Abstract
Antibodies of the immunoglobulin A (IgA) class react with capsular polysaccharides of Streptococcus pneumoniae and support complement-dependent opsonophagocytosis (OPC) of the organism by phagocytes. We characterized the biologic impact of the molecular forms of human monoclonal capsule-specific IgA (monomeric IgA [mIgA], polymeric IgA [pIgA], and secretory IgA [SIgA]) on OPC and susceptibility to cleavage by IgA1 protease. The efficiency of SIgA in support of OPC of S. pneumoniae was comparable to that of pIgA, and both forms exceeded that of mIgA by a fivefold margin. This structure-function relationship was associated with three factors. First, the avidities, or functional affinities, of both pIgA and SIgA for pneumococcal capsules exceeded those of mIgA. Second, both pIgA and SIgA required less complement to achieve similar levels of bacterial OPC than did mIgA, indicating that secretory component does not hinder the effect of complement. Third, both pIgA and SIgA mediated agglutination of the organism, whereas mIgA did not. All three forms of capsule-specific IgA showed comparable susceptibilities to cleavage and functional inhibition by bacterial IgA1 protease, demonstrating that secretory component does not prevent the proteolytic degradation of IgA1 by IgA1 protease. IgA1 cleavage results in formation of identical Fab fragments for each of the molecular forms, thereby abolishing the contribution of multivalence of pIgA and SIgA. In summary, the polymeric forms of IgA (both pIgA and SIgA) provide a substantial advantage in binding, agglutination, and OPC of the organism.
Streptococcus pneumoniae infection is an important cause of both mucosal disease and systemic infection in children and adults. Each syndrome most often begins with binding to and colonization of the upper respiratory tract and nasopharynx. Limitations in mucosal defense can result in the development of otitis media, sinusitis, and pneumonia, which can be complicated by bacteremia and meningitis. Immunoglobulin A (IgA) may provide both local defense against mucosal infection and activity in local tissues to prevent dissemination of the infection. The ability of capsule-specific IgA in combination with complement to mediate phagocytosis of S. pneumoniae is supported by in vitro data (28, 32). Secretory IgA (SIgA) likely hinders disease transmission through control of mucosal infection and reduction of bacterial shedding (63). Such interruption of colonization and transmission may underlie the association of pneumococcal conjugate vaccine use among children with decreased rates of invasive pneumococcal disease in adults (36, 62, 63). The ability of mucosal IgA to prevent colonization has been shown by the lack of protection in mice deficient in the polymeric Ig (pIg) receptor (54). The pIgA receptor binds pIgA, transports pIgA across epithelial surfaces, and is cleaved to release the non-membrane-associated portion of pIg receptor (secretory component [SC]) bound to pIgA, forming SIgA in the lumen. However, the functional differences in the compartment-specific molecular forms of IgA present in blood and tissue, monomeric IgA (mIgA) and pIgA, and that present at mucosal surfaces, SIgA, have not been characterized for S. pneumoniae.
pIgA has been shown to enhance virus- and toxin-neutralizing capabilities compared with monomeric mIgA (47, 53). The presence of SC in SIgA has been shown to protect the antibody against cleavage by selected proteases (12, 37, 45), and the SC has been proposed to block binding sites required for IgA phagocytic function (22). We propose that characterizing the role of SIgA in the clearance of S. pneumoniae will enhance our understanding of the basic requirements for phagocyte activation and function at mucosal sites. We present evidence of phagocytosis of S. pneumoniae by three forms of IgA (mIgA, pIgA, and SIgA), together with comparative avidity studies and complement requirements for this function. Revealing the mechanisms by which IgA acts at mucosal surfaces and in mucosal tissues will clarify its role as a mediator of mucosal defense and provide a rationale for the development of vaccines that elicit robust antibody responses at mucosal sites.
MATERIALS AND METHODS
hMAb production.
As previously described (61), B cells were purified (>95% CD19+) by negative selection (StemCell Technologies, Vancouver, Canada) from adult human subjects 1 week after intramuscular immunization with 23-valent capsular pneumococcal polysaccharide (PPS) vaccine (PNU-IMMUNE; a generous gift from Lederle-Praxis Biologicals, Pearl River, NY) and fused with the K6H6/B5 heteromyeloma fusion partner (1:2) (8, 9). Written informed consent of human subjects was obtained per Institutional Review Board protocols of the University of Minnesota and the Minneapolis VA Medical Center. Fused cells were grown in medium containing hypoxanthine, aminopterin, and thymidine (Sigma Chemical Co., St. Louis, MO) at 1 × 105 to 3 × 105 cells per 200-μl well. Cells from wells with supernatants reactive with only one of three pooled capture antigens of 3 or 4 serotypes each (11 total) of PPS (ATCC, Manassas, VA) and not cell wall polysaccharide (CPS; Statens Serum Institut, Copenhagen, Denmark) as determined by enzyme-linked immunosorbent assay (ELISA) were plated at 0.6 cells/well on 1 × 105 washed and irradiated (2,500 rads) feeder peripheral blood mononuclear cells and rescreened at 2 to 3 weeks for each of the 4 serotypes in each well. Those reactive with only one serotype were replated at 0.6 cells/well. According to Poisson distribution calculations, this double-cloning approach should yield a 99% probability that the selected cell progeny are clonal (35, 55). Clones 2A01 (IgA2), 2A02 (IgA1), 6BA01 (IgA1), and 8A01 (IgA1) were grown in RPMI 1640 with 4 mM l-glutamine (Invitrogen Corp., Carlsbad, CA) and 15% fetal bovine serum (HyClone Laboratories, Logan, UT) and supernatants harvested for antibody purification. Purified PPS-specific monoclonal antibody was obtained by passing culture supernatant through an anti-human IgA affinity column prepared with goat anti-human IgA (Southern Biotechnology Associates, Inc., Birmingham, AL) coupled to CNBr-activated Sepharose 4B (Amersham Biosciences, Piscataway, NJ), which bound MAb. The human capsule-specific monoclonal antibody (hMAb) was then eluted from the column using 50 mM glycine (Invitrogen Corp.), pH 2.7, and the elution fraction neutralized as it is collected. The quantity, specificity, and IgA subclass of the purified antibody were determined by ELISA (see below). The heterogeneity of molecular forms produced was characterized by 6% nondenaturing polyacrylamide gel electrophoresis (PAGE).
