Abstract
Despite inducing a strong host cellular and humoral immune response, the helminth Echinococcus granulosus is a highly successful parasite that develops, progresses, and ultimately causes chronic disease. Although surgery remains the preferred therapeutic option, pharmacological research now envisages antihelminthic strategies. To understand the mechanisms that E. granulosus uses to escape host immunosurveillance and promote chronic infection, we investigated how two hydatid cyst components, purified antigen B (AgB) and sheep hydatid fluid (SHF), act on host dendritic cell (DC) differentiation from monocyte precursors and how they influence maturation of DC that have already differentiated. We evaluated the immunomodulatory potential of these antigens by performing immunochemical and cytofluorimetric analyses of monocyte-derived DCs from healthy human donors. During monocyte differentiation, AgB and SHF downmodulated CD1a expression and upregulated CD86 expression. Compared with immature DCs differentiated in medium alone (iDCs), AgB- and SHF-differentiated cells stimulated with lipopolysaccharide included a significantly lower percentage of CD83+ cells (P < 10−4) and had weaker costimulatory molecule expression. When stimulated with AgB and SHF, iDCs matured and primed lymphocytes towards the Th2 response typical of E. granulosus infection. SHF and particularly AgB reduced the production of interleukin-12p70 (IL-12p70) and tumor necrosis factor alpha in lipopolysaccharide-stimulated iDCs. Anti-IL-10 antibodies increased the levels of IL-12p70 secretion in AgB- and SHF-matured DCs. AgB and SHF induced interleukin-1 receptor-associated kinase phosphorylation and activated nuclear factor-κB, suggesting that Toll-like receptors could participate in E. granulosus-stimulated DC maturation. These results suggest that E. granulosus escapes host immunosurveillance in two ways: by interfering with monocyte differentiation and by modulating DC maturation.
Helminth parasites live for long times in the hostile medium of the host. In their struggle for life, these organisms have developed various strategies that allow them to feed, reproduce, and defend themselves from host immune attacks (29). Although older models suggested that parasites play a passive role in immune evasion, later studies suggested that they actively interfere with the host immune response (15). Helminths penetrate and establish themselves in the host tissues, incorporate metabolites from the host, and modulate the host immune response. Proteins secreted by parasites and proteins expressed on their surfaces as membrane-bound proteins participate in a wide range of parasite functions (52). Little is known about parasitic molecules that behave as immunomodulatory antigens and the mechanisms that they use to evade the host's immune response (29).
Cystic echinococcosis (CE) is a widespread chronic endemic helminthic disease caused by infection with metacestodes (larval stage) of the tapeworm Echinococcus granulosus. CE affects humans and a wide range of livestock species (35, 65). During the larval stage the parasite forms a cyst that is filled with liquid, hydatid fluid (HF), and is surrounded by three membrane layers, and it grows in the liver, lungs, or other organs of the host. Because parasite molecules secreted from the hydatid cyst are exposed to the host's immune system, the various components help in understanding the mechanisms that E. granulosus uses for adapting to its host (54). HF is a complex mosaic of antigens having different features and functions. Although various immunomodulatory proteins have been isolated and characterized, the signature E. granulosus antigens in hydatid cyst fluid are still antigen 5 and antigen B (AgB) (39, 40, 58). Antigen 5, a 67-kDa glycoprotein, and especially AgB, a 160-kDa lipoprotein, are the major immunodominant antigens and are thought to be responsible for the immunomodulatory activities of E. granulosus, promoting its survival within a mammalian host (3, 10, 13, 22, 28, 32). In our previous studies investigating the cellular response in human CE, we found that sheep hydatid fluid (SHF) elicits both T helper 1 (Th1) and Th2 cell activation (49, 50). Th1 cell activation is related to protective immunity, whereas Th2 cell activation is related to susceptibility to disease and to escape mechanisms. AgB intervenes in early natural immunity, inhibiting neutrophil recruitment (56), and activates Th cells, thereby eliciting a nonprotective Th2 cell response (48).
The mechanisms triggering Th1 and Th2 cell activation during helminth infections remain unclear. While exogenous “danger” signals, such as lipopolysaccharides (LPS), activate antigen-presenting cells (APCs) to promote a Th1 response, less is known about the cell types or molecules involved in initiating a Th2 response (9, 64).
Dendritic cells (DCs) are professional APCs that represent the link between innate immunity and adaptive immunity (5, 60). DC-parasite interactions are pivotal in triggering and regulating parasite-induced immunity. DC function is itself modulated during parasitic infection for the mutual benefit of the host and the parasite (18, 57). The function of DCs is to capture antigens at peripheral sites and to migrate to T-cell areas in lymphoid organs, where they elicit a specific T-cell response by presenting antigen (17). During this process, activated DCs undergo distinct changes in phenotype and function, termed DC maturation (59). Subsequently, DCs exist in various states of polarization, which is reflected in the DC polarization of T-cell responses (38, 53). Among the various factors known to alter and contribute to DC polarization are the surface levels of costimulatory molecules and the local cytokine milieu in which DCs develop (21, 26). DC function depends on pathogen components that DCs recognize. For example, upon bacterial or viral infection, DCs sense pathogens through a group of transmembrane receptors, Toll-like receptors (TLRs), and direct T-cell differentiation towards Th1 cells (24, 43, 44, 45, 55). In a few studies workers have examined the role of TLRs in immunity to parasites (4, 34). Distinct parasite molecules have been shown to signal through TLR2 and TLR4 (8, 31, 41, 63). Data obtained previously have shown that upon helminth infection Th2 cell differentiation predominates, but how DCs intervene in the immune responses is unclear (4, 38).
Investigating what happens when E. granulosus antigens interact with monocytic precursors and DCs might therefore help us understand E. granulosus-induced immunity and possibly explain how DCs participate. In a recent study Kanan and Chain (23) observed that E. granulosus HF modulates DC differentiation and cytokine secretion.
In this study, to expand our understanding of E. granulosus AgB interference with host immune responses, we investigated the effects of purified AgB and SHF on host DC differentiation from monocytes and on DC maturation from cells that have already differentiated. To evaluate the immunomodulatory potential of AgB, we analyzed, by using flow cytometry and immunochemistry, phenotypic and functional changes in human monocytes and DCs from healthy donors. To find out whether TLRs participate in DC maturation, we analyzed interleukin-1 (IL-1) receptor-associated kinase (IRAK) phosphorylation and nuclear factor-κB (NF-κB) activation.
MATERIALS AND METHODS
Antigens.
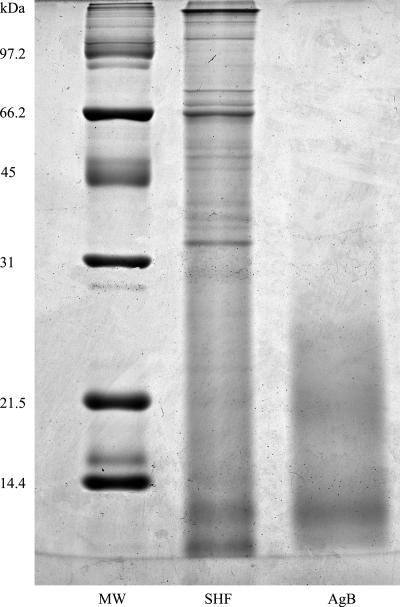
SHF was collected from several ovine fertile cysts for subsequent use as a specific parasite antigen. SHF was clarified by centrifugation at 10,000 × g and 4°C for 60 min, dialyzed against phosphate-buffered saline (PBS) (pH 7.2), concentrated 10-fold with a collodion bag ultrafiltration apparatus (Sartorius GmbH, Gottingen, Germany), and lyophilized until it was used. A purified AgB preparation was obtained after SHF was heated at 100°C as described by Rogan et al. (51). The same batch of pooled SHF and AgB was used in all experiments. The total protein content was determined by the Bio-Rad protein assay, performed as indicated by the manufacturer (Bio-Rad, Richmond, CA). During 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) in reducing conditions, SHF produced a characteristic complex protein banding pattern with bands at positions ranging from 200 to 8 kDa, including bands at 38 and 22 to 25 kDa, corresponding to antigen 5, and bands at 8, 16, and 20 kDa, corresponding to AgB. Purified AgB contained the immunodominant subunit located at 8 and 12 kDa and other larger subunits (Fig. 1). The two antigenic preparations were filtered through a 0.45-μm membrane filter (Millex-HA; Millipore S.A, Bedford, MA) for subsequent use in cellular cultures. The level of endotoxin contamination in SHF and AgB, as determined by the quantitative chromogenic Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD), was <0.03 endotoxin unit/ml.
FIG. 1.
