Abstract
Environmental shedding of genetically manipulated microorganisms is an issue impeding the development of new live vaccines. We have investigated the immunogenicity of a number of novel Salmonella enterica serotype Typhimurium oral vaccine candidates that express the fragment C (TetC) component of tetanus toxin and harbor combinations of additional mutations in genes shdA, misL, and ratB that contribute to the persistence of serotype Typhimurium's colonization of the intestine. Serotype Typhimurium aroA (TetC) derivatives harboring additional mutations in either shdA or misL or combinations of these mutations exhibited a marked decrease in shedding of the vaccine strain in the feces of orally vaccinated mice. However, equivalent levels of anti-TetC and anti-Salmonella lipopolysaccharide immunoglobulin G (IgG), IgG1, IgG2a, and IgA were detected in sera of the vaccinated but not of the control mice. Cellular immune responses to TetC were detected in all vaccinated mice, regardless of the presence of the additional mutations in shdA or misL. Further, immunization with serotype Typhimurium aroA candidate vaccines harboring shdA and misL afforded complete protection against challenge with a virulent strain of serotype Typhimurium.
Genetically manipulated, attenuated microorganisms are emerging as candidates for the development of live vaccines. However, there are health and safety concerns associated with the release of genetically modified microorganisms into the environment (15). Ideally, it is desirable to limit the number of genetically modified microorganisms entering the environment, without decreasing vaccine immunogenicity or efficacy. Derivatives of Salmonella enterica that express heterologous antigens are promising candidate polyvalent live oral vaccines. The degree to which viable Salmonella cells are shed with feces after oral inoculation varies considerably between different Salmonella serotypes. S. enterica serotype Typhi (serotype Typhi) and S. enterica serotype Typhimurium cause diseases in humans that represent two ends of the spectrum in this respect. Serotype Typhi is the causative agent of typhoid fever, a syndrome characterized by systemic spread of the microorganism (20). A licensed live oral typhoid vaccine, serovar Typhi Ty21a, is shed in the stools of most vaccinees for 1 to 4 days (21). The results of volunteer studies with novel live oral serovar Typhi vaccine candidates, such as a phoP-phoQ deletion strain (10), an aromatic amino acid auxotroph strain (17), and a serovar Typhi derivative harboring mutations in aroC and ssaV, reveal similar durations of fecal shedding. In contrast to serovar Typhi, serotype Typhimurium causes a disease typified by a high level of colonization of the intestine and associated inflammatory diarrhea in humans and some farm animals. The results of volunteer trials of candidate serotype Typhimurium live oral vaccine strains show markedly greater intestinal colonization and shedding with feces for these strains than for serovar Typhi strains. For example, a serotype Typhimurium phoP-phoQ strain was shed with feces for at least 10 days in some cases, at which stage antibiotic therapy was used to clear the vaccine bacteria (3). In the results of a second volunteer study with a serotype Typhimurium aroA ssaV vaccine candidate, heavy colonization of the intestine and shedding of the vaccine-associated bacteria with feces for 2 to 3 weeks were reported (9). Similar findings were reported in a third study using a serotype Typhimurium ΔphoP-phoQ ΔaroA Δasd ΔstrA-strB strain (16). The high levels of shedding exhibited by genetically manipulated candidate serotype Typhimurium vaccine strains have precluded both their development as live Salmonella vaccines in humans and their use as live vectors for delivering heterologous antigens.
The limited intestinal persistence of serovar Typhi during infection may result from the degradation of its genome compared to the genomes of nontyphoidal serotypes, such as serotype Typhimurium. Several genes implicated in intestinal colonization and persistence in serotype Typhimurium, including shdA, ratB, sivH (12, 14), misL (8), and some fimbrial operons (22), are pseudogenes in serovar Typhi (19). Serovar Typhi has 12 putative usher-chaperone-family fimbrial operons (22), of which 5 (bcf, stb, stc, std, and sth) have homologous operons in serotype Typhimurium that are required for prolonged intestinal colonization (24). Two of these, bcf and sth, contain one or more pseudogenes in the operon that are likely to abrogate fimbrial biogenesis (22). The shdA and misL genes encode proteins of the autotransporter family of secreted proteins. Both ShdA and MisL bind fibronectin but differ in their binding to additional extracellular matrix proteins, collagen I and collagen IV, respectively (8, 13). The ratB gene of serotype Typhimurium is predicted to encode a secreted protein of unknown function that has a profound effect on persistence in the ceca of genetically resistant mice (12).
Here, we address the hypothesis that prolonged intestinal colonization is required for a robust immune response to the vector and heterologous antigen expressed from a plasmid. We determined the immune response to a heterologous antigen (TetC) expressed by live serotype Typhimurium vaccine strains containing mutations in a subset of the genetic loci previously implicated in shedding of serotype Typhimurium with feces in the murine host. Our aim was to test a proof in principle that vaccines with reduced shedding levels could still make effective vaccines. These considerations are important for the development of improved live attenuated multivalent vaccines with a reduced impact on the environment.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
SL3261 is an aroA hisS vaccine strain (11). AH9 (ΔshdA::aph) has been described previously (12). Bacteria were cultured aerobically at 37°C in Luria-Bertani (LB) broth or LB with 1% agar supplemented with antibiotics as appropriate at the following concentrations: ampicillin (Amp), 100 mg/liter (LB + Amp); chloramphenicol (Chl), 30 mg/liter (LB + Chl); and kanamycin (Kan), 30 mg/liter (LB + Kan). Bacteriophage P22 HT105/1 int was used for generalized transduction of antibiotic resistance genes, the Chl acetyltransferase (cat) gene, or the aminoglycoside phosphotransferase (aph) gene between serotype Typhimurium strains. Transductants were routinely purified of contaminating phage by streaking the strain twice for single colonies on Evans blue uridine plates (4).