PPS type-specific IgA ELISA.
MaxiSorp plates (Nunc, Inc., Naperville, IL) were coated overnight at 4°C with 100 μl of 5 μg/ml PPS in phosphate-buffered saline. Standards, controls, and samples were incubated overnight with CPS (4 μg/ml) and pneumococcal type 22F PPS (2 μg/ml) (11) to absorb interfering nonspecific antibodies and then applied for 2 h to PPS-coated (see below) and washed plates. Affinity-purified goat anti-human IgA horseradish peroxidase (HRPO) conjugate (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) followed by ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Sigma Chemical Co.) was used to detect bound antibody. Absorbance was read at 405 nm.
Specificity or cross-reactivity of IgA monoclonal antibodies determined by ELISA.
Cloned monoclonal antibodies were retested individually against 11 PPSs (types 1, 2, 3, 4, 6B, 8, 9V, 12F, 14, 19A, and 19F), CPS, and six other antigens (thyroglobulin [Calbiochem-Novabiochem Corp., San Diego, CA], human double-stranded DNA and lipopolysaccharide from Escherichia coli serotype O127:B8 [Sigma Chemical Co.], tetanus toxoid [a gift from W. Latham, Massachusetts Department of Health, Boston], cholera toxin from Vibrio cholerae Inaba 569B [List Biological, Campbell, CA], and Haemophilus influenzae type B capsule polyribosyl ribitol phosphate [Connaught Labs, Swiftwater, PA] linked to tyramine hydrochloride [Sigma]) as ELISA captures to exclude polyreactive and cross-reactive antibodies. The hMAbs were applied after coating and washing, and binding was detected using affinity-purified goat anti-human IgA HRPO conjugate followed by ABTS substrate.
IgA subclass ELISA.
MaxiSorp plates were coated with unlabeled goat anti-human IgA (Jackson ImmunoResearch) diluted in 50 mM carbonate buffer (pH 9.6) and incubated overnight at 4°C. Standards of purified human myeloma IgA1 (Calbiochem-Novabiochem) or purified human myeloma IgA2 (Calbiochem-Novabiochem) and samples were applied for 2 h and detected using mouse anti-human IgA1-biotin or mouse anti-human IgA2-biotin (both Southern Biotechnology Associates) followed by streptavidin-HRPO (Zymed Laboratories Inc., South San Francisco, CA) and TMB substrate (BD Biosciences, San Diego, CA).
Fractionation of IgA molecular forms.
Immunopurified IgA was fractionated according to molecular size using a Sephacryl S300 HR gel filtration column (Amersham Biosciences) (28, 30) calibrated with pIgA and mIgA. Fractions corresponding to each form were pooled, and the pooled fractions were concentrated and analyzed using 6% nondenaturing PAGE to determine fraction purity (see Fig. 2A). Standards included serum IgA and SIgA (MP Biomedicals, Aurora, IL).
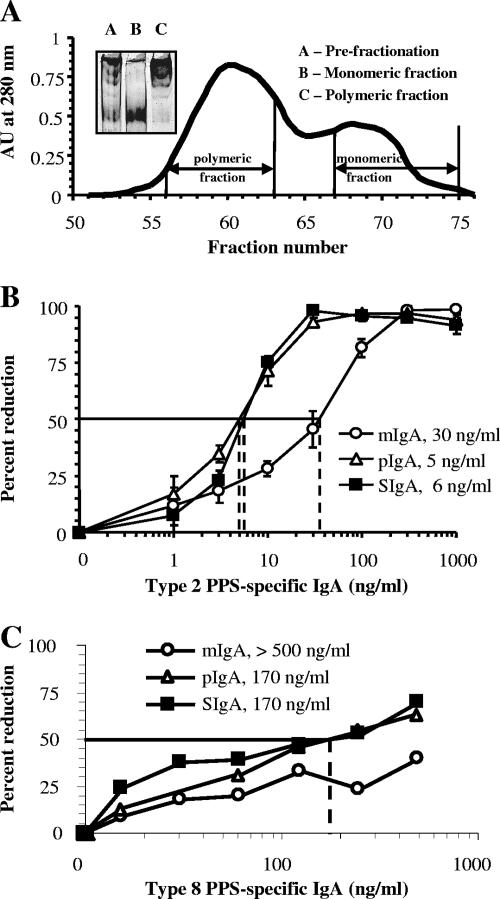
FIG. 2.
(A) Affinity-purified MAb 2A01 supernatant (inset; lane A) was fractionated by gel filtration to yield mIgA (inset; lane B) and pIgA (inset; lane C). AU, absorbance units. (B) Enhanced opsonophagocytosis with MAb 2A01 (IgA2) pIgA and SIgA compared to that seen with mIgA as measured by OPC-CFU (n = 3; error bars = 1 ± standard error of the mean; key indicates the opsonophagocytic index for each molecular form). (C) MAb 8A01 showed results similar to those obtained with MAb 2A01.
