Abstract
Francisella tularensis is a gram-negative facultative intracellular pathogen and the causative agent of tularemia. Little is known about the immunopathogenesis of oral infection with this pathogen. Here, for the first time, we examined the susceptibility of mice to intragastric inoculation with virulent type A F. tularensis and characterized the course of infection and the associated host responses. Both immunocompetent and immunodeficient mice were relatively susceptible to intragastric inoculation of type A F. tularensis with a 50% lethal dose (LD50) of 106 organisms, which was 100,000-fold higher than the LD100 for intradermal or respiratory routes of infection. Mice deficient in gamma interferon or tumor necrosis factor receptors 1 and 2 were more susceptible than wild-type controls to oral infection with a high dose of the pathogen. After oral inoculation, F. tularensis appeared first in the mesenteric lymph nodes (MLN) and then rapidly spread to the livers and spleens, where the organism multiplied to high numbers and induced marked neutrophilic infiltration and severe tissue necrosis. Infected mice showed rapid increases in tissue cytokine mRNA expression, which peaked in the MLN at 2 days postinfection (dpi) and in the liver and spleen at 3 dpi. The levels of gamma interferon, interleukin-1β (IL-1β), IL-6, tumor necrosis factor alpha, macrophage inflammatory protein 1α, KC, interferon-inducible protein 10, and monocyte chemotactic protein 1 were elevated from day 2 postinoculation onward. Moreover, mice intradermally immunized with the live vaccine strain of F. tularensis showed little survival advantage over naive mice after oral challenge with type A F. tularensis. These results suggest that type A F. tularensis is an effective oral pathogen that can cause fatal systemic infection and could pose a public health concern, particularly to immunocompromised individuals, if ingested in contaminated water and food.
Francisella tularensis is a gram-negative facultative intracellular pathogen and the causative agent of tularemia. The two predominant subspecies of F. tularensis (30), F. tularensis subsp. tularensis (type A F. tularensis) and F. tularensis subsp. holarctica (type B F. tularensis), are both highly infectious to humans, but only type A F. tularensis causes a life-threatening disease, especially when inhaled (10). Naturally, humans acquire the infection through arthropod bites, direct contact with infected tissues, inhalation, or ingestion of contaminated food or water.
Although aerosol- or skin-initiated infections are the most common forms of naturally occurring tularemia and aerosol infection is considered the prime choice for a bioterrorist attack (10), incidental or deliberate contamination of drinking water and the food supply could be an alternative means to initiate infection with this pathogen (2). In fact, natural outbreaks of food- and waterborne tularemia, mainly caused by the type B strain F. tularensis, have occurred recently in Europe, Russia, and the United States (15, 20, 24, 29), and gastrointestinal tularemia is a recognized, although rare, clinical form of F. tularensis infection (18). Experimentally, oral administration of 1010 CFU of virulent type A F. tularensis, strain SCHU S4, to human volunteers resulted in enlarged painful cervical lymph nodes, and all recipients required prompt antibiotic therapy to prevent further morbidity (18). The oral 50% infectious dose of strain SCHU S4 for the monkey is approximately 107 CFU (34). At a dose of 106 CFU or greater, some or all animals became infected and died if antibiotics were not promptly administered (34).
The majority of our current knowledge on the pathogenesis of F. tularensis infection has been derived from studies of mice infected with either the attenuated live vaccine strain (LVS) or virulent strains of F. tularensis by the intradermal (i.d.) or respiratory route (9, 12, 14, 26, 33, 38). Little is known about host susceptibility to and the pathogenesis of virulent F. tularensis infection initiated through the gastrointestinal tract. As the first step to further our understanding of these issues, in the present study we examined the susceptibility of immunocompetent and immunocompromised mice to intragastric inoculation with type A F. tularensis, determined the bacterial burden and associated pathology in various tissues during the course of the infection, and analyzed the cytokine or chemokine responses to the infection. In addition, we utilized this mouse model to examine the ability of the LVS immunization to protect against oral challenge with virulent type A F. tularensis.
(These findings were presented in part on 1 to 4 November 2006 at the 5th International Conference on Tularemia, Woods Hole, MA.)
MATERIALS AND METHODS
Mice.
Eight- to twelve-week-old specific-pathogen-free female C57BL/6, BALB/c, and C.B-17 SCID mice were purchased from Charles Rivers Laboratories (St. Constant, Quebec, Canada). Transgenic mice (B6.129S7-Ifngtm1 Agt (IFN-γ−/−), B6;129S-Tnfrsf1atm1 Imx Tnfrsf1btm1 Imx (TNFR1R2−/−), B6.129S2-Igh-6tm1 Cgn (B cell−/−), B6.129P2-Tcrbtm1 Mom (T cell−/−), and B6.129S7-Rag1tm1 Mom (Rag1−/−)) of similar ages were purchased from Jackson Laboratories (Bar Harbor, ME). The animals were housed under specific-pathogen-free conditions in a federally licensed small animal containment level 3 facility and given free access to sterile water and certified mouse chow. The animals were maintained and used in accordance with the recommendations of the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals, and the experimental procedures were approved by the institutional animal care committee.
F. tularensis and oral inoculation.
F. tularensis LVS (ATCC 29684) was obtained from American Type Culture Collection (Manassas, VA). Type A F. tularensis strain FSC033/snMF (strain FSC033) was originally isolated from a squirrel in Georgia (United States) (21). Strain FSC033 appeared to be subtly, and sometimes statistically, more virulent than the virulent SCHU S4 isolate in our hands when given i.d. or by the aerosol route. However, for the most part, the observed virulence differences seemed not to be biologically significant (35). For oral inoculation, thawed F. tularensis stocks were diluted in phosphate-buffered saline, and 0.5 ml of the inoculum was given to each mouse by using an 18-gauge gavage needle (5). Actual inoculum concentrations were determined by plating 10-fold serial dilutions on cystine heart agar supplemented with 1% (wt/vol) hemoglobin (9). In some experiments, mice were fasted overnight and administered 0.2 ml of 3% sodium bicarbonate to neutralize gastric acidity 10 min prior to oral inoculation.
Quantitative bacteriology and histopathology.
