Abstract
Pertussis toxin (PT), a secreted virulence factor of Bordetella pertussis, ADP ribosylates mammalian Gi proteins and plays an important early role in respiratory tract infection by this pathogen in a mouse intranasal infection model. To test the hypothesis that PT targets resident airway macrophages (AM) to promote this infection, we depleted AM by intranasal administration of liposome-encapsulated clodronate prior to bacterial inoculation. This treatment enhanced respiratory tract infection by B. pertussis, even though it also induced a rapid influx of neutrophils to the airways. Strikingly, AM depletion also enhanced infection by mutant strains deficient in PT production or activity to the same level as the wild-type infection, indicating that AM may be the primary target cells for PT in promoting infection. The enhancing effect of clodronate-liposome treatment on infection (i) was shown to be due to macrophage depletion rather than neutrophil influx; (ii) was observed for both tracheal infection and lung infection; (iii) was observed during the early and peak phases of the infection but was lost by day 14 postinoculation, during clearance of the infection; (iv) persisted for at least 1 week (prior to bacterial inoculation); and (v) was equivalent in magnitude to the effect of PT pretreatment and the effects were not additive, consistent with the idea that PT targets AM. We found that PT efficiently ADP ribosylated AM G proteins both in vitro and after intranasal administration of PT in mice and that the duration of G protein modification in vivo was equivalent to the duration of the enhancing effect of PT treatment on the bacterial infection. Collectively, these observations indicate that PT targets AM to promote early infection of the respiratory tract by B. pertussis.
Pertussis toxin (PT) is uniquely produced by Bordetella pertussis, a gram-negative bacterial pathogen of the human respiratory tract, and is an important virulence factor in mouse models of B. pertussis infection. PT is an AB5 toxin comprising an enzymatically active A subunit (S1) that ADP ribosylates the alpha subunit of heterotrimeric Gi proteins in mammalian cells (22, 28) and a B heteropentamer that binds unidentified glycoconjugate receptors on cells (3, 38). G protein modification by PT leads to many different effects on signaling pathways in mammalian cells (34), and PT is the factor that causes systemic symptoms of pertussis disease, including lymphocytosis, insulinemia, and histamine sensitivity (27, 29, 32). However, the specific role that PT plays in promotion of B. pertussis infection of the airways has not been determined. Our recent studies using a mouse model have elucidated an important early role for PT in establishing respiratory tract infection by B. pertussis. The numbers of bacteria recovered from the lungs and trachea are significantly lower for a mutant strain that has an in-frame deletion of the genes encoding PT (ΔPT) or produces an enzymatically inactive version of PT (PT*) than for the wild-type strain, and this defect is apparent as early as 24 h postinoculation (5, 7). In addition, PT acts as a soluble factor that can enhance B. pertussis respiratory tract infection even when it is administered intranasally 14 days prior to bacterial inoculation, but not when it is administered just 24 h after bacterial inoculation (7). Together, these observations indicate that PT acts at an early time following bacterial inoculation and that the target cells for this activity of PT are likely to be resident cells of the airways.
Our working hypothesis is that PT targets cells of the innate immune response involved in protection of the airways against bacterial pathogens by inactivating or suppressing Gi protein-coupled signaling pathways that contribute to this arm of host defense. In support of this hypothesis, we and other workers have found that PT activity during B. pertussis infection delays the recruitment of neutrophils to the respiratory tract (5, 7, 24), an activity that may promote early bacterial infection. Neutrophil chemotaxis is known to be sensitive to PT, since almost all chemokine receptors signal through Gi proteins (31). Whether the early delay of neutrophil recruitment after B. pertussis infection is due to a direct effect of PT on neutrophils in the circulation or due to an indirect effect on another cell type has not been determined. In this study, we sought to determine the role of airway macrophages (AM) in host defense against B. pertussis infection and as possible targets for PT activity in promoting this infection. Previous studies have shown that in vitro macrophage migration, like the migration of neutrophils, is sensitive to PT (25). PT has also been found in some studies to inhibit various other macrophage activities, including phagocytosis (26) and responses to cytokines (13, 18) or lipopolysaccharide (10, 20, 39). However, these studies were not performed with AM and did not involve B. pertussis, and so their relevance to early events after B. pertussis infection of the respiratory tract is unclear. Here we show that depletion of AM not only exacerbates B. pertussis infection but also eliminates the need for PT production by the bacteria to achieve maximal infection levels, suggesting that AM may be the primary target cells for PT activity in this host-pathogen interaction.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The B. pertussis background strains used in this study were streptomycin- and nalidixic acid-resistant derivatives of Tohama (21) or 18323 (= ATCC 9797). Strains WT and ΔPT (having an in-frame deletion of the PT genes) were described previously (7). B. pertussis was grown on Bordet-Gengou agar (Becton-Dickinson) plates containing 15% defibrinated sheep blood and the following antibiotics when necessary: streptomycin, 400 μg/ml; nalidixic acid, 20 μg/ml; or gentamicin, 10 μg/ml.
Mouse infection.
Six-week-old female BALB/c mice (Charles River Laboratories) were used for infection experiments. Inocula were prepared and intranasal inoculation (20 μl) of anesthetized mice was performed as previously described (7). At different times, mice were euthanized by carbon dioxide inhalation, the lower respiratory tract (trachea plus lungs) was surgically removed and homogenized in 2 ml of phosphate-buffered saline (PBS), and dilutions were plated on Bordet-Gengou agar plates containing blood and streptomycin. Four days later the number of CFU per respiratory tract was determined. A statistical analysis to compare groups was performed using a t test. Some mice were pretreated by intranasal administration of PT in 20 μl. PT was purified from B. pertussis culture supernatant as previously described (8, 23).
BAL.