Binding of secretory component.
pIgA and recombinant human SC (44) were combined at a weight ratio of 5:1, respectively, and incubated for 1 h at room temperature as described previously (12). Stable association of recombinant human SC and pIgA was confirmed by ELISA. Samples were applied to MaxiSorp plates (Nunc Inc.) coated with PPS as described above and incubated for 2 h. Rabbit anti-human SC (Dako Corp., Carpenteria, CA) followed by goat anti-rabbit biotin (Dako Corp.) and streptavidin-HRPO (Zymed) was used with ABTS substrate to detect bound antibody associated with SC.
OPC-CFU assay.
We compared the abilities of three molecular forms of IgA to mediate opsonophagocytosis (OPC) of type 2 S. pneumoniae isolates (IgA1 protease-producing P210 and IgA1 protease-deficient P354 strains) (60) by freshly isolated neutrophils (19, 27, 28, 48). Log-phase S. pneumoniae isolates (2 × 103 CFU/well) were opsonized with antibody for 30 min followed by the addition of 1 × 106 human neutrophils (effector/target ratio, 500:1) purified from heparinized blood and complement source (baby rabbit complement; Cedarlane Laboratories Limited, Hornby, Ontario, Canada) and incubated for an additional hour. To determine the percentages of bacterial reduction in the presence of test antibodies, the numbers of CFU remaining in each assay well were determined and divided by the number of CFU from control wells containing complement and neutrophils but lacking antibody. The opsonophagocytic index was established as the amount of antibody required to effect a 50% reduction in CFU compared with the results obtained with the control well (complement but no antibody). To test for the effects of complement on OPC, wells with or without hMAb IgAs were tested without complement, with 1%, 3%, or 10% complement, or with 10% heat-inactivated (HI) complement. We determined whether reductions in CFU were mediated by antibody-dependent agglutination and/or by OPC of the organism with the combination of antibody, complement, and phagocytes.
Avidity measurement.
Avidity was measured by two ELISA-based methods: (i) dissociation of antibody binding to the solid-phase antigen in the presence of the mild denaturing agent ammonium thiocyanate (Sigma Chemical Co.) (20, 46) and (ii) soluble antigen competitive inhibition (29, 31). For the former method, PPS-specific hMAb was diluted to give an optical density of 1.0 at 405 nm on a PPS-coated plate. After incubation for 2 h, plates were washed and increasing concentrations (0, 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, and 4.0 M) of ammonium thiocyanate were applied for 30 min and washed. Affinity-purified goat anti-human IgA HRPO conjugate followed by ABTS substrate was used to detect any remaining bound antibody. Absorbance was read at 405 nm. The “avidity index” (AI) was determined as the molar concentration of the ammonium thiocyanate required to inhibit 50% of antibody binding to solid-phase capsular polysaccharides at submaximal concentration (initial optical density of 0.8 to 1.1 at 405 nm; i.e., ∼20 ng/ml). For the competitive inhibition assay, we incubated increasing concentrations (0.0001 to 10 μg/ml) of soluble PPS antigen overnight at 4°C with IgA also at submaximal concentration and compared the binding of the same concentration of IgA without soluble antigen incubation to solid-phase PPS. Disassociation constants (KD) were calculated by the method of Friguet et al. (15). The absorbance value for antibody without soluble antigen (Ao), the absorbance value for antibody at the concentration of soluble antigen which inhibited 50% of IgA binding (A), and the molar concentration of soluble antigen at that 50% inhibition point (ao) were used to determine the antigen-antibody equilibria using the equation KD = ao{[Ao/(Ao − A)] − 1}.
Binding and opsonophagocytosis (OPC-flow) assay.
The ability of IgA to mediate binding and uptake of S. pneumoniae strain P210 by freshly isolated neutrophils was tested by flow cytometry (39, 49). Briefly, heat-killed fluorescein isothiocyanate (FITC)-labeled bacteria (2.5 × 106 CFU) were combined with IgA molecular forms and indicated amounts of complement followed by the addition of 2.5 × 105 human neutrophils purified from blood. The suspension was analyzed for bacterial fluorescence associated with neutrophils at 530 nm by use of a FACSVantage SE cytometer (BD Biosciences). The FITC mean fluorescent intensity reflects the magnitude of binding and uptake of the bacteria by neutrophils for each condition.
Bacterial agglutination.
Washed log-phase bacteria (strain P210) (1 × 106 CFU) were incubated with 1 μg PPS type 2-specific mIgA, pIgA, or SIgA for 30 min at room temperature. The ability of the molecular forms of IgA to induce bacterial agglutination was then measured by both the increase in forward scatter measurement by flow cytometry (FACSVantage SE), which reflects particle size, and the formation of clumps as visualized by standard microscopy at ×1,000 total magnification. Human MAb binding to bacteria was confirmed by staining bacterial suspensions with goat F(ab′)2 anti-human IgA-phycoerythrin (Southern Biotechnology Associates) and analyzed for fluorescence at 575 nm.
Protease digestion.