For bacterial kinetic analysis, groups of five mice were sacrificed by CO2 asphyxiation at day 1, 2, or 3 postinoculation (dpi). Mesenteric lymph nodes (MLN), spleens, livers, and lungs were aseptically removed and homogenized in aerosol-proof homogenizers, and the number of viable bacteria in the respective organs was determined by quantitative bacteriology (9). Additional groups of four mice were sacrificed at 0, 1, 2, and 3 dpi. The MLN, stomach, intestines, lung, liver, thymus, and spleen was removed from each animal; fixed immediately in 10% neutral buffered formalin; and processed by standard paraffin-embedding methods (Dept of Pathology and Laboratory Medicine, University of Ottawa, Ottawa, Ontario). Sections were cut 4 μm thick, stained with hematoxylin and eosin, and examined for the nature and extent of the tissue damage and inflammation by using a light microscope.
In vitro susceptibility of F. tularensis to pH.
The in vitro susceptibility of the type A and LVS strains of F. tularensis to acidity was assessed based on the method of Pasquali et al. (27). Briefly, LVS or type A F. tularensis (107 CFU/ml) was incubated in 5 ml of sterile saline. The pH was adjusted as required with 1 N HCl. All liquid cultures were incubated at 37°C for 90 min. The total number of viable bacteria in each duplicate tube was evaluated by plating aliquots on cystine heart agar supplemented with 1% (wt/vol) hemoglobin (9).
Tissue RNA extraction, quantitative reverse transcription-PCR analysis, and measurement of serum cytokines and chemokines.
For cytokine or chemokine analysis, groups of five to eight C57BL/6 mice were sacrificed 0, 1, 2, and 3 days after oral inoculation with ∼108 CFU of type A F. tularensis. Blood samples were collected for serum separation, and the spleen, liver, and MLN were dissected and immersed immediately in RNAlater (QIAGEN, Germantown, MD). The serum and the tissues were stored at −80°C until analyzed.
Relative amounts of cytokine and chemokine mRNA in the MLN, liver, and spleen over the course of infection were estimated by using a real-time PCR-based method essentially as described elsewhere (1, 22). Briefly, total RNA was extracted from tissues, and cDNA was prepared, amplified, and quantified by using primers and probes designed with the Primer3 program available at http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi. The levels of PCR product were normalized to a housekeeping gene (β2-microglobulin), and data are presented as the average of relative expression values compared to those in the corresponding tissues of mice killed at dpi 0 (1, 22).
The serum levels of cytokines and chemokines were determined by using the mouse multiplex cytokine detection system 2 and the mouse chemokine 5-Plex (Upstate, Charlottesville, VA, and BioSource, Camarillo, CA) on a Luminex 100 IS system (Luminex, Austin, TX). Serum samples (25 μl) were diluted 1:2 and analyzed as specified by the manufacturer. The analysis was done in duplicate, and the cytokine or chemokine concentrations were calculated against the standards by using Beadview software (version 1.03; Upstate).
Statistical analysis.
Data are presented as means ± the standard deviation for each group. Differences in quantitative measurements were assessed by one-way or two-way analysis of variance, followed by post hoc multiple comparison tests. Differences in nonparametric data were analyzed by the Kruskal-Wallis nonparametric analysis of variance and Mann-Whitney U test. The Fisher exact test and the chi-square test were used for comparison of categorical variables. Differences were considered significant when the P value was <0.05.
RESULTS
Susceptibility of immunocompetent mice to oral inoculation with type A F. tularensis.
To determine the relative susceptibility of mice to oral infection with type A F. tularensis, a total of 67 8- to-10-week-old female C57BL/6 mice were gavaged with various numbers of type A F. tularensis (ca. 104 to 108 CFU) in three separate experiments with or without preadministration of 3% sodium bicarbonate to neutralize gastric acidity. Survival of the inoculated mice was monitored for 14 days. Oral gavage of mice with 106 CFU of virulent type A F. tularensis resulted in slightly more than 50% mortality by day 5 after inoculation. All mice except one that received the lowest inoculum (104 CFU) survived the infection, whereas all mice that received the highest inoculum (108 CFU) died by dpi 5 (Fig. 1). A similar susceptibility was also observed in BALB/c mice (data not shown). These results suggest that the 50% lethal dose (LD50) for oral type A F. tularensis infection in mice is about 106 CFU.
FIG. 1.
Survival of C57BL/6 mice after oral inoculation with different doses of virulent type A F. tularensis. A total of 67 mice were inoculated by gavage with the highly virulent type A F. tularensis strain FSC033 at day 0, and their survival was monitored for 14 days. The data are compiled from three independent experiments with similar results.
The reason for the relatively high oral LD50 versus inhaled or intradermal LD50 for type A F. tularensis in mice is unknown but is unlikely to be due to the inhibitory effect of gastric acidity on the pathogen. Compared to other bacteria such as B. abortus and Y. pestis where viability reduced substantially at pH 3.8 (4, 27), both type A F. tularensis and LVS are relatively resistant to acid stress in vitro in that there was no substantial reduction in the bacterial viability of either strain of the pathogen at pH 3 (Table 1) . This tolerance to acidified conditions is in agreement with our findings that neutralization of gastric acidity with 3% sodium bicarbonate does not increase the susceptibility of mice to the infection and the fact that viable F. tularensis can be recovered from the gastric juices of patients with tularemia (18, 34). These results imply that most orally inoculated F. tularensis organisms failed to overcome other natural defenses of the gastrointestinal barrier.
TABLE 1.
Susceptibility of type A F. tularensis and F. tularensis LVS to acid conditions in vitroa
| F. tularensis strain | Avg log10 CFU ± SD at pH:
|
||||||
|---|---|---|---|---|---|---|---|
| 7 | 6 | 5 | 4 | 3.5 | 3 | 2.5 | |
| Type A (FSC033) | 7.07 ± 0.06 | 7.12 ± 0.05 | 7.02 ± 0.03 | 7.07 ± 0.06 | 6.76 ± 0.03 | 6.72 ± 0.03 | 6.27 ± 0.38 |
| LVS | 7.33 ± 0.03 | 7.30 ± 0.02 | 7.29 ± 0.08 | 7.10 ± 0.08 | 7.32 ± 0.04 | 7.01 ± 0.01 | <5.70 |
Five milliliters of sterile saline was inoculated with 107 CFU of type A F. tularensis strain FSC033 or LVS. After 90 min of incubation at 37°C, aliquots of different suspensions were plated to assess the viability of bacteria. Data are presented as the average of duplicate cultures.