A serotype Typhimurium aroA strain in which the misL gene was replaced with a cat gene was constructed by allelic exchange using a method previously described, with minor modifications (7). Primers 5′ TTATTGCGATGTGGAAGACGCTTTACGCCATAATGCAGGAGGCAGATGTGTAGGCTGGAGCTGCTTC 3′ and 5′ TCCGGGTAAAAGCCGCTGAAGATCAGCGGCTCTGTTGTTACCTGAACATATGAATATCCTCCTTAG 3′ were used to amplify the cat gene from pKD3 (7). These primers contain a 46-nucleotide sequence that is identical to the flanking sequence of the intended deletion (+1 to +3116 of the misL open reading frame). The resulting PCR product was introduced by electrotransformation into serotype Typhimurium strain SL3261 containing plasmid pKD46. Recombination of the PCR product into the serotype Typhimurium chromosome was selected for by plating transformants on LB + Chl agar. The cat gene was transferred to serotype Typhimurium SL3261 by P22 transduction. One colony was selected and designated serotype Typhimurium MFA12. Colony PCR using primer pairs that annealed within the deletion and flanking the deletion was used to confirm the correct structure of the mutation (data not shown). In addition, genomic DNA prepared from MFA12 was digested with restriction endonuclease EcoRV, transferred to a nylon membrane by Southern transfer, and probed with a cloned fragment of misL. These data also confirmed the misL deletion in MFA12 (data not shown).
A serotype Typhimurium aroA strain in which the ratB gene was replaced with an aph gene was constructed by a methodology similar to that described above. Primers 5′ AGCTGGCGCTGTTCCGGTAGCGATGTATTTTTACGTTTTTGTATTGTGTAGGCTGGAGCTGCTTC 3′ and 5′ GCGTCGCCGCGGGTAATCACGAAATCAACGTTGCCCTCCGGTTCGCCTTACATATGAATATCCTCCTTAG 3′ were used to amplify the aph gene from pKD4 (7). These primers contain a 46-nucleotide sequence that is identical to the flanking sequence of the intended deletion (−50 to +752 of the ratB open reading frame). The resulting PCR product was introduced by electrotransformation into serotype Typhimurium SL3261 containing plasmid pKD46. Recombination of the PCR product into the serotype Typhimurium chromosome was selected for by plating transformants on LB + Kan agar. The aph gene was transferred to serotype Typhimurium SL3261 by P22 transduction. A colony was selected and designated serotype Typhimurium MFA16. Colony PCR using primer pairs that annealed within the deletion and flanking the deletion was used to confirm the correct structure of the mutation (data not shown). In addition, genomic DNA prepared from MFA16 was digested with restriction endonuclease EcoRV, transferred to a nylon membrane by Southern transfer, and probed with a cloned fragment of ratB. These data also confirmed the ratB deletion in MFA16 (data not shown).
Serotype Typhimurium strain AH9, in which the shdA gene has been replaced by the aph gene, was previously described (12). A serotype Typhimurium aroA strain in which the shdA gene was replaced by the aph gene was constructed by transferring the aph gene from AH9 into SL3261 by P22 transduction. This strain was designated MFA13. Serotype Typhimurium strain AJB715, in which the phoN gene has been replaced by the aph gene, was previously described (12). A serotype Typhimurium aroA strain in which the phoN gene was replaced by the aph gene was constructed by transferring the aph gene from AJB715 into strain SL3261 by P22 transduction. This derivative was designated MFA25. A serotype Typhimurium aroA strain in which the shdA gene was replaced by the aph gene and the misL gene with the cat gene was constructed by transferring the cat gene from MFA12 into MFA13 by P22 transduction. This derivative was designated MFA17.
Determination of TetC expression.
Serotype Typhimurium strains were transformed with pTetCnirB15 by electroporation. In order to determine the expression of TetC from pTetCnirB15-containing strains, LB broth cultures were harvested by centrifugation and suspended in phosphate-buffered saline (PBS) (pH 7.4) to an optical density at 600 nm (OD600) of 1. This suspension was mixed with an equal volume of 2× sodium dodecyl sulfate loading buffer and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a 4- to 20%-gradient gel (Bio-Rad). Proteins were transferred to polyvinylidene fluoride membranes (Immobilon-P) by Western transfer and detected with rabbit anti-TetC antibody.
Experimental infections of mice.
For inoculation of mice, serotype Typhimurium strains were grown statically in LB broth supplemented with Amp as appropriate. Bacteria were harvested by centrifugation, washed, and then suspended in PBS (pH 7.4) to approximately 1 × 109 CFU per ml. Groups of 6- to 8-week-old female BALB/c ByJ mice were inoculated orally by gavage with 0.2 ml of bacterial suspension each. Viable counts were determined in the inoculum and on days 2, 4, 7, 11, and 14 in freshly recovered fecal pellets suspended in 0.5 ml PBS (pH 7.4) by serial 10-fold dilution and culture on LB agar supplemented with the appropriate antibiotic. Serotype Typhimurium cells were selectively cultured and distinguished from normal flora by the addition of Kan or Chl (MFA12) to the culture medium. For the calculation of the means of the CFU, in cases where no CFU were detected, the limit of detection (50 CFU) was used for the calculation. The two-tailed Student t test was used to determine whether the mean of the CFU recovered from each test group was significantly different from that of the group inoculated with strain MFA25. On day 21 postinoculation, peripheral blood was collected by tail bleed and sera were prepared by standard techniques and stored at −20°C for further analysis. All mice except the group inoculated with the plasmidless MFA25 were boosted by intranasal administration of 0.01 mg of six-His-tagged TetC and 0.001 mg of wild-type heat-labile enterotoxin (LT) in 0.05 ml of PBS (pH 7.4). At 14 days postboost, mice were sacrificed, blood was collected by cardiac puncture, and spleens were asceptically removed for splenocyte preparation.
To determine the maintenance of pTetCnirB15 in serotype Typhimurium in vivo, a group of five mice were inoculated orally by gavage with 1 × 109 CFU of serotype Typhimurium MFA25(pTetCnirB15). Serial dilutions of freshly collected fecal pellets homogenated in PBS (pH 7.4) were cultured on LB + Kan and LB + Amp in order to determine the proportion of serotype Typhimurium MFA25 containing pTetCnirB15.
Determination of total IgG and IgG subtypes in mouse sera.