To investigate the functional effect of IgA1 protease digestion of hMAbs, PPS-specific pIgA and SIgA (2.5 μg) were incubated for 18 h at 37°C with purified His-tagged H. influenzae type 1 IgA1 protease (0.75 μg) (provided by A. Plaut). The protease was then removed using nickel-nitrilotriacetic acid agarose binding (QIAGEN, Valencia, CA), and the antibody was tested in the OPC-CFU assay as described above. Examination of the functional effect of pneumococcal IgA1 protease on IgA was accomplished by extending the incubation of encapsulated type 2 S. pneumoniae (wild-type strain P210 or IgA1 protease-deficient P354) and antibody (pIgA or SIgA) in the OPC-CFU assay to 2 h. This allowed time for the bacterial surface-bound enzyme to act on IgA before phagocytes and complement were added. To examine the effect of proteases on IgA heavy chain structure, pIgA or SIgA (0.15 pmol) was incubated with purified His-tagged H. influenzae type 1 IgA1 protease (0.03, 0.15, 0.3, or 1.5 pmol), cell-associated pneumococcal IgA1 protease (IgA1 protease-producing unencapsulated R6x and IgA1 protease-deficient unencapsulated P262) (1.5 × 106 CFU), human neutrophil elastase (Sigma Chemical Co.) (0.15, 1.5, or 15 pmol), or human neutrophil cathepsin G (Calbiochem, La Jolla, CA) (0.15, 1.5, or 15 pmol) at 37°C for indicated times and separated by denaturing 12% PAGE. The amount of remaining heavy chain was examined by use of silver stain or Western blot probed with mouse anti-human IgA1 (Southern Biotechnology Associates) and mouse anti-human kappa chain (Southern Biotechnology Associates). A low-molecular-weight protein standard (Bio-Rad Laboratories, Hercules, CA) was included for each denaturing gel. The activity of human neutrophil elastase and human neutrophil cathepsin G was confirmed by their ability to hydrolyze the synthetic chromogenic substrates N-methoxysuccinyl-alanyl-alanyl-prolyl-valine p-nitroanilide and N-succinyl-alanyl-alanyl-prolyl-phenylalanine p-nitroanilide (Sigma Chemical), respectively, measured at 405 nm (data not shown).
RESULTS
Monoclonal antibodies are serotype specific.
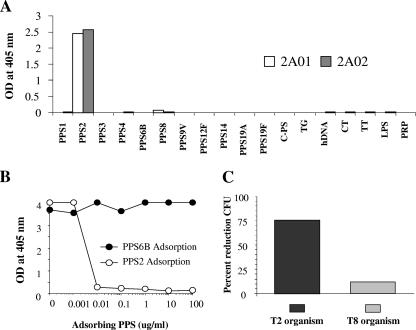
The four hMAbs generated from healthy vaccinated adults were tested extensively to determine their monospecificities for individual pneumococcal capsular polysaccharides. Antibodies purified from supernatants were tested by ELISA for reactivity with 18 antigens (11 PPS, 1 CPS, and 6 other antigens), several of which are recognized by polyreactive antibodies (6, 29). Each of the hMAbs described herein reacted with only one solid-phase antigen (the results for 2A01 and 2A02 are shown in Fig. 1A). Monoreactivity was further confirmed in representative data by competitive inhibition showing that only the homologous antigen (PPS2) to the antibody (hMAb 2A01) inhibited binding to the PPS2 solid antigen, whereas a heterologous antigen (PPS6B) did not (Fig. 1B). Similarly, in the functional opsonophagocytosis assay (OPA-CFU), only antibody to an organism of the homologous serotype (hMAb 2A01 with a type 2 organism) induced a substantial reduction in CFU in the presence of complement and phagocytes whereas a heterologous hMAb to type 8 did not (Fig. 1C). Thus, as confirmed by three methods, the hMAbs are specific for only one serotype of S. pneumoniae.
FIG. 1.
Monoclonal antibody specificities. (A) MAb 2A01 and 2A02 cross-reactivity examined using 18 coating antigens. C-PS, cell wall polysaccharide; TG, thyroglobulin; hDNA, human double-stranded DNA; CT, whole cholera toxin; TT, tetanus toxoid; LPS, lipopolysaccharide; PRP, polyribosyl ribitol phosphate. (B) Competitive inhibition ELISA using MAb 2A01 and PPS type 2 or PPS type 6B as an adsorbing antigen. (C) OPC-CFU using MAb 2A01 against two serotypes of S. pneumoniae.
pIgA and SIgA support opsonophagocytosis of S. pneumoniae more efficiently than mIgA.
Type 2-specific hMAb 2A01 produced a mixture of molecular forms of hMAb IgA (Fig. 2A). Wild-type S. pneumoniae (type 2; strain P210) was incubated with increasing amounts of purified mIgA, pIgA, and SIgA hMAb 2A01 followed by addition of complement (10%) and phagocytes for 1 h, after which the numbers of CFU were determined. Although all forms of hMAb possessed identical antigen-binding regions, fivefold less pIgA and SIgA (5 to 6 ng/ml) were required to produce a 50% reduction of S. pneumoniae CFU compared with mIgA results (30 ng/ml) (Fig. 2B). A comparable pattern of results was found with a type 8-specific hMAb (Fig. 2C) and with polyclonal IgA purified from human serum (data not shown). Results obtained with SIgA were similar to those seen with pIgA, suggesting that the presence of the SC does not interfere with the functional activity of the antibody.
Avidity of PPS-specific hMAb IgA forms varies with valence.