Susceptibility of immunocompromised mice to oral inoculation with type A F. tularensis.
We next determined whether immunocompromised mice are more susceptible to oral inoculation with type A F. tularensis than are immunocompetent mice. Groups of mice lacking functional T and B lymphocytes (SCID), B cells (Igh−/−), IFN-γ (IFN-γ−/−), or TNF receptors 1 and 2 (TNFR1R2−/−), as well as wild-type (WT) C57BL/6, and their survival rates and median times to irreversible moribundity (MTM) were monitored. As shown in Fig. 2A, mice with various global or defined immunodeficiencies displayed similar susceptibilities to oral inoculation with this dose of type A F. tularensis (P > 0.05).
FIG. 2.
Survival of immunocompromised mice after oral inoculation with a sublethal (A) or lethal (B) dose of type A F. tularensis. Groups of immunodeficient or WT control C57BL/6 (B6) mice (n = 5 to 10) were inoculated by gavage with 5 × 105 CFU (sublethal) or 5 × 107 CFU (lethal) of the highly virulent type A F. tularensis strain FSC033 at day 0, and their survival was monitored for 15 days.
It has been previously reported that increased susceptibility of immunodeficient mice to certain bacterial pathogens may only be apparent when a high inoculum is used (6, 28, 32). To examine this possibility, groups of 10 mice deficient in B cells, IFN-γ, Rag-1, TCR-β, or TNFR1R2, as well as WT control mice, were gavaged with 5 × 107 CFU (∼50 LD50) of type A F. tularensis, and their survival rate and MTM were compared (Fig. 2B). In contrast to WT mice, which showed an MTM of 5 days, mice with various immunodeficiencies had a shortened MTM (3 to 4 days). In particular, mice deficient in IFN-γ or TNFR1R2 became sick more rapidly and all IFN-γ−/− mice and 70% TNFR1R2−/− mice died of infection by dpi 3 (Fig. 2B). These results suggest that IFN-γ and TNF signaling play only a subtle role in host resistance against orally initiated tularemia.
Course of oral type A F. tularensis infection in mice.
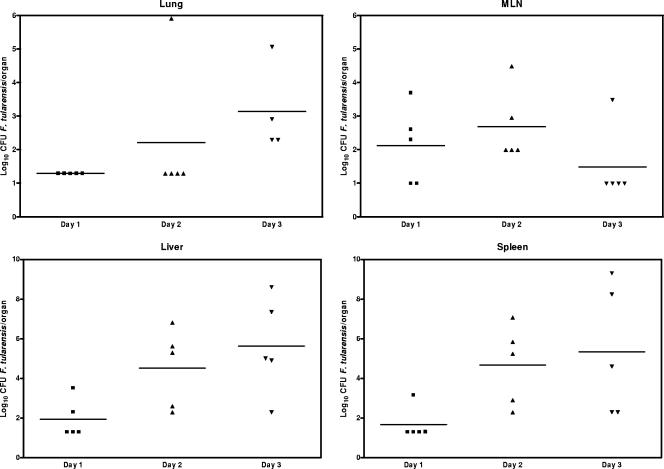
As the first step toward a better understanding of the pathogenesis of oral infection with virulent type A F. tularensis, we determined the bacterial burdens and examined the associated pathology in different organs of C57BL/6 mice gavaged with 108 CFU of type A F. tularensis over the course of infection. As shown in Fig. 3, small numbers of F. tularensis were cultured from the MLN, spleen, and liver at dpi 1. The bacterial numbers continued to increase significantly (P < 0.05) in the liver and spleen between dpi 2 and 3 and reached log10 9.0 in some of the infected mice by dpi 3. In contrast, the number of bacteria in the MLN increased more slowly than in the spleen and liver, peaked at dpi 2, and then fell to undetectable levels in most of the mice by dpi 3. F. tularensis was not cultured from the lung at dpi 1, was present in one mouse at dpi 2, and was only detected occasionally in small numbers at dpi 3 (Fig. 3).
FIG. 3.
Bacterial burdens in the MLN, livers, spleens, and lungs of mice inoculated by gavage with 108 CFU of type A F. tularensis strain FSC033. Each symbol represents the log10 of the viable count of an individual mouse. Horizontal lines indicate the mean of each group of mice on the indicated postinoculation days. The detection limits for the bacterial burdens were 0.9, 1.2, 2.2, and 2.2 log10 CFU/organ for the MLN, lung, liver, and spleen, respectively.
Pathology of orally initiated tularemia.
The pathological changes seen in the different tissue organs after oral type A F. tularensis infection were consistent with the kinetics of bacterial dissemination and growth. Apparent gross changes in the liver, spleen, and MLN were not seen until dpi 3. By this time, the liver was pale yellowish in color with a moderately friable and flaccid texture. The MLN showed slight to moderate decrease in size. The spleens from some mice showed multiple white-gray foci on their surface but remained normal in size. The lungs showed no remarkable changes and remained floating in the fixative even at dpi 3. Similarly, there was no gross change in the stomach, kidney, thymus, or small or large intestine.