Mice were euthanized by carbon dioxide inhalation, and the respiratory tract was exposed by dissection. A small incision was made near the top of the trachea, and a blunt-end 20-gauge needle was inserted and tied in place with surgical thread around the trachea. Bronchoalveolar lavage (BAL) fluid was obtained by four rounds of filling the lungs with 0.7 ml PBS and withdrawing as much of the liquid as possible. BAL samples were centrifuged to pellet the cells, erythrocytes were lysed in ACK lysing buffer (Quality Biologicals, Inc.), cells were washed and resuspended in 1 ml PBS, and aliquots were removed for counting with a hemocytometer and for cytospin centrifugation onto a microscope slide, followed by staining with modified Wright's stain for identification of cell types. To determine the numbers of macrophages and neutrophils in the samples, 100 cells from several microscopy fields were identified.
Airway macrophage depletion.
Clodronate was a gift from Roche Diagnostics GmbH, Mannheim, Germany, and was incorporated into liposomes from a 250-mg/ml solution as previously described (37). Anesthetized mice were inoculated intranasally with 100 μl clodronate-containing liposomes (CL) or PBS-containing liposomes (PL). Depletion was confirmed by analysis of BAL fluid cells as described above.
ADP ribosylation assay.
BAL cells were washed in PBS, pelleted by centrifugation, and lysed in Triton X-100 buffer, and postnuclear supernatant samples were prepared as previously described (8). Giα protein ADP ribosylation assays with these samples were performed using [32P]NAD and PT, and samples were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and autoradiography, as previously described (8).
Western blotting.
Samples were electrophoresed on 12% SDS-PAGE gels and transferred to nitrocellulose membranes. To detect Giα proteins, blocked filters were incubated with polyclonal anti-Giα3 antibody (Santa Cruz Biotechnology), followed by peroxidase-conjugated anti-rabbit immunoglobulin G secondary antibody (Amersham). Blots were developed using ECL Plus (Amersham) and were exposed to X-ray film.
CHO cell clustering assay.
Chinese hamster ovary (CHO) cells were seeded into 24-well tissue culture plates at a concentration of 5 × 104 cells/well. After 16 h of incubation at 37°C samples were added. After 24 to 36 h of incubation the cells were stained with Giemsa stain and examined with a microscope to determine clustering morphology, as first described by Hewlett et al. (16).
RESULTS
Intranasal CL administration depletes airway macrophages but induces neutrophil influx.
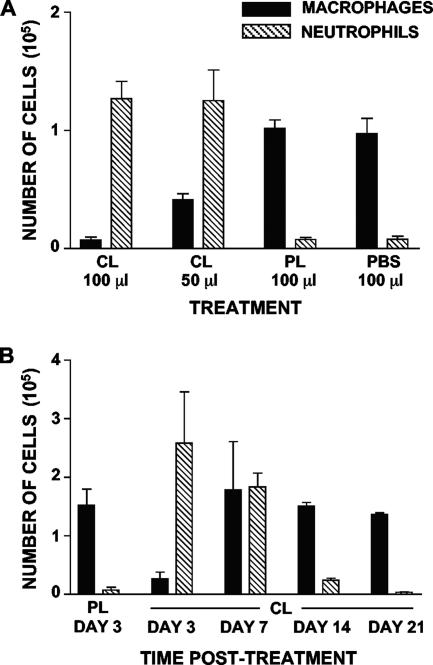
To determine the extent of AM depletion by CL, 6-week-old BALB/c mice (n = 3) were inoculated intranasally with either 50 or 100 μl of CL. Control mice received 100 μl of PL or PBS. Mice were euthanized 24 h later, and the cell content of the BAL fluid was analyzed. As shown in Fig. 1A, CL administration effectively depleted AM (93% reduction with 100 μl and 60% reduction with 50 μl), whereas PL administration had no effect and the results were similar to the results with the PBS control. Surprisingly, a high number of neutrophils (>105 cells) was present in the BAL fluid of CL-treated mice but not in the BAL fluid of PL-treated mice (Fig. 1A). We examined the kinetics of macrophage depletion and neutrophil influx in the airways of CL-treated mice by euthanizing mice (n = 3) at various times after CL administration and analyzing the cell contents of the BAL fluid (Fig. 1B). AM were still depleted 3 days posttreatment (85% reduction compared with PL-treated mice), recovered to normal levels by 7 days posttreatment (although with some variability in numbers), and regained homeostatic levels by 14 days posttreatment. The number of neutrophils peaked at day 3 posttreatment, was still high at 7 days posttreatment, and returned to nearly normal low levels by 14 days posttreatment.
FIG. 1.
Intranasal administration of CL depletes AM but causes neutrophil influx. (A) Numbers of different types of cells in the BAL fluid from groups of three mice 1 day after different treatments. (B) Kinetics of BAL cell content changes after intranasal administration of CL (or PL) to groups of three mice.
CL treatment exacerbates B. pertussis respiratory tract infection and obviates the role for PT.