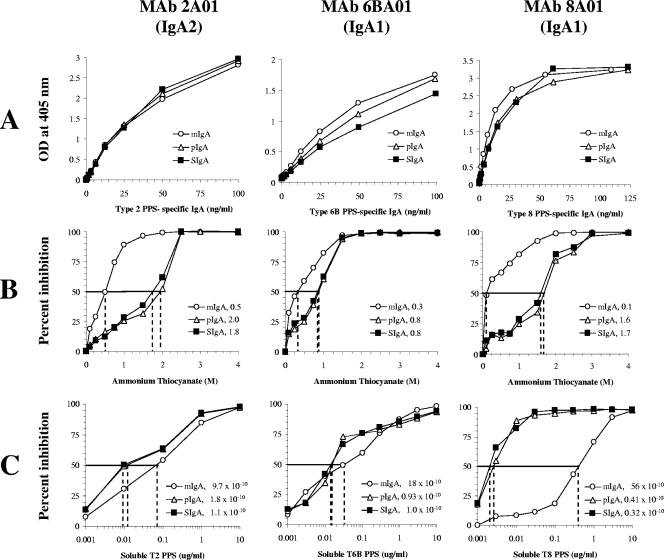
The increased functional efficiency observed with pIgA and SIgA versus mIgA may be related to differences in the avidity of antigen binding. Although binding curves with respect to the solid-phase antigen were comparable by ELISA with the results seen with three molecular forms of the hMAbs (Fig. 3A), both the ammonium thiocyanate avidity measurement (Fig. 3B) and soluble antigen competitive inhibition (Fig. 3C) showed consistently that pIgA and SIgA (valence of at least four antigen binding sites) possess increased avidity (higher AI and smaller KD) compared with mIgA (two antigen binding sites). Moreover, the AI and KD of the pIgA and SIgA forms were very similar, indicating that the presence of the SC does not diminish antibody avidity.
FIG. 3.
Solid-phase ELISA was used to examine affinity and avidity for the three molecular forms of IgA. Antibody binding curves for mIgA, pIgA, and SIgA of one IgA2 (MAb 2A01) and two IgA1s (MAb 6BA01 and MAb 8A01). (A) ELISA binding curves show similar antigen affinities. (B) More ammonium thiocyanate is needed to disrupt pIgA and SIgA antigen binding than is required for disruption of mIgA binding for each of the three monoclonal antibodies (keys indicate the molar amounts of ammonium thiocyanate which result in a 50% reduction of antigen binding for each molecular form). (C) Lower amounts of soluble antigen are able to competitively bind 50% of pIgA and SIgA compared to the amounts of soluble antigen required for mIgA for each of the three monoclonal antibodies (keys indicate the calculated disassociation constant expressed in molar concentration for each molecular form).
IgA-mediated uptake and opsonophagocytosis (OPA-CFU) are complement dose dependent for all IgA forms.
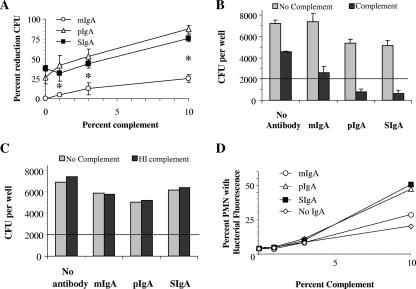
To further examine the impact of molecular form on IgA function, we determined the contribution of complement in induction of OPA-CFU of S. pneumoniae type 2 (strain P210) in the presence of the mIgA, pIgA, and SIgA forms of hMAb 2A01 at a single antibody concentration (30 ng/ml). All three forms demonstrated a progressive increase in the percentage of reduction of CFU with polymorphonuclear leukocytes as complement concentrations rose (Fig. 4A). Reductions in CFU with 10% complement significantly exceeded those in the presence of 0% and 1% complement for mIgA (P < 0.02 and 0.036, respectively), as did reductions in CFU for both pIgA and SIgA at 10% complement compared to the results seen with mIgA at 0%, 1%, and 3% complement (P < 0.005 for all).
FIG. 4.
Opsonophagocytic activity, binding, and phagocytosis are dependent on the presence of complement. (A) Opsonophagocytosis (OPC-CFU) was tested with 30 ng/ml 2A01 mIgA, pIgA, or SIgA at four concentrations of complement (0%, 1%, 3%, and 10%). All three forms showed a direct relationship between complement concentration and amount of opsonophagocytosis (n = 3). Asterisks designate significant differences between pIgA or SIgA and mIgA (Student's t test; P < 0.05). (B) CFU per well after OPC-CFU for a control lacking complement (light bars) or when 10% complement is present (dark bars) for each molecular form of MAb 2A01 or in the absence of antibody (n = 3; error bars = 1 ± standard error of the mean; the line across the chart at 2,000 CFU represents the starting bacterial inoculum). Control results show that the presence of complement alone did result in fewer CFU at the end of the assay, while numbers of CFU seen with a combination of complement and antibody were lower than those seen with either alone. (C) CFU per well after OPC-CFU, comparing the results obtained in the absence of complement (light bars) to those obtained with 10% heat-inactivated complement (dark bars) for each molecular form of MAb 2A01 or in the absence of antibody (n = 1; the line across the chart at 2,000 CFU represents the starting bacterial inoculum). Control findings show that heat-inactivated complement gave CFU results similar to those seen under conditions without complement, demonstrating a lack of nonspecific organism inhibitors in the complement preparation. (D) Binding and phagocytosis were tested at 30 ng/ml 2A01 mIgA, pIgA, and SIgA or in the absence of antibody at four concentrations of complement (0%, 1%, 3%, and 10%) (n = 1). OPC-flow analysis confirmed that for each molecular form the binding or phagocytosis was complement dependent. PMN, polymorphonuclear leukocyte.