Protein content of SHF and AgB. SHF (15 μg total protein) and AgB (3.0 μg total protein) were subjected to 12% SDS-PAGE in nonreducing conditions and stained with Coomassie blue. Molecular masses are indicated on the left (lane MW).
Monocyte differentiation towards DCs.
Immature DCs (iDCs) were generated from human monocytes as previously described (7). In brief, human peripheral blood mononuclear cells from 10 healthy human donors (buffy coats obtained from the Transfusion Center of the Università degli Studi “La Sapienza,” Rome, Italy) were isolated by density gradient separation (Lympholite, Cedarlane, Ontario, Canada). Informed consent was obtained for the use of blood samples according to the declaration of Helsinki. CD14+ monocytes were purified by incubation with anti-CD14-coated microbeads (Miltenyi Biotec, Bergish Gladbach, Germany), followed by sorting with a magnetic device (MiniMacs separation unit; Miltenyi Biotec), according to the manufacturer's instructions. Monocytes were induced to differentiate to DCs in 6-day cultures with or without SHF (0.1, 50, and 100 μg/ml), AgB (0.1, 10, and 100 μg/ml), and LPS (100 ng/ml; Escherichia coli strain 0111:B4; Sigma-Aldrich, Milan, Italy) grown in the presence of 100 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (R & D Systems, Abingdon, United Kingdom) and 1,000 U/ml recombinant IL-4 (R & D Systems) in RPMI 1640 supplemented with 1% nonessential amino acids, 1% sodium pyruvate, 50 U/ml penicillin, 50 μg/ml streptomycin (Gibco, Grand Island, NY), 5 × 10−5 M 2-mercaptoethanol (Merck, West Point, PA), and 10% fetal calf serum (HyClone Laboratories, Inc., Logan, UT). To rule out endotoxin contamination of SHF and AgB, the same experiments were performed in the presence of polymyxin B (10 μg/ml; Sigma-Aldrich). Cell viability was determined microscopically by trypan blue dye exclusion. Morphological changes in monocytes were observed with an inverted microscope. DC differentiation was routinely checked by flow cytometric analysis. The SHF- and AgB-differentiated DCs were checked for the ability to mature in response to LPS.
DC maturation.
Preliminary dose-response experiments (0.1 to 100 μg/ml) established that parasite antigen effects were dose dependent, and we chose 50 μg/ml for SHF and 10 μg/ml for AgB as the optimal reagent concentrations for DC stimulation. Human iDCs were stimulated with or without SHF (50 μg/ml), AgB (10 μg/ml), and LPS (100 ng/ml) for 18 h and then collected and washed. SHF-differentiated DCs (50 μg/ml) and AgB-differentiated DCs (10 μg/ml) were stimulated with LPS (100 ng/ml; E. coli strain 0111:B4; Sigma-Aldrich) for 18 h and then collected and washed. To check the influence of E. granulosus antigens on DC maturation induced by LPS, SHF (50 μg/ml) and AgB (10 μg/ml) were added to LPS-stimulated DCs. To rule out endotoxin contamination of SHF and AgB, the same experiments were performed in the presence of polymyxin B (10 μg/ml; Sigma-Aldrich). Phenotypic DC maturation was assessed by flow cytometric analysis.
Flow cytometric analysis.
For phenotypic analysis, monocytes and DCs were stained with phycoerythrin-conjugated monoclonal antibodies (MAbs) to CD1a, CD80, CD86, and HLA-DR and fluorescein isothiocyanate (FITC)-conjugated MAbs to CD14, CD83, and CD40 (Becton Dickinson-PharMingen Co. Biosciences, San Jose, CA) for 30 min at 4°C before analysis by flow cytometry with a FACScan using the CellQuest software (Becton Dickinson-PharMingen Co.). Typically, iDCs cultures contained >90% CD1a+ CD14− cells. To avoid reproducibility problems in this study, we used the same lots of specific antibodies.
Endocytosis assay.
Cells (2 × 105 cells per sample) were incubated in RPMI 1640 medium with FITC-dextran (1 mg/ml; molecular weight, 40,000; Sigma-Aldrich) for 60 min at 37°C or at 4°C (for control binding). After incubation, DCs were washed twice with PBS and fixed with 1% formaldehyde. The uptake of FITC-dextran by DCs, cultivated previously under different conditions, was determined by flow cytometry. At least 5,000 cells per sample were analyzed.
Purification of T cells.
CD4+ T cells were purified from peripheral blood mononuclear cells by negative selection using an untouched CD4+ T-cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. Negatively selected CD4+ T cells were depleted of CD45RO+ cells using anti-CD45RO-coupled magnetic beads and LD negative selection columns (Miltenyi Biotec) to obtain negatively selected CD45RA+ T cells. The purity of negatively selected CD4+ CD45RA+ cells was determined by direct staining for membrane expression of CD45RA and CD4 using phycoerythrin-conjugated MAb to CD45RA and FITC-conjugated MAb to CD4 (Becton Dickinson-PharMingen Co.). A portion of the negatively selected CD4+ CD45RA+ T cells was cryopreserved for later use in T-cell priming experiments.
Cytokine production assay.
Levels of IL-10, tumor necrosis factor alpha (TNF-α), IL-12p70, and IL-6 in stimulated and unstimulated DC culture supernatants collected at 18 h were determined by performing enzyme-linked immunosorbent assays (ELISA) (OptEIA kits; Becton Dickinson-PharMingen Co.) according to the manufacturer's instructions. The limits of detection were as follows: IL-10 and TNF-α, 16 pg/ml; and IL-12p70 and IL-6, 2.2 pg/ml. To block endogenous IL-10, neutralizing mouse anti-human IL-10 MAb (R&D Systems) or mouse immunoglobulin was added to unstimulated DC cultures or at the time of stimulation with AgB and SHF, and cells were analyzed after 18 h. Preliminary dose-response experiments indicated that the optimal neutralizing dose of anti-IL-10 MAb was 10 μg/ml. The level of endotoxin contamination of anti-IL-10 MAb, as determined by the quantitative chromogenic Limulus amebocyte lysate assay (BioWhittaker), was < 0.03 endotoxin unit/ml.
Mixed lymphocyte reaction (MLR).
The ability of DCs to stimulate allogeneic T cells was assessed, and irradiated DCs (30 Gy) were used as stimulator cells. Allogeneic T lymphocytes (1 × 105 cells/well) were incubated with the irradiated DCs for 3 days at different responder/stimulator ratios (DC/T cell ratios, 1:4 to 1:64) in a 96-well round-bottom plate. On day 2, 0.5 μCi/well [methyl-3H]thymidine (Amersham Life Science, Buckinghamshire, United Kingdom) was added to each well. After incubation for another 18 h at 37°C, cells were harvested on glass fiber filter paper (Wallac, EG&G Company, Turku, Finland), using an automatic cell harvester (Harvester 96, MACH III M; TOMTEC, Orange, CT). [methyl-3H]thymidine uptake into cell DNA was measured by reading samples with a beta counter (1450 Microbeta Plus; Wallac, EG&G Company). The net cpm for triplicate cultures were determined.
T-cell priming.
To determine whether E. granulosus antigen-matured DCs primed naïve T lymphocytes, negatively selected naïve allogeneic T cells were cultured with SHF- and AgB-stimulated DCs at a ratio of 20:1. Zymosan- and LPS-matured DCs were used as positive controls to prime IL-4- or gamma interferon (IFN-γ)-expressing T cells. Activated T cells were expanded for 10 days with recombinant IL-2 (30 U/ml; Roche Molecular Biochemicals, Mannheim, Germany), added on day 5, in a 24-well plate containing complete medium to obtain polyclonal T-cell lines whose IL-4 expression and IFN-γ expression were analyzed by flow cytometry. In brief, 106 cells were stimulated with 10−7 M phorbol 12-myristate 13-acetate plus 1 μg/ml ionomycin for 4 h in the presence of 10 μg/ml brefeldin A (all reagents were obtained from Sigma-Aldrich). Cells were labeled with anti-CD3 peridinin-chlorophyll-protein (5 μl/104 cells; Becton Dickinson-PharMingen Co.) for 30 min on ice, and cells were then fixed with fluorescence-activated cell sorting lysing solution, treated with fluorescence-activated cell sorting permeabilizing solution (Becton Dickinson-PharMingen Co.), stained with a predetermined optimal concentration of anticytokine MAb or appropriate isotype MAb control (Becton Dickinson-PharMingen Co.), and analyzed with a FACScan. The variables evaluated included the pattern of cytokine expression in the CD3+ population. Cells were gated according to light scattering properties to exclude cell debris. A minimum of 10,000 viable cells were analyzed for each sample. Results were processed using the CellQuest software (Becton Dickinson-PharMingen Co.).