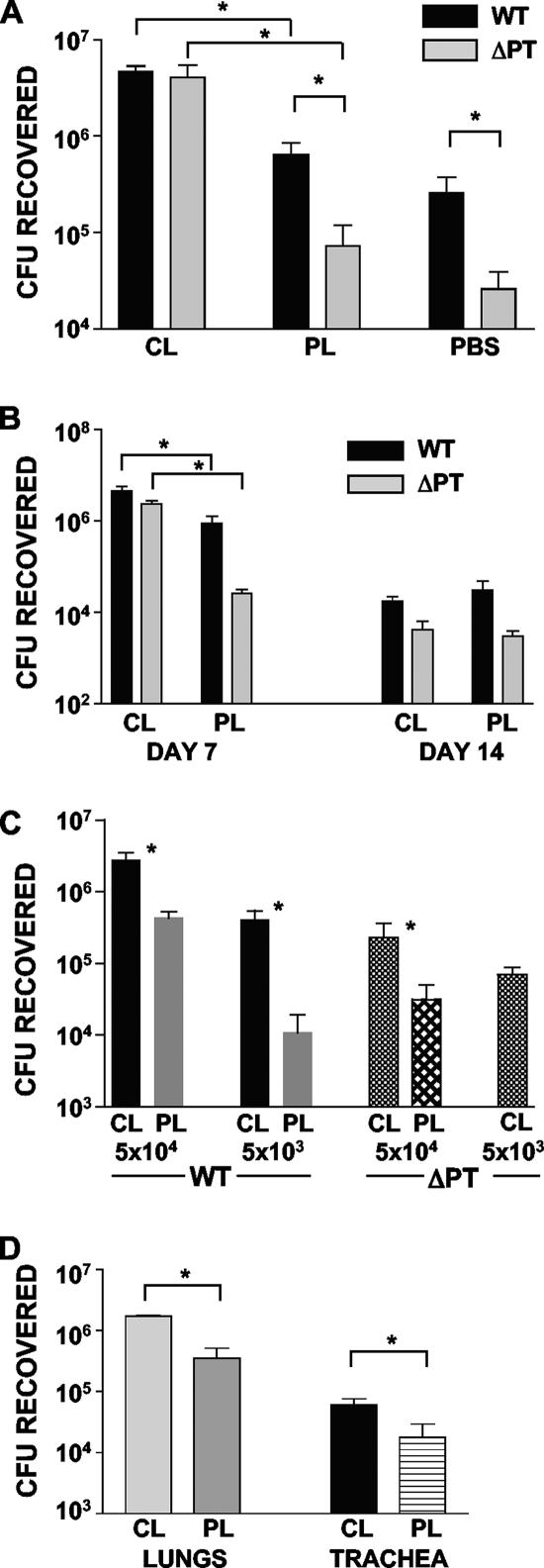
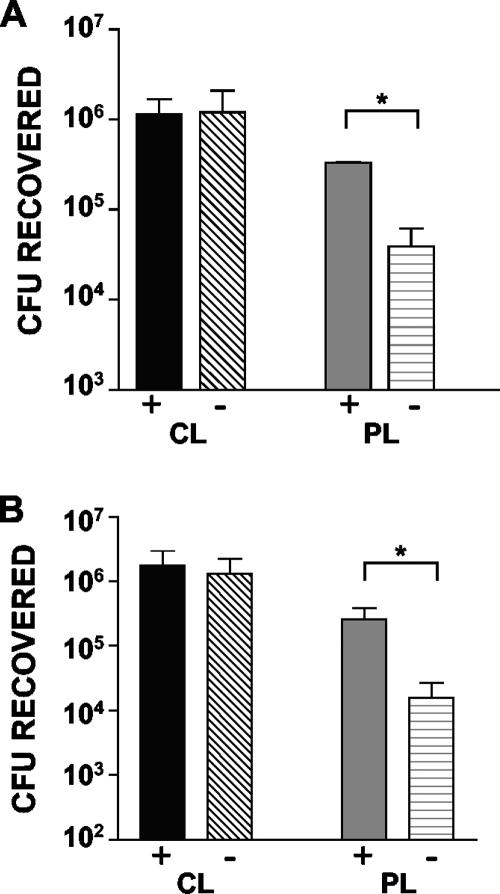
To test the hypothesis that AM protect against B. pertussis infection of the respiratory tract and that PT may target these cells to promote B. pertussis infection, we performed infection experiments with CL- and PL-treated mice. Mice were given 100 μl CL or PL (or PBS as a further control) intranasally, and then 48 h later groups of these mice (n = 4) were inoculated with 5 × 105 CFU of strain Tohama WT or ΔPT. Mice were euthanized 4 days after bacterial inoculation, and bacterial loads in the respiratory tract were determined. As shown in Fig. 2A, in the PL- and PBS-treated control mice, ΔPT was defective for respiratory tract infection, and the numbers of bacteria were >1 log lower than the numbers of strain WT bacteria, as we have previously observed with untreated mice (5, 6, 7). However, in CL-treated mice, two important results were observed: (i) the level of WT infection was enhanced compared with the level in control mice, with an almost 1-log increase in the numbers of bacteria (P = 0.002); and (ii) the level of ΔPT infection was dramatically enhanced (by almost 2 logs; P = 0.03), with the same high numbers of bacteria observed in the WT-infected CL-treated mice. From these data we concluded that resident AM help protect against B. pertussis infection of the respiratory tract and that an important role for PT is to combat the protective activity of these macrophages. To determine the longevity of this enhancing effect after bacterial inoculation, we repeated the experiment with CL- and PL-treated mice inoculated with either Tohama WT or ΔPT, but we euthanized the mice at days 7 and 14 postinoculation, which corresponded to the peak and clearance phases of infection, respectively. As shown in Fig. 2B, the enhancing effect of AM depletion was still apparent 7 days postinoculation, with a fivefold increase in the numbers of bacteria for WT (P = 0.02) and a 2-log increase for ΔPT (P = 0.0003). However, by day 14 postinoculation the enhancing effect was no longer present, indicating that the effect is an effect on early and peak infection but not on clearance. CL treatment also enhanced B. pertussis infection at lower inoculum doses. When a dose of 5 × 104 CFU was used, the bacterial load was 1 log greater in CL-treated mice than in PL-treated mice at 4 days after inoculation with either WT (P = 0.02) or ΔPT (P = 0.04) (Fig. 2C). With a dose of 5 × 103 CFU CL treatment enhanced WT infection 50-fold (P = 0.04), and for ΔPT infection no bacteria were recovered from PL-treated mice, while CL-treated mice had a mean bacterial load of 7 × 104 CFU (Fig. 2C), clearly demonstrating the enhancing power of AM depletion for infection and for overcoming the lack of PT production.
FIG. 2.
CL treatment enhances B. pertussis infection. (A) Groups of four mice were treated as indicated on the y axis and then inoculated with 5 × 105 CFU of strain WT or ΔPT 2 days later. The data indicate the bacterial loads in the airways 4 days postinoculation. (B) Groups of four mice were treated as indicated on the y axis and then inoculated with 5 × 105 CFU of strain WT or ΔPT 2 days later. The data indicate the bacterial loads in the airways 7 and 14 days postinoculation. (C) Groups of four mice were treated as indicated on the y axis and then inoculated with 5 × 104 or 5 × 103 CFU of strain WT or ΔPT 2 days later. The data indicate the bacterial loads in the airways 4 days postinoculation. (D) Groups of four mice were treated as indicated on the y axis and then inoculated with 5 × 105 CFU of Tohama ΔPT 2 days later. The data indicate the bacterial loads in the lungs and trachea 4 days postinoculation. An asterisk indicates that the P value is <0.05, as determined by a t test.