Nunc Maxisorp plates were coated overnight at 4°C with 0.05 ml of a 0.002-mg/ml solution of purified six-His-tagged TetC or lipopolysaccharide (LPS) in coating buffer (0.1 M Na2HPO4 at a pH of 9). Wells were washed with PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T), and then plates were blocked with 0.1 ml of 3.0% bovine serum albumin or nonfat dried milk in PBS (pH 7.4) at room temperature for 60 min. Plates were then washed with PBS-T, and serum was added as follows. A total of 0.003 ml of serum was added to 0.027 ml of PBS with 0.2% bovine serum albumin and 0.05% Tween 20 (PBS-BT); 0.0125 ml of this was then added to 0.125 ml of PBS-BT in the top well and diluted (with PBS-BT) 5-fold in subsequent wells. Each plate contained control wells with preimmune sera, PBS (pH 7.4), or a known positive immune serum and incubated for 2 h at room temperature. Wells were washed three times with PBS-T and then anti-mouse immunoglobulin G (IgG) or IgA antibody conjugated to horseradish peroxidase (HRP) (Serotec) diluted 1/1,000 in PBS-BT was added at 0.1 ml per well. Plates were incubated for 1 h at room temperature. Wells were washed five times with PBS-T and the level of HRP associated with each well was determined using Sigma Fast o-phenylenediamine dihydrochloride (100 μl per well) colorimetric substrate. The reaction was stopped after 20 min by the addition of 0.02 ml of 2.5 M H2SO4. The OD490 was determined using an enzyme-linked immunosorbent assay (ELISA) plate reader, and the titer was expressed as the reciprocal of the dilution giving an OD of 0.3.
The protocol was modified as follows to determine the IgG subclass titers in mouse sera. Rat monoclonal antibodies that specifically bind mouse IgG subclasses, conjugated to biotin (PharMingen), were used as the secondary antibody. Anti-IgG1 and anti-IgG2a were used at dilutions of 1:4,000 and 1:1,000, respectively. Subclass conjugate antibodies were calibrated against purified isotype antibodies as antigen to enable direct comparisons. To detect the biotin-conjugated antibodies, plates were washed four times in PBS-T and then 0.05 ml of streptavidin-HRP diluted at 1:1,000 in PBS-T was added to each well. Plates were developed and titers measured as described above.
Th1/Th2 cytometric bead array.
Mice were sacrificed and their spleens removed aseptically into RPMI medium supplemented with 10% fetal calf serum, 2 mM glutamine, 10−5 2-mercaptoethanol, 100 U/ml penicillin, and 0.1 mg/ml streptomycin (RPMI+ medium). Single-cell suspensions were prepared by pushing spleens through a 100-μm cell strainer (Becton Dickinson). Cells were centrifuged at 1,500 rpm for 5 min, followed by incubation with 0.5% Tris-ammonium chloride (pH 7.2) solution for 5 min to remove erythrocytes. Cells were washed with RPMI medium twice and then suspended in 1 ml RPMI+ medium. Viable splenocytes were counted by a trypan blue exclusion assay.
Cells were seeded, in duplicate, into round-bottomed, 96-well tissue culture plates at a concentration of 5 × 105 cells/well in a volume of 0.2 ml. Splenocytes were stimulated with either purified fragment C (0.005 mg/ml) or concanavalin A or RPMI+ medium. Plates were incubated at 37°C and 5% CO2 for 24 h, when supernatants were stored at −80°C for subsequent cytometric bead array cytokine analysis.
Assays were performed per the manufacturer's instructions (BD Biosciences). Briefly, 0.05 ml of mixed capture beads was added to 0.05 ml of splenocyte culture supernatant and mixed. A total of 0.05 ml of phycoerythrin detection reagent was added to each sample tube and incubated for 2 h at room temperature. A 1-ml volume of wash buffer (BD Biosciences) was added to each tube. This was then mixed thoroughly using a vortex and centrifuged at 200 × g for 5 min. After the supernatant was discarded, a further 0.3 ml of wash buffer was added and the pellet was suspended. Samples were then analyzed on an Aria flow cytometer (BD Biosciences).
RESULTS
Mutations in shdA and misL decrease shedding of a serotype Typhimurium aroA vaccine candidate into feces.
Serotype Typhimurium derivatives harboring mutations in shdA (12, 14), ratB (12), or misL (8) exhibit reduced persistence in the murine cecum. These strains are also shed with the feces in lower numbers and for a shorter time period than the isogenic parental strains. We therefore tested the hypothesis that the introduction of these mutations into vaccine strain serotype Typhimurium SL3261 (aroA) would decrease the level and duration of fecal shedding. In order to enumerate SL3261 in the feces, it was necessary to introduce a selectable marker so that SL3261 could be distinguished from the normal flora. To this end, the aph gene, which confers Kan resistance, was used to replace the phoN gene by allelic exchange. The phoN gene has previously been shown to be dispensable for the intestinal and systemic phases of murine salmonellosis (12). Furthermore, in order to compare the abilities of the candidate vaccine strains to elicit an immune response to heterologous antigen, each strain was transformed with pTetCnirB15. This plasmid encodes the nontoxic fragment C (TetC) of tetanus toxin and directs the expression of TetC under the control of the nirB15 promoter (6). All of the vaccine strains (Table 1) transformed with plasmid pTetCnirB15 expressed similar amounts of TetC when grown under anaerobic conditions, as determined by Western blot analysis of whole-cell lysates using rabbit anti-TetC antibody (data not shown).
TABLE 1.