IRAK phosphorylation assay.
Cell-free lysates from unstimulated DCs or DCs stimulated with SHF, AgB, and LPS for 45 min at 37°C in the presence of 5% CO2 were immunoprecipitated with a polyclonal anti-IRAK antibody (MBL, Woburn, MA). In brief, cells were lysed in lysis buffer (20 mM HEPES [pH 7.2], 1% Nonidet P-40, 10% glycerol, 50 mM NaF) containing protease inhibitors. To preclear nonspecific binding, cell-free lysates were mixed with protein A-acrylic beads (Bio-Rad) and stirred in a rotary shaker for 1 h at 4°C. After centrifugation (500 × g for 1 min), the supernatant was immunoprecipitated with the anti-IRAK antibody (2 μg) or non-IRAK-specific immunoglobulin G (IgG) (irrelevant) plus protein A-acrylic beads. The immunoprecipitates were subjected to 7.5% SDS-PAGE. The proteins were electrophoretically transferred to nitrocellulose membranes (Bio-Rad) and then, after blocking with PBS containing 3% albumin, probed with antiphosphoserine MAb (Sigma-Aldrich). Bound antibodies were visualized with horseradish peroxidase-conjugated anti-mouse IgG (Sigma-Aldrich), and immunoreactivity was assessed by the chemiluminescence reaction using the enhanced chemoluminescence Western blotting system (Amersham). To confirm that the positive band was IRAK, the antiphosphoserine antibody was stripped from the nitrocellulose, and the membrane was then reprobed with polyclonal anti-IRAK antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). As a control for nonspecific reactivity, parallel SDS-PAGE gels were blotted as described above using an anti-mouse IgG (Sigma-Aldrich).
NF-κB assay.
An NF-κB p65/p50 transcription factor assay kit (Active Motive, San Diego, CA) was used to monitor NF-κB activation. Unstimulated DCs and DCs stimulated for 45 min at 37°C in the presence of 5% CO2 with SHF, AgB, and LPS were lysed, the protein was quantified, and equal amounts of lysates were used to test activated levels of p50 and p65 subunits with the antibodies directed against the subunits bound to the oligonucleotide containing the NF-κB consensus binding site. As a positive control, we used a HeLa cell extract, and to monitor the specificity of the assay, we used NF-κB wild-type and mutated consensus oligonucleotides according to the manufacturer's instructions.
Statistical analysis.
Statistically significant differences were tested with Student's paired t test. P values of <0.05 were considered significant. All culture experiments were performed in triplicate.
RESULTS
Effects of E. granulosus antigens on monocyte differentiation toward DCs.
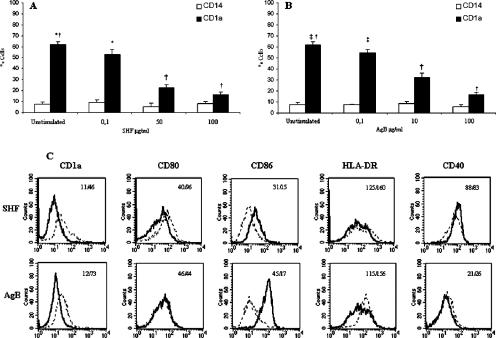
For monocytes differentiated into immature DC in medium without parasite antigens (iDCs), trypan blue exclusion showed that the level of cell viability was 90%; for SHF- and AgB-differentiated DCs (up to 100 μg/ml) the percentage was the same. Microscopic analysis showed that cells that differentiated in the presence of parasite antigens had fewer dendritic processes than iDC, but they did not have the macrophage-like appearance. iDCs acquired the typical immature phenotype (CD14− CD1a+, mannose receptor positive, CD80+ CD83−). AgB- and SHF-differentiated cells could not express CD14, but they were predominantly CD1a−, indicating that only a few differentiated into iDCs (Fig. 2). In all donors tested (n = 7), AgB and SHF significantly reduced the percentage of CD1a+ cells compared with the percentage of unstimulated iDCs in a dose-related manner (the P values as determined by Student's paired t test for comparisons with iDCs were as follows: for AgB, P = 0.02 at 0.1 μg/ml and P < 10−4 at 10 and 100 μg/ml; and for SHF, P = 0.03 at 0.1 μg/ml and P < 10−4 at 50 and 100 μg/ml) (Fig. 2). Analysis of the mean fluorescence intensity showed that both antigens significantly inhibited the upregulation of CD1a expression (P = 0.049 for comparisons of SHF- and AgB-stimulated cells with unstimulated iDCs) and increased the upregulation of CD86 expression (P = 0.014 for a comparison of AgB-stimulated and unstimulated iDCs; P = 0.003 for a comparison of SHF-stimulated and unstimulated iDCs) (Table 1).
FIG. 2.
Effect of AgB and SHF on the differentiation of human monocytes into DCs. Human monocytes were cultured with GM-CSF plus IL-4 with or without SHF (0.1, 50, or 100 μg/ml) and AgB (0.1, 10, or 100 μg/ml). Expression of cell surface molecules was assessed by flow cytometry as described in Materials and Methods. (A and B) SHF (A) and AgB (B) induced selective failure in CD1a expression in a dose-dependent manner. The data are the means ± standard deviations for the percentages of positive cells from seven independent experiments. Significant differences for comparisons with unstimulated DCs as determined by Student's paired t test are indicated by asterisks (P = 0.03), daggers (P < 10−4), and double daggers (P = 0.02). (C) SHF (50 μg/ml) and AgB (10 μg/ml) decreased CD1a expression and increased CD86 expression. The results are expressed as mean fluorescence intensities. The results of one of seven experiments are shown; comparable results were obtained in the other experiments. Dotted lines, control DCs; solid lines, SHF- or AgB-differentiated DCs.
TABLE 1.
Phenotypic characterization of AgB- and SHF-differentiated cells not stimulated and stimulated with LPSa
| Stimulus | GM-CSF + IL-4 | % of cells positive for CD83 | Fluorescence intensities for:
|
|||
|---|---|---|---|---|---|---|
| CD40 | CD80 | CD86 | HLA-DR | |||
| None | − | 3.2 ± 1.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.0 ± 10.0 | 83.2 ± 21.2 |
| None | + | 2.6 ± 1.8 | 30.1 ± 25.5 | 38.7 ± 31.1 | 19.2 ± 8.8 | 97.1 ± 67.1 |
| SHF | + | 10.6 ± 3.4 | 31.7 ± 29.4 | 41.4 ± 7.3 | 55.4 ± 24.6b | 80.5 ± 51.1 |
| AgB | + | 8.2 ± 2.5 | 26.8 ± 26.2 | 34.1 ± 17.6 | 35.1 ± 16.7c | 66.1 ± 51.4 |
| LPS | + | 73.5 ± 18.4 | 48.8 ± 24.8 | 121.6 ± 59.7 | 156.6 ± 71.5 | 119.6 ± 49.3 |
| SHF + LPS | + | 12.4 ± 4.2d | 48.6 ± 19.3 | 82.4 ± 55.3d | 53.4 ± 23.4d | 35.8 ± 14.2d |
| AgB + LPS | + | 4.5 ± 2.2d | 43.1 ± 17.3 | 84.5 ± 73.7d | 13.8 ± 5.1d | 51.2 ± 18.1d |
Monocytes were induced to differentiate to immature DCs in 6-day cultures with or without AgB (10 μg/ml) or SHF (50 μg/ml). The immature DCs were stimulated with LPS for an additional 18 h, and surface markers were analyzed by flow cytometry. The results are expressed as the percentage of cells positive for CD83 and as the fluorescence intensities for CD14, CD1a, CD40, CD80, CD86, and HLA-DR; the data are means ± standard deviations for seven independent experiments.
Significantly different (P = 0.003) than the value for unstimulated iDCs as determined by Student's paired t test.
Significantly different (P = 0.014) than the value for unstimulated iDCs as determined by Student's paired t test.
Significantly different (P < 10−4) than the value for LPS-stimulated DCs as determined by Student's paired t test.