To demonstrate the role which complement plays in OPA-CFU, the numbers of CFU were compared in wells containing no antibody or containing mIgA (30 ng/ml), pIgA (30 ng/ml), or SIgA (30 ng/ml), each tested with or without 10% complement (Fig. 4B). CFU numbers were consistently lower in the presence of antibody and complement compared with the results seen with all forms of antibody alone or complement alone. However, in the absence of antibody, the presence of complement did modestly reduce the numbers of CFU (∼35%). These effects obtained with complement alone may have been due to the presence of complement itself or to that of small amounts of residual antibody in the commercial preparation. Although each commercial lot of complement is screened and chosen for adequate potency and low toxicity before use (26), we have typically found some similar effects with the complement source alone for every serotype of S. pneumoniae used (E. N. Janoff and C. Fasching, unpublished data). For this reason, in the OPA-CFU assay testing the effects of human antibody, the percentages of CFU reductions were calculated using control wells that contained the same amount of complement without antibody. In addition to complement control wells, wells without either complement or antibody (for confirmation of adequate pneumococcal growth) and wells which contained both a dilution of human serum from a vaccinated subject and complement (for confirmation of adequate neutrophil performance) were included in each experiment.
To determine whether active factors other than complement were present in the commercial complement, we compared the numbers of CFU in wells containing no antibody or containing mIgA (30 ng/ml), pIgA (30 ng/ml), or SIgA (30 ng/ml) in the presence or absence of 10% HI baby rabbit complement and no other complement source (Fig. 4C). The determinations of CFU per well for each set of antibody conditions showed equivalent results in wells without complement and in wells containing 10% HI complement, indicating that the commercial complement preparation does not contain pneumococcal inhibitors. The absence of reduction of CFU in tests containing antibody and HI complement (Fig. 4C) compared with CFU levels in these tests performed with 10% complement (Fig. 4B) also supports the idea of a requirement of complement for opsonophagocytosis of pneumococci by neutrophils. That CFU reductions in wells when both complement and antibody were present were greater than the combined reductions observed with either complement or antibody alone further confirms the requirement for both factors to effectively mediate this process. Additionally, we have previously reported both bacterial internalization and evidence of bacterial lysis in phagolysosomes as determined by electron microscopy (28), further supporting the evidence indicating that bacterial phagocytosis and, most likely, killing are dependent on both antibody and complement.
Comparison of molecular forms of hMAb 2A01 IgA revealed that SIgA and pIgA reduced S. pneumoniae CFU equivalently and that both reduced CFU to a greater extent than mIgA at each complement concentration (Fig. 4A). Greater reductions in CFU of S. pneumoniae at 10% complement were apparent with pIgA (87%) and SIgA (76%) compared with the results seen with mIgA (25%) (P < 0.00002 and P < 0.0001, respectively). In contrast, results with pIgA and SIgA were comparable (Fig. 4A). We also noted that, in the absence of complement, a significant reduction in CFU was also seen with pIgA (27%) and SIgA (38%) compared with mIgA results (0%) (P < 0.028) (Fig. 4A) and that lower numbers of CFU per well were seen with pIgA and SIgA than were seen with mIgA or without antibody (Fig. 4B).
To investigate these findings, we performed an alternate assay which measured neutrophil binding and phagocytosis of FITC-labeled heat-killed type 2 S. pneumoniae by flow cytometry rather than reduction in CFU. The results of this flow-based assay verified that pIgA- and SIgA-opsonized S. pneumoniae produced increased binding and uptake compared to mIgA results and that this process was complement dependent for all three molecular forms (Fig. 4D). However, the alternate flow cytometry-based assay did not replicate findings that pIgA and SIgA were different from mIgA with respect to binding and phagocytosis in the absence of complement (Fig. 4D). That the two assays employed different endpoints in the presence of the same substrates (organisms, hMAb IgA, complement, and neutrophils) suggested that a second mechanism might have affected the outcome obtained in the absence of complement.
SIgA and pIgA agglutinate bacteria, but mIgA does not.
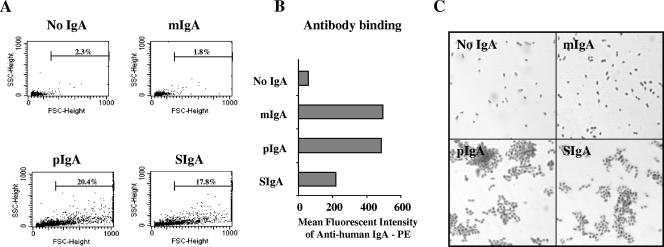
The ability of the pIgA and SIgA forms, but not the mIgA form, of 2A01 MAb to agglutinate S. pneumoniae may explain, in part, the decrease in the effective phagocytic function of mIgA. Bacterial agglutination was assessed by use of flow cytometry to measure an increase in forward scatter, which is used to estimate particle size. S. pneumoniae incubated with pIgA or SIgA (although not with mIgA) demonstrated a pronounced increase in particle size as determined by forward scatter measurement compared to the results obtained without antibody (Fig. 5A). The absence of bacterial agglutination seen with mIgA was not due to a lack of antibody binding to the bacterial surface, as phycoerythrin-labeled anti-human IgA detected the presence of mIgA bound to bacteria (Fig. 5B). Agglutination of bacteria by pIgA and SIgA but not mIgA was confirmed by the clumping pattern observed by light microscopy (Fig. 5C). The effect of the presence of nonspecific antibody and complement on agglutination was also examined. Table 1 shows the increase in the percentage of forward scatter in the presence of the serotype-matched antibody-organism pair alone (2A01-serotype 2 bacteria). No agglutination was observed when serotype 8 organisms were combined with PPS type 2-specific antibody. The addition of complement or HI complement had no effect under conditions in which no agglutination was seen; therefore, the complement source itself did not support agglutination of either serotype of S. pneumoniae.