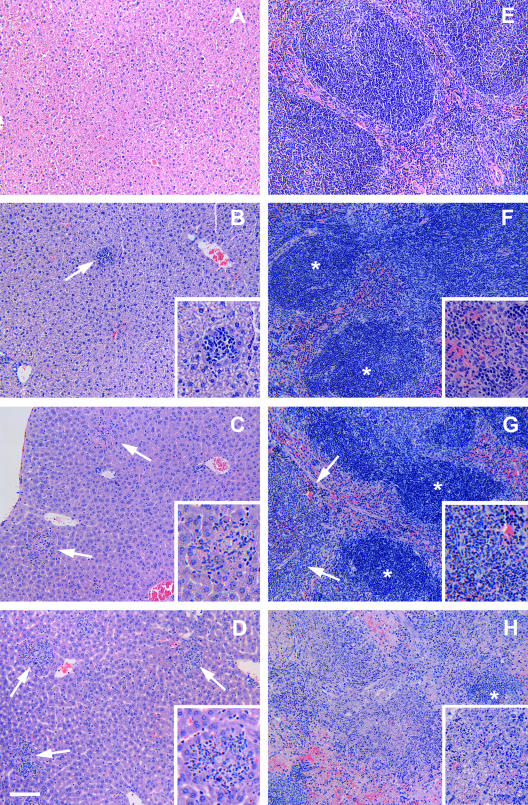
As expected, all of the tissues collected from mice killed at dpi 0 showed normal histological appearances (Table 2 and Fig. 4A and E). However, the histopathological changes in the liver and spleen were evident in some mice as early as dpi 1 and consisted of single or multiple, randomly distributed small foci of inflammatory infiltrates with mixed mononuclear cells and a few neutrophils (Fig. 4B). The number and size of these inflammatory foci increased substantially on dpi 2 and 3. At dpi 2, there were multiple inflammatory foci of medium size throughout the section with the occasional presence of necrotic hepatocytes (Fig. 4C). The hepatocytes immediately adjacent to the infiltrates were usually moderately swollen with a basophilic cytoplasm. There was also an increase in the number and size of Kupffer cells in some areas. Occasionally, the portal areas were infiltrated with small to moderate numbers of macrophages and lymphocytes. By dpi 3, livers from most mice showed multiple large foci or areas of inflammation with a poor demarcation from the adjacent tissue and hepatic necrosis (Fig. 4D).
TABLE 2.
Histological examination of livers and spleens from mice orally inoculated with virulent type A F. tularensisa
| Time point (dpi) | Histopathological changes in
|
|
|---|---|---|
| Liverb | Spleenc | |
| 0 | 0, 0, 0, 0d | 0, 0, 0, 0 |
| 1 | 1, 1, 0, 1 | 1, 1, 0, 1 |
| 2 | 2, 1, 2, 2 | 3, 2, 2, 2 |
| 3 | 3, 1, 3, 3 | 3, 4, 2, 4 |
The oral inoculum was 108 CFU of type A F. tularensis strain FSC033 per mouse.
Liver lesion: 0, no remarkable change; 1, small numbers of focal inflammatory infiltrates with occasional degeneration and necrosis of individual hepatocytes; 2, moderate numbers of small to medium-size inflammatory infiltrates with occasional degeneration and necrosis of individual or clusters of hepatocytes; 3, moderate numbers of large inflammatory infiltrates with aggregates of hepatocyte necrosis; 4, extensive infiltration with large numbers of inflammatory cells and large areas of hepatocyte necrosis with loss of normal liver anatomic architecture.
Spleen lesion: 0, no remarkable change; 1, occasional inflammatory infiltrates in the junction between white and red pulp region; 2, small numbers of inflammatory infiltrates in the white pulp without loss of normal lymphoid follicle structure; 3, severe inflammatory infiltrates in the white pulp and medulla with some loss of lymphoid follicle structure; 4, extensive inflammatory infiltration with severe necrosis of lymphocytes and loss of normal spleen anatomic architecture.
Individual scores are given for liver or spleen samples of four mice for each time point.
FIG. 4.
Histopathological findings of the livers (A to D) and spleens (E to H) of mice killed at 0, 1, 2, and 3 days after inoculation by gavage with 108 CFU of type A F. tularensis strain FSC033. The liver (A) and spleen (E) from a mouse killed at dpi 0 showed normal histological appearance. Mild, focal monocytic, and neutrophilic infiltration (arrow) was observed in the liver (B) and spleen (F) of a mouse killed at dpi 1. (C) At dpi 2, marked inflammatory infiltration and necrosis of individual hepatocytes (arrows) was observed in the liver. (G) The spleen showed moderate infiltration of neutrophils in the red pulp regions (arrows) as well as within some lymphoid follicles. (D and H) At dpi 3, the liver showed multiple foci and areas of inflammation (arrows) (D), and the spleen showed the severe destruction of lymphoid tissues and neutrophil infiltration, with only the remnants of normal lymphoid follicles (*) evident in some severely affected areas (H). *, Lymphoid follicles. Hematoxylin and eosin staining was used. Bar, 40 μm for the main images and 20 μm for all of the inset images.
The spleen showed mild expansion of the red pulp together with accumulations of small numbers of neutrophils and macrophages at dpi 1 (Fig. 4F). The severity of spleen changes varied in the mice killed at dpi 2, ranging from an increased number of neutrophils in the red pulp to a moderate infiltration of neutrophils in the white pulp, lymphocyte necrosis, and the destruction of entire lymphoid follicles (Fig. 4G). By dpi 3, the spleen showed multifocal infiltrations of neutrophils admixed with large numbers of degenerated and necrotic lymphoid cells in the red pulp and the peripheral areas of the white pulp. In severely affected areas, adjacent necrotic areas became coalescing and resulted in the loss of entire lymphoid follicles (Fig. 4H).
The MLN showed only mild infiltration of small numbers of neutrophils in the medulla with little or no involvement of lymphoid follicles at dpi 1 and 2 (Fig. 5A). By dpi 3, the MLN showed moderately severe depletion of lymphocytes and focal infiltration of neutrophils throughout the medullar regions and the destruction of lymphoid follicles in some areas (Fig. 5B).
FIG. 5.
Histopathological findings of the MLN (A and B), lungs (C), and small intestines (D) of mice killed at different days after inoculation by gavage with 108 CFU of type A F. tularensis strain FSC033. (A) The MLN from a mouse killed at dpi 2 shows an the almost-normal appearance with only occasional accumulation of neutrophils in small venules (inset). (B) The MLN from a mouse killed at dpi 3 shows the severe neutrophil infiltration and lymphocyte necrosis in the medullar region, whereas the lymphoid follicles (*) in the cortical region were relatively unaffected. (C) The lung from a mouse killed at dpi 3 shows almost-normal architecture with only mild nonsuppurative pneumonitis. (D) The ileum from a mouse killed at dpi 3 shows the mild infiltration of mononuclear cells in the lamina propria and the presence of occasional necrotic neutrophils in the crypt lumen. Hematoxylin and eosin staining was used. Bar = 40 μm for the main images and 10 μm for the inset image.