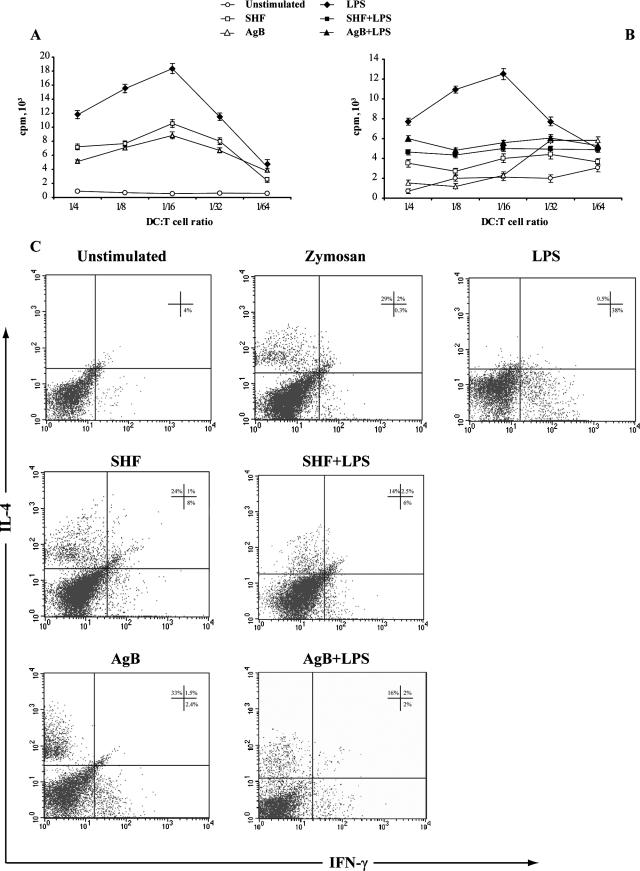
To obtain insight into the functional effects of CD86 upregulation induced by AgB and SHF during the differentiation from monocytes to DCs, we investigated the functional ability of these cells to induce an alloproliferative T-cell response in MLR. Irradiated AgB- and SHF-differentiated cells induced no proliferative response in an MLR (mean cpm) and did not significantly (P > 0.05) block the effects of LPS, indicating that E. granulosus antigens had no effect on the antigen-presenting activity of these cells (Fig. 3). MLR was chosen because, although it is not specific for a given antigen, it provides adequate information concerning the overall antigen-presenting function of DCs.
FIG. 3.
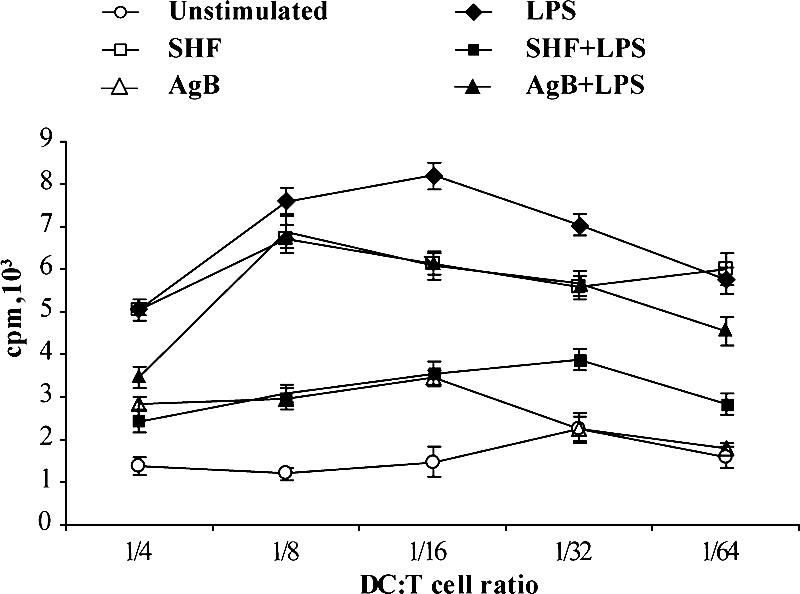
Effects of AgB and SHF during the differentiation of human monocytes into DCs on the ability to induce a proliferative response. Six-day human monocytes that were differentiated with AgB (10 μg/ml), SHF (50 μg/ml), and LPS (100 ng/ml) or were not stimulated were extensively washed and cultured with allogeneic T lymphocytes (1 × 105 cells/well) for 3 days at different stimulator/responder ratios (DC/T cell ratio, 1:4 to 1:64). Proliferation of allogeneic T cells was measured by determining [methyl-3H]thymidine incorporation. The data are means ± standard deviations for triplicate cultures. The results of one of three experiments are shown; comparable results were obtained in the other experiments.
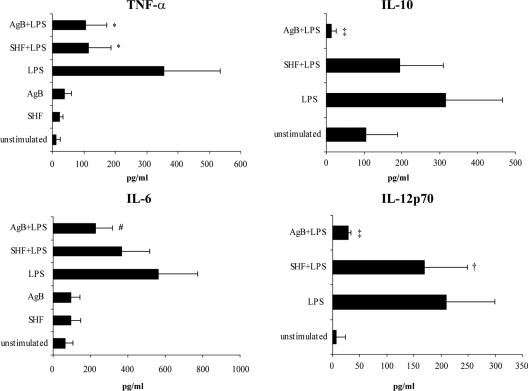
To investigate whether the in vitro impairment of monocyte precursor differentiation into iDCs influenced the ability of these cells to produce cytokines, we evaluated the cytokine production in culture supernatants of AgB- and SHF-differentiated cells by performing ELISA (Fig. 4).
FIG. 4.
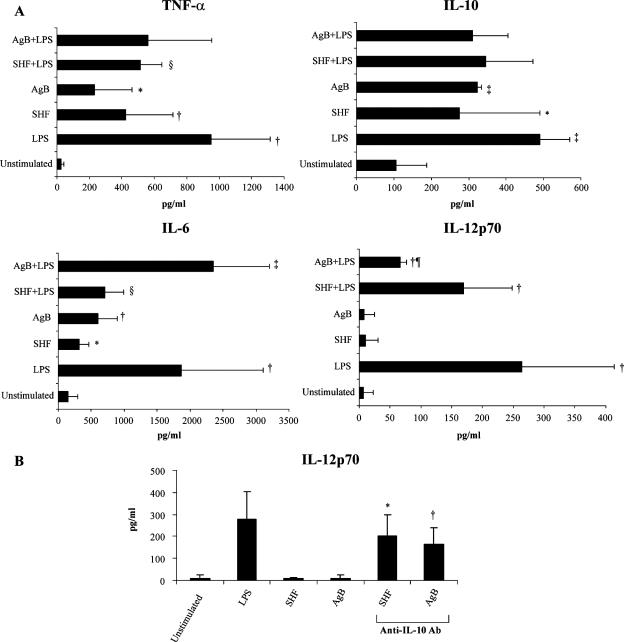
Effects of AgB and SHF on cytokine production by differentiated DCs. Monocytes were induced to differentiate to immature DCs in 6-day cultures with or without AgB (10 μg/ml) or SHF (50 μg/ml). The immature DCs were stimulated with LPS for an additional 18 h, and then cytokine production was analyzed. Cytokine concentrations in culture supernatants were determined by ELISA. The data are means ± standard deviations for seven independent experiments. Significant differences for comparisons with LPS-stimulated DCs as determined by Student's paired t test are indicated by asterisks (P = 0.01), a dagger (P = 0.04), a double dagger (P < 10−4), and a number sign (P = 0.002).
To examine the ability of AgB- and SHF-differentiated cells to mature in response to the TLR4 ligand LPS, we determined phenotypic and functional characteristics of AgB- and SHF-differentiated cells stimulated with LPS for an additional 18 h. AgB- and SHF-differentiated cells stimulated with LPS included a significantly lower percentage of CD83+ cells (P < 10−4) and exhibited significantly lower expression of CD80, CD86, and HLA-DR (P < 10−4) than iDCs stimulated with LPS (Table 1). After stimulation with LPS, AgB-differentiated cells produced significantly smaller amounts of cytokine than iDCs stimulated with LPS produced (for TNF-α, P = 0.01; for IL-6, P = 0.002; for IL-10 and IL-12p70, P < 10−4) (Fig. 4). After stimulation with LPS, SHF-differentiated cells produced smaller amounts of cytokines than iDCs stimulated with LPS produced (for TNF-α, P = 0.01; for IL-12p70, P = 0.04) (Fig. 4). Together, these results suggest that exposing cells to AgB and SHF impairs phenotypic and functional differentiation of monocytes into iDCs, thereby arresting DC maturation.
Effects of E. granulosus antigens on iDC maturation.
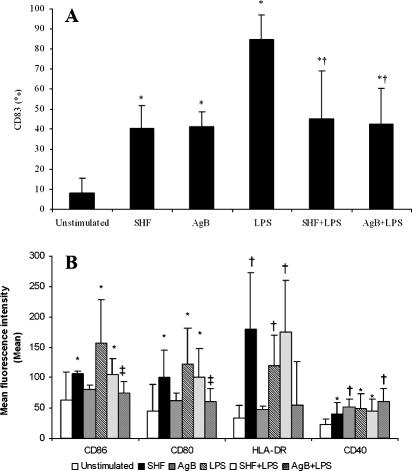
The parasite antigens induced final DC maturation, as shown by the decreased endocytic activity (data not shown) and the increased percentage of CD83+ cells (P < 10−4) (Fig. 5A). SHF upregulated CD80, CD86, CD40, and HLA-DR. In contrast to SHF, AgB upregulated only CD40 (Fig. 5B).