FIG. 5.
SIgA and pIgA cause antibody-mediated agglutination of S. pneumoniae. (A) Bacterial agglutination measured by an increase in forward scatter (particle size) using flow cytometry was seen for hMAb 2A01 pIgA and SIgA but absent for mIgA. Percentages of bacteria which exceed single-cell values are indicated in each panel, including a control panel (No IgA). (B) Analysis of the mean fluorescent intensity of antibody binding to bacteria confirms that even though the mIgA results showed no agglutination, there was mIgA bound to the bacteria. (C) Light microscopy at ×1,000 total magnification also demonstrated the agglutinating capabilities of pIgA and SIgA.
TABLE 1.
Agglutination levels measured by flow cytometry
| Antibody added | % Forward scatter over baselinea
|
|||||
|---|---|---|---|---|---|---|
| Serotype 2 organism
|
Serotype 8 organism
|
|||||
| No C | C | hiC | No C | C | hiC | |
| No antibody | 2.06 | 2.39 | 2.21 | 3.27 | 2.51 | 2.64 |
| 2A01 mIgA | 2.72 | 2.77 | 2.52 | 4.08 | 2.30 | 3.16 |
| 2A01 pIgA | 11.13 | 14.82 | 19.40 | 2.78 | 3.37 | 2.72 |
| 2A01 SIgA | 15.98 | 21.60 | 24.82 | 2.90 | 2.38 | 2.56 |
No C, complement absent; C, complement present; hiC, heat-inactivated complement.
SIgA is not protected from IgA1 protease digestion.
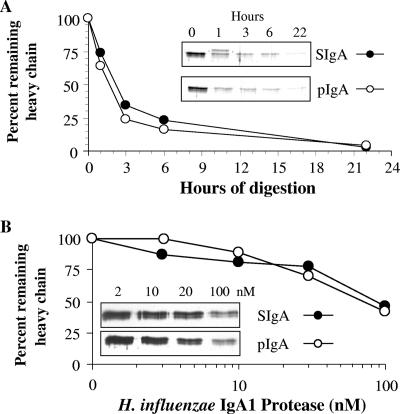
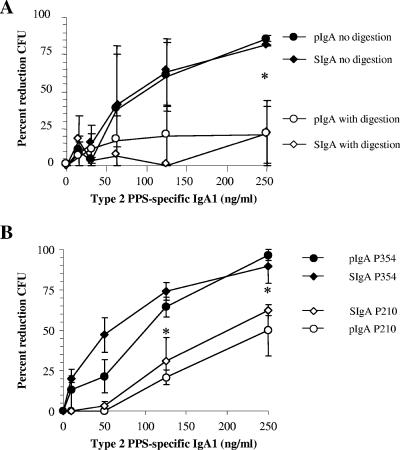
IgA1 protease specifically cleaves the hinge region of IgA1, the predominant IgA subclass found in the upper and lower respiratory tracts (40). We characterized the effect of the presence of bacterial IgA1 protease on 2A02 pIgA and SIgA forms to determine the potential role of SC in providing proteolytic protection to this particular hMAb. Recombinant IgA1 protease from H. influenzae type 1 was incubated with either pIgA or SIgA for increasing intervals of time (Fig. 6A) or with increasing amounts of protease (Fig. 6B). The two molecular forms demonstrated equivalent decrements in the amounts of intact heavy chain either with increasing exposure time to the enzyme or with increasing amounts of enzyme. We also characterized the functional impact of IgA1 protease digestion of hMAb IgA1 to determine whether Fab fragments alone could support bacterial OPC with complement and phagocytes. Percent OPC was significantly lower with IgA1 protease-digested pIgA and SIgA than with undigested antibodies at 250 ng/ml (P < 0.05; Student's t test), indicating a loss of function (Fig. 7A). Recombinant IgA1 protease from H. influenzae type 1 was used, because the purified recombinant protein is not available from S. pneumoniae. However, to more specifically characterize the effect of the presence of pneumococcal IgA1 protease, pIgA and SIgA were tested for killing of an IgA1 protease-producing strain (P210; parental) and a congenic IgA1 protease-negative strain (P354; mutant) of S. pneumoniae after a 2-h bacterium-antibody exposure time. Again, the percentage of OPC (reduction in CFU) seen with IgA1 protease-producing S. pneumoniae P210 for both SIgA and pIgA was less than that observed for the IgA1 protease-negative mutant P354 (P < 0.05 at 125 ng/ml and 250 ng/ml) (Fig. 7B). No difference in killing was observed between the two bacterial strains when pIgA and SIgA of subclass IgA2 (hMAb 2A01) were used (data not shown). These data demonstrate that SC does not protect IgA1 2A02 from degradation by IgA1 protease produced by either H. influenzae or S. pneumoniae. Unlike bacterial IgA1 proteases, the host-derived proteases cathepsin G and neutrophil elastase showed no degradation of the IgA heavy chain (data not shown). Cleavage of IgA1 by pneumococcal IgA1 protease was inhibited by the divalent cation chelator EDTA (10 mM).
FIG. 6.
SIgA and pIgA are similarly digested by H. influenzae type 1 IgA1 protease. (A) A 0.15-pmol volume of either hMAb 2A02 SIgA or pIgA was digested at 37°C with 0.15 pmol H. influenzae IgA1 protease for 0, 1, 3, 6, or 22 h. (B) SIgA or pIgA (10 nM) was digested at 37°C with H. influenzae type 1 IgA1 protease (2, 10, 20, or 100 nM) for 2 h. Samples were run on a 12% denaturing PAGE and silver stained, and percent remaining heavy chain was quantified by an inverse luminosity measurement in Photoshop.