Since pertussis is considered to be primarily a tracheobronchial infection rather than a deeper lung infection (although pneumonia can occur) (14), we analyzed infection of the trachea and lungs separately in CL-treated mice. CL- and PL-treated mice were inoculated 2 days later with 5 × 105 CFU of Tohama WT or ΔPT and then euthanized 4 days postinoculation to determine the bacterial loads in the lungs and trachea. As shown in Fig. 2D (for ΔPT infection), CL treatment significantly enhanced the infection at both locations (P < 0.05), and the same effect was observed for the WT infection (data not shown), demonstrating that this is not a lung-specific phenomenon but occurs throughout the lower respiratory tract.
AM depletion by CL treatment also significantly enhanced respiratory tract infection by WT and ΔPT derivatives of B. pertussis strain 18323 (the ATCC type strain), as well as infection by Tohama PT*, a strain producing an enzymatically inactive version of PT (7, 33), confirming that this is not a strain-specific phenomenon (data not shown).
B. pertussis infection is enhanced by AM depletion and not by neutrophil influx.
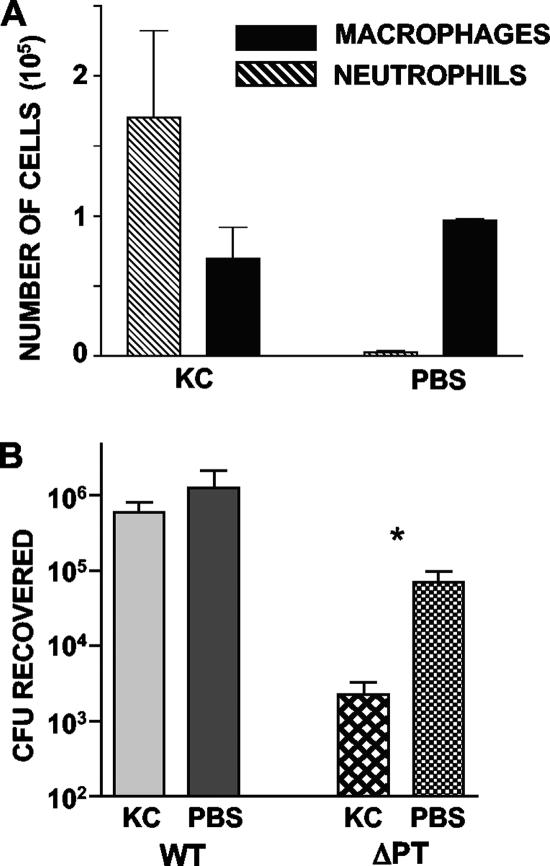
Since CL treatment of BALB/c mice resulted in both macrophage depletion and neutrophil influx in the airways (Fig. 1), we wanted to rule out the unlikely possibility that neutrophil influx rather than AM depletion was responsible for the enhancing effect on infection. Mice were treated by intranasal administration of 1 μg KC (PeproTech), a chemokine that recruits neutrophils to the airways (36). Control mice were treated with an equal volume of PBS. Twenty-four hours later, three mice per group were euthanized, and BAL cells were analyzed to confirm neutrophil influx to the airways; the remaining mice (n = 5) were inoculated with 5 × 105 CFU of either Tohama WT or ΔPT. Inoculated mice were euthanized 4 days postinoculation for assessment of infection levels. As shown in Fig. 3A, KC administration caused a large influx of neutrophils, comparable to the influx caused by CL treatment, but did not significantly deplete AM. In contrast to CL treatment, KC treatment had no effect on WT infection and reduced the level of infection of Tohama ΔPT (by approximately 20-fold) (Fig. 3B). From these data we concluded that macrophage depletion, and not neutrophil influx, is responsible for enhancing B. pertussis infection of the airways.
FIG. 3.
Neutrophil influx without macrophage depletion does not enhance B. pertussis infection. (A) Groups of three mice were treated intranasally with 1 μg KC or PBS, and the data indicate the BAL cell content 1 day later. (B) Groups of five mice were treated intranasally with 1 μg KC or PBS and then inoculated with 5 × 105 CFU of Tohama WT or ΔPT 1 day later. The data indicate the bacterial loads in the airways 4 days postinoculation. The asterisk indicates that the P value is <0.05, as determined by a t test.
Longevity of the enhancing effect of AM depletion on B. pertussis infection.
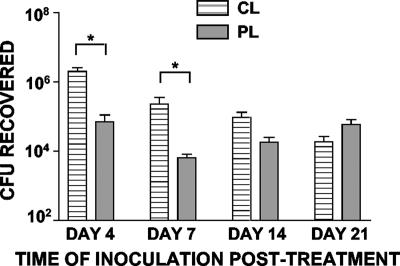
To determine the longevity prior to bacterial inoculation of the enhancing effect of AM depletion on B. pertussis infection, we inoculated CL- and PL-treated mice with 2 × 105 CFU Tohama ΔPT at various times after liposome treatment. In preliminary experiments, we determined that bacterial inoculation on day 1, 2, or 3 after CL treatment resulted in equivalent enhancing effects on infection (data not shown). In addition, as shown in Fig. 4, CL treatment had an enhancing effect when bacterial inoculation occurred 4 or 7 days after liposome treatment, but there was no longer a significant enhancing effect by day 14 after liposome treatment. This may have been due to the recovery of numbers (Fig. 1) and activity of AM by this time after CL-mediated depletion.
FIG. 4.
Longevity of the enhancing effect of AM depletion prior to bacterial inoculation. Groups of four mice were treated with CL or PL and then inoculated with 5 × 105 CFU of Tohama ΔPT at different times after treatment. The data indicate the bacterial loads in the airways 4 days postinoculation. An asterisk indicates that the P value is <0.05, as determined by a t test.
PT and CL enhance B. pertussis infection by acting on the same targets.