FIG. 5.
Effects of AgB and SHF on the maturation of human monocyte-derived DCs. Human DCs were stimulated for 18 h with or without SHF (50 μg/ml), AgB (10 μg/ml), and LPS (100 ng/ml). Expression of surface molecules was analyzed by flow cytometry as described in Materials and Methods. Phenotypic maturation of DCs was detected by the appearance of CD83 (A) and by the expression of costimulatory molecules (B). (A) Percentages of positive cells from seven independent experiments (means ± standard deviations). Significant differences for comparisons with unstimulated DCs as determined by Student's paired t test are indicated by asterisks (P < 10−4), and significant differences for comparisons with LPS-stimulated DCs as determined by Student's paired t test are indicated by daggers (P < 10−4). (B) Mean fluorescence intensities from seven independent experiments. Significant differences for comparisons with unstimulated DCs as determined by Student's paired t test are indicated by asterisks (P < 0.05) and daggers (P < 0.003), and significant differences for comparisons with LPS-stimulated DCs as determined by Student's paired t test are indicated by double daggers (P < 0.03).
Because mature DCs are powerful producers of key inflammatory and immunoregulatory cytokines that can modulate the type of T-cell response induced (Th1 or Th2), we investigated the cytokine pattern promoted by AgB and SHF in immature DCs. Compared with iDCs, AgB- and SHF-matured DCs produced significantly larger amounts of TNF-α (for AgB, P < 0.05; for SHF, P < 0.004), IL-10 (for AgB, P < 10−4; for SHF, P < 0.05), and IL-6 (for AgB, P < 0.004; for SHF, P < 0.05). Neither SHF nor AgB induced DC to produce IL-12p70 (Fig. 6A).
FIG. 6.
(A) Effects of AgB and SHF on cytokine production by mature DCs. Six-day human DCs, differentiated in medium supplemented with GM-CSF and IL-4, were stimulated with or without AgB (10 μg/ml), SHF (50 μg/ml), and LPS (100 ng/ml) for an additional 18 h. Supernatants were collected after 18 h to measure IL-6, IL-12p70, IL-10, and TNF-α by specific ELISA. The data are the means ± standard deviations of seven independent experiments. Significant differences for comparisons with unstimulated DCs as determined by Student's paired t test are indicated by asterisks (P < 0.05), daggers (P < 0.004), and double daggers (P < 10−4), and significant differences for comparisons with LPS-stimulated DCs as determined by Student's paired t test are indicated by a section sign (P < 0.04) and a paragraph sign (P < 10−4). (B) Preexposure to anti-IL-10 antibody restored IL-12p70 production by AgB- and SHF-matured DCs. DCs were stimulated with AgB (10 μg/ml) and SHF (50 μg/ml) in the presence or absence of neutralizing anti-human IL-10 MAb. After 18 h, the amounts of IL-12p70 in the supernatants were determined by ELISA. Unlike the anti-mouse IL-10 MAb, mouse immunoglobulin had no effect on IL-12p70 secretion. The data are means ± standard deviations of three independent experiments. Significant differences are indicated by an asterisk (P = 0.028) and a dagger (P = 0.024).
Because IL-10 is a known regulator of IL-12 production (37) and plays a key role in Th2 polarization, we examined whether E. granulosus-stimulated production of IL-10 could account for the E. granulosus-mediated inhibition of IL-12 production. Although SHF and AgB failed to induce IL-12 secretion by DCs directly, the presence of anti-IL-10 antibody resulted in greatly increased levels of IL-12 secretion in supernatants of AgB- and SHF-pulsed DCs (Fig. 6B), consistent with an effective role of IL-10 in the inhibition of IL-12 production. These results indicate that exposure to AgB and SHF confers to sentinel iDC the propensity to generate a Th2 response.
Effects of E. granulosus antigens on LPS-induced DC maturation.
The diminutive IL-12p70 response from E. granulosus-stimulated iDC suggested that parasite products may specifically block production of this Th1 key cytokine. We therefore examined whether exposure of iDC to AgB and SHF during LPS-induced iDC maturation interfered with subsequent inflammatory responses to the TLR4 ligand LPS, a stimulus associated with Th1 responses. Addition of AgB and SHF to LPS-stimulated-iDCs significantly decreased the percentage of CD83+ cells (P < 10−4) (Fig. 5A) and the expression of CD86 (for AgB, P < 0.03) and CD80 (for AgB, P < 0.03) (Fig. 5B). Besides phenotypic impairment, AgB and SHF reduced TNF-α production (for SHF, P < 0.04) in LPS-stimulated iDCs (Fig. 6A). AgB was more effective than SHF in inhibiting IL-12p70 production by LPS-stimulated iDCs (P < 10−4) and left IL-6 production unchanged (for SHF, P < 0.04). These results indicate that exposure to parasite antigens impairs the ability of sentinel iDCs to respond to inflammatory stimuli, such as LPS.
E. granulosus antigens increase the allostimulatory ability of DCs and license them for priming and polarizing naïve T lymphocytes.
When irradiated iDCs prestimulated with AgB, SHF, and LPS were tested in an MLR, the relatively low proliferative ability (mean cpm) of resting allogeneic T cells that could be obtained with unstimulated DCs increased, starting from a DC/T cell ratio of 1:4 (Fig. 7A), indicating that E. granulosus antigens potentiate the antigen-presenting activity of DCs in an allogeneic MLR. AgB and SHF significantly (P < 0.05) blocked the effects of LPS on DC. When stimulated by allogeneic AgB- and SHF-matured DCs in the MLR assay, most naïve T cells differentiated into IL-4-producing cells, as they did in response to zymosan A (24 and 33% versus 29%). A small percentage (8%) of SHF-matured DCs differentiated into IFN-γ-producing cells (Fig. 7C). Both antigens could reduce the LPS-induced Th1 profile stimulating IL-4 expression, thus supporting a mixed Th1 and Th2 response. These data provide evidence that E. granulosus SHF and particularly AgB have important immunomodulating effects on DC function and skew immunity, inducing a DC-mediated Th2 response.
FIG. 7.
Effects of AgB and SHF on the ability of DCs to induce a proliferative response (A and B) and to prime and polarize allogeneic naïve human T cells (C). (A and B) Six-day human DCs were stimulated with AgB (10 μg/ml), SHF (50 μg/ml), and LPS (100 ng/ml) or were not stimulated. After 18 h, DCs were extensively washed and cultured with allogeneic T lymphocytes (1 × 105 cells/well) for 3 days at different stimulator/responder ratios (DC/T cell ratio, 1:4 to 1:64). Proliferation of allogeneic T cells was determined by measuring [methyl-3H]thymidine incorporation. The data are means ± standard deviations of triplicate cultures. In five (A) and three (B) independent experiments comparable results were obtained, and the results of representative experiments are shown. (C) Human DCs were stimulated with or without AgB (10 μg/ml), SHF (50 μg/ml), LPS (100 ng/ml), and zymosan A (10 μg/ml) for 18 h. Zymosan, which classically activates DC to prime for a Th2-type response, was used as a positive control for IL-4 expression. A total of 5 × 104 DCs were used to stimulate 1 × 106 allogeneic naïve negatively selected CD4+ CD45RA+ T cells. Activated T cells were expanded with recombinant human IL-2 (30 U/ml) added on day 5. On day 10, polyclonal T-cell lines were stimulated with phorbol 12-myristate 13-acetate and ionomycin for 4 h in the presence of brefeldin A. Cells were stained with anti-human CD3 peridinin-chlorophyll-protein and processed for intracellular labeling with anti-human IFN-γ-FITC and anti-human IL-4-phycoerythrin. The values are the percentages of activated CD3+ cells producing the cytokine. The results of one of five experiments are shown; comparable results were obtained in the other experiments.
Analysis of IRAK phosphorylation and NF-κB activation in E. granulosus antigen-matured DC.