The lung showed no remarkable change at dpi 1 and 2 and only mild, focal infiltration of neutrophils and hypercellularity of alveolar septa at dpi 3 (Fig. 5C). There was surprisingly little change in the stomach and intestine except for the presence of small numbers of necrotic neutrophils in the crypt epithelium in some mice killed at dpi 3 (Fig. 5D). The stomach and thymus showed only incidental change consisting of submucosal gastritis and thymus atrophy and thymocyte necrosis, respectively, in one mouse killed at dpi 3.
Cytokine and chemokine responses after oral infection with type A F. tularensis.
mRNA expression of selected inflammatory chemokines and cytokines was assessed by real-time reverse transcription-PCR in the MLN, liver, and spleen samples of the mice killed at dpi 0, 1, 2, and 3 (Table 3). Among the tested cytokines and chemokines, gamma interferon (IFN-γ), interleukin-1β (IL-1β), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and interferon-inducible protein 10 (IP-10) mRNA expression gradually increased in the spleen over the course of infection and peaked at dpi 3. IL-6, MIP-2, and monocyte chemotactic protein 1 (MCP-1) mRNA expression increased significantly and peaked at dpi 2 and remained elevated at dpi 3, whereas tumor necrosis factor alpha (TNF-α) mRNA expression did not significantly increase until dpi 3. The IFN-γ, MIP-1α, MIP-1β, TNF-α, and IP-10 mRNA expression profiles in the liver were more or less similar to those in the spleen.However, IL-1β, IL-6, MIP-2, and MCP-1 mRNA expression peaked at dpi 1 and declined gradually on dpi 2 and 3. In contrast, the expression pattern and kinetics of cytokine or chemokine mRNA in the MLN were substantially different from those in the liver and spleen. In this tissue, the expression of most cytokine and chemokine mRNA peaked at dpi 2 and subsided, although remaining elevated, at dpi 3 (Table 3).
TABLE 3.
Relative levels of proinflammatory chemokine and cytokine mRNA expression in mice orally inoculated with type A F. tularensisa
| Chemokine or cytokine | Avg relative expression (range) ± SD in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spleen
|
Liver
|
MLN
|
|||||||
| 1 dpi | 2 dpi | 3 dpi | 1 dpi | 2 dpi | 3 dpi | 1 dpi | 2 dpi | 3 dpi | |
| IFN-γ | 4.20 (3.1-5.8) | 12.38 (5.0-30.9) | 129.8 (56.1-300.3) | 0.62 (0.3-1.4) | 1.68 (0.5-5.3) | 4.44 (0.2-128.9) | 0.97 (0.6-1.6) | 7.84 (2.3-26.5) | 4.96 (1.2-25.3) |
| IL-1β | 2.79 (1.3-6.1) | 8.46 (7.1-10.1) | 16.11 (9.3-27.9) | 4.53 (1.8-11.2) | 1.27 (0.2-10.3) | 1.32 (0.4-4.2) | 5.66 (0.7-46.9) | 72 (10.0-519.1) | 1.62 (0.8-3.5) |
| IL-6 | 5.35 (1.0-27.5) | 28.84 (26.4-31.6) | 26.72 (10.4-68.6) | 3.2 (1.9-5.5) | 0.68 (0.1-7.9) | 0.12 (0.01-1.3) | 10.6 (0.7-153.28) | 259.6 (15.3-4389.9) | 4.79 (1.8-12.8) |
| TNF-α | 1.28 (1.0-1.6) | 1.64 (1.1-2.4) | 12.38 (7.4-20.7) | 1.2 (0.6-2.2) | 3.48 (0.8-14.3) | 3.56 (0.3-40.5) | 0.98 (0.7-0.4) | 1.11 (0.4-1.9) | 0.52 (0.4-0.7) |
| MIP-1α | 1.34 (1.0-1.8) | 7.21 (3.6-14.3) | 70.03 (51.3-95.7) | 1.16 (0.7-1.9) | 2.71 (0.9-8.6) | 4.44 (0.3-63.6) | 1.11 (0.5-2.3) | 5.06 (1.1-23.6) | 1.28 (0.7-2.4) |
| MIP-1β | 1.12 (0.7-1.8) | 2.17 (1.4-3.4) | 12.73 (3.8-43.1) | 1.61 (1.0-2.6) | 1.79 (0.9-3.6) | 3.36 (0.5-23.8) | 1.46 (0.6-3.4) | 4.66 (2.8-7.8) | 1.74 (1.0-3.18) |
| MIP-2 | 1.12 (0.5-2.4) | 11.63 (8.9-15.2) | 14.32 (9.8-19.6) | 2.39 (1.6-3.6) | 1.21 (0.7-1.9) | 0.38 (0.1-1.5) | 8.22 (0.5-145.0) | 34.54 (4.5-268.7) | 1.69 (0.7-3.97) |
| IP-10 | 2.38 (1.5-3.7) | 2.30 (1.1-6.1) | 17.51 (3.8-80.4) | 1.49 (0.7-3.1) | 13.27 (1.0-181.0) | 7.01 (0.4-122.8) | 0.84 (0.6-1.2) | 2.27 (0.4-13.5) | 5.54 (1.9-15.7) |
| MCP-1 | 1.55 (0.7-3.5) | 15.35 (12.1-19.4) | 18 (12.0-27.1) | 2.45 (1.6-3.8) | 0.43 (0.04-4.1) | 0.14 (0.02-1.12) | 3.56 (0.2-72.5) | 25.3 (2.6-245.6) | 1.42 (0.8-2.5) |
Groups of mice (n = 5) were inoculated by gavage with 2 × 107 CFU of type A F. tularensis strain FSC033 at day 0, and the MLN, liver, and spleen were collected from each animal at 0, 1, 2, and 3 dpi. The relative levels of cytokine and chemokine mRNA expression were determined by real-time PCR analysis as described in Materials and Methods. Mouse β2-microglobulin RNA was measured and used to calculate relative expression using the formula Rel Exp = 2−ΔΔCt. The results shown are the average of relative expression values determined using cDNA from three mice, with ranges indicated in parentheses.