The effect of macrophage depletion on enhancement of B. pertussis infection of the airways is reminiscent of the enhancing effect of intranasal administration of purified PT, as we previously reported (7). In the previous study, we found that administration of PT along with the bacterial inoculum allowed Tohama ΔPT to infect at WT levels. In addition, administration of PT up to 14 days prior to bacterial inoculation had the same enhancing effect, indicating that the effect of PT was relatively long lived. Since AM depletion appears to obviate the need for PT activity during early B. pertussis infection, we hypothesized that intranasally administered PT targets AM and inactivates some aspect of their antibacterial activity, in effect mimicking the AM depletion caused by CL treatment. To test this hypothesis, we determined whether PT treatment had any enhancing effect in addition to that provided by CL treatment. Mice that were treated with CL and PL (n = 4) were inoculated 2 days later with 2 × 105 CFU Tohama ΔPT either with or without 100 ng PT in the inoculum and were euthanized on day 4 postinoculation for assessment of infection levels. As shown in Fig. 5A, PT had no additional enhancing effect on infection in CL-treated mice, while it was still able to enhance the lower level of infection in PL-treated mice. We performed a similar experiment but with prior intranasal treatment of mice with 100 ng PT (or PBS) 5 days before CL or PL treatment (instead of coadministration of PT with the bacterial inoculum) and obtained similar results (Fig. 5B). Thus, PT and CL enhancement of B. pertussis infection is not additive, suggesting that PT and CL have the same targets and consistent with the hypothesis that PT targets AM to enhance early infection.
FIG. 5.
CL and PT enhancing effects are not additive. (A) Groups of four mice were treated with CL or PL and then inoculated 2 days later with 2 × 105 CFU of Tohama ΔPT either with (+) or without (−) 100 ng PT. The data indicate the bacterial loads in the airways 4 days postinoculation. (B) Groups of four mice were treated intranasally with 100 ng PT (+) or with PBS (−), treated 5 days later with CL or PL, and then inoculated 2 days later with 2 × 105 CFU of Tohama ΔPT. The data indicate the bacterial loads in the airways 4 days postinoculation. An asterisk indicates that the P value is <0.05, as determined by a t test.
PT ADP ribosylates G proteins in AM.
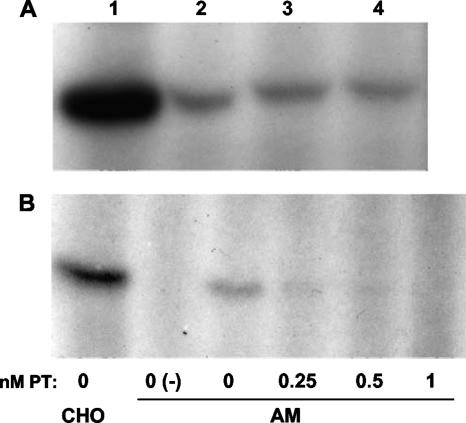
As a further test of the hypothesis that PT targets AM, we sought to determine whether the longevity of the enhancing effect of PT for B. pertussis infection correlated with the longevity of ADP ribosylation of AM G proteins in PT-treated mice. In a preliminary experiment, we determined whether AM from BALB/c mice express PT target G proteins. BAL was performed with six uninfected mice, cells from the BAL fluid (>98% macrophages) were pelleted and lysed, and the postnuclear supernatant (PNS) containing G proteins was used as the substrate in an in vitro ADP ribosylation assay. As shown in Fig. 6A, ADP-ribosylated PT target G proteins (molecular mass, approximately 41 kDa) were detected in these AM PNS samples. Furthermore, while pooled cells from three mice provided abundant substrate for PT ADP ribosylation (Fig. 6A, lane 1), the cells from a single mouse (approximately 105 cells) provided sufficient target substrate to be detected by this assay (lanes 2 to 4). We next confirmed that PT could enter and modify the G proteins of intact AM (recovered from untreated mice by BAL) in vitro. BAL cells were resuspended in Dulbecco modified Eagle medium and plated in six-well culture plates, PT was added, and the plates were incubated at 37°C in the presence of 5% CO2 for 3 h. Cells were recovered from the wells, washed, and lysed, and PNS samples were prepared and used in an in vitro ADP ribosylation assay. As shown in Fig. 6B, while untreated AM retained G proteins that were ADP ribosylated by PT in the in vitro assay (Fig. 6B, lane 3), incubation of intact AM with increasing concentrations of PT progressively reduced the level of available PT-modifiable G proteins (73% with 0.25 nM PT, 87% with 0.5 nM PT, and >95% with 1 nM PT) in the in vitro ADP ribosylation assay, demonstrating that PT can enter and modify target G proteins in intact AM in culture. Western blot analysis of the PNS samples confirmed that PT target G proteins were expressed by PT-treated AM at normal levels (data not shown), ruling out the possibility that PT treatment resulted in a loss of G protein expression (rather than ADP ribosylation).
FIG. 6.
PT ADP ribosylates G proteins in AM. (A) PNS was prepared from pooled AM from the BAL fluid of three untreated mice (lane 1) or from AM from the BAL fluid of individual untreated mice (lanes 2 to 4), used in an ADP ribosylation assay, and analyzed by SDS-PAGE and autoradiography. The band (ADP ribosylated by PT in vitro) is the Giα subunit (molecular mass, approximately 41 kDa). (B) AM were recovered from the BAL fluid of six untreated mice, pooled, plated in a six-well culture dish (105 cells/well), and treated for 3 h with different doses of PT. PNS was prepared from these samples (and CHO cells as a control), used in an ADP ribosylation assay, and analyzed by SDS-PAGE and autoradiography. Lane 0(−) contained a control in vitro ADP ribosylation reaction mixture with no PT added (to demonstrate PT-specific labeling of Giα).