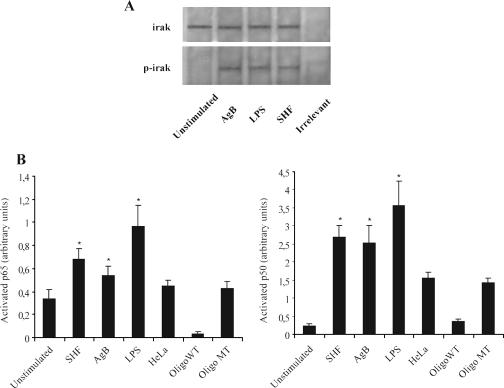
To investigate the intracytoplasmic signaling events associated with E. granulosus stimulation in DCs, we first examined by Western blot analysis the effects of AgB and SHF treatment on IRAK activation in DC lysates. In immunoprecipitates from AgB-, SHF-, and LPS-matured DCs IRAK phosphorylation increased, suggesting that AgB and SHF, as well as LPS, induced IRAK phosphor ylation (Fig. 8A). Immunoprecipitates obtained using non-IRAK-specific IgG and iDCs produced only a weak band in immunoblots. The identity of the IRAK band was verified by Western blotting using a polyclonal anti-IRAK antibody.
FIG. 8.
Effects of AgB and SHF on IRAK phosphorylation (A) and NF-κB activation (B). DCs were stimulated with AgB (10 μg/ml), SHF (50 μg/ml), or LPS (100 ng/ml) or were not stimulated for 45 min. (A) Phosphorylated levels of IRAK were determined by Western blotting with antiphosphoserine MAb. Bound antibodies were visualized with horseradish peroxidase-conjugated anti-mouse IgG, and the immunoreactivity was assessed by enhanced chemiluminescence. Immunoprecipitates obtained using unstimulated cells and non-IRAK-specific IgG (irrelevant) yielded only a weak band. To confirm that the positive band was IRAK, the antiphosphoserine antibody was stripped from the nitrocellulose, and the membrane was then reprobed with polyclonal anti-IRAK antibody. Bound antibodies were visualized with horseradish peroxidase-conjugated anti-goat IgG, and the immunoreactivity was assessed by enhanced chemiluminescence. The results of one of three experiments are shown; similar results were obtained in the other experiments. (B) After cell lysis, protein was quantified, and equal amounts of lysates were used to determine the activated levels of p65 and p50 subunits with antibodies directed against the subunits bound to the oligonucleotide containing the NF-κB consensus binding site. A HeLa cell extract was used as a positive control alone or in the presence of a wild-type (Oligo WT) or mutated (Oligo MT) consensus oligonucleotide. The data are the means ± standard deviations for three different experiments. Significant differences between unstimulated samples and samples stimulated with LPS, AgB, and SHF are indicated by asterisks (P < 10−4).
Second, we evaluated the rates of NF-κB activation in total lysates from untreated or AgB-, SHF-, or LPS-treated DCs by determining the levels of both NF-κB p50 and p65 subunits capable of binding an oligonucleotide containing the NF-κB consensus binding site. AgB- and SHF-matured DCs activated significantly higher p65 and p50 levels than iDCs activated (for AgB, 1.5-fold for p65 and 12-fold for p50; for SHF, 1.9-fold for p65 and 13-fold for p50) (P < 10−4) (Fig. 8B). These levels were only about 1.5-fold lower than those in LPS-stimulated DCs. The assay was specific, because a HeLa extract incubated with a nonbound wild-type consensus oligonucleotide abolished binding of both subunits, whereas the HeLa extract incubated with the mutated consensus oligonucleotide left NF-κB binding unchanged. These results suggest that a member of the TLR family may be involved in the human DC maturation induced by E. granulosus antigens.
DISCUSSION
The results of this study provide new evidence that E. granulosus AgB interferes with host DC functions through two strategies. First, it impairs monocyte precursor differentiation into iDCs, rendering them unable to mature when they are stimulated with LPS. Even though suppressing monocyte differentiation alone might be sufficient to prevent or impair the host immune response, AgB also modulates sentinel DC maturation, priming DCs to polarize lymphocytes into Th2 cells that benefit the parasite. We also obtained evidence that DCs matured by parasite AgB have a reduced ability to respond to inflammatory stimuli, such as LPS. Our experiments investigating IRAK phosphorylation and NF-κB activation raise the possibility that TLRs participate in E. granulosus human DC maturation.
In this study AgB and SHF interfered with DC differentiation by downmodulating CD1a expression; in contrast to the results of Kanan and Chain (23), in our study AgB and SHF had no effect on CD14 expression, indicating that parasite antigens did not force monocytes to differentiate into macrophage-like cells, which occurs in other infections (33). Interestingly, we found that during monocyte differentiation AgB and SHF upregulated CD86 expression. Although controversial, some evidence suggests that CD80 and CD86 expressed on APCs regulate the development of Th1 and Th2 responses by delivering differential signals to helper T cells (62). CD80 preferentially polarizes the immune response toward Th1, whereas CD86, the natural ligand of CD28, drives the response into a Th2 pathway (27). Precisely how E. granulosus antigens modulate CD86 expression and whether this effect has functional importance in polarizing T-cell responses remain unclear. In our experiments, CD86 upregulation nevertheless did not confer the APC function to these cells.
Our flow cytometry experiments showing that there was a significantly reduced percentage of CD83+ cells and that there was reduced expression of all costimulatory molecules on AgB- and SHF-differentiated cells stimulated with LPS also provided evidence that E. granulosus antigens interact with monocytic precursors, thus impairing their response to LPS. In line with our results, Jenne et al. (19) found that Echinococcus multi locularis parasite antigens prevented monocyte precursors from maturing into DCs. They also found that E. multilocularis antigens left CD83, CD86, CD80, CD40, and major histocompatibility complex class I and II expression unaltered.
A distinctive finding was that besides altering the cell phenotype, E. granulosus antigens alter cytokine production, thus inducing distinct functional changes in human monocytes. Interestingly, when we stimulated AgB- and SHF-differentiated cells with LPS, production of IL-12p70 and production of the proinflammatory cytokine TNF-α decreased. Hence, if E. granulosus antigens encounter monocytes while they are still differentiating into iDCs, they render them unable to mature in response to the TLR stimulus LPS and prevent LPS-induced inflammation, favoring a Th2 environment. These results are in line with the recent finding of Kanan and Chain (23) that cells that differentiated in the presence of bovine HF had a reduced ability to release IL-12 and IL-6 in response to LPS.
In addition to suppressing monocyte differentiation, the second pathway that E. granulosus AgB uses to prevent or impair the host immune response is to induce sentinel iDC maturation, priming iDCs to polarize T lymphocytes into Th2 cells that benefit the parasite. We found that AgB matures DCs, thus inducing an exclusive Th2 response. Conversely, SHF, a mixture containing various soluble components, matures DCs, thus polarizing a predominant Th2 response along with a Th1 response. These new in vitro findings obtained with healthy human donors are in line with our previous in vitro observations obtained with patients with CE showing that AgB exploits Th cell activation by eliciting Th2 cytokine production, whereas SHF elicits a Th1/Th2/Th0 balance dominated by Th2 cells (47, 50).
Our findings for DC maturation in response to E. granulosus antigens corroborate the results of previous studies performed with mice and humans, indicating that helminths or their signature molecules induce biased immune responses by directly priming DCs (11). The ability of DCs to recognize a pathogen and mobilize the most appropriate immune response is a key paradigm in immunology (43, 45). Whereas the pathway that DCs use to promote Th1 induction is well known (24, 53), the pathway for Th2 induction is less clear (64). The default hypothesis suggests that the failure of DCs to mature produces a Th2 outcome (25, 57, 60). A more mechanistic version is that when IL-12p70 is absent, Th1 responses cannot occur and Th2 responses do occur, irrespective of other functional features displayed by DCs (for example, costimulatory molecules and cytokine production). Here we provide a rationale for this polarization of the immune response to E. granulosus by showing that if cyst fluid antigens, such as AgB, encounter iDCs, they suppress IL-12p70 production by inducing the immunoregulatory cytokine IL-10. A possible reason why parasite antigen-matured DCs failed to produce IL-12 is that they upregulate IL-10R expression on DCs, thus increasing IL-10 inhibitory activity. In contrast to our findings, Kanan and Chain (23) found that predifferentiated DCs, matured with bovine HF, released some IL-12. These differences presumably depend on differences in the antigens studied (source and composition) and on the reagents used for cytokine detection. Whether other DC negative regulators besides IL-10 are involved in suppressing IL-12 production was beyond the scope of this study. Collectively, our results suggest that E. granulosus infection may actively induce Th2 polarization by promoting the development of distinct phenotypes. The finding that SHF exerts actions on DCs that AgB cannot exert is hardly surprising because SHF contains a complex mixture of immunosuppressive, immunostimulatory, and immunomodulatory components. One way to find out what these other components do would be to use SHF with AgB depleted.