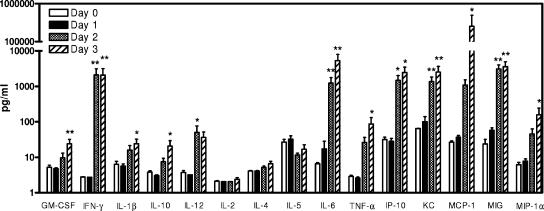
In addition to tissue cytokine or chemokine mRNA levels, the serum levels of a panel of ten cytokines and five chemokines were determined over the course of the infection. Compared to dpi 0, there was little, if any, detectable change in the serum levels of these cytokines or chemokines at dpi 1 (Fig. 6). As observed with the tissue mRNA expression patterns, the serum levels of IFN-γ, IL-6, TNF-α, IL-12, IP-10, MIG, and MCP-1 were significantly increased; the KC, MIP-1α, IL-1β, and IL-10 levels were moderately increased at dpi 2; and most of these cytokines or chemokines maintained similar levels at dpi 3 except IL-6 and MCP-1, which increased further. On the other hand, there was little or no change in the serum IL-2, IL-4, IL-5, or granulocyte-macrophage colony-stimulating factor level throughout the course of the infection.
FIG. 6.
Cytokine and chemokine levels in sera from mice orally inoculated with type A F. tularensis. Groups of C57BL/6 mice were orally gavaged with 2 × 107 CFU type A F. tularensis strain FSC033 on day 0, and blood samples were collected at dpi 0, 1, 2, and 3. Cytokine and chemokine levels in the serum were determined by using the Beadlyte mouse multiplex cytokine detection system 2 and mouse chemokine 5-Plex assay kits on a Luminex 100 IS instrument. The data are expressed as means ± the standard deviations of eight mice at each time point. The detection limits of the assays were 2.5 to 5 pg/ml. *, P < 0.05; **, P < 0.01 (versus dpi 0).
Intradermal LVS immunization provides poor protection against oral infection with virulent type A F. tularensis in mice.
Previous studies from several laboratories including ours have shown that i.d. immunization of mice with LVS protects them against subsequent i.d. challenge with virulent type A F. tularensis but is less effective against respiratory challenge (7, 38). Therefore, we were interested to determine whether this vaccination strategy would be effective against orally initiated tularemia. To this end, groups of BALB/c and C57BL/6 mice were immunized i.d. with 2 × 105 CFU LVS on day 0 (7) and challenged by gavage with 108 CFU type A F. tularensis 4 weeks later. As can be seen in Fig. 7, i. d. immunization of mice with LVS failed to protect against an oral infection with virulent type A F. tularensis since immunized mice showed no substantial survival advantage over unvaccinated naive mice after the challenge, despite the fact that these mice were fully protected against i.d. challenge with 103 CFU (approximately 100 LD100) type A F. tularensis (data not shown).
FIG. 7.
Lack of protection against oral challenge with type A F. tularensis in mice i.d. immunized with LVS. Groups of C57BL/6 and BALB/c mice were i.d. immunized with approximately 2 × 105 CFU of F. tularensis LVS (n = 10) or sham immunized (n = 5) at day 0. Mice were challenged orally with 108 CFU type A F. tularensis strain FSC033 4 weeks after immunization, and their survival and MTM were monitored.
DISCUSSION
F. tularensis has been shown to be extremely lethal to mammals (including mice and humans) by skin and respiratory routes of exposure, but little is known about the potential of F. tularensis as an oral pathogen. Hornick et al. (18) and Tulis (34) showed that monkeys and humans are susceptible to oral challenge with type A F. tularensis strain SCHU S4 (18, 34). In the present study, for the first time, we described and characterized a mouse model of oral infection with another highly virulent type A strain of F. tularensis (strain FSC033). Our results suggest that the LD50 for oral type A F. tularensis infection in mice is about 106 CFU. This is about 100,000-fold higher than the LD100 for this organism administered i.d. or via respiratory routes (9, 38). Nevertheless, F. tularensis is more virulent for mice via the oral route than many other common gastrointestinal pathogens such as Yersinia enterocolitica, Escherichia coli O157:H7, Listeria monocytogenes, Salmonella enterica serovar Typhimurium, and Brucella abortus, the oral LD50 of which are generally higher than 107 CFU (16, 23, 25, 27, 36). On the other hand, the oral LD50 for type A F. tularensis in mice is very similar to that of Y. pestis, another gram-negative, intracellular pathogen with high virulence by systemic or respiratory routes of inoculation (4).
The present study has shown that there was minimal change in the intestinal mucosa during the course of oral F. tularensis infection, and the changes seen in the intestinal epithelium, Peyer's patches, and MLN were comparable to those seen in mice inoculated by respiratory or i.d. route (19, 38; W. Chen and J. W. Conlan, unpublished data). Compared to aerosol infection, changes in the lungs of mice after oral inoculation with type A F. tularensis were relatively minimal (9). In particular, there was no bacterial colonization, cellular and fibrinous exudates in the alveolar space or airway lumens, or dilatation of the perivascular spaces. The changes in the liver, and particularly in the spleen, were evident somewhat earlier in orally infected mice than in mice infected by aerosol with the same strain of the pathogen.
The lack of tissue damage and inflammatory response in the gastrointestinal tract in the early stages of orally initiated tularemia supports the possibility that it is the extraintestinal bacterial dissemination and resulting damage to systemic tissues, rather than local enteric infection, that contribute to the associated morbidity and mortality. Indeed, in contrast to the pathology associated with other oral bacterial infections in mice (16), which produces acute inflammation and necrosis in the intestinal mucosa and its associated lymphoid tissues, F. tularensis produced minimal local gastrointestinal pathology. Mice showed no clinical signs of acute enteritis, and the MLN revealed little change at dpi 1 to 2. Instead, orally inoculated mice showed histopathological changes typical of overwhelming systemic infection, consisting of heavy infection and acute inflammation and necrosis of the spleen and liver. The lesions in these tissues were identical to those seen in mice infected by the systemic and respiratory route (9, 13, 38). Thus, the gastrointestinal tract, like the lungs and skin, acts primarily as a portal of entry for F. tularensis rather than as the critical site of infection. In this regard, we have previously speculated that, in aerosol-initiated F. tularensis infection, it is the disseminated rather than the pulmonary infection that kills the host (9). Taken together, these data would postulate a single mechanism by which this pathogen causes disease, regardless of the route of infection. However, this postulation does not explain the observation that mice intradermally immunized with F. tularensis LVS can control i.d. challenge with up to 1,000 CFU type A F. tularensis but struggle to contain a 100-fold smaller aerosol inoculum, despite the fact that the LD100 for naive mice is similar by either route (7), suggesting that different immune responses are required to combat systemically initiated versus inhalation-initiated tularemia. It is possible that, in the immunization challenge model, the immune responses elicited by LVS vaccination might be less well expressed in the lungs than elsewhere or be less able to combat pulmonary versus systemic tularemia.