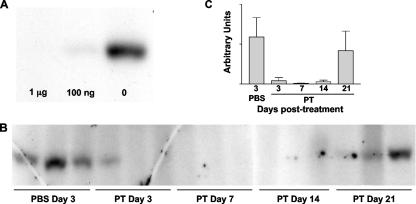
Next we determined whether intranasally administered PT could modify target G proteins of resident AM in live mice. Mice (n = 3) were treated with 1 μg or 100 ng PT or an equivalent volume of PBS, and 2 days later BAL cells were recovered, pooled in groups, washed, and lysed for the ADP ribosylation assay. As shown in Fig. 7A, G proteins in AM from mice treated with 1 μg PT were almost completely (>99%) modified (no unmodified proteins available for labeling in the in vitro assay), and G proteins in AM from mice treated with 100 ng PT were also substantially modified (>95%), while G proteins in AM from mice treated with PBS were unmodified and fully labeled in the in vitro assay. Western blot analysis once again confirmed that PT target G proteins were expressed normally in these AM (data not shown). Therefore, intranasal administration of PT efficiently targets and modifies AM in live mice. To determine the longevity of G protein modification in AM after PT treatment of mice, 12 mice were treated intranasally with 100 ng PT, and three control mice were treated with an equivalent volume of PBS. Groups of three mice were then euthanized on day 3 (including control mice), day 7, day 14, and day 21 posttreatment, and BAL cells were recovered, washed, and lysed for the ADP ribosylation assay. As shown in Fig. 7B, whereas AM from PBS-treated mice retained unmodified G proteins (labeled in the in vitro assay), AM from PT-treated mice were ADP ribosylated by day 3 posttreatment, and this modification was retained through day 14 posttreatment, with virtually no unmodified G proteins detectable (<5%) (Fig. 7C) in these samples. By day 21 posttreatment, however, unmodified G proteins were detected in samples from each of the three mice (mean, 71%) (Fig. 7C), indicating that in vivo G protein modification by PT was reduced or lost by this time. These data indicate that ADP ribosylation of AM G proteins by PT in vivo lasts for 2 to 3 weeks, which correlates closely with the longevity of the enhancing effect of PT treatment on respiratory tract infection by B. pertussis (7), providing further support for the hypothesis that PT targets AM to promote this bacterial infection. We assumed that G protein modification at days 7 and 14 was due to the activity of PT immediately after intranasal administration rather than to continuous PT activity over the course of the experiment, since PT activity was undetectable (by the CHO cell clustering assay) in the BAL fluid of mice treated with 100 ng PT at days 7 and 14 posttreatment (data not shown).
FIG. 7.
Kinetics of recovery of AM G proteins in vivo after ADP ribosylation by PT. (A) Groups of three mice were treated intranasally with 1 μg or 100 ng PT or with an equivalent volume of PBS, and 3 days later BAL cells were recovered and pooled in groups. PNS samples were prepared, used in an ADP ribosylation assay, and analyzed by SDS-PAGE and autoradiography, and the results are shown. (B) Mice were treated intranasally with 100 ng PT (n = 12) or an equivalent volume of PBS (n = 3), and at different times BAL cells were recovered. Then PNS samples were prepared, used in an ADP ribosylation assay, and analyzed by SDS-PAGE and autoradiography. Results for individual mice are shown. (C) Densitometric quantitation of the results in panel B, expressed as group mean values in arbitrary units.
DISCUSSION
In this project we are attempting to determine the role played by PT in the early phase of respiratory tract infection by B. pertussis in a mouse model. In the study reported here, we tested the hypothesis that AM protect against this infection and are the target cells for the infection-promoting activity of PT. Our results provide several lines of evidence that support this hypothesis: (i) B. pertussis infection was enhanced by AM depletion; (ii) the need for PT activity for full infection was obviated by AM depletion; (iii) the enhancing effects of AM depletion and PT treatment were equivalent and not additive; (iv) PT intoxicated AM in vivo; and (v) the longevity of in vivo AM intoxication by PT was equivalent to the longevity of the enhancement of infection by PT treatment of mice.
We used intranasal administration of CL to deplete AM, and our conclusions rest on the assumption that this is the major activity of this treatment that influences B. pertussis infection in our model. Although CL treatment is widely used as a tool to deplete macrophages specifically (1, 4, 37), it is conceivable that this treatment has other unanticipated effects, and indeed we were surprised to find that while it was effective at macrophage depletion, it also induced marked neutrophil influx into the airways. This phenomenon has not been reported often after intranasal CL treatment of mice, although it has been observed previously in some studies (1, 4) and may depend on the age and strain of mice and other differences in the state of the animals. However, CL is thought to induce apoptosis of macrophages (37), and macrophage apoptosis has been found to induce production of neutrophil-recruiting chemokines both in the airways (30) and in the peritoneum (17). Therefore, the presence of large numbers of apoptotic macrophages in the airways after CL treatment is likely the cause of the influx of neutrophils. How then is B. pertussis able to thrive in the presence of large numbers of neutrophils? Our data from KC-treated mice show that neutrophil influx in the absence of macrophage depletion does not increase B. pertussis infection, ruling out the unlikely explanation that bacterial replication is enhanced by the presence of neutrophils. B. pertussis secretes adenylate cyclase toxin, which can inhibit neutrophil activity (9, 11), and mutants lacking this toxin infect the mouse respiratory tract at reduced levels (5, 12), so this may be a primary mechanism of defense against neutrophils. Intriguingly, KC treatment significantly reduced infection by ΔPT but not by WT in our study, suggesting that PT may also play a role in combating neutrophil activity once the cells are present in the airways. However, we have recently observed that B. pertussis infection is unaltered in naïve BALB/c mice depleted of neutrophils (C. Andreasen and N. Carbonetti, unpublished results), bringing into question the role of neutrophils in protection against this infection in nonimmune animals.