Another distinct finding for AgB was that it reduced LPS-induced production of IL-12p70 but not LPS-induced production of IL-6, providing further evidence that this E. granulosus antigen actively modulates DC responsiveness in a manner favoring a Th2 outcome. These findings strengthen the hypothesis that AgB has direct anti-inflammatory effects on the innate immune response. Recent studies investigating the ability of helminths to subvert the immune response have provided important information on how parasites generate anti-inflammatory responses and have indicated that it may be possible to use pathogen products for immunotherapy (12). Insofar as our study showed that AgB inhibits the human DC inflammatory response to LPS, the biological importance of this E. granulosus antigen in preventing or controlling proinflammatory pathological responses deserves investigation.
Despite the known potent anti-inflammatory actions of the cytokine IL-10 (6, 30), we unexpectedly found that AgB and SHF downregulated IL-10 production in response to LPS. Whether the two antigens reduced expression of the genes coding for inflammatory cytokines through an IL-10-independent mechanism warrants a functional study designed to block IL-10 or IL-10R with monoclonal antibodies.
In this study AgB and SHF seemed to activate a DC signaling cascade resembling the cascade triggered by TLRs (1, 2, 36, 46, 55). Most studies have focused on the role of TLRs and components of their signaling pathways in the control of Th1-type immune responses. Although controversial, some evidence also suggests that TLRs may trigger effector DCs that promote Th2-type responses (61). The ability of parasite molecules to trigger TLR-dependent events may therefore depend on the complex interplay with other receptors that mediate DC-pathogen binding and on the spatial organization of TLR ligands at the parasite surface (42). Apart from AgB, a component of SHF that could bind to TLRs is the phosphorylcholine-containing glycoprotein antigen 5. The antigen 5 hypothesis has received support from molecular studies of DCs in other helminth parasites (14, 16). Because other signal transduction pathways can lead to activation of NF-κB and IRAK during DC stimulation with SHF and AgB, further studies considering the use of TLR or MyD88 knockout mice are necessary to confirm definitively the involvement of TLRs (20). Two other open questions are which TLRs are involved and how the intracellular activity of TLRs in E. granulosus antigen-conditioned DCs can determine the ability to suppress LPS-induced proinflammatory signals and trigger Th2 cell differentiation.
In conclusion, our findings support the hypothesis that during CE, AgB and possibly other soluble molecules released by the hydatid cyst escape host immunosurveillance and promote chronic E. granulosus infection by altering host monocyte precursor differentiation and by modulating DC maturation towards Th2 priming. In addition to increasing knowledge about E. granulosus infection, our study may help workers understand the cellular and molecular mechanisms that other helminth parasites use to modulate human host immune responses. These findings are of interest in ongoing research to develop antihelminthic treatment strategies and novel anti-inflammatory strategies that have wider therapeutic interest.
Acknowledgments
R.R. and B.B. contributed equally to this study.
The assistance of Massimo Delle Femmine for drawing is gratefully acknowledged. This work was supported by a research grant from the Italian Ministry of Health (project no. 6ACF/1).
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nature Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 2.Ardeshna, K. M., A. R. Pizzey, S. Devereux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-KappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039-1046. [PubMed] [Google Scholar]
- 3.Arend, A. C., A. Zaha, F. J. Ayala, and K. L. Haag. 2004. The Echinococcus granulosus antigen B shows a high degree of genetic variability. Exp. Parasitol. 108:76-80. [DOI] [PubMed] [Google Scholar]
- 4.Balic, A., Y. Harcus, M. J. Holland, and R. M. Maizels. 2004. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur. J. Immunol. 34:3047-3059. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 6.Butcher, B. A., L. Kim, A. D. Panopoulos, S. S. Watowich, P. J. Murray, and E. Y. Denkers. 2005. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J. Immunol. 174:3148-3152. [DOI] [PubMed] [Google Scholar]
- 7.Buttari, B., E. Profumo, V. Mattei, A. Siracusano, E. Ortona, P. Margutti, B. Salvati, M. Sorice, and R. Rigano. 2005. Oxidized beta2-glycoprotein I induces human dendritic cell maturation and promotes a T helper type 1 response. Blood 106:3880-3887. [DOI] [PubMed] [Google Scholar]
- 8.Campos, M. A., I. C. Almeida, O. Takeuchi, S. Akira, E. P. Valente, D. O. Procopio, L. R. Travassos, J. A. Smith, D. T. Golenbock, and R. T. Gazzinelli. 2001. Activation of toll-like receptor-2 by glycosylphosphatidyl inositol anchors from a protozoan parasite. J. Immunol. 167:416-423. [DOI] [PubMed] [Google Scholar]
- 9.Cervi, L., A. S. MacDonald, C. Kane, F. Dzierszinski, and E. J. Pearce. 2004. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J. Immunol. 172:2016-2020. [DOI] [PubMed] [Google Scholar]
- 10.Chemale, G., H. B. Ferreira, J. Barrett, P. M. Brophy, and A. Zaha. 2005. Echinococcus granulosus antigen B hydrophobic ligand binding properties. Biochim. Biophys. Acta 1747:189-194. [DOI] [PubMed] [Google Scholar]
- 11.Colonna, M., B. Pulendran, and A. Iwasaki. 2006. Dendritic cells at the host-pathogen interface. Nat. Immunol. 7:117-120. [DOI] [PubMed] [Google Scholar]
- 12.Dunne, D. W., and A. Cooke. 2005. A worm's eye view of the immune system: consequences for evolution of human autoimmune disease. Nat. Rev. Immunol. 5:420-426. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Sapienza, G., and R. E. Cachau. 2003. Identification of critical residues of an immunodominant region of Echinococcus granulosus antigen B. J. Biol. Chem. 278:20179-20184. [DOI] [PubMed] [Google Scholar]
- 14.Goodridge, H. S., G. Stepek, W. Harnett, and M. M. Harnett. 2005. Signalling mechanisms underlying subversion of the immune response by the filarial nematode secreted product ES-62. Immunology 115:296-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harnett, W. 2005. Parasite modulation of the immune response. Parasite Immunol. 27:357-359. [DOI] [PubMed] [Google Scholar]
- 16.Harnett, W., H. S. Goodridge, and M. M. Harnett. 2005. Subversion of immune cell signal transduction pathways by the secreted filarial nematode product, ES-62. Parasitology 130:S63-S68. [DOI] [PubMed] [Google Scholar]
- 17.Hart, D. N. 1997. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90:3245-3287. [PubMed] [Google Scholar]
- 18.Jankovic, D., S. Steinfelder, M. C. Kullberg, and A. Sher. 2006. Mechanisms underlying helminth-induced Th2 polarization: default, negative or positive pathways? Chem. Immunol. Allergy 90:65-81. [DOI] [PubMed] [Google Scholar]
- 19.Jenne, L., J. F. Arrighi, B. Sauter, and P. Kern. 2001. Dendritic cells pulsed with unfractionated helminthic proteins to generate antiparasitic cytotoxic T lymphocyte. Parasite Immunol. 23:195-201. [DOI] [PubMed] [Google Scholar]
- 20.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 21.Kalinski, P., C. M. Hilkens, E. A. Wierenga, and M. L. Kapsenberg. 1999. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20:561-567. [DOI] [PubMed] [Google Scholar]
- 22.Kamenetzky, L., P. M. Muzulin, A. M. Gutierrez, S. O. Angel, A. Zaha, E. A. Guarnera, and M. C. Rosenzvit. 2005. High polymorphism in genes encoding antigen B from human infecting strains of Echinococcus granulosus. Parasitology 131:805-815. [DOI] [PubMed] [Google Scholar]
- 23.Kanan, J. H., and B. M. Chain. 2006. Modulation of dendritic cell differentiation and cytokine secretion by the hydatid cyst fluid of Echinococcus granulosus. Immunology 118:271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapsenberg, M. L. 2003. Dendritic-cell control of pathogen-driven T cell polarization. Nat. Rev. Immunol. 3:984-993. [DOI] [PubMed] [Google Scholar]
- 25.Kelsall, B. L., C. A. Biron, O. Sharma, and P. M. Kaye. 2002. Dendritic cells at the host-pathogen interface. Nat. Immunol. 3:699-702. [DOI] [PubMed] [Google Scholar]