Strains constructed in this study
| Name | Genotype |
|---|---|
| MFA25 | Serovar Typhimurium ΔaroA ΔphoN::aph |
| MFA13 | Serovar Typhimurium Δaro ΔshdA::aph |
| MFA12 | Serovar Typhimurium ΔaroA ΔmisL::cat |
| MFA17 | Serovar Typhimurium ΔaroA ΔmisL::cat ΔshdA::aph |
| MFA16 | Serovar Typhimurium ΔaroA ΔratB::aph |
Approximately 1 × 109 CFU of serotype Typhimurium MFA25 aroA ΔphoN::aph, MFA25(pTetCnirB15) aroA ΔphoN::aph, MFA13(pTetCnirB15) aroA ΔshdA::aph, MFA16 (pTetCnirB15) aroA ΔratB::aph, MFA12 (TetCnirB15) aroA ΔmisL::cat, or MFA17(pTetCnirB15) aroA ΔmisL::cat ΔshdA::aph was inoculated orally into groups of five female BALB/c ByJ mice (Table 2). No significant difference in the number of CFU shed with feces was observed between groups of mice inoculated with MFA25, MFA25(pTetCnirB15), and MFA16(pTetCnirB15) ΔratB. The mean numbers of CFU/mg feces shed by these groups of mice were 1 × 105 to 1 × 106. In contrast, groups of mice inoculated with MFA13(pTetCnirB15) shdA or MFA12(pTetCnirB15) misL had a mean shedding with feces approximately two orders of magnitude lower than that of MFA25(pTetCnirB15) on days 4, 7, and 14. Furthermore, the number of mice shedding vaccine CFU with feces was lower in groups of mice inoculated with MFA13(pTetCnirB15) shdA or MFA12(pTetCnirB15) misL. All five mice in the group inoculated with MFA25(pTetCnirB15) shed this vaccine with feces at levels above the detectable limit on each of the 4 days tested. In contrast, on just a single occasion (day 10) did all five mice inoculated with MFA13(pTetCnirB15) shdA shed detectable numbers of CFU with their feces. On none of the 4 days tested did all five mice in the group inoculated with MFA12 (pTetCnirB15) misL shed this strain. Indeed, on day 14 postinoculation, CFU were detectable in only a single mouse. On days 4 and 14 postinoculation, the mean shedding of CFU from groups of mice inoculated with MFA13(pTetCnirB15) shdA or MFA12(pTetCnirB15) misL was significantly (P < 0.05) lower than the mean shedding from mice inoculated with MFA25(pTetCnirB15). There was no significant difference between these groups on day 10 postinoculation. We next tested whether mutations in shdA and misL have synergistic effects when combined in the same serotype Typhimurium derivative. A group of five mice orally inoculated with approximately 1 × 109 CFU of serotype Typhimurium MFA17(pTetCnirB15) misL shdA shed this vaccine with feces at levels similar to the levels of MFA13 and MFA12 (Table 2). However, unlike these derivatives, MFA17 was shed at significantly fewer mean CFU (P < 0.05) on day 10 postinoculation in addition to days 4, 7, and 14. Furthermore, on days 4 and 14, only a single mouse in the group was shedding detectable numbers of CFU and only two from the group of five were shedding detectable numbers of CFU on days 7 and 10.
TABLE 2.
Shedding of serovar Typhimurium strains in feces of mice following oral inoculationa
| Strain and day postinoculation | Log10 CFU per 100 mg feces for mouseb
|
Mean log10 CFU | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| MFA25 ΔaroA ΔphoN::aph | ||||||
| 4 | 4.7 | 4.9 | 4.3 | 3.7 | 4.0 | 4.5 |
| 7 | 3.4 | 4.1 | 3.7 | 4.0 | 4.9 | 3.9 |
| 10 | 4.2 | 3.4 | 3.4 | 4.2 | 3.4 | 3.9 |
| 14 | 4.0 | 3.7 | 3.7 | 3.0 | 2.7 | 3.6 |
| MFA25(pTetCnirB15) ΔaroA ΔphoN::aph | ||||||
| 4 | 4.4 | 5.4 | 3.9 | 4.4 | 4.4 | 4.8 |
| 7 | 4.4 | 3.7 | 4.0 | 4.0 | 4.4 | 4.2 |
| 10 | 3.9 | 3.9 | 3.4 | 3.4 | 3.4 | 3.7 |
| 14 | 3.9 | 3.9 | 3.7 | 3.0 | 2.7 | 3.6 |
| MFA13(pTetCnirB15) ΔaroA ΔshdA::aph | ||||||
| 4 | 3.4 | 2.7 | 2.9 | ND | 2.4 | 2.6 |
| 7 | 2.9 | 2.9 | 3.0 | ND | 3.2 | 2.7 |
| 10 | 3.3 | 3.2 | 2.7 | 3.4 | 4.0 | 3.5 |
| 14 | 2.4 | ND | 2.4 | ND | 2.4 | 2.1 |
| MFA12(pTetCnirB15) ΔaroA ΔmisL::cat | ||||||
| 4 | 2.4 | 3.1 | 2.4 | 2.4 | ND | 2.4 |
| 7 | ND | 2.7 | ND | 2.4 | 2.7 | 2.2 |
| 10 | 2.4 | ND | ND | 3.4 | ND | 2.2 |
| 14 | ND | ND | ND | ND | 2.4 | 1.8 |
| MFA17(pTetCnirB15) ΔaroA ΔmisL::cat ΔshdA::aph | ||||||
| 4 | ND | ND | ND | 3.0 | ND | 2.0 |
| 7 | ND | ND | 2.7 | 2.7 | ND | 2.1 |
| 10 | ND | 4.1 | ND | 3.7 | ND | 2.6 |
| 14 | ND | ND | ND | 3.0 | ND | 2.0 |
| MFA16(pTetCnirB15) ΔaroA ΔratB::aph | ||||||
| 4 | 5.4 | 4.7 | 5.0 | 3.9 | 4.7 | 5.0 |
| 7 | 5.5 | 4.4 | 5.7 | 4.7 | 4.4 | 5.3 |
| 10 | 5.4 | 4.7 | 3.7 | 3.7 | 4.1 | 4.8 |
| 14 | 5.4 | 3.7 | 3.0 | 3.0 | 3.4 | 4.7 |
Groups of five mice were inoculated orally with serovar Typhimurium MFA25 aroA phoN, serovar Typhimurium MFA25(pTetCnirB15) aro phoN, serovar Typhimurium MFA16(pTetCnirB15) aroA ratB, serovar Typhimurium MFA13(pTetCnirB15) aroA shdA, serovar Typhimurium MFA12(pTetCnirB15) aroA misL, or serovar Typhimurium MFA17(pTetCnirB15) aroA shdA misL.
The limit of detection was 50 (log10, 1.7) CFU per sample. ND, not detected.
Attenuation of intestinal persistence of serotype Typhimurium does not detectably affect elicitation of humoral or cellular immune responses to heterologous antigen.