Our observations that macrophage depletion enhances B. pertussis infection in this model and obviates the need for PT strongly support the hypothesis that AM are involved in innate immune protection against this pathogen and are the primary target cells for PT. PT can intoxicate virtually any mammalian cell in culture, and specific cell surface receptors for PT have not been identified, but which cells are intoxicated in vivo during infection is unknown. In this study we showed that PT intoxicates AM in vivo after intranasal administration of purified toxin. Interestingly, we observed that the modification of G proteins by PT in these cells lasted for at least 14 days, which is very similar to the longevity of the enhancing effect of intranasally administered PT on B. pertussis infection in this model, as we previously reported (7), which again is consistent with the hypothesis that PT intoxication of AM enhances B. pertussis infection. However, the life span of AM in mice was reported to be 6 to 7 days (2), so it is unclear how the effect of PT on AM after one treatment lasted longer than this. One possibility is that PT inhibits AM turnover, either by prolonging viability or by preventing migration of cells. Another possibility is that active PT persists in the respiratory tract after administration, but we were unable to detect any active PT in the BAL fluid of treated mice at 7 and 14 days posttreatment. The effect of PT on AM in vivo was observed after the administration of purified PT, whereas a more relevant question is whether the effect can be observed after B. pertussis infection. We were unable to detect such an effect consistently (data not shown), but this experiment was complicated by the influx of neutrophils and other cells after infection, and so the cells recovered from BAL fluid were not exclusively resident AM. Purification of AM after infection may be necessary to answer this question. Another interesting question is, which aspect of AM function does PT inhibit? PT may suppress macrophage migration to sites of bacterial growth, phagocytosis and killing of bacteria, or cytokine and chemokine production and downstream immune responses. Various immunosuppressive activities of PT have been described (6, 20, 24, 29, 35), and suppression of AM activities may yet be added to the list.
The longevity of the enhancing effect of CL treatment on infection also supports a role for AM in protection and as targets for PT. We observed that CL treatment enhanced the early and peak phases of infection (up to day 7 postinoculation) but did not increase the bacterial load during the clearance phase (day 14 postinoculation), indicating that it had an effect on innate immunity but not on adaptive immunity. The enhancing effect of CL treatment was relatively long lived prior to bacterial inoculation, and a clear enhancing effect was maintained beyond 7 days posttreatment. This was despite our observation that AM numbers had recovered to normal, although variable, levels by 7 days posttreatment (Fig. 1). This may have been due to the relatively poor antibacterial activity of newly recruited AM, which may have needed a period of adaptation to the new environment before maturation into fully functional cells. We are currently attempting to assay the antibacterial functions of AM recovered from mice after various treatments, and the results of our experiments should shed light on these questions.
An important question that bears on these studies is, how relevant is this mouse model to early events of pertussis infection in humans? The primary site of bacterial growth in human infection is thought to be the ciliated epithelium of the trachea and bronchi (14), although this belief is due primarily to observations from animal model and organ culture infections. Macrophages are more numerous in the alveoli than at higher locations in the airways, although they are also present in the trachea and bronchi (19). We found that AM depletion enhanced infection of both lungs and trachea in our model, demonstrating that this effect is not confined to the lungs, which are thought to be less frequently involved in the human infection. In addition, PT was found to inhibit phagocytosis of B. pertussis by human monocytes isolated from peripheral blood (35), indicating a possible mechanism of inhibition of AM activity by PT.
Pertussis continues to be an important infectious disease worldwide, and the incidence of pertussis has been on the rise in the United States in recent years (15). A greater understanding of the interplay between the virulence mechanisms employed by the bacteria and the protective immune responses of the host should be beneficial in developing new therapeutics and vaccines to reduce the incidence of infection and disease caused by B. pertussis. In the absence of human volunteer studies of this infection, the mouse airway infection model may provide important clues for progress toward such an understanding.
Acknowledgments
This work was supported by NIH grants AI050022 and AI063080.
We thank Zoë Worthington, Roger Plaut, and Charlotte Andreasen for critical comments on the manuscript.
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 22 January 2007.
REFERENCES
- 1.Benoit, A., Y. Huang, J. Proctor, G. Rowden, and R. Anderson. 2006. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV). Clin. Exp. Immunol. 145:147-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blusse van Oud Alblas, A., and R. van Furth. 1983. A quantitative evaluation of pulmonary macrophage kinetics. Cell Tissue Kinet. 16:211-219. [PubMed] [Google Scholar]
- 3.Brennan, M. J., J. L. David, J. G. Kenimer, and C. R. Manclark. 1988. Lectin-like binding of pertussis toxin to a 165 kilodalton Chinese hamster ovary cell glycoprotein. J. Biol. Chem. 263:4895-4899. [PubMed] [Google Scholar]
- 4.Broug-Holub, E., G. B. Toews, J. F. van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine III, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbonetti, N. H., G. V. Artamonova, C. Andreasen, and N. Bushar. 2005. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect. Immun. 73:2698-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carbonetti, N. H., G. V. Artamonova, C. Andreasen, E. Dudley, R. M. Mays, and Z. E. V. Worthington. 2004. Suppression of serum antibody responses by pertussis toxin after respiratory tract colonization by Bordetella pertussis and identification of an immunodominant lipoprotein. Infect. Immun. 72:3350-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbonetti, N. H., G. V. Artamonova, R. M. Mays, and Z. E. V. Worthington. 2003. Pertussis toxin plays an early role in respiratory tract colonization by Bordetella pertussis. Infect. Immun. 71:6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carbonetti, N. H., R. M. Mays, G. V. Artamonova, R. D. Plaut, and Z. E. Worthington. 2005. Proteolytic cleavage of pertussis toxin S1 subunit is not essential for its activity in mammalian cells. BMC Microbiol. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Confer, D. L., and J. W. Eaton. 1982. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science 217:948-950. [DOI] [PubMed] [Google Scholar]
- 10.Daniel-Issakani, S., A. M. Spiegel, and B. Strulovici. 1989. Lipopolysaccharide response is linked to the GTP binding protein, Gi2, in the promonocytic cell line U937. J. Biol. Chem. 264:20240-20247. [PubMed] [Google Scholar]
- 11.Friedman, R. L., R. L. Fiederlein, L. Glasser, and J. N. Gagliani. 1987. Bordetella pertussis adenylate cyclase: effects of affinity-purified adenylate cyclase on human polymorphonuclear leukocyte functions. Infect. Immun. 55:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodwin, M. S., and A. A. Weiss. 1990. Adenylate cyclase toxin is critical for colonization and pertussis toxin is critical for lethal infection by Bordetella pertussis in infant mice. Infect. Immun. 58:3445-3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, Y. X., E. Hewlett, D. Temeles, and P. Quesenberry. 1988. Inhibition of interleukin 3 and colony-stimulating factor 1-stimulated marrow cell proliferation by pertussis toxin. Blood 71:1187-1195. [PubMed] [Google Scholar]
- 14.Hewlett, E. L. 1997. Pertussis: current concepts of pathogenesis and prevention. Pediatr. Infect. Dis. J. 16:S78-S84. [DOI] [PubMed] [Google Scholar]
- 15.Hewlett, E. L., and K. M. Edwards. 2005. Clinical practice. Pertussis—not just for kids. N. Engl. J. Med. 352:1215-1222. [DOI] [PubMed] [Google Scholar]
- 16.Hewlett, E. L., K. T. Sauer, G. A. Myers, J. L. Cowell, and R. L. Guerrant. 1983. Induction of a novel morphological response in Chinese hamster ovary cells by pertussis toxin. Infect. Immun. 40:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hohlbaum, A. M., M. S. Gregory, S. T. Ju, and A. Marshak-Rothstein. 2001. Fas ligand engagement of resident peritoneal macrophages in vivo induces apoptosis and the production of neutrophil chemotactic factors. J. Immunol. 167:6217-6224. [DOI] [PubMed] [Google Scholar]
- 18.Hume, D. A., and Y. M. Denkins. 1989. Activation of macrophages to express cytocidal activity correlates with inhibition of their responsiveness to macrophage colony-stimulating factor (CSF-1): involvement of a pertussis toxin-sensitive reaction. Immunol. Cell Biol. 67:243-249. [DOI] [PubMed] [Google Scholar]
- 19.Hume, D. A., V. H. Perry, and S. Gordon. 1984. The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80: macrophages associated with epithelia. Anat. Rec. 210:503-512. [DOI] [PubMed] [Google Scholar]
- 20.Jakway, J. P., and A. L. DeFranco. 1986. Pertussis toxin inhibition of B cell and macrophage responses to bacterial lipopolysaccharide. Science 234:743-746. [DOI] [PubMed] [Google Scholar]
- 21.Kasuga, T., Y. Nakase, K. Ukishima, and K. Takatsu. 1954. Studies on Haemophilus pertussis. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch. Exp. Med. 27:57-62. [PubMed] [Google Scholar]
- 22.Katada, T., M. Tamura, and M. Ui. 1983. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch. Biochem. Biophys. 224:290-298. [DOI] [PubMed] [Google Scholar]
- 23.Kimura, A., K. T. Mountzouros, P. A. Schad, W. Cieplak, and J. L. Cowell. 1990. Pertussis toxin analog with reduced enzymatic and biological activities is a protective immunogen. Infect. Immun. 58:3337-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirimanjeswara, G. S., L. M. Agosto, M. J. Kennett, O. N. Bjornstad, and E. T. Harvill. 2005. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J. Clin. Investig. 115:3594-3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meade, B. D., P. D. Kind, J. B. Ewell, P. P. McGrath, and C. R. Manclark. 1984. In vitro inhibition of murine macrophage migration by Bordetella pertussis lymphocytosis-promoting factor. Infect. Immun. 45:718-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mork, T., and R. E. Hancock. 1993. Mechanisms of non-opsonic phagocytosis of Pseudomonas aeruginosa. Infect. Immun. 61:3287-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse, S. I., and J. H. Morse. 1976. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J. Exp. Med. 143:1483-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moss, J., S. J. Stanley, D. L. Burns, J. A. Hsia, D. A. Yost, G. A. Myers, and E. L. Hewlett. 1983. Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J. Biol. Chem. 258:11879-11882. [PubMed] [Google Scholar]
- 29.Munoz, J. J., H. Arai, R. K. Bergman, and P. L. Sadowski. 1981. Biological activities of crystalline pertussigen from Bordetella pertussis. Infect. Immun. 33:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neff, T. A., R. F. Guo, S. B. Neff, J. V. Sarma, C. L. Speyer, H. Gao, K. D. Bernacki, M. Huber-Lang, S. McGuire, L. M. Hoesel, N. C. Riedemann, B. Beck-Schimmer, F. S. Zetoune, and P. A. Ward. 2005. Relationship of acute lung inflammatory injury to Fas/FasL system. Am. J. Pathol. 166:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olson, T. S., and K. Ley. 2002. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R7-R28. [DOI] [PubMed] [Google Scholar]
- 32.Pittman, M. 1979. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev. Infect. Dis. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 33.Pizza, M., A. Covacci, A. Bartoloni, M. Perugini, L. Nencioni, M. T. DeMagistris, L. Villa, D. Nucci, R. Manetti, M. Bugnoli, F. Giovannoni, R. Olivieri, J. T. Barbieri, H. Sato, and R. Rappuoli. 1989. Mutants of pertussis toxin suitable for vaccine development. Science 246:497-500. [DOI] [PubMed] [Google Scholar]
- 34.Reisine, T. 1990. Pertussis toxin in the analysis of receptor mechanisms. Biochem. Pharmacol. 39:1499-1504. [DOI] [PubMed] [Google Scholar]
- 35.Schaeffer, L. M., and A. A. Weiss. 2001. Pertussis toxin and lipopolysaccharide influence phagocytosis of Bordetella pertussis by human monocytes. Infect. Immun. 69:7635-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai, W. C., R. M. Strieter, J. M. Wilkowski, K. A. Bucknell, M. D. Burdick, S. A. Lira, and T. J. Standiford. 1998. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J. Immunol. 161:2435-2440. [PubMed] [Google Scholar]
- 37.Van Rooijen, N., and A. Sanders. 1994. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174:83-93. [DOI] [PubMed] [Google Scholar]
- 38.Witvliet, M. H., D. L. Burns, M. J. Brennan, J. T. Poolman, and C. R. Manclark. 1989. Binding of pertussis toxin to eukaryotic cells and glycoproteins. Infect. Immun. 57:3324-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, X., and D. C. Morrison. 1993. Pertussis toxin-sensitive factor differentially regulates lipopolysaccharide-induced tumor necrosis factor-alpha and nitric oxide production in mouse peritoneal macrophages. J. Immunol. 150:1011-1018. [PubMed] [Google Scholar]