- 26.Kourilsky, P., and P. Truffa-Bachi. 2001. Cytokine fields and the polarization of the immune response. Trends Immunol. 22:502-509. [DOI] [PubMed] [Google Scholar]
- 27.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 28.Lorenzo, C., J. A. Last, and G. G. Gonzalez-Sapienza. 2005. The immunogenicity of Echinococcus granulosus antigen 5 is determined by its post-translational modifications. Parasitology 131:669-677. [DOI] [PubMed] [Google Scholar]
- 29.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89-116. [DOI] [PubMed] [Google Scholar]
- 30.Maizels, R. M., and M. Yazdanbakhsh. 2003. Immune regulation of the immune response by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733-743. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado-Bernal, C., C. J. Kirschning, Y. Rosenstein, L. M. Rocha, N. Rios-Sarabia, M. Espinosa-Cantellano, I. Becker, I. Estrada, R. M. Salazar-Gonzalez, C. Lopez-Macias, H. Wagner, J. Sanchez, and A. Isibasi. 2005. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol. 27:127-137. [DOI] [PubMed] [Google Scholar]
- 32.Mamuti, W., Y. Sako, M. Nakao, N. Xiao, K. Nakaya, Y. Ishikawa, H. Yamasaki, M. W. Lightowlers, and A. Ito. 2006. Recent advances in characterization of Echinococcus antigen B. Parasitol. Int. 55:S57-S62. [DOI] [PubMed] [Google Scholar]
- 33.Mariotti, S., R. Teloni, E. Iona, L. Fattorini, G. Romagnoli, M. C. Gagliardi, G. Orefici, and R. Nisini. 2004. Mycobacterium tuberculosis diverts alpha interferon-induced monocyte differentiation from dendritic cells into immunoprivileged macrophage-like host cells. Infect. Immun. 72:4385-4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McGuinness, D. H., P. K. Dehal, and R. J. Pleass. 2003. Pattern recognition molecules and innate immunity to parasites. Trends Parasitol. 19:312-319. [DOI] [PubMed] [Google Scholar]
- 35.McManus, D. P., W. Zhang, J. Li, and P. B. Bartley. 2003. Echinococcosis. Lancet 362:1295-1304. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed] [Google Scholar]
- 37.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 38.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 39.Ortona, E., P. Margutti, F. Delunardo, S. Vaccari, R. Rigano, E. Profumo, B. Buttari, A. Teggi, and A. Siracusano. 2003. Molecular and immunological characterization of the C-terminal region of a new Echinococcus granulosus heat shock protein 70. Parasite Immunol. 25:119-126. [DOI] [PubMed] [Google Scholar]
- 40.Ortona, E., P. Margutti, F. Delunardo, V. Nobili, E. Profumo, R. Rigano, B. Buttari, G. Carulli, A. Azzara, A. Teggi, F. Bruschi, and A. Siracusano. 2005. Screening of an Echinococcus granulosus cDNA library with IgG4 from patients with cystic echinococcosis identifies a new tegumental protein involved in the immune escape. Clin. Exp. Immunol. 142:528-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ouaissi, A., E. Guilvard, Y. Delneste, G. Caron, G. Magistrelli, N. Herbault, N. Thieblemont, and P. Jeannin. 2002. The Trypanosoma cruzi Tc52-released protein induces human dendritic cell maturation, signals via Toll-like receptor 2, and confers protection against lethal infection. J. Immunol. 168:6366-6374. [DOI] [PubMed] [Google Scholar]
- 42. Pearce, E. J., C. M. Kane, and J. Sun. 2006. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem. Immunol. Allergy 90:82-90. [DOI] [PubMed] [Google Scholar]
- 43.Pulendran, B. 2004. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol. Res. 29:187-196. [DOI] [PubMed] [Google Scholar]
- 44.Pulendran, B., K. Palucka, and J. Banchereau. 2001. Sensing pathogens and tuning immune responses. Science 293:253-256. [DOI] [PubMed] [Google Scholar]
- 45.Reis e Sousa, C. 2001. Dendritic cells as sensors of infection. Immunity 14:495-498. [DOI] [PubMed] [Google Scholar]
- 46.Rescigno, M., M. Martino, C. L. Sutherland, M. R. Gold, and P. Ricciardi-Castagnoli. 1998. Dendritic cell survival and maturation are regulated by different signaling pathways. J. Exp. Med. 188:2175-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riganò, R., B. Buttari, E. De Falco, E. Profumo, E. Ortona, P. Margutti, C. Scottà, A. Teggi, and A. Siracusano. 2004. Echinococcus granulosus-specific T-cell lines derived from patients at various clinical stages of cystic echinococcosis. Parasite Immunol. 26:45-52. [DOI] [PubMed] [Google Scholar]
- 48.Riganò, R., E. Profumo, F. Bruschi, G. Carulli, A. Azzara, S. Ioppolo, B. Buttari, E. Ortona, P. Margutti, A. Teggi, and A. Siracusano. 2001. Modulation of human immune response by Echinococcus granulosus antigen B and its possible role in evading host defenses. Infect. Immun. 69:288-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riganò, R., E. Profumo, G. Di Felice, E. Ortona, A. Teggi, and A. Siracusano. 1995. In vitro production of cytokines by peripheral blood mononuclear cells from hydatid patients. Clin. Exp. Immunol. 99:433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riganò, R., E. Profumo, S. Ioppolo, S. Notargiacomo, E. Ortona, A. Teggi, and A. Siracusano. 1995. Immunological markers indicating the effectiveness of pharmacological treatment in human hydatid disease. Clin. Exp. Immunol. 102:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rogan, M. T., P. S. Craig, E. Zeyhle, T. Romig, G. M. Lubano, and L. Deshan. 1991. Evaluation of a rapid dot-ELISA as a field test for the diagnosis of cystic hydatid disease. Trans. R. Soc. Trop. Med. Hyg. 85:773-777. [DOI] [PubMed] [Google Scholar]
- 52.Rosenzvit, M. C., F. Camicia, L. Kamenetzky, P. M. Muzulin, and A. M. Gutierrez. 2006. Identification and intra-specific variability analysis of secreted and membrane-bound proteins from Echinococcus granulosus. Parasitol. Int. 55:S63-S67. [DOI] [PubMed] [Google Scholar]
- 53.Sallusto, F., B. Palermo, D. Lenig, M. Miettinen, S. Matikainen, I. Julkunen, R. Forster, R. Burgstahler, M. Lipp, and A. Lanzavecchia. 1999. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 29:1617-1625. [DOI] [PubMed] [Google Scholar]
- 54.Schantz, P. M., and B. Gottstein. 1985. Echinococcosis (hydatidosis), p. 69-107. In Immunodiagnosis of parasitic disease, vol. 1. Academic Press Inc., Orlando, FL.
- 55. Schnare, M., G. M. Barton, A. C. Holt, K. Takeda, S. Akira, and R. Medzhitov. 2001. Toll-like receptors control activation of adaptive immune responses. Nat. Immunol. 2:947-950. [DOI] [PubMed] [Google Scholar]
- 56.Shepherd, J. C., A. Aitken, and D. P. McManus. 1991. A protein secreted in vivo by Echinococcus granulosus inhibits elastase activity and neutrophil chemotaxis. Mol. Biochem. Parasitol. 44:81-90. [DOI] [PubMed] [Google Scholar]
- 57.Sher, A., E. Pearce, and P. Kaye. 2003. Shaping the immune response to parasites: role of dendritic cells. Curr. Opin. Immunol. 15:421-429. [DOI] [PubMed] [Google Scholar]
- 58.Siracusano, A., E. Ortona, and R. Rigano. 2002. Molecular and cellular tools in human cystic echinococcosis. Curr. Drug Targets Immune Endocr. Metab. Disord. 2:235-245. [DOI] [PubMed] [Google Scholar]
- 59.Steinman, R. M. 1991. The dendritic cell system and its role in immuno genicity. Annu. Rev. Immunol. 9:271-296. [DOI] [PubMed] [Google Scholar]
- 60.Steinman, R. M. 1999. Dendritic cells, p. 547-573. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 61. Sun, J., M. Walsh, A. V. Villarino, L. Cervi, C. A. Hunter, Y. Choi, and E. J. Pearce. 2005. TLR ligands can activate dendritic cells to provide a MyD88-dependent negative signal for Th2 cell development. J. Immunol. 174:742-751. [DOI] [PubMed] [Google Scholar]
- 62.Thompson, C. B. 1995. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell 81:979-982. [DOI] [PubMed] [Google Scholar]
- 63.van der Kleij, D., E. Latz, J. F. Brouwers, Y. C. Kruize, M. Schmitz, E. A. Kurt-Jones, T. Espevik, E. C. de Jong, M. L. Kapsenberg, D. T. Golenbock, A. G. Tielens, and M. Yazdanbakhsh. 2002. A novel host-parasite lipid cross talk: schistosomal lyso-phosphatidylserine activates Toll-like receptor-2 and affects immune polarization. J. Biol. Chem. 277:48122-48129. [DOI] [PubMed] [Google Scholar]
- 64.Whelan, M., M. M. Harnett, K. M. Houston, V. Patel, W. Harnett, and K. P. Rigley. 2000. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J. Immunol. 164:6453-6460. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, W., and D. P. McManus. 2006. Recent advances in the immunology and diagnosis of echinococcosis. FEMS Immunol. Med. Microbiol. 47:24-41. [DOI] [PubMed] [Google Scholar]