Based on the knowledge from other well-studied oral bacterial infection models, orally administered bacteria may disseminate to systemic tissues from the intestinal lumen through one of the following avenues: use of M cells and epithelial cells as a portal of entry with or without the colonization of intestinal lymphoid tissues (such as Peyer's patches and MLN) (3, 17) or transport by CD18-expressing phagocytes directly across the epithelial layer (37). Although it is not clear from the present study exactly how F. tularensis crossed the intestinal epithelium to cause fatal systemic infection, the presence of relatively low numbers of bacteria in the MLN during the course of infection and large numbers of the pathogen in the liver and spleen at dpi 2 and 3 also suggest that F. tularensis introduced by the oral route traversed the gastrointestinal tract and proceeded to infect systemic organs with very little local bacterial replication. In addition, the absence of F. tularensis in the lung during the first 2 days rules out incidental inhalation or aspiration of the gavage inoculum as the mode of infection.
The magnitude of mRNA expression and cytokine levels was similar in pattern to that of the bacterial burdens in the tissue, which peaked in the MLN at dpi 2 and in the liver and spleen at dpi 3. Overall, the cytokine and chemokine profiles seen in orally infected mice were similar to those reported after systemic or aerosol infection with virulent type A. F. tularensis and the attenuated LVS strain (1, 8, 11, 14, 31). For the latter organism, early production of several of these cytokines by cells of the innate immune system, particularly TNF-α, IFN-γ, and IL-12, has been shown to be critical for effectively combating primary infection (11, 31). In contrast, we and others have previously shown that mice that produce these cytokines in response to low-dose systemic or aerosol challenge with type A F. tularensis still rapidly succumb to the ensuing infection (1, 9, 14, 38). In other words, virulent strains of F. tularensis that infect via the skin or lungs thrive despite a vigorous innate immune response by the murine host. This also appears to be the case when the pathogen enters the host via the oral route. However, it is interesting that IFN-γ−/− mice died sooner than WT mice when a high dose of the pathogen was used (Fig. 2), indicating a role for this cytokine in early protection, whereas in WT mice the significant increase in the mRNA expression and production of IFN-γ on dpi 2 and 3 suggest that overproduction of IFN-γ after infection contribute to the subsequent death of the animal. These findings suggest that IFN-γ may play a divergent, although subtle, role in the pathogenesis of oral infection with virulent type A F. tularensis.
It is worth noting that, under natural circumstances, the oropharynx is probably a major interface of the pathogen with the host in oral F. tularensis infection. However, it is not practical to simply feed mice with type A F. tularensis without the risk of concomitant respiratory tract infection given the fact that the LD100 for the latter route is approximately 10 CFU (9, 38). To avoid this, the F. tularensis inoculum was delivered by gavage in the present study, which bypassed the oropharynx region. Thus, the mouse model described probably does not represent all features of human oral F. tularensis infection but rather those that occur once the pathogen passes beyond the oropharynx. Nevertheless, this mouse model will be a useful tool for future studies on the immunopathogenesis of orally initiated tularemia and for the development and evaluation of prophylactic and therapeutic agents against this form of the disease.
Acknowledgments
This study was supported by the U.S. National Institutes of Health (R21AI59064) and by the National Research Council Canada.
We thank Tom Devecseri for assistance in the preparation of the photomicrography.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Andersson, H., B. Hartmanova, R. Kuolee, P. Ryden, W. Conlan, W. Chen, and A. Sjostedt. 2006. Transcriptional profiling of host responses in mouse lungs following aerosol infection with type A Francisella tularensis. J. Med. Microbiol. 55:263-271. [DOI] [PubMed] [Google Scholar]
- 2.Atlas, R. M. 2002. Bioterrorism: from threat to reality. Annu. Rev. Microbiol. 56:167-185. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, P. D., M. A. Bergman, J. Mecsas, and R. R. Isberg. 2006. Yersinia pseudotuberculosis disseminates directly from a replicating bacterial pool in the intestine. J. Exp. Med. 203:1591-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler, T., Y. S. Fu, L. Furman, C. Almeida, and A. Almeida. 1982. Experimental Yersinia pestis infection in rodents after intragastric inoculation and ingestion of bacteria. Infect. Immun. 36:1160-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, W., J. A. Harp, A. G. Harmsen, and E. A. Havell. 1993. Gamma interferon functions in resistance to Cryptosporidium parvum infection in severe combined immunodeficient mice. Infect. Immun. 61:3548-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, W., R. Kuolee, H. Shen, M. Busa, and J. W. Conlan. 2005. Toll-like receptor 4 (TLR4) plays a relatively minor role in murine defense against primary intradermal infection with Francisella tularensis LVS. Immunol. Lett. 97:151-154. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., H. Shen, A. Webb, R. KuoLee, and J. W. Conlan. 2003. Tularemia in BALB/c and C57BL/6 mice vaccinated with Francisella tularensis LVS and challenged intradermally or by aerosol with virulent isolates of the pathogen: protection varies depending on pathogen virulence, route of exposure, and host genetic background. Vaccine 21:3690-3700. [DOI] [PubMed] [Google Scholar]
- 8.Cole, L. E., K. L. Elkins, S. M. Michalek, N. Qureshi, L. J. Eaton, P. Rallabhandi, N. Cuesta, and S. N. Vogel. 2006. Immunologic consequences of Francisella tularensis live vaccine strain infection: role of the innate immune response in infection and immunity. J. Immunol. 176:6888-6899. [DOI] [PubMed] [Google Scholar]
- 9.Conlan, J. W., W. Chen, H. Shen, A. Webb, and R. KuoLee. 2003. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 34:239-248. [DOI] [PubMed] [Google Scholar]
- 10.Dennis, D. T., T. V. Inglesby, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, M. Layton, S. R. Lillibridge, J. E. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2001. Tularemia as a biological weapon: medical and public health management. JAMA 285:2763-2773. [DOI] [PubMed] [Google Scholar]
- 11.Elkins, K. L., A. Cooper, S. M. Colombini, S. C. Cowley, and T. L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12p70. Infect. Immun. 70:1936-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins, K. L., S. C. Cowley, and C. M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135-142. [DOI] [PubMed] [Google Scholar]
- 13.Fortier, A. H., M. V. Slayter, R. Ziemba, M. S. Meltzer, and C. A. Nacy. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golovliov, I., K. Kuoppa, A. Sjostedt, A. Tarnvik, and G. Sandstrom. 1996. Cytokine expression in the liver of mice infected with a highly virulent strain of Francisella tularensis. FEMS Immunol. Med. Microbiol. 13:239-244. [DOI] [PubMed] [Google Scholar]
- 15.Greco, D., G. Allegrini, T. Tizzi, E. Ninu, A. Lamanna, and S. Luzi. 1987. A waterborne tularemia outbreak. Eur. J. Epidemiol. 3:35-38. [DOI] [PubMed] [Google Scholar]
- 16.Handley, S. A., P. H. Dube, P. A. Revell, and V. L. Miller. 2004. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect. Immun. 72:1645-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Handley, S. A., R. D. Newberry, and V. L. Miller. 2005. Yersinia enterocolitica invasin-dependent and invasin-independent mechanisms of systemic dissemination. Infect. Immun. 73:8453-8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornick, R. B., A. T. Dawkins, H. T. Eigelsbach, and J. J. Tulis. 1967. Oral tularemia vaccine in man. Antimicrob. Agents Chemother. 1966:11-14. [DOI] [PubMed] [Google Scholar]
- 19.Ito, M., K. Nishiyama, S. Hyodo, S. Shigeta, and T. Ito. 1985. Weight reduction of thymus and depletion of lymphocytes of T-dependent areas in peripheral lymphoid tissues of mice infected with Francisella tularensis. Infect. Immun. 49:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jellison, W. L., D. C. Epler, E. Kuhns, and G. M. Kohls. 1950. Tularemia in man from a domestic rural water supply. Public Health Rep. 65:1219-1226. [PubMed] [Google Scholar]
- 21.Johansson, A., A. Ibrahim, I. Goransson, U. Eriksson, D. Gurycova, J. E. Clarridge III, and A. Sjostedt. 2000. Evaluation of PCR-based methods for discrimination of Francisella species and subspecies and development of a specific PCR that distinguishes the two major subspecies of Francisella tularensis. J. Clin. Microbiol. 38:4180-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, L. L., K. N. Berggren, F. M. Szaba, W. Chen, and S. T. Smiley. 2003. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 197:801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judge, N. A., H. S. Mason, and A. D. O'Brien. 2004. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect. Immun. 72:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manenkova, G. M., L. V. Rodina, L. A. Tsvil, and P. Solodovnikov Iu. 1996. A milk-borne outbreak of tularemia in Moscow. Zh. Mikrobiol. Epidemiol. Immunobiol. 5:123-124. (In Russian.) [PubMed] [Google Scholar]
- 25.Okamoto, M., A. Nakane, and T. Minagawa. 1994. Host resistance to an intragastric infection with Listeria monocytogenes in mice depends on cellular immunity and intestinal bacterial flora. Infect. Immun. 62:3080-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oyston, P. C., A. Sjostedt, and R. W. Titball. 2004. Tularaemia: bioterrorism defense renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2:967-978. [DOI] [PubMed] [Google Scholar]
- 27.Pasquali, P., A. Rosanna, C. Pistoia, P. Petrucci, and F. Ciuchini. 2003. Brucella abortus RB51 induces protection in mice orally infected with the virulent strain B. abortus 2308. Infect. Immun. 71:2326-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 29.Reintjes, R., I. Dedushaj, A. Gjini, T. R. Jorgensen, B. Cotter, A. Lieftucht, F. D'Ancona, D. T. Dennis, M. A. Kosoy, G. Mulliqi-Osmani, R. Grunow, A. Kalaveshi, L. Gashi, and I. Humolli. 2002. Tularemia outbreak investigation in Kosovo: case control and environmental studies. Emerg. Infect. Dis. 8:69-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjostedt, A., and D. J. Brenner. 2002. Genus 1. Francisella Dorofe'ev 1947, 176AL, p. 200-210. In Bergey's manual of systematic bacteriology, vol. 2, part B, 2nd ed. Springer-Verlag, New York, N.Y. [Google Scholar]
- 31.Sjostedt, A., R. J. North, and J. W. Conlan. 1996. The requirement of tumour necrosis factor-alpha and interferon-gamma for the expression of protective immunity to secondary murine tularaemia depends on the size of the challenge inoculum. Microbiology 142:1369-1374. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 33.Tarnvik, A., and L. Berglund. 2003. Tularaemia. Eur. Respir. J. 21:361-373. [DOI] [PubMed] [Google Scholar]
- 34.Tulis, J. J., H. T. Eigelsbach, and R. B. Hornick. 1969. Oral vaccination against tularemia in the monkeys. Proc. Soc. Exp. Biol. Med. 132:893-897. [DOI] [PubMed] [Google Scholar]
- 35.Twine, S. M., H. Shen, J. F. Kelly, W. Chen, A. Sjostedt, and J. W. Conlan. 2006. Virulence comparison in mice of distinct isolates of type A Francisella tularensis. Microb. Pathog. 40:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uren, T. K., O. L. Wijburg, C. Simmons, F. E. Johansen, P. Brandtzaeg, and R. A. Strugnell. 2005. Vaccine-induced protection against gastrointestinal bacterial infections in the absence of secretory antibodies. Eur. J. Immunol. 35:180-188. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 38.Wu, T. H., J. A. Hutt, K. A. Garrison, L. S. Berliba, Y. Zhou, and C. R. Lyons. 2005. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect. Immun. 73:2644-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]