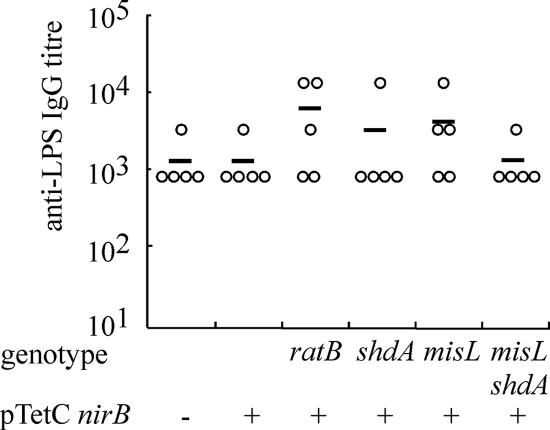
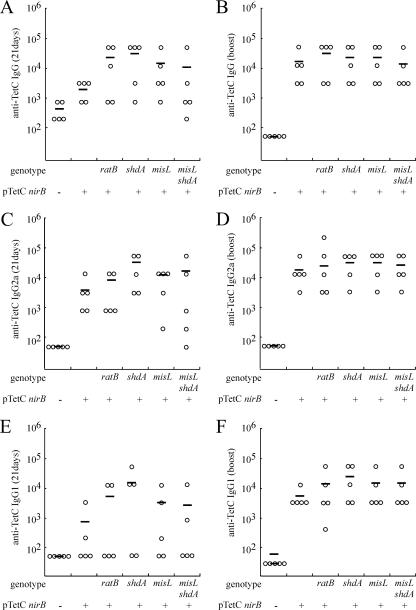
To address the question as to whether decreased intestinal colonization and persistence impact the immunogenicity of serotype Typhimurium vaccine candidates, we determined the humoral and cellular immune responses to LPS and the heterologous antigen TetC. To determine the humoral immune response to serotype Typhimurium somatic antigen and TetC, peripheral blood from the groups of mice described in the previous section was collected at 21 days postinoculation by tail bleed. Anti-serotype Typhimurium LPS IgG was detected in all groups of mice (Fig. 1), with no significant difference (P > 0.05) in the mean titers between the six groups. All of the groups inoculated with serotype Typhimurium harboring pTetCnirB15 had a greater titer of anti-TetC IgG (Fig. 2) than mice inoculated with MFA25 lacking the plasmid. No significant difference was detected in the mean titers of anti-TetC IgG between the group inoculated with MFA25(pTetCnirB15) and the groups inoculated with serotype Typhimurium MFA13(pTetCnirB15) aroA ΔshdA::aph, MFA16(pTetCnirB15) aroA ΔratB::aph, MFA12(pTetCnirB15) ΔaroA ΔmisL::cat, or MFA17(pTetCnirB15) ΔaroA ΔmisL::cat ΔshdA::aph. The same trends were evident for anti-TetC IgG2a (Fig. 2C) and IgG1 (Fig. 2E) subtypes. However, in all groups, the IgG2a anti-TetC immune response was greater than that of IgG1, suggesting a bias toward a Th1-type immune response.
FIG. 1.
IgG response to serotype Typhimurium LPS in mice 21 days following inoculation with serovar Typhimurium vaccine strains. Anti-LPS IgG was quantified from serum from peripheral blood by ELISA. The titer for each animal (circle) and the mean titer for each group (horizontal bar) are indicated. The mutation(s) of the serotype Typhimurium strain tested in each group is indicated below the graph. All are aroA. − and + indicate the absence or presence of pTetCnirB.
FIG. 2.
IgG response to serotype Typhimurium LPS and TetC in animals 21 days following inoculation with serotype Typhimurium vaccine strains and following intranasal boost. IgG specific for TetC (A), IgG specific for TetC following intranasal boost (plasmidless MFA25 group was not boosted) (B), IgG2a specific for TetC (C), IgG2a specific for TetC following intranasal boost (plasmidless MFA25 group was not boosted) (D), IgG1 specific for TetC (E), or IgG1 specific for TetC following intranasal boost (plasmidless MFA25 group was not boosted) (F) was quantified from serum derived from peripheral blood by ELISA. The titer for each animal (circle) and the mean titer for each group (horizontal bar) are indicated. The mutation(s) of the serotype Typhimurium strain tested in each group is indicated below each graph. All are aroA. − and + indicate the absence or presence of pTetCnirB.
The humoral and cellular immune responses to TetC were determined following intranasal boost with TetC and LT as the adjuvant. Fourteen days following this boost, sera were collected by cardiac puncture, the mice were sacrificed, and the spleens aseptically removed. Similar trends were observed in the total IgG and IgG subtype responses between postboost sera and preboost sera (Fig. 2). All immune responses were greater and had more uniformity following intranasal boost, especially for the IgG1 subclass. However, the IgG2a subclass response was still greater than that with IgG1. No significant difference was detected in the mean titer of anti-TetC IgG between the group inoculated with MFA25 (pTetCnirB15) and the groups inoculated with serotype Typhimurium MFA13 (pTetCnirB15) aroA ΔshdA::aph, MFA16 (pTetCnirB15) aroA ΔratB::aph, MFA12 (pTetCnirB15) ΔaroA ΔmisL::cat, or MFA17 (pTetCnirB15) ΔaroA ΔmisL::cat ΔshdA::aph. The same trends were evident for anti-TetC IgG2a (Fig. 2D) and IgG1 (Fig. 2F) subtypes.
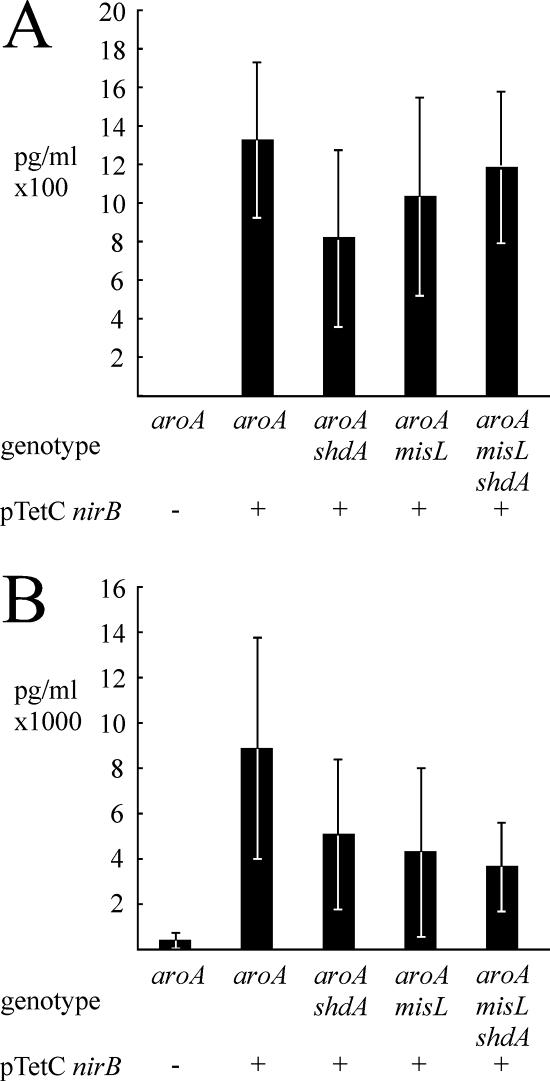
The concentrations of interleukin-2 (IL-2), gamma interferon (IFN-γ), tumor necrosis factor alpha, and IL-4 in the cell culture supernatant of splenocytes stimulated with TetC were determined. All of the groups inoculated with serotype Typhimurium harboring pTetCnirB15 had elevated concentrations of IL-2 and IFN-γ compared to mice inoculated with MFA25 lacking the plasmid (Fig. 3A and B). In contrast, no detectable TetC-specific production of TNF-α or IL-4 was observed (data not shown). No significant differences in IL-2 and IFN-γ production by splenocytes from the groups inoculated with MFA25(pTetCnirB15) and the groups inoculated with serotype Typhimurium MFA13(pTetCnirB15) aroA ΔshdA::aph, MFA16(pTetCnirB15) aroA ΔratB::aph, MFA12 (pTetCnirB15) ΔaroA ΔmisL::cat, or MFA17(pTetCnirB15) ΔaroA ΔmisL::cat ΔshdA::aph were observed.
FIG. 3.
Production of IL-2 (A) and gamma interferon (B) by splenocytes derived from mice vaccinated with serotype Typhimurium vaccine strains in response to stimulation with recombinant TetC. Groups of five mice were vaccinated with serotype Typhimurium MFA25 aroA phoN, serotype Typhimurium MFA16(pTetCnirB15) aroA phoN, serotype Typhimurium MFA16(pTetCnirB15) aroA ratB, serotype Typhimurium MFA13(pTetCnirB15) aroA shdA, serotype Typhimurium MFA12(pTetCnirB15) aroA misL, or serotype Typhimurium MFA17(pTetCnirB15) aroA shdA misL. − and + indicate the absence or presence of pTetCnirB.
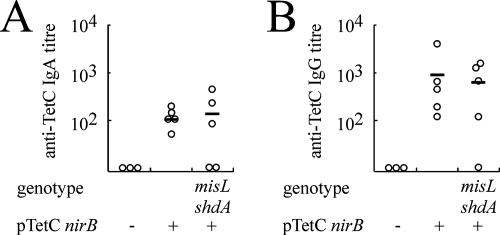
In order to determine the mucosal immune response to TetC, three groups of BALB/c ByJ mice were inoculated with serotype Typhimurium MFA25(pTetCnirB15), MFA17 (pTetCnirB15), or MFA25. Shedding of the vaccine strains from these mice was similar to that observed in the experiment previously described (data not shown). Significantly, on day 1 postinoculation, similar numbers of CFU were detected in feces of mice inoculated with MFA25(pTetCnirB15) and MFA17(pTetCnirB15), indicating that initial colonization was not affected by the shdA and misL mutations. The mean anti-TetC serum IgA and IgG immune responses were similar in the groups inoculated with the vaccines containing the plasmid (Fig. 4). However, the immune responses of mice vaccinated with strain MFA17(pTetCnirB15) were more variable than the responses of those vaccinated with MFA25(pTetCnirB15), with one of the five mice having no measurable IgA response and another mouse having no IgA or IgG response.
FIG. 4.
IgA response to serotype Typhimurium TetC in animals 21 days following inoculation with serotype Typhimurium vaccine strains. IgA specific for TetC (A) or IgG specific for TetC (B) was quantified from serum derived from peripheral blood by ELISA. The titer for each animal (circle) and the mean titer for each group (horizontal bar) are indicated. The mutations of the serotype Typhimurium strains tested are indicated below the graph. All groups are aroA. − and + indicate the absence or presence of pTetCnirB.
Mice vaccinated with the serotype Typhimurium ΔaroA ΔmisL::cat ΔshdA::aph strain are protected against subsequent challenge with virulent serotype Typhimurium.
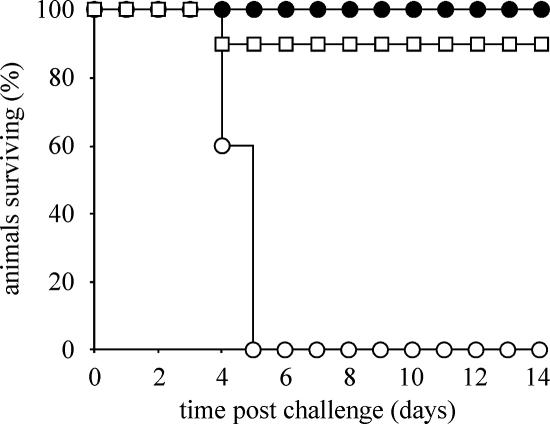
To determine if serotype Typhimurium MFA17 ΔaroA ΔmisL::cat ΔshdA::aph is efficacious as a single-dose vaccine for murine salmonellosis, we compared its ability to protect against a challenge with a virulent strain of serotype Typhimurium, SL1344, to that of serotype Typhimurium MFA25 (Fig. 5). A group of 13 mice were orally inoculated with 1 × 109 CFU of MFA25(pTetCnirB15) and a second group of 15 mice were orally inoculated with 1 × 109 CFU MFA17(pTetCnirB15) ΔaroA ΔmisL::cat ΔshdA::aph. On day 56 postinoculation, three mice from the MFA25 and five mice from the MFA17 groups of mice were sacrificed and the number of CFU of the vaccine strain in organ homogenates determined. No CFU of either strain were detectable in the feces, cecum, Peyer's patch, mesenteric lymph nodes, liver, or spleen. On day 70 postinoculation, mice were challenged with 1 × 108 CFU of serotype Typhimurium SL1344, a virulent isolate. As expected, a group of five age-matched mice that were not vaccinated succumbed to infection with serotype Typhimurium SL1344 within 5 days after oral inoculation. In the group of 10 mice vaccinated with MFA25, one mouse was scored as moribund and sacrificed; the 9 other mice in this group remained healthy for the duration of the experiment. In the group of 10 mice vaccinated with MFA17, all 10 mice remained healthy throughout the course of the experiment (Fig. 5).
FIG. 5.
Survival curve of naïve mice and mice vaccinated with serotype Typhimurium. Approximately 1 × 108 CFU of serotype Typhimurium SL1344 was inoculated orally into a group of 10 mice previously vaccinated with strain MFA26(pTetCnirB15) (ΔaroA ΔphoN::aph) (open squares), a group of 10 mice previously vaccinated with strain MFA17(pTetCnirB15) (ΔaroA ΔmisL::cat ΔshdA::aph) (solid circles), or a group of 5 naïve mice (open circles); the health of the mice was monitored twice daily, and the mice were sacrificed when they appeared moribund.
DISCUSSION
The use of genetically modified and attenuated Salmonella enterica strains that express one or more heterologous antigens is a promising strategy to develop relatively cheap, single-oral-administration polyvalent vaccines. The success of these vaccine strains in stimulating a robust humoral and cellular immune response may be due to their colonization of mucosal surfaces and the immune cells of the underlying tissues. Prolonged colonization of these sites correlates with shedding of S. enterica into the environment with feces, a factor that is considered undesirable from a health and safety point of view. In this study, we have tested the hypothesis that a robust immune response is dependent on prolonged intestinal colonization. We did this by comparing the humoral and cellular immune responses as well as the Th1/Th2 biases of the immune responses of mice vaccinated with the well-characterized serotype Typhimurium aroA vaccine with those of strains that exhibit reduced intestinal colonization due to mutations in shdA and misL.
All of the live attenuated strains characterized in this study induced similar cellular immune responses of the Th1 type. Cytokine production of splenocytes contributes to a cellular response biased to the Th1 or Th2 type. The Th1 response is characterized by the production of IL-2 and IFN-γ, while a Th2 response is characterized by the production of IL-4 and other cytokines. Salmonella infections typically generate a Th1-type cellular response, and as expected, splenocytes from vaccinated mice expressing TetC produced significantly greater amounts of IL-2 and IFN-γ than mice vaccinated with a strain not expressing this antigen when stimulated in vitro with recombinant TetC. In contrast, the levels of IL-4 production from stimulated splenocytes were similar in mice vaccinated with the strain expressing TetC and mice vaccinated with the strain not expressing TetC, indicating a relatively low Th2-type cellular response. Th1 cells direct cell-mediated immunity and promote IgG isotype class switching to IgG2a. The mean anti-TetC IgG2a titer was not significantly greater than the anti-TetC IgG1 titer for any of the vaccination groups. However, two to three animals in each group of five mice had no response above the background IgG1 titer. In contrast, all but one mouse had a measurable anti-TetC IgG2a titer, suggesting that if larger groups of mice had been used, significant differences in the titers of these two IgG isotypes might have been observed. An intranasal boost with TetC and LT adjuvant resulted in an increased IgG titer, but with a similar Th1 bias. In summary, the predominance of a Th1-type response was the same for each of the test vaccine strains, regardless of the presence of additional mutations in the shdA, misL, or ratB genes. In light of the similar immune responses to serotype Typhimurium LPS antigen generated by serotype Typhimurium MFA25 (ΔaroA ΔphoN::aph) and MFA17 (ΔaroA ΔmisL::cat ΔshdA::aph), it is perhaps not surprising that both these strains were able to protect against an otherwise lethal challenge with a virulent strain of serotype Typhimurium.
We did not determine whether the anti-TetC immune response resulted in protection against challenge with wild-type tetanus toxin. However, several studies have concluded unambiguously that protection against tetanus toxin challenge correlates with anti-TetC IgG response (1, 2, 18, 23). Since there was no difference in the anti-TetC IgG response regardless of the presence of mutations in shdA, misL, or ratB genes on the vaccine strain, we therefore would not expect there to be a difference in protection against tetanus toxin challenge.
In addition to shdA and misL, a number of putative fimbrial operons have been implicated in persistent colonization of the murine intestine. These include lpf, which encodes long polar fimbriae; bcf, previously identified as a bovine colonization factor; and four previously uncharacterized putative fimbrial operons, stb, stc, std, and sth (24). The effects on immune responses to the vector or heterologous antigen for strains lacking these factors are currently unknown. It is possible that the incorporation of additional mutations in one or more of these loci, in addition to shdA and misL, will further decrease shedding of attenuated serotype Typhimurium strains with feces. It remains to be seen whether further attenuation of intestinal persistence will begin to impact the immunogenicity and effectiveness of the vaccine strain.
In light of the unacceptably high level of fecal shedding of serotype Typhimurium vaccine strains in two human volunteer trials following oral administration (3, 9, 16), there is a clear need for additional attenuation of intestinal persistence in future vaccines intended for use in humans. There are a number of considerations in transferring these observations to the use of similar vaccine strains in humans. The data presented here are proof of the principle that, at least in the case of the combination of the TetC antigen expressed in the serotype Typhimurium vaccine strain during natural infections of the murine host, decreased persistence in the intestine does not negatively impact immunogenicity or protection. This is in line with previous findings that the critical event in determining the immune response to a heterologous antigen may be the initial amount of the antigen that primes the inductive site and not the persistence of the vector (5). However, it is possible that differences in the murine and human immune systems result in different temporal requirements for the priming of the immune system. Furthermore, it remains to be seen whether the same combination of mutations (i.e., deletion of shdA and misL) is the most suitable for attenuation of fecal shedding in the human host, since mechanisms of intestinal persistence may be host specific.