FIG. 7.
IgA1 proteases from H. influenzae type 1 and S. pneumoniae inhibit IgA-mediated opsonophagocytosis of S. pneumoniae in the presence of complement. (A) Digestion of hMAb 2A02 pIgA and SIgA with H. influenzae IgA1 protease before use in opsonophagocytosis assays caused loss of the ability to effect bacterial killing compared to the results seen with undigested pIgA and SIgA. (B) Prolonged exposure of pIgA and SIgA to a protease-producing strain of S. pneumoniae (P210) inhibited IgA-mediated killing compared with the results seen with pIgA and SIgA exposed to a protease-negative mutant (P354). For each graph, data represent the results of three separate experiments. Asterisks designate significant differences between digested and nondigested antibody results (Student's t test; P < 0.05). No significant differences between the effects on capsule-specific pIgA and SIgA were seen.
DISCUSSION
We have characterized a potentially important effector function of SIgA, antibody-mediated phagocytosis. With human polyclonal antibodies (14, 28) and now highly specific hMAb antibodies to S. pneumoniae, SIgA supports phagocytic effector function. Using isogenic molecular forms of hMAbs with identical antigen-binding variable regions specific for the capsule of S. pneumoniae type 2, we show that both pIgA and SIgA more effectively mediated antibody-dependent and complement-dependent phagocytosis than did mIgA. Earlier studies suggested that SIgA-mediated phagocytic responses were better (18, 44), equivalent (41), or absent (59) compared with pIgA results. These discrepancies may relate to differences in IgA preparations, characteristics of the organisms tested, and endpoints. The activity of the specific SIgA we report resulted from the confluence of several related functions: avidity, interactions with complement, and agglutination.
Avidity, or functional affinity, is determined by both the intrinsic affinity of the antigen-binding variable region and the structure and valence of the entire antibody molecule. Both multivalent forms (pIgA and SIgA) of hMAbs 2A01 (type 2), 6BA01 (type 6B), and 8A01 (type8) showed increased avidity compared with the bivalent monomeric form (mIgA). The intrinsic affinities (on-off rates constant) of the variable regions of each of these clonal mIgA, pIgA, and SIgA molecules were identical, so the difference in avidity we observed within serotypes derived from the increased valence of the polymeric molecules. The avidity of pIgA was neither diminished nor enhanced by the addition of SC. This observation is supported by the work of Lüllau et al. (38), who found that adding SC to pIgA does not modify the affinity of IgA to Vibrio cholerae lipopolysaccharide, and by Berdoz and Corthésy (4), who found that pIgA and SIgA of the same specificity had equivalent Helicobacter pylori urease-inhibiting capabilities. Increased avidity of IgG has been associated with increased functional activity against viruses (2) and bacteria (49), the ability to activate complement (13), and increased phagocytosis of S. pneumoniae (57). The increased avidities of our monoclonal pIgA and SIgA antibodies are also associated with the increased functional capability of these forms.
Complement activation and opsonization link the adaptive and innate immune systems, directly influencing the level of phagocytic killing of pathogens. Using bacterium-antibody complexes containing native human IgA, we found that the amount of opsonophagocytic activity increased as the complement concentration rose and as the IgA concentration increased. The ability of IgA to directly activate complement is debatable. Whole IgA has been shown by others to activate complement via an alternative pathway (5, 51) and the mannose binding lectin pathway (50). Under restricted experimental conditions, IgA fragments F(ab′)2 or F(abc)2 (24), Fcα, and free SC (42), but not Fab (42), bound to the solid-phase fixed complement by an alternative pathway. In the context of our data, pneumococcal capsular and cell wall polysaccharides have a direct impact on C3-dependent opsonization of the organism (25, 64), and complement receptor 3 (CR3) of neutrophils is a major phagocytic receptor for pneumococci (17). Although the specific biochemical interactions between IgA and complement have not been defined, both pIgA and SIgA can support opsonophagocytosis of S. pneumoniae in the presence of complement.
At equal antibody concentrations, both pIgA and SIgA required less complement than mIgA to achieve similar levels of organism reduction. The increased avidities of pIgA and SIgA may moderate the amount of complement needed for pathogen phagocytosis. This feature may be important at mucosal sites where complement concentrations are limited (34), although human complement components can be produced by alveolar type II epithelial cells (52) and alveolar macrophages (43). Studies in which SIgA did not mediate killing (59) did not include complement. We demonstrate that SIgA opsonizes S. pneumoniae in a complement-dependent fashion. However, whether pIgA or SIgA actually fixes and activates complement by the alternate or mannose binding lectin pathways, and the mechanism of such putative activation, remains to be verified.
In the absence of complement, IgA-mediated agglutination accounts for a portion of the reductions in CFU in the OPA-CFU assay. Such agglutination accounts for lower estimations of bacterial viability and increased CFU reduction in the absence of complement with pIgA and SIgA but not mIgA. As determined both by flow cytometric particle size analysis and by basic light microscopy, pIgA and SIgA each agglutinated S. pneumoniae whereas mIgA did not. This binding and cross-linking activity is similar to that described by Renegar et al. (47), who showed that SIgA was five times more effective than mIgA in hemagglutination inhibition of influenza virus, and by Stubbe et al. (53), who found superior Clostridium difficile toxin neutralization by pIgA compared with mIgA results. We suggest that the reductions in CFU of S. pneumoniae seen with pIgA and SIgA in the absence of complement derive from an increase in agglutination based on increased valence and avidