Abstract
Macrophages ingest the fungus Cryptococcus neoformans only in the presence of opsonins, and this provides a remarkably clean system for the detailed analysis of phagocytosis. This system is also unusual in that antibody-mediated phagocytosis involves ingestion through both Fc and complement receptors in the absence of complement. Mathematical modeling was used to analyze and explain the experimental data that the macrophage phagocytic index increased with increasing doses of antibody despite saturating concentrations and declined at high concentrations. A model was developed that explains the increase in phagocytic index with increasing antibody doses, differentiates among the contributions from Fc and complement receptors, and provides a tool for estimating antibody concentrations that optimize efficacy of phagocytosis. Experimental results and model calculations revealed that blocking of Fc receptors by excess antibody caused a reduction in phagocytic index but increased phagocytosis through complement receptors rapidly compensated for this effect. At high antibody concentrations, a further reduction in phagocytic index was caused by interference with complement receptor ingestion as a consequence of saturation of the fungal capsule. The ability of our model to predict the antibody dose dependence of the macrophage phagocytic efficacy for C. neoformans strongly suggest that the major variables that determine the efficacy of this process have been identified. The model predicts that the affinity constant of the opsonic antibody for the Fc receptor and the association-dissociation constant of antibody from the microbial antigen are critical parameters determining the efficacy of phagocytosis.
Phagocytosis is a process by which certain types of cells are able to ingest particles and microbes. Some protozoa, like amoebae, use phagocytosis for the acquisition of food. Among the metazoa, many animals have specialized cells for host defense that can ingest and destroy microbes. In mammals, tissue macrophages are highly specialized cells that ingest, destroy, and digest microbes and present peptide antigens to lymphocytes. Macrophage phagocytosis is dependent on cellular receptors and can be enhanced by the presence of antibody or complement opsonins (2). In many infectious diseases, the production of opsonic antibody is associated with immunity. Therefore, this phylogenetically ancient process represents a critical component of host defense against microbial infections.
Cryptococcus neoformans is a pathogenic yeast that is a relatively frequent cause of life-threatening meningoencephalitis, especially in immunocompromised individuals (10). This fungus is unusual in that it has a polysaccharide capsule that is antiphagocytic. Consequently, the interaction of C. neoformans and macrophages rarely results in phagocytosis unless specific antibody and/or complement-derived opsonins are present. Phagocytosis of C. neoformans by macrophages has been studied with cells derived from various sources, including the J774 murine macrophage-like cell line (8). In this system, the phagocytic index was shown to depend on several variables, including the concentration and type of opsonin, the size of the capsule expressed by the C. neoformans strain, and the relative ratio of macrophages to yeast cells (8). This system has several advantages for the study of phagocytosis, including the fact that yeast cells are relatively large and can be easily counted by light microscopy and the qualitative outcome of the interaction whereby there is no significant phagocytosis in the absence of opsonins. Hence, it is possible to define the variables that affect the outcome of the interaction between C. neoformans and macrophages in a manner that would be very difficult for other microbial-macrophage systems.
We are particularly interested in the mechanisms of antibody-mediated protection against C. neoformans and the relationship between antibody dose and protective efficacy. Passive immunization with antibody to the capsule is protective, but administration of large amounts of antibody abrogates protection (13, 15) and can actually enhance the course of infection. This phenomenon has been called a “prozone-like” effect. While studying the interaction of macrophages and C. neoformans in vitro, we noted that the phagocytic index declines at higher antibody concentrations (13, 15). Given that this observation could be associated with the prozone-like phenomenon observed in passive protection experiments, we decided to study it in more detail and construct a mathematical model of phagocytosis of C. neoformans that would allow us to better understand the contribution of the various parameters to opsonic efficacy.
There have been several attempts to generate mathematical models of phagocytosis in the literature (11, 16). Phagocytosis is an attractive process for mathematical modeling because many of the variables are relatively well understood. However, none of the models available have addressed the critical contribution of opsonin concentration, and the subject was last investigated almost two decades ago.
We propose and test a mathematical model based on understanding of the underlying principles and mechanisms of phagocytosis. We identify the main variables and parameters of the model and analyze their impact on the outcomes of our experiments. Our mathematical description of phagocytosis is based on the differential equation which describes the rate of change of the total number of ingested microbes, PI, as a function of the population of noningested (free) microbes, PF, and the rate of phagocytosis, rT. The rate of phagocytosis depends on the amount of antibody bound to the C. neoformans capsule and the number of receptors available, and we use this model to explore the dependence of the phagocytic index on the concentration of free antibody by combining mathematical analysis and experimental work. We discuss the issues that arise from the analysis of the model and propose experimental and theoretical questions for further refinement of the model.
MATERIALS AND METHODS
Phagocytosis assays.
The assay used to study phagocytosis involved minor modifications to the previously described methods (8, 9). For all experiments, we used the J774.16 murine macrophage cell line, which faithfully reproduces the interaction of C. neoformans with murine macrophages (6). Briefly, on the day prior to the phagocytosis experiment, approximately 5 × 104 J774.16 cells were added to 96-well polystyrene tissue culture plates (BD Falcon, Franklin Lakes, NY) in media consisting of 10% heat-inactivated fetal bovine serum (Gemini Bio-Products, Woodland, CA), 10% NCTC-109 (Gibco, Grand Island, NY), and 1% nonessential amino acids (Mediatech, Herndon, VA) in Dulbecco's modified Eagle media (Gibco) containing 50 U/ml of gamma interferon. After overnight incubation at 37°C, the media were removed, the monolayer was washed three times with fresh media, and 105 C. neoformans cells were added in media. All experiments used C. neoformans strain 24067 (American Type Tissue Collection, Rockville, MD). Monoclonal antibody (MAb) 18B7 is a murine immunoglobulin G1 (IgG1) that binds the C. neoformans capsular component glucuronoxylomannan and has been extensively characterized and used in a human clinical trial (3, 5). MAb 18B7 was added to the C. neoformans suspension in variable amounts, and the macrophage-yeast suspension was incubated for 2 h at 37°C. In this experimental system, the macrophages are immobilized at the bottom by virtue of their capacity to grow as an adherent monolayer. The C. neoformans is added in suspension, the yeast settle rapidly by gravity, and phagocytosis begins within minutes. Afterwards, the monolayer was washed three times with phosphate-buffered saline, fixed with cold methanol, and stained with Giemsa (Sigma-Aldrich, St. Louis, MO). The phagocytic index was determined by counting ingested yeast with an inverted microscope at a magnification of ×100. Under these conditions, it is very easy to distinguish attached from ingested yeast cells, since the latter reside in discernible intracellular vacuoles. The phagocytic index under the various conditions was determined by counting internalized yeast cells per 100 macrophages in various fields (4 fields were counted). In our system and conditions, antibody-mediated opsonization results in almost complete ingestion, such that the overwhelming majority of yeast cells are inside macrophages. In prior studies, we have validated our ability to distinguish between attached and ingested yeast cells using fluorescence dyes that stain only attached yeast cells. Antibody-mediated opsonization in this system is highly efficient and leads to ingestion. In all conditions, more than 90% of the attached yeast cells internalized irrespective of antibody concentration.