Editor: A. Camilli
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Abreu, P. A., P. A. Miyasato, M. M. Vilar, W. O. Dias, P. L. Ho, M. Tendler, and A. L. Nascimento. 2004. Sm14 of Schistosoma mansoni in fusion with tetanus toxin fragment C induces immunoprotection against tetanus and schistosomiasis in mice. Infect. Immun. 72:5931-5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R., X. M. Gao, A. Papakonstantinopoulou, M. Roberts, and G. Dougan. 1996. Immune response in mice following immunization with DNA encoding fragment C of tetanus toxin. Infect. Immun. 64:3168-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angelakopoulos, H., and E. L. Hohmann. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar Typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 68:2135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bochner, B. R. 1984. Curing bacterial cells of lysogenic viruses by using UCB indicator plates. BioTechniques 2:234-240. [Google Scholar]
- 5.Cardenas, L., U. Dasgupta, and J. D. Clements. 1994. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine 12:833-840. [DOI] [PubMed] [Google Scholar]
- 6.Chatfield, S. N., I. G. Charles, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsey, C. W., M. C. Laarakker, A. D. Humphries, E. H. Weening, and A. J. Baumler. 2005. Salmonella enterica serotype Typhimurium MisL is an intestinal colonization factor that binds fibronectin. Mol. Microbiol. 57:196-211. [DOI] [PubMed] [Google Scholar]
- 9.Hindle, Z., S. N. Chatfield, J. Phillimore, M. Bentley, J. Johnson, C. A. Cosgrove, M. Ghaem-Maghami, A. Sexton, M. Khan, F. R. Brennan, P. Everest, T. Wu, D. Pickard, D. W. Holden, G. Dougan, G. E. Griffin, D. House, J. D. Santangelo, S. A. Khan, J. E. Shea, R. G. Feldman, and D. J. Lewis. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 70:3457-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohmann, E. L., C. A. Oletta, and S. I. Miller. 1996. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine 14:19-24. [DOI] [PubMed] [Google Scholar]
- 11.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live oral vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 12.Kingsley, R. A., A. D. Humphries, E. H. Weening, M. R. De Zoete, S. Winter, A. Papaconstantinopoulou, G. Dougan, and A. J. Baumler. 2003. Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype Typhimurium: identification of intestinal colonization and persistence determinants. Infect. Immun. 71:629-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsley, R. A., A. M. Keestra, M. R. de Zoete, and A. J. Baumler. 2004. The ShdA adhesin binds to the cationic cradle of the fibronectin 13FnIII repeat module: evidence for molecular mimicry of heparin binding. Mol. Microbiol. 52:345-355. [DOI] [PubMed] [Google Scholar]
- 14.Kingsley, R. A., K. van Amsterdam, N. Kramer, and A. J. Baumler. 2000. The shdA gene is restricted to serotypes of Salmonella enterica subspecies I and contributes to efficient and prolonged fecal shedding. Infect. Immun. 68:2720-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotton, C. N., and E. L. Hohmann. 2004. Enteric pathogens as vaccine vectors for foreign antigen delivery. Infect. Immun. 72:5535-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotton, C. N., A. J. Lankowski, N. Scott, D. Sisul, L. M. Chen, K. Raschke, G. Borders, M. Boaz, A. Spentzou, J. E. Galan, and E. L. Hohmann. 2006. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella Type III secretion system. Vaccine 24:6216-6224. [DOI] [PubMed] [Google Scholar]
- 17.Levine, M. M., D. Herrington, J. R. Murphy, J. G. Morris, G. Losonsky, B. Tall, A. A. Lindberg, S. Svenson, S. Baqar, M. F. Edwards, et al. 1987. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J. Clin. Investig. 79:888-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medaglini, D., A. Ciabattini, M. R. Spinosa, T. Maggi, H. Marcotte, M. R. Oggioni, and G. Pozzi. 2001. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 19:1931-1939. [DOI] [PubMed] [Google Scholar]
- 19.Parkhill, J., G. Dougan, K. D. James, N. R. Thomson, D. Pickard, J. Wain, C. Churcher, K. L. Mungall, S. D. Bentley, M. T. Holden, M. Sebaihia, S. Baker, D. Basham, K. Brooks, T. Chillingworth, P. Connerton, A. Cronin, P. Davis, R. M. Davies, L. Dowd, N. White, J. Farrar, T. Feltwell, N. Hamlin, A. Haque, T. T. Hien, S. Holroyd, K. Jagels, A. Krogh, T. S. Larsen, S. Leather, S. Moule, P. O'Gaora, C. Parry, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 413:848-852. [DOI] [PubMed] [Google Scholar]
- 20.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 21.Tacket, C. O., G. Losonsky, D. N. Taylor, L. S. Baron, D. Kopecko, S. Cryz, and M. M. Levine. 1991. Lack of immune response to the Vi component of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a in human studies. J. Infect. Dis. 163:901-904. [DOI] [PubMed] [Google Scholar]
- 22.Townsend, S. M., N. E. Kramer, R. Edwards, S. Baker, N. Hamlin, M. Simmonds, K. Stevens, S. Maloy, J. Parkhill, G. Dougan, and A. J. Bäumler. 2001. Salmonella enterica serovar Typhi possesses a unique repertoire of fimbrial gene sequences. Infect. Immun. 69:2894-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tregoning, J. S., S. Clare, F. Bowe, L. Edwards, N. Fairweather, O. Qazi, P. J. Nixon, P. Maliga, G. Dougan, and T. Hussell. 2005. Protection against tetanus toxin using a plant-based vaccine. Eur. J. Immunol. 35:1320-1326. [DOI] [PubMed] [Google Scholar]
- 24.Weening, E. H., J. D. Barker, M. C. Laarakker, A. D. Humphries, R. M. Tsolis, and A. J. Baumler. 2005. The Salmonella enterica serotype Typhimurium lpf, bcf, stb, stc, std, and sth fimbrial operons are required for intestinal persistence in mice. Infect. Immun. 73:3358-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]