of C3 α chain by C3 convertase or GelE are marked by arrows, and the vertical bar represents a disulfide bond between the C3 α chain and C3 β chain. (C) SDS-PAGE performed with purified C3b. Lane M, molecular mass marker; lane 1, C3b incubated with PBS; lane 2, C3b incubated with GelE. (D) Tricine SDS-PAGE performed with purified C3a. Lane M, molecular mass marker; lane 1, C3a incubated with PBS; lane 2, C3a incubated with GelE. The gels were stained with Coomassie blue.
DISCUSSION
The greater wax moth, G. mellonella, has been shown to be killed as the result of infection with a host of human pathogenic microbes. Therefore, the moth has been employed in investigations into the virulence mechanisms of several human pathogens, including P. aeruginosa (19, 31), Proteus mirabilis (9), E. coli (18), Bacillus cereus (16), Cryptococcus neoformans (35), and Candida albicans (10).
In this study, we purified two proteases (GelE and SprE) secreted by E. faecalis GM, which was isolated from the cadavers of G. mellonella larvae. Several proteinases secreted from a variety of pathogenic bacteria were previously shown to be capable of degrading an antimicrobial peptide such as LL-37 and abolishing its activity (43), which indicated that proteolytic degradation of an antimicrobial peptide might be a common virulence mechanism. Accordingly, we first evaluated the virulence effects of GelE and SprE on antimicrobial peptides and on the complement system, which were principally responsible for the clearance of invading microbes at the early phase of microbial infection from insect hemolymph and from human blood, respectively. In several animal infection models, E. faecalis GelE and SprE were identified as critical virulence factors of E. faecalis, although the mechanisms underlying their virulence remain to be fully elucidated. GelE/SprE-producing E. faecalis was shown, in a mouse peritonitis model, to elicit increased mortality compared to the GelE/SprE-defective mutant (47). Similarly, E. faecalis GelE and SprE were determined to contribute in concert to pathogenesis in a rabbit endophthalmitis model (11) and a C. elegans-killing model (46). In particular, Creti et al. (6) reported that when a collection of E. faecalis strains from clinical isolates was screened for the presence of virulence factor genes, the GelE gene (gelE) was found to be the most common factor. It was also previously determined that E. faecalis expressed gelE at the second highest level among several enterococcal virulence genes upon culture in human serum (44). In parallel with these previous results, our data (Fig. 3) showed that E. faecalis GelE negated an inducible antimicrobial activity of the G. mellonella hemolymph, which was attributed to the degradation of Gm cecropin by GelE. Collectively, these findings suggested that the extracellular GelE protein of E. faecalis may perform a critical role in overcoming the immune systems inherent to human serum, thereby perpetuating bacterial survival in vivo. In order to confirm this hypothesis, we evaluated the effects of E. faecalis GelE on the complement system of human serum.
Complement activation, via the classical, lectin, or alternative pathway, frequently results in the activation of C3, which results in the generation of C3b and iC3b for the opsonization of the target and consequently causes the formation of the membrane attack complex (MAC). In order to resist opsonophagocytosis and MAC-mediated killing, pathogenic microbes have developed an array of virulence mechanisms. Three groups of streptococci have been the most extensively studied with regard to bacterial complement resistance mechanisms (22). Streptococcus pyogenes and S. agalactiae evade complement attack via the acquisition of host regulators of complement amplification, including factor H and C4bp (20, 49). In addition, S. pyogenes secretes streptococcal inhibitor of complement, which binds to the host complement regulator clusterin and thereby inhibits the reactive hemolysis of guinea pig erythrocytes resultant from complement activation (1, 48). Pneumococcal surface protein A (PspA) has also been identified as a virulence factor of S. pneumoniae, which also appears to function as a complement inhibitor (39). PspA was demonstrated, using mouse models, to reduce the quantity of C3b deposited onto the surfaces of pneumococci (39). Accordingly, PspA has been suggested to affect complement activation and to inhibit the formation of C3 convertase (51). However, the precise mechanism employed by PspA in the inhibition of complement activation remains to be elucidated. Unlike streptococcal virulence factors, E. faecalis GelE exerts an inhibitory effect against the complement system via its proteolytic properties. Thus far, GelE has been shown to be capable of degrading several substrates, including gelatin, insulin B chain, human endothelin, collagen, and several bioactive peptides (30). However, the hydrolysis of an antimicrobial peptide and C3a by E. faecalis GelE is a novel finding in this study. C3a, also referred to as anaphylatoxin, has been reported to perform an important function in host defense against microbial infections as well as in immune regulation. A variety of the functions of a host of immune cells, including macrophages, eosinophils, basophils, mast cells, and B lymphocytes, have been demonstrated to be modulated by C3a (28). Interestingly, it was recently shown that C3a and C3a-derived peptides also evidenced antibacterial properties and were capable of killing several bacteria, as has been observed with many other antimicrobial peptides (36). Therefore, the degradation of C3a by GelE permitted us to postulate that GelE may contribute to the paralysis of a variety of C3a-mediated immune reactions occurring in human blood. Also, this finding emphasized the importance of GelE as a virulence factor of E. faecalis. However, the α chain of the purified C3b was not affected by GelE (Fig. 7C), although the α chain of C3 in NHS was degraded completely upon incubation with NHS and GelE (Fig. 6A). According to these data, we now surmise that the α chain of C3b may be destroyed via the action of an as-yet-unknown factor in human serum, which is apparently activated by GelE.
In conclusion, we have demonstrated the virulence effects of E. faecalis GelE on the immune systems of insects and the complement system inherent to human serum. GelE directly hydrolyzed a cecropin-like antimicrobial peptide and C3a. It was also determined to inhibit C3b-mediated immune reactions (opsonization and MAC formation) via the stimulation of the degradation of the α chain of C3b in human serum. In contrast, extracellular SprE, which has also been considered a putative virulence factor of E. faecalis, did not affect the immune systems evaluated in the present study.
Acknowledgments
This work was supported by a grant from the Korea Science and Engineering Foundation (grant no. R01-2004-000-10878-1).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Akesson, P., A. G. Sjoholm, and L. Bjorck. 1996. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J. Biol. Chem. 271:1081-1088. [DOI] [PubMed] [Google Scholar]
- 2.Azghani, A. O., L. D. Gray, and A. R. Johnson. 1993. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect. Immun. 61:2681-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldassarri, L., R. Creti, L. Montanaro, G. Orefici, and C. R. Arciola. 2005. Pathogenesis of implant infections by enterococci. Int. J. Artif. Organs 28:1101-1109. [DOI] [PubMed] [Google Scholar]
- 4.Choi, J. Y., C. D. Sifri, B. C. Goumnerov, L. G. Rahme, F. M. Ausubel, and S. B. Calderwood. 2002. Identification of virulence genes in a pathogenic strain of Pseudomonas aeruginosa by representational difference analysis. J. Bacteriol. 184:952-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coburn, P. S., and M. S. Gilmore. 2003. The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 5:661-669. [DOI] [PubMed] [Google Scholar]
- 6.Creti, R., M. Imperi, L. Bertuccini, F. Fabretti, G. Orefici, R. Di Rosa, and L. Baldassarri. 2004. Survey for virulence determinants among Enterococcus faecalis isolated from different sources. J. Med. Microbiol. 53:13-20. [DOI] [PubMed] [Google Scholar]
- 7.Di Rosa, R., R. Creti, M. Venditti, R. D'Amelio, C. R. Arciola, L. Montanaro, and L. Baldassarri. 2006. Relationship between biofilm formation, the enterococcal surface protein (Esp) and gelatinase in clinical isolates of Enterococcus faecalis and Enterococcus faecium. FEMS Microbiol. Lett. 256:145-150. [DOI] [PubMed] [Google Scholar]
- 8.Dougherty, S. H. 1984. Role of enterococcus in intraabdominal sepsis. Am. J. Surg. 148:308-312. [DOI] [PubMed] [Google Scholar]
- 9.Dunphy, G. B., and J. S. Chadwick. 1989. Effects of selected carbohydrates and the contribution of the prophenoloxidase cascade system to the adhesion of strains of Pseudomonas aeruginosa and Proteus mirabilis to hemocytes of nonimmune larval Galleria mellonella. Can. J. Microbiol. 35:524-527. [DOI] [PubMed] [Google Scholar]
- 10.Dunphy, G. B., U. Oberholzer, M. Whiteway, R. J. Zakarian, and I. Boomer. 2003. Virulence of Candida albicans mutants toward larval Galleria mellonella (Insecta, Lepidoptera, Galleridae). Can. J. Microbiol. 49:514-524. [DOI] [PubMed] [Google Scholar]
- 11.Engelbert, M., E. Mylonakis, F. M. Ausubel, S. B. Calderwood, and M. S. Gilmore. 2004. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect. Immun. 72:3628-3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson, D. L., J. L. Lines, E. C. Pesci, V. Venturi, and D. G. Storey. 2004. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 72:5638-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frobius, A. C., M. R. Kanost, P. Gotz, and A. Vilcinskas. 2000. Isolation and characterization of novel inducible serine protease inhibitors from larval hemolymph of the greater wax moth Galleria mellonella. Eur. J. Biochem. 267:2046-2053. [DOI] [PubMed] [Google Scholar]
- 14.Galli, D., R. Wirth, and G. Wanner. 1989. Identification of aggregation substances of Enterococcus faecalis cells after induction by sex pheromones. An immunological and ultrastructural investigation. Arch. Microbiol. 151:486-490. [DOI] [PubMed] [Google Scholar]
- 15.Guzman, C. A., C. Pruzzo, G. LiPira, and L. Calegari. 1989. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect. Immun. 57:1834-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajaij-Ellouze, M., S. Fedhila, D. Lereclus, and C. Nielsen-Leroux. 2006. The enhancin-like metalloprotease from the Bacillus cereus group is regulated by the pleiotropic transcriptional activator PlcR but is not essential for larvicidal activity. FEMS Microbiol. Lett. 260:9-16. [DOI] [PubMed] [Google Scholar]
- 17.Hancock, L. E., and M. Perego. 2004. The Enterococcus faecalis fsr two-component system controls biofilm development through production of gelatinase. J. Bacteriol. 186:5629-5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanschke, R., and M. Hanschke. 1977. Studies on the activation of humoral defense factors against bacteria in larvae of the wax moth Galleria mellonella. Acta Biol. Med. Ger. 36:267-273. [PubMed] [Google Scholar]
- 19.Hendrickson, E. L., J. Plotnikova, S. Mahajan-Miklos, L. G. Rahme, and F. M. Ausubel. 2001. Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice. J. Bacteriol. 183:7126-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horstmann, R. D., H. J. Sievertsen, J. Knobloch, and V. A. Fischetti. 1988. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc. Natl. Acad. Sci. USA 85:1657-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 182:3843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarva, H., T. S. Jokiranta, R. Wurzner, and S. Meri. 2003. Complement resistance mechanisms of streptococci. Mol. Immunol. 40:95-107. [DOI] [PubMed] [Google Scholar]
- 23.Jett, B. D., M. M. Huycke, and M. S. Gilmore. 1994. Virulence of enterococci. Clin. Microbiol. Rev. 7:462-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, H., E. Wagner, H. Zhang, and M. M. Frank. 2001. Complement 1 inhibitor is a regulator of the alternative complement pathway. J. Exp. Med. 194:1609-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaito, C., N. Akimitsu, H. Watanabe, and K. Sekimizu. 2002. Silkworm larvae as an animal model of bacterial infection pathogenic to humans. Microb. Pathog. 32:183-190. [DOI] [PubMed] [Google Scholar]
- 26.Kayaoglu, G., and D. Orstavik. 2004. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit. Rev. Oral Biol. Med. 15:308-320. [DOI] [PubMed] [Google Scholar]
- 27.Kim, C. H., J. H. Lee, I. Kim, S. J. Seo, S. M. Son, K. Y. Lee, and I. H. Lee. 2004. Purification and cDNA cloning of a cecropin-like peptide from the great wax moth, Galleria mellonella. Mol. Cells 17:262-266. [PubMed] [Google Scholar]
- 28.Kohl, J. 2001. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol. Immunol. 38:175-187. [DOI] [PubMed] [Google Scholar]
- 29.Lehrer, R. I., M. Rosenman, S. S. Harwig, R. Jackson, and P. Eisenhauer. 1991. Ultrasensitive assays for endogenous antimicrobial polypeptides. J. Immunol. Methods 137:167-173. [DOI] [PubMed] [Google Scholar]
- 30.Makinen, P. L., D. B. Clewell, F. An, and K. K. Makinen. 1989. Purification and substrate specificity of a strongly hydrophobic extracellular metalloendopeptidase (“gelatinase”) from Streptococcus faecalis (strain 0G1-10). J. Biol. Chem. 264:3325-3334. [PubMed] [Google Scholar]
- 31.Miyata, S., M. Casey, D. W. Frank, F. M. Ausubel, and E. Drenkard. 2003. Use of the Galleria mellonella caterpillar as a model host to study the role of the type III secretion system in Pseudomonas aeruginosa pathogenesis. Infect. Immun. 71:2404-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montravers, P., A. Andremont, L. Massias, and C. Carbon. 1994. Investigation of the potential role of Enterococcus faecalis in the pathophysiology of experimental peritonitis. J. Infect. Dis. 169:821-830. [DOI] [PubMed] [Google Scholar]
- 33.Montravers, P., J. Mohler, L. Saint Julien, and C. Carbon. 1997. Evidence of the proinflammatory role of Enterococcus faecalis in polymicrobial peritonitis in rats. Infect. Immun. 65:144-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murry, B. E. 1990. The life and times of the enterococcus. Clin. Microbiol. Rev. 3:46-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mylonakis, E., R. Moreno, J. B. El Khoury, A. Idnurm, J. Heitman, S. B. Calderwood, F. M. Ausubel, and A. Diener. 2005. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect. Immun. 73:3842-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordahl, E. A., V. Rydengard, P. Nyberg, D. P. Nitsche, M. Morgelin, M. Malmsten, L. Bjorck, and A. Schmidtchen. 2004. Activation of the complement system generates antibacterial peptides. Proc. Natl. Acad. Sci. USA 101:16879-16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinheiro, E. T., B. P. Gomes, C. C. Ferraz, E. L. Sousa, F. B. Teixeira, and F. J. Souza-Filho. 2003. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 36:1-11. [DOI] [PubMed] [Google Scholar]
- 38.Reeves, E. P., C. G. Messina, S. Doyle, and K. Kavanagh. 2004. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia 158:73-79. [DOI] [PubMed] [Google Scholar]
- 39.Ren, B., M. A. McCrory, C. Pass, D. C. Bullard, C. M. Ballantyne, Y. Xu, D. E. Briles, and A. J. Szalai. 2004. The virulence function of Streptococcus pneumoniae surface protein A involves inhibition of complement activation and impairment of complement receptor-mediated protection. J. Immunol. 173:7506-7512. [DOI] [PubMed] [Google Scholar]
- 40.Rich, R. L., B. Kreikemeyer, R. T. Owens, S. LaBrenz, S. V. Narayana, G. M. Weinstock, B. E. Murray, and M. Hook. 1999. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J. Biol. Chem. 274:26939-26945. [DOI] [PubMed] [Google Scholar]
- 41.Rumbaugh, K. P., J. A. Griswold, and A. N. Hamood. 1999. Contribution of the regulatory gene lasR to the pathogenesis of Pseudomonas aeruginosa infection of burned mice. J. Burn Care Rehabil. 20:42-49. [DOI] [PubMed] [Google Scholar]
- 42.Sannomiya, P., R. A. Craig, D. B. Clewell, A. Suzuki, M. Fujino, G. O. Till, and W. A. Marasco. 1990. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc. Natl. Acad. Sci. USA 87:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidtchen, A., I.-M. Frick, E. Endersson, H. Tapper, and L. Bjorck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 44.Shepard, B. D., and M. S. Gilmore. 2002. Differential expression of virulence-related genes in Enterococcus faecalis in response to biological cues in serum and urine. Infect. Immun. 70:4344-4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sifri, C. D., A. Baresch-Bernal, S. B. Calderwood, and C. von Eiff. 2006. Virulence of Staphylococcus aureus small colony variants in the Caenorhabditis elegans infection model. Infect. Immun. 74:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sifri, C. D., E. Mylonakis, K. V. Singh, X. Qin, D. A. Garsin, B. E. Murray, F. M. Ausubel, and S. B. Calderwood. 2002. Virulence effect of Enterococcus faecalis protease genes and the quorum-sensing locus fsr in Caenorhabditis elegans and mice. Infect. Immun. 70:5647-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 178:1416-1420. [DOI] [PubMed] [Google Scholar]
- 48.Stockbauer, K. E., D. Grigsby, X. Pan, Y. X. Fu, L. M. Mejia, A. Cravioto, and J. M. Musser. 1998. Hypervariability generated by natural selection in an extracellular complement-inhibiting protein of serotype M1 strains of group A Streptococcus. Proc. Natl. Acad. Sci. USA 95:3128-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thern, A., L. Stenberg, B. Dahlback, and G. Lindahl. 1995. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J. Immunol. 154:375-386. [PubMed] [Google Scholar]
- 50.Towbin, H., T. Staehelin, and J. Gordon. 1992. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology 24:145-149. [PubMed] [Google Scholar]
- 51.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeng, J., F. Teng, and B. E. Murray. 2005. Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infect. Immun. 73:1606-1612. [DOI] [PMC free article] [PubMed] [Google Scholar]