In some experiments, yeast cells were incubated with 18B7 for an hour or an hour and a half to obtain a near-saturation occupancy of the yeast capsule before addition of the antibody-coated cells to the macrophage monolayer. In some experiments, we blocked the complement receptors by adding antibody to CD18, CD11b, and CD11c (BD Biosciences Pharmigen, San Jose, CA), which are expressed by J774.16 cells (14). For these experiments, the above protocol was modified by incubating the macrophage monolayer with blocking antibodies (50 μg/ml) for 1 h prior to the phagocytosis experiment. After washing and addition of the C. neoformans suspension, MAb 18B7 blocking was maintained by including the blocking antibodies (10 μg/ml) in the macrophage-yeast suspension. It is noteworthy that this protocol was arrived at after modeling results revealed that incubation of J774 cells with 10 μg/ml of blocking antibody was not sufficient to block the complement receptors.
Mathematical modeling.
Our mathematical model of phagocytosis is based on the differential equation
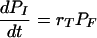 |
(1) |
which describes the rate of change of the total number of ingested microbes, PI, as a function of the population of free microbes, PF, and the rate of phagocytosis, rT. The detailed mathematical analysis of processes involved in phagocytosis is given in the supplemental material, where we construct a mathematical model of this process. The main variables in the model are listed and described in Table 1. The main goal of this paper is to model the function of rT, which we call the efficacy of phagocytosis and which depends on the amount of antibody bound to the C. neoformans capsule and the number of receptors available.
TABLE 1.
Main variables in the model
| Variable | Description |
|---|---|
| M | No. of macrophages per well, assumed constant is our simulation M = M(0) = 0.75 × 105 |
| PF | Population of free (not ingested) microbes, in microbes/well, PF(0) = P0 = 1.5 × 105;  F(0) = F(0) =  0 = 200 the normalized (per 100 macrophages) initial population of free microbes 0 = 200 the normalized (per 100 macrophages) initial population of free microbes |
| PI | Population of microbes ingested by macrophages, in microbes/well, PI(0) = 0 |
We built our mathematical model of the phagocytic efficacy on two main assumptions. The first assumption is that phagocytic efficacy increases as the number of binding sites on the C. neoformans capsule for the appropriate type of receptors increases, and the second is that there is a maximum efficacy of phagocytosis for a given number of available receptors. Both of these assumptions were motivated and confirmed by the experimental results.
The simplest, and naturally the first, model to consider is a model based on a linear increase in the phagocytic efficacy with the number of sites, s, on the C. neoformans capsule available to bind the appropriate receptors on the macrophage surface. This model is described by the differential equation 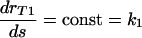 and is therefore based on the assumption that the rate at which the phagocytic efficacy increases does not depend on the amount of antibody already bound to the C. neoformans capsule. Moreover, the solution of this model, assuming rT1(0) = 0, is rT1 = k1s and is proportional to the rate at which the antibody is binding to a given (constant) number of receptors. Combining this model with the assumption that there is a maximum phagocytic efficacy led to our first mathematical model of phagocytic efficacy:
and is therefore based on the assumption that the rate at which the phagocytic efficacy increases does not depend on the amount of antibody already bound to the C. neoformans capsule. Moreover, the solution of this model, assuming rT1(0) = 0, is rT1 = k1s and is proportional to the rate at which the antibody is binding to a given (constant) number of receptors. Combining this model with the assumption that there is a maximum phagocytic efficacy led to our first mathematical model of phagocytic efficacy:
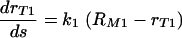 |
(2) |
The constant k1 is the initial growth rate of phagocytic efficacy, and the term (RM1 − rT1) represents the reduction in the rate at which the efficacy of phagocytosis increases as it approaches the maximum phagocytic efficacy, RM1, for the given conditions. Both k1 and RM1 depend on the number of available receptors and may also depend on the health of macrophages and vary slightly depending on the experimental conditions.
Our experimental results in the case of fully blocked complement receptors strongly supported this model. The number of binding sites for Fc receptors, s, is equal to the amount of antibody bound to the C. neoformans capsule AP, and therefore, equation 2 leads to the differential equation
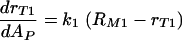 |
(3) |
whose solution is
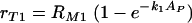 |
(4) |
The data of the phagocytic efficacy versus the amount of antibody bound to the C. neoformans capsule indicate that the same type of a model
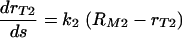 |
(5) |
does not fully account for the increase in the phagocytic index when the complement receptors are involved. Consequently, to explain the efficacy of phagocytosis when the complement receptors are involved, we consider a mathematical model where the increase in the phagocytic efficacy is a linear function of the number of the complement receptor binding sites s on the C. neoformans capsule. The differential equation applicable in this case is
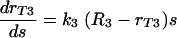 |
(6) |
where s is the number of complement receptor binding sites on the C. neoformans capsule. This mathematical model indicates a possibility of cooperation among receptors at high density of antibody bound to C. neoformans capsule. The general solution of the above differential equation is
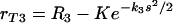 |
(7) |
where K is a constant. We combined the two above models (equations 5 and 7) and obtained a mathematical model that incorporates both effects: the constant increase in phagocytic efficacy at lower concentrations and the more rapid increase in the efficacy of phagocytosis at a higher density of antibody bound to C. neoformans capsule. We assumed that equation 7 describes the rate of increase of the maximum phagocytic efficacy due to cooperation between receptors. The constant K was determined from the assumption that, when there is no cooperation (s = 0), the maximum phagocytic efficacy is rT3 = RM2. We therefore obtained 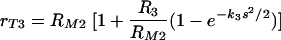 . We replaced the constant maximum phagocytic efficacy RM2 in equation 5 with rT3 and therefore modeled phagocytosis through complement receptors by the following equation
. We replaced the constant maximum phagocytic efficacy RM2 in equation 5 with rT3 and therefore modeled phagocytosis through complement receptors by the following equation
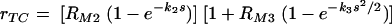 |
(8) |
where 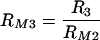 . Our experimental results also indicated that, when both types of receptors are available, the phagocytosis through Fc and phagocytosis through complement receptors are not independent processes, that is, the cumulative result is not simply the sum of their respective contributions. Our model is set up so that this effect can be easily incorporated. A natural extension of our model for phagocytosis through complement receptors is the following equation:
. Our experimental results also indicated that, when both types of receptors are available, the phagocytosis through Fc and phagocytosis through complement receptors are not independent processes, that is, the cumulative result is not simply the sum of their respective contributions. Our model is set up so that this effect can be easily incorporated. A natural extension of our model for phagocytosis through complement receptors is the following equation:
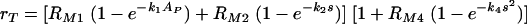 |
(9) |
where s is the number of complement receptor binding sites on the C. neoformans capsule. Note that we introduced RM4 and k4 in this model instead of RM3 and k3. While RM3 and k3 formally described the effect of cooperation among complement receptors, RM4 and k4 account for the effect of cooperation among complement receptors as well as complement and Fc receptors. We assumed that the number of complement receptor binding sites on the C. neoformans capsule increases quadratically with the amount of antibody bound to the capsule, reaches a maximum, and then decreases with binding of the additional antibody. The assumption of the quadratic increase is justified by the experimental results, which show that the total increase in phagocytic efficacy is more rapid than the linear increase in the number of binding sites s in equation 9 would imply. We were primarily interested in modeling the phagocytic efficacy in the range of concentrations of free antibody where the number of complement receptor binding sites on the C. neoformans capsule increases. In that range, our model leads to the following dependence of the phagocytic efficacy, rT, of phagocytosis on the amount of antibody bound to C. neoformans capsule:
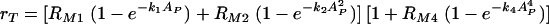 |
(10) |
Simulations of the model.
We used MatLab 7.4 (The MathWorks, Natick, MA) in simulations of our model.
RESULTS
Dependence of phagocytosis on antibody concentration and time.
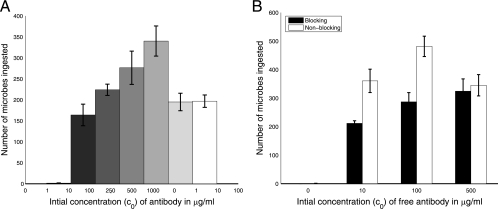
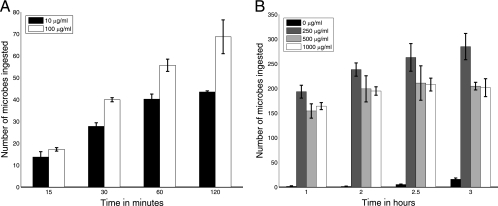
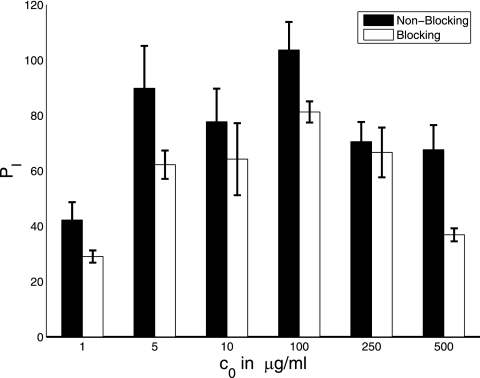
The efficacy of phagocytosis was studied as a function of antibody concentration (Fig. 1) and time (Fig. 2) in the presence and absence of complement receptor blockade. Although this system does not include complement opsonins, phagocytosis can occur through the complement receptor as a result of an antibody-mediated change in the fungal polysaccharide capsule that allows interaction of polysaccharide with the complement receptor. Hence, in the absence of complement receptor blockage, phagocytosis occurs through both Fc and complement receptors, whereas in the presence of blockage, phagocytosis occurs only through the Fc receptor. In the absence of complement receptor blockage, the number of ingested microbes increased as a function of antibody concentration until 250 μg/ml but sharply decreased thereafter (Fig. 2A). At most antibody concentrations studied, phagocytosis is essentially completed or considerably slowed down after 2 h.
FIG. 1.
Experimental counts of the number of phagocytized microbes (per 100 macrophages) as a function of antibody concentration (A) and in the setting of blocked complement (C3R) receptors (B). Error bars represent standard deviations.
FIG. 2.
Experimental counts of the number of phagocytized microbes (per 100 macrophages) as a function of time for antibody concentrations of 10 and 100 μg/ml (A) and 0, 250, 500, and 100 μg/ml (B). Error bars represent standard deviations.
Mathematical analysis of antibody binding to C. neoformans and Fc receptors.
C. neoformans capsule has 1.1 × 106 binding sites (4), which implies that the 1.5 × 105 microbes per well used in our experiments can bind 0.04 μg of IgG. It is known that each macrophage cell has approximately 105 binding sites (see references 7 and 18), and therefore, 0.75 × 105 macrophages would bind approximately 0.004 μg IgG (for 2 × 105 binding sites per macrophage as given in reference 7). This implies that in our experiments the amount of free antibody is well above the total (Cryptococcus neoformans capsule and macrophage together) capacity for binding of antibody. In such a case, standard biochemical equations imply that the amount, AM, of the antibody bound to the Fc receptors is given by the exponential function
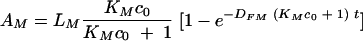 |
(11) |
LM is the total capacity (the number of receptors) of Fc receptors for binding the antibody, KM is the affinity constant, and DFM is the corresponding dissociation rate constant. c0 is the initial concentration of free antibody. A summary of the variables and parameters as well as their values and units are given in Tables 2 and 3. A full derivation and a discussion of these equations is given in the supplemental material.
TABLE 2.
Antibody binding variables and parameters
| Variable or | Description |
|---|---|
| parameter | |
| cF | Concn of free antibody, in mol/liter, cF (0) = c0 is initial concn of antibody |
| AM | No. (per well) of antibody molecules bound to Fc receptors, AM (0) = 0 |
| LM | No. of binding sites on surface of macrophages, approximately 2 × 105 Fc receptors per macrophage gives a total of 1.5 × 1010 sites per well (7) |
| AP | No. (per well) of antibody molecules bound to microbes, AP (0) = 0 |
| LP | No. of binding sites on C. neoformans cell capsule, 1.1 × 106 binding sites per cell amounts to 1.6 × 1011 sites per well |
| X | No. of free (not occupied by antibody) Fc receptors per well, X(0) = LM, where LM is the total no. of receptors on the macrophages in one well |
| Z | No. of complement receptors per well, assumed constant in our experiments |
TABLE 3.
Affinity and rate constants for antibody binding
| Parameter | Description and value |
|---|---|
| KM | Association (affinity constant) for free antibody binding to macrophages, 2.9 × 107 M−1, reported in reference 18 |
| DFM | Dissociation constant for free antibody binding to macrophages, 0.26 min−1, as reported in reference 18 |
| kFM | on rate constants for free antibody binding to macrophages, kFM = KPDFM = 7.54 × 108 M−1 min−1 |
| KP | Association (affinity constant) for free antibody binding to microbes, KP = 2.2 × 109 M−1 |
| DFP | Dissociation constant for binding of free antibody binding to microbes, we used 0.9 × 10−5 min−1, slightly lower then the value 10−5 min−1 of the dissociation constant measured for binding of antibody to oligosaccharide by surface plasmon resonance (unpublished); since the binding of antibody to oligosaccharide is monovalent while the IgG molecule is divalent and capsular polysaccharide is likely to have many repeats per molecule, this number is likely to be an overestimate of DFP |
| kFP | on rate constant for free antibody binding to microbes, kFP = KPDFP = 1.98 × 104 M−1 min−1 |
Similarly, we determined that the amount of the antibody, AP, bound to the Cryptococcus neoformans capsule is given by
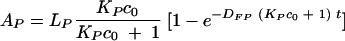 |
(12) |
LP is the total capacity of microbes for binding the antibody (number of binding sites in the capsule), KP is the affinity constant, and DFP is the corresponding dissociation rate constant.
From the above equations, we can calculate that the half-time for antibody-macrophage binding is 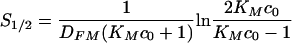 , and 90% of Fc receptors are occupied after
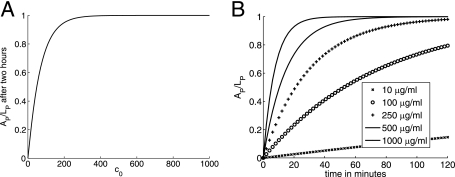
, and 90% of Fc receptors are occupied after 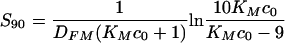 . This implies that 90% of Fc receptors would be occupied in less than 1 min at concentrations of 100, 250, and 500 μg/ml of free antibody, assuming that KM = 2 × 107 M−1. At 10 μg/ml, approximately 80% of the total number of Fc receptors are occupied in a long run, and saturation is reached within the first 5 min. The predictions for the amount of free antibody bound to the C. neoformans capsule are depicted in Fig. 3 and indicate that this process will reach saturation within 2 h.
. This implies that 90% of Fc receptors would be occupied in less than 1 min at concentrations of 100, 250, and 500 μg/ml of free antibody, assuming that KM = 2 × 107 M−1. At 10 μg/ml, approximately 80% of the total number of Fc receptors are occupied in a long run, and saturation is reached within the first 5 min. The predictions for the amount of free antibody bound to the C. neoformans capsule are depicted in Fig. 3 and indicate that this process will reach saturation within 2 h.
FIG. 3.
Graphs of the antibody binding to the microbe capsule: the amount bound after 2 h as a function of the initial concentration (A) and the dynamics of binding (B).
The above binding dynamics are consistent with our experimental data on the time course of phagocytosis at low concentrations (Fig. 2A) as well as at high concentrations. There was no increase in the number of ingested microbes after 1 h with high initial concentrations of free antibody (Fig. 2B). However, the experimental data also revealed a progressive increase in phagocytosis after 1 h for assays using an initial antibody concentration of 250 μg/ml. This was unexpected because, at this concentration, our calculations showed that most of the Fc receptors on the macrophage surface and antibody binding sites in the capsule are occupied (saturated) at that time.
The increase in the phagocytic index at antibody concentrations of 100 and 250 μg/ml (Fig. 1A) was also rather surprising, considering that most Fc receptors are occupied very rapidly and that the number of sites on C. neoformans available for binding of antibody already bound to Fc receptors is reduced with higher concentrations. This was unexpected because, at this concentration, our calculations showed that most of the Fc receptors on the macrophage surface and antibody binding sites in the capsule are occupied (saturated).
It is noteworthy that these results indicate that there is no replication of ingested yeast. That result is consistent with the fact that intracellular yeast replication occurs several hours after ingestion (17), while the experiments studied and modeled here were limited to 2 h.
The above experimental data and the analysis of the binding process indicated that the dynamics of binding in this experimental setup had a major influence on the outcome of these experiments. We hypothesized that phagocytosis at lower concentrations of antibody was facilitated by both Fc and complement receptors, that at higher concentrations the contribution of the complement receptors would be more significant than at low concentrations, and that phagocytosis through Fc receptors is negligible since free antibody would block most of them very quickly. Consequently, we proposed a mathematical description of the efficacy of phagocytosis and designed several new experiments to analyze and quantify its dependence on the amount of antibody and further develop the mathematical formalism based on the experimental results obtained.
We performed three additional sets of experiments, each set including two conditions, one with and the other without blocking complement receptors. In all three sets, C. neoformans was incubated with IgG1 for at least 1 h to obtain near-saturation occupancy of the binding sites on the C. neoformans capsule. This provided us with a set of data where the amount of antibody bound to the C. neoformans capsule did not change significantly over the 2 h during which the phagocytosis experiments were conducted and was therefore suitable for modeling phagocytic efficacy and validating our models and assumptions.
Experiments designed to investigate phagocytic efficacy.
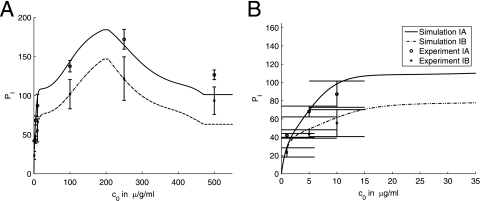
In the first set of experiments, C. neoformans cells were incubated with antibody for 1 h, separated from remaining free antibody present in the solution, and added to the macrophage monolayer in the fresh medium. This experimental setup eliminated the possibility of blocking of Fc receptors by excess free antibody. In this set, we conducted experiments without blocking complement receptors as well as experiments with (partial) blocking of complement receptors. The experimental results are shown in Fig. 4. We blocked the complement receptor using antibodies to the complement receptor subunit components CD11b, CD11c, and CD18. These antibodies do not affect the Fc receptor but inhibit phagocytosis through the complement receptor. The experimental methodology used had been developed in a prior study that explored mechanisms by which IgM promoted phagocytosis in the absence of complement (14). Initially, we applied those conditions to the system studied, but the inability of our modeling equations to adequately describe the dependence of the phagocytic index on the antibody concentration suggested that this protocol, which had been devised to block IgM-mediated phagocytosis through the complement receptor, was inadequate for fully inhibiting IgG-mediated phagocytosis through that receptor. Hence, the protocol was modified to increase the amount of antibody in the initial blocking step and to maintain blocking by adding antibody to the complement receptor to the phagocytosis conditions. Phagocytosis data generated with the enhanced blocking conditions were well simulated by our proposed model. Hence, the model proposed here was predictive of incomplete blocking and generated a hypothesis that could be tested by altering the conditions of the experiment.
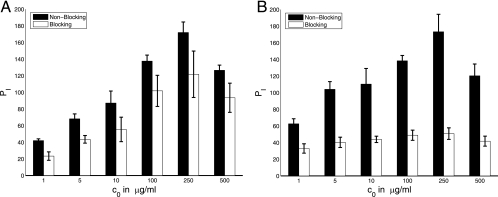
FIG. 4.
Experimental counts of the number of phagocytized microbes, PI (per 100 macrophages), as a function of the initial antibody concentration c0. IgG1 was incubated with C. neoformans cells for 1 h to obtain near-saturation occupancy of the binding sites on the C. neoformans capsule. C. neoformans cells were then separated from antibody and added to a macrophage monolayer in the fresh medium (see Materials and Methods). The two sets of results represent experiments without and with blocking of complement receptors. The results of experiments with blocking represented in panel A were obtained with partial blocking of complement receptors, and those in panel B were obtained with complement receptors fully blocked. Error bars represent standard deviations.
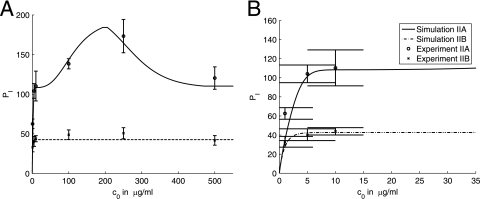
In the second set of experiments, C. neoformans cells were again incubated with IgG1 for 1 h to obtain near-saturation occupancy of the binding sites on the C. neoformans capsule. C. neoformans cells were then separated from remaining free antibody present in the solution and added to the macrophage monolayer in the fresh medium. We first conducted a control experiment without blocking of complement receptors and under the same experimental conditions as the experiment without blocking of complement receptors in the first set. The experiment with blocking of complement receptors was also conducted under the same experimental conditions as the corresponding experiment in the first set, except for the enhanced blocking of complement receptors. For these experiments, the protocol of the experiment with blocking was modified by incubating the macrophage monolayer with (complement) blocking antibodies (50 μg/ml) for 1 h prior to the phagocytosis experiment. After washing and addition of the C. neoformans suspension and MAb 18B7, complement receptor blocking was maintained by including the blocking antibodies (10 μg/ml) in the macrophage-yeast suspension. The experimental results are shown in Fig. 4B. The experimental results show a considerable reduction in the phagocytic index compared to the experiment with blocking in the first set and, in particular, almost no increase in the phagocytic index with increasing concentrations of free antibody above 10 μg/ml. In our simulations, we assume that complement receptors in this experiment were fully blocked.
In the third set of experiments, we incubated antibody with C. neoformans for an hour and a half. The mixture of antibody and C. neoformans was added to macrophages, and phagocytized C. neoformans cells were counted after 2 h. In this set of experiments, we again examined conditions with and without blocking of complement receptors. The experiment with blocking of complement receptors was done under the same conditions as the experiment without blocking except for the (partial) blocking of complement receptors (by adding 10 μg/ml complement blocking antibodies, as discussed above). The experimental results are shown in Fig. 5. Our model reveals that a slight, but noticeable, drop in the number of ingested microbes observed in experimental results at 10 μg/ml is a result of blocking Fc receptors by excess free antibody. A summary of the experimental conditions in the above described three sets of experiments is given in Table 4.
FIG. 5.
Experimental counts of the number of phagocytized microbes, PI (per 100 macrophages), as a function of the initial antibody concentration, c0. IgG1 was incubated with C. neoformans cells for an hour and a half to obtain near-saturation occupancy of the binding sites on the C. neoformans capsule. C. neoformans (in the solution) was then added to the macrophage monolayer (see Materials and Methods). The two sets of results represent experiments without and with (partial) blocking of complement (C3R) receptors. Error bars represent standard deviations.
TABLE 4.
Summary of experiments designed to model efficacy of phagocytosis and corresponding simulations
| Expt no. and summary | Simulation and parameters |
|---|---|
| IA (Fig. 4), phagocytosis without blocking complement receptors, | |
| microbes incubated with antibody for 1 h, excess free | |
| antibody removed prior to adding antibody coated | |
| microbes to macrophages | IA (Fig. 7), maximum values for RM1, k1, RM2, RM4, and k4; k2 |
| IB (Fig. 4A), phagocytosis with partial blocking of complement | reduced due to experimental variations |
| receptors, microbes incubated with antibody for 1 h and | |
| excess free antibody removed prior to adding | |
| antibody-coated microbes to macrophages | IB (Fig. 7), maximum values for RM1 and k1; k2 reduced due to |
| IIA (Fig. 4B), phagocytosis without blocking complement receptors, | experimental variations; complement and cooperation |
| microbes incubated with antibody for 1 h, excess free | parameters RM2, k2, RM4, and k4 reduced |
| antibody removed prior to adding antibody-coated | |
| microbes to macrophages | IIA (Fig. 8), maximum values for RM1, k1, RM2, k2, RM4, and k4 |
| IIB (Fig. 4B), phagocytosis with complete blocking of complement | |
| receptors, microbes incubated with antibody for 1 h and | |
| excess free antibody removed prior to adding | |
| antibody-coated microbes to macrophages | IIB (Fig. 8), maximum values for RM1, k1; RM2 = 0 and RM4 = 4 |
| IIIA (Fig. 5), phagocytosis without blocking complement receptors, | due to complete blocking of complement receptors and |
| microbes incubated with antibody for one and a half hours, | cooperation parameters |
| suspension containing microbes and excess free antibody | |
| added to macrophages | IIIA (Fig. 9), values of RM1 and RM2 reduced by factor of 0.9 |
| IIIB (Fig. 5), phagocytosis with partial blocking of complement | due to exptl variation; RM1 and RM4 reduced [by a factor |
| receptors, microbes incubated with antibody for one and a | of f(c0)] due to blocking of Fc receptors by excess free |
| half hours, suspension containing microbes and excess free | antibody |
| antibody added to macrophages | IIIB (Fig. 9), values of RM1 and RM2 reduced by factor of 0.9 due to exptl variation; RM1 and RM4 reduced [by a factor of f(c0)] due to blocking of Fc receptors by excess free antibody; complement parameters reduced due to blocking of complement receptors |
Modeling the efficacy of phagocytosis and simulations of the model.
We used our proposed mathematical model and the above-described experimental results to investigate the dependence of the phagocytic efficacy on the antibody bound to the capsule and to generate simulations that could be compared to the experimental data. The main variables and parameters of the model are listed in Tables 1, 2, and 3, and a detailed mathematical derivation is given in the supplemental material.
In our experiments, C. neoformans was incubated with IgG1 for at least 1 h to obtain near-saturation occupancy of the binding sites on the C. neoformans capsule. This provided us with a set of data where the amount of antibody bound to the C. neoformans capsule did not change significantly over the 2 h during which the phagocytic efficacy was observed. In addition to that, since the number of ingested microbes per macrophage is small compared to the number of microbes a macrophage can ingest, we can also assume that the number of receptors does not change significantly in our first two sets of experiments (12). Consequently, we can consider that the phagocytic efficacy, rT, does not change during the time of the experiments (120 min) and solve the main equation in our model (equation 1) analytically. Therefore, after 120 min, the number of ingested microbes (per 100 macrophages) is given by
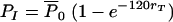 |
(13) |
In our simulations of the model, we will assume that the initial population of microbes is 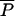 0 = 200, which corresponds to the population of microbes (200) per 100 macrophages in our experiments, therefore normalizing the number of cells involved with respect to the number of macrophages.
0 = 200, which corresponds to the population of microbes (200) per 100 macrophages in our experiments, therefore normalizing the number of cells involved with respect to the number of macrophages.
We were primarily interested in modeling the phagocytic efficacy in the range of concentrations of free antibody where the number of complement receptor binding sites on C. neoformans capsule increases. It that range our model, mathematically derived in Materials and Methods, leads to the following expression of the phagocytic efficacy, rT, of phagocytosis
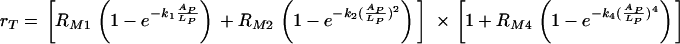 |
(14) |
where AP/LP is the fraction of antibody binding sites on C. neoformans capsule that are occupied. The units for rT, RM1, and RM2 are min−1, and the parameters k1, k2, k4, and RM4 are dimensionless parameters. Descriptions, values, and units of AP and LP are given in Table 2.
This model is based on the assumption that there is a maximum phagocytic efficacy, RM1, for Fc as well as for complement receptors (RM2) but that high concentrations of antibody bound to C. neoformans capsule and the presence of complement receptors provide an additional increase in both the maximum phagocytic efficacy and the rate at which this maximum is reached. The first two terms describe the increase in phagocytic efficacy due to Fc [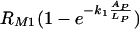 ] and complement receptors [
] and complement receptors [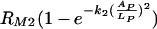 ] when there is no cooperation among receptors. The rates k1 and k2 quantify the rates at which the efficacy of phagocytosis increases with the increasing amount of antibody bound the C. neoformans capsule. The factor
] when there is no cooperation among receptors. The rates k1 and k2 quantify the rates at which the efficacy of phagocytosis increases with the increasing amount of antibody bound the C. neoformans capsule. The factor 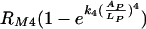 accounts for the increase in the phagocytic efficacy due to cooperation among different receptors.
accounts for the increase in the phagocytic efficacy due to cooperation among different receptors.
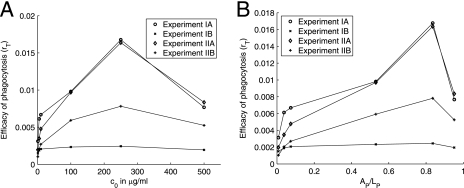
We assumed that a maximum number of complement receptor binding sites is reached for a certain occupancy of C. neoformans capsule and that, after that point, the number of sites decreases quadratically with the increasing amount of the antibody bound to the capsule. Our model provides an estimate for the fraction of antibody binding sites on a C. neoformans capsule occupied that results in the maximum phagocytic efficacy of phagocytosis through complement receptors: data presented in Fig. 6 (computed from the experimental results presented in Fig. 4 and 5) indicate that the maximum number of complement receptor binding sites on C. neoformans capsule is reached when between 70 and 80% of the sites are occupied. However, we do not have enough data to set up a simple model for the events that occur after the maximum efficacy is reached.
FIG. 6.
Plots of efficacy of phagocytosis computed from the experimental data. We computed the efficacy using the formula rT = −ln(1 − PI/200)/120 and experimental data from the three sets of experiments presented in Fig. 4 and 5. The efficacy of phagocytosis is plotted as a function of the initial concentration, c0, of antibody (A) and as a function of the fraction (AP/LP) of the antibody binding sites on the C. neoformans capsule occupied by IgG (B). A summary of experimental conditions in given in Table 4.
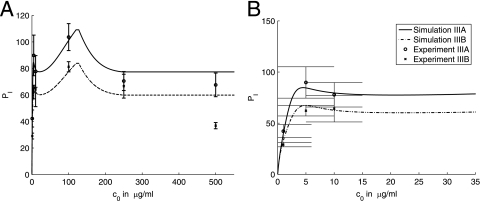
We therefore use equation 14 to describe the increase in phagocytic efficacy involving both Fc and complement receptors and in the range in which the number of complement receptor binding sites on the C. neoformans capsule increases. We provide an approximate model based on equation 9 and assuming a quadratic decrease in the number of complement receptor binding sites on the C. neoformans capsule after the maximum number is reached. This approximation fits well to the data. The experimental results and simulations are presented in Fig. 7, 8, and 9.
FIG. 7.
Comparison of the model simulations and experimental results presented in Fig. 4A. Panels A and B present the same data and simulations but using different scales. The same legend applies to both panels. Error bars represent standard deviations. Summaries of experimental conditions and simulation parameters are given in Tables 4 and 5.
FIG. 8.
Comparison of the model simulations and experimental results presented in Fig. 4B. Panels A and B present the same data and simulations but using different scales. The same legend applies to both panels. Error bars represent standard deviations. Summaries of experimental conditions and simulation parameters are given in Tables 4 and 5.
FIG. 9.
Comparison of the model simulations and experimental results presented in Fig. 5. Panels A and B present the same data and simulations but using different scales. The same legend applies to both panels. Error bars represent standard deviations. Summaries of experimental conditions and simulation parameters are given in Tables 4 and 5.
Simulation parameters.
The data for rT computed from the experimental results and presented in Fig. 6 determine the maximum efficacy of phagocytosis as well as the appropriate rates of increase. We first determined the parameters RM1 and k1 by fitting the model to the data points depicted in Fig. 6 (experiment IIB: experiment with [full] blocking in our second set of experiments). The model and experimental results for phagocytosis through Fc receptors and with full complement receptors completely blocked are presented in Fig. 8. The parameters RM2, RM4, k2, and k4 were then determined by fitting the model to the data points computed from the experimental results (Fig. 4B) for phagocytosis without blocking complement receptors (in the same set) and are depicted in Fig. 6 (experiment IIA). The model and experimental results for phagocytosis through Fc receptors and without complement receptors blocking are also presented in Fig. 8.
Simulations of the model for the experimental conditions in our first set of experiments presented in Fig. 7 were obtained by reducing the appropriate parameters in our model for the phagocytosis through complement receptors, as indicated in Tables 4 and 5. We note that the outcomes of the experiments without blocking, which were conducted under the same experimental conditions, were slightly different. We attributed this difference to small variations in the experimental procedure; for example, state of activation of macrophages and different media lots, etc., and accounted for those variations first by adjusting the parameters (k2) of the model to fit the experimental results of the control experiment. A similar adjustment was done when fitting the parameters to simulate the results of the experiments in our third set, where we reduced the total maximum efficacy of phagocytosis by a factor of 0.9. Results (not shown) of earlier experiments conducted under the same conditions justify this adjustment and its attribution to the experiment-to-experiment variation.
TABLE 5.
Simulation parameters
| Simulation | RM1 (min−1) | k1 | RM2 (min−1) | k2 | RM4 | k4 |
|---|---|---|---|---|---|---|
| IA | 0.002 | 125 | 0.0045 | 308 | 6.5 | 1.3 |
| IB | 0.002 | 125 | 0.0045 × 0.45 | 308 × 0.45 | 6.5 × 0.85 | 1.3 × 0.85 |
| IIA | 0.002 | 125 | 0.0045 | 1,418 | 6.5 | 1.3 |
| IIB | 0.002 | 125 | 0 | NAa | 0 | NA |
| IIIA | 0.9 × 0.002 × f(A0) | 125 | 0.9 × 0.0045 | 1,418 | 6.5 × [f(A0) + 1]/2 | 1.3 × [f(A0) + 1]/2 |
| IIIB | 0.9 × 0.002 × f(A0) | 125 | 0.9 × 0.0045 × 0.73 | 1,418 × 0.73 | 6.5 × [f(A0) + 0.92]/2 | 1.3 × [f(A0) + 0.92]/2 |
NA, not applicable.
Simulations of the results of the experiments in our third set, conducted without separating excess free antibody from the suspension containing microbes, are presented in Fig. 9. The model parameters in this case were obtained by introducing the dependence of the parameters RM1 and RM4 on the initial amount of free antibody. While the assumption that the number of receptors does not significantly change in the course of our experiments is reasonable in our first two sets of experiments, it does not apply to the third set, since our computations showed that a large fraction of Fc receptors were blocked by excess free antibody. Since the conditions in our experiments were uniform and controlled, we know that most yeast cells reach the macrophage layer within minutes. Moreover, our computations indicated that most Fc receptors are blocked by excess antibody also within minutes at all initial concentrations of free antibody used in our experiments except the lowest one. Therefore, we assumed that the phagocytic efficacy in our experiment was mostly influenced by the number of Fc receptors that were available at the moment when the yeast cells reached the macrophage layer. Consequently, when applying our model to the experimental results of our third set of experiments, we accounted for this reduction in the number of Fc receptors by making an appropriate adjustment to phagocytic efficacy. We therefore introduced a decrease in the maximum phagocytic efficacy through Fc receptor RM1 by a factor 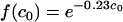 , where c0 is in μg/ml. This decrease corresponds to the increase in blocking of Fc receptors as the concentration of free antibody is increasing. We also reduce RM4 by a factor which characterizes the contribution of Fc receptors to the increase in phagocytic efficacy due to cooperation among the receptors.
, where c0 is in μg/ml. This decrease corresponds to the increase in blocking of Fc receptors as the concentration of free antibody is increasing. We also reduce RM4 by a factor which characterizes the contribution of Fc receptors to the increase in phagocytic efficacy due to cooperation among the receptors.
This is a very rough approximation, but it gives a surprisingly good fit to the experimental data. The rationale for such an estimate is that the fraction of the Fc receptors that will bind free antibody increases exponentially as a function of the initial concentration and that the phagocytic efficacy is considerably reduced at the point when a large fraction of Fc receptors are occupied very early in the process.
The summary of experimental conditions in the experiments modeled and the corresponding adjustments of the parameters in the simulations is given in the Table 4. The values of model parameters used in simulations are given in Table 5.
DISCUSSION
Experimental studies of dependence of the phagocytic index on IgG concentration revealed a peculiar aspect of this process that was difficult to explain: while the phagocytic index increased steadily in the 10- to 100-μg/ml IgG range, at antibody concentrations greater than 250 μg/ml, there was a consistent net reduction in phagocytic index. This phenomenon is called the prozone-like effect, and it is noteworthy that a similar type of nonlinear dose dependence is observed in the experiments with the survival rate of mice subject to passive immunization where the high doses of antibody are associated with reduced efficacy and protection (13). Moreover, the higher concentrations used in our experiments and studied in our mathematical analysis are comparable to those found when antibody is administered passively or therapeutically. The lower concentrations of antibody used in our studies are comparable to those found after an immune response or vaccination.
To understand the mechanisms of phagocytosis and, in particular, those responsible for the above-described increase and subsequent drop in phagocytic index, we turned to mathematical modeling, which allows quantitative analysis of causal relationships and the testing of assumptions. There are several mathematical models of phagocytosis in the literature (11, 16), but none has considered the mechanism of attachment of microbes to macrophages and the dependence of the processes involved in both attachment and ingestion on the amount of antibody and the number of available receptors. We described the phenomenon of IgG-mediated phagocytosis by a system of seven differential equations. We started with very general assumptions that a biological process of this type would satisfy and constructed a model that reproduced the experimental results very well.
The results of our mathematical analysis of the dependence of phagocytic index on the concentration of antibody in our initial experiments brought to light a perplexing phenomenon: the concentrations where the increase in the phagocytic index was observed in our initial experiments were considerably higher than the binding capacity of both macrophages and microbes, and there was a high likelihood that both antigen binding sites in the cryptococcal capsule and Fc receptors were saturated. This suggested additional experiments that provided insights into these effects and enabled us to construct a mathematical model that reproduced the experimental data.
More precisely, our calculations indicated that, in our initial set of experiments, Fc receptors would be rapidly saturated at concentrations higher than 10 μg/ml. That is, we obtained from equation 11 that, at concentrations of 100, 250, and 500 μg/ml of free antibody, half of the Fc receptors are occupied within a minute and 90% of them are occupied in less then 5 min. As we already pointed out in our initial experiments, we observed an increase in phagocytic index through the range of concentration up to 250 μg/ml of free antibody.
Our mathematical analysis indicated that the conditions in the experiments whose outcomes we analyzed were quite extreme: the concentrations of free antibody were much higher than the binding capacity of not only macrophages but also microbes. The fact that the range of concentrations studied is well above the binding capacity of microbes and macrophages and yet we still observed an increase in the phagocytic index over that range put an emphasis on understanding the dynamics of phagocytosis and, in particular, the contribution of phagocytosis through complement receptors to the phagocytic index. Our analysis of binding of antibody to the C. neoformans capsule predicted that the timing of mixing the antibody with microbes and macrophages was essential for the outcomes of our experiments.
More precisely, the analysis indicated that the time necessary for the binding sites on C. neoformans capsule to reach saturation was comparable to the time during which the phagocytosis was taking place and was observed (e.g., 2 h). Consequently, this indicated that the amount of antibody bound to C. neoformans capsule changed considerably throughout the duration of our experiments. To understand and model the dependence of phagocytic index on the amount of antibody bound to C. neoformans capsule, we designed and conducted a new set of experiments where the amount of antibody would not change significantly during the time when phagocytosis is in progress and used those results to construct our mathematical model.
In particular, we introduced and studied the efficacy of phagocytosis, which we define as the fraction of the free microbes that is ingested as a function of the amount of antibody bound to C. neoformans capsule and the number of receptors available (see reference 1). We discussed and tested mathematical models for this function and constructed a mathematical model for phagocytic efficacy through Fc and complement receptors separately as well as for phagocytic efficacy when both types of receptors are available.
By studying the reduction in phagocytic index that resulted from blocking complement receptors by antibodies to CD11 and CD18, we distinguished among the contributions from the two different types of receptors: Fc receptors and complement receptors. In fact, our modeling simulations predicted that the blocking protocol used in earlier studies (13, 14) was inadequate to fully inhibit phagocytosis through the complement receptor at higher antibody concentrations. Consequently, the protocol for blocking complement receptors was modified to use higher amounts of blocking antibody, leading to improved concordance between experimental results and mathematical predictions. The ability of the mathematical model to discriminate between receptor binding and to yield insight on the relative contribution of the two receptors to phagocytosis provides confidence in the relevance and correctness of the proposed equations.
We considered IgG concentration effects on both the microbe and the macrophage. Our model predicts that more free antibody results in more rapid binding to the yeast capsule, producing a higher saturation level and therefore rapidly enhancing phagocytosis through Fc receptors. However, too much antibody rapidly reduces the number of available Fc receptors. Phagocytosis through complement receptors rapidly compensates for this effect, and after a slight but noticeable drop in the phagocytic efficacy (at the concentration of free antibody, around 10 μg/ml in our experiments), phagocytic index continues to increase. However, eventually there is too much antibody bound to the capsule, which considerably reduces phagocytosis through complement receptors, possibly through steric effects and/or additional changes to the capsule. Additional experimental information that could contribute to the refinement of the model includes more quantitative data on the involvement of different types of receptors, the extent to which there is cooperation between them, and a better understanding of the interaction between antibody-modified capsular polysaccharide and the complement receptors. Particularly interesting would be experiments with different biological systems whereby antibody-mediated phagocytosis occurs through more than one cellular receptor.
Given that antibody-mediated phagocytosis through the complement receptor has recently been shown to be associated with protection (19), interference with this mechanism by high antibody concentrations may contribute to the decline in efficacy observed in passive protection studies with large antibody doses (13, 15). We expect that, in all conditions, the efficacy of phagocytosis will involve a subtle balance between enough free antibody available to bind to the yeast capsule and yet not so much as to saturate the capsule and Fc receptors.
We established that with higher initial concentrations of free antibody the affinity constant of Fc receptors for binding of free antibody is an essential parameter of the model and might influence how the phagocytic index depends on the concentrations of free antibody. This in turn implies that modeling the efficacy of phagocytosis will strongly depend on the type of the phagocytic cell and the types, affinities, and numbers of Fc receptors involved. Furthermore, the model predicts that the magnitude of the affinity and dissociation constants of the antibody from binding sites on the microbe is a major contributor to the phagocytic efficacy, especially for the phagocytosis through the complement receptors.
In summary, we propose a mathematical model for the phagocytosis of C. neoformans that can simulate and explain experimental results obtained through a range of antibody concentrations. The ability of our model to predict the experimental outcome on the basis of the assumed parameters provides confidence that the major variables and parameters in this system are well understood. The model proposed successfully predicts the dependence of the phagocytic index on the antibody concentration and explains the paradoxical reduction in phagocytic index at high antibody concentrations. Hence, our model provides an explanation for the processes involved, establishes the relative importance of those variables and parameters, and provides a starting point for incorporating additional complexity. Although the equations presented were derived to explain the experimental data for C. neoformans, they could serve as a basis for future mathematical modeling of phagocytosis of other microbes, especially those systems in which opsonization and/or cooperation among different types of receptors plays a significant role. The development of a more accurate model for C. neoformans phagocytosis would require additional information that is not currently available, including the rates of microbe-macrophage attachment as a function of antibody concentration, macrophage receptor density, and microbial binding sites. In this regard, the mathematical modeling has identified gaps in the current knowledge of C. neoformans phagocytosis and calls for further studies, experimental and theoretical, of the mechanisms of attachment and ingestion of this and other microbes to macrophages, the signaling pathways involved, and the potential for cooperation between different types of opsonic receptors.
Supplementary Material
Acknowledgments
A.C. is supported by NIH grants AI033142, AI033774, and HL059842.
N.M. thanks W. Weckesser for many valuable discussions. We also thank The Mathematical Biosciences Institute at Ohio State University for organizing the interdisciplinary workshop on Host-Pathogen Interactions in Disease Models in June 2004 where we began our collaboration. We are also grateful to the reviewers for their valuable comments.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 5 February 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Camner, P., M. Lundborg, L. Lastbom, P. Gerde, N. Gross, and C. Jarstrand. 2002. Experimental and calculated parameters on particle phagocytosis by alveolar macrophages. J. Appl. Physiol. 92:2608-2616. [DOI] [PubMed] [Google Scholar]
- 2.Casadevall, A. 2004. The methodology for determining the efficacy of antibody-mediated immunity. J. Immunol. Methods 291:1-10. [DOI] [PubMed] [Google Scholar]
- 3.Casadevall, A., W. Cleare, M. Feldmesser, A. Glatman-Freedman, D. L. Goldman, T. R. Kozel, N. Lendvai, J. Mukherjee, L. Pirofski, J. Rivera, A. L. Rosas, M. D. Scharff, P. Valadon, K. Westin, and Z. Zhong. 1998. Characterization of a murine monoclonal antibody to Cryptocococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob. Agents Chemother. 42:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dadachova, E., R. A. Bryan, C. Apostolidis, A. Morgenstern, T. Zhang, T. Moadel, M. Torres, X. Huang, E. Revskaya, and A. Casadevall. 2006. Interaction of radiolabeled antibodies with fungal cells and components of the immune system in vitro and during radioimmunotherapy for experimental fungal infection. J. Infect. Dis. 193:1427-1436. [DOI] [PubMed] [Google Scholar]
- 5.Larsen, R. A., P. G. Pappas, J. R. Perfect, J. A. Aberg, A. Casadevall, G. A. Cloud, R. James, S. Filler, and W. E. Dismukes. 2005. A phase I evaluation of the safety and pharmacodynamic activity of a murine-derived monoclonal antibody 18b7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother 49:952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo, Y., S. C. Tucker, and A. Casadevall. 2005. Fc- and complement-receptor activation stimulates cell cycle progression of macrophage cells from G1 to S. J. Immunol. 174:7226-7233. [DOI] [PubMed] [Google Scholar]
- 7.Mellman, I. S., H. Plutner, R. M. Steinman, J. C. Unkeless, and Z. A. Cohn. 1983. Internalization and degradation of macrophage Fc receptors during receptor-mediated phagocytosis. J. Cell Biol. 96:887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukherjee, S., M. Feldmesser, and A. Casadevall. 1996. J774 murine macrophage-like cell interactions with Cryptococcus neoformans in the presence and absence of opsonins. J. Infect. Dis. 173:1222-1231. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee, S., S. C. Lee, and A. Casadevall. 1995. Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect. Immun. 63:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perfect, J. R., and A. Casadevall. 2002. Cryptococcosis. Infect. Dis. Clin. N. Am. 16:837-874, v-vi. [DOI] [PubMed] [Google Scholar]
- 11.Petri, I., R. Egerer, A. Stelzner, J. Suss, and H. Schutz. 1987. Development of mathematical models for an in vitro-phagocytosis test system. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 267:217-227. [DOI] [PubMed] [Google Scholar]
- 12.Rivera, J., and A. Casadevall. 2005. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J. Immunol. 174:8017-8026. [DOI] [PubMed] [Google Scholar]
- 13.Taborda, C. P., and A. Casadevall. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100-2107. [DOI] [PubMed] [Google Scholar]
- 14.Taborda, C. P., and A. Casadevall. 2002. CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16:791-802. [DOI] [PubMed] [Google Scholar]
- 15.Taborda, C. P., J. Rivera, O. Zaragoza, and A. Casadevall. 2003. More is not necessarily better: prozone-like effects in passive immunization with IgG. J. Immunol. 170:3621-3630. [DOI] [PubMed] [Google Scholar]
- 16.Tran, C. L., and A. D. Jones, and K. Donaldson. 1995. Mathematical model of phagocytosis and inflammation after the inhalation of quartz at different concentrations. Scand. J. Work Environ. Health 21(Suppl. 2):50-54. [PubMed] [Google Scholar]
- 17.Tucker, S. C., and A. Casadevall. 2002. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc. Natl. Acad. Sci. USA 99:3165-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Unkeless, J. C., and H. N. Eisen. 1975. Binding of monomeric immunoglobulins to Fc receptors of mouse macrophages. J. Exp. Med. 142:1520-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang, F., A. Nakouzi, M. Alvarez, O. Zaragoza, R. H. Angeletti, and A. Casadevall. 2006. Structural and functional characterization of glycosylation in an immunoglobulin G1 to Cryptococcus neoformans glucuronoxylomannan. Mol. Immunol. 3:987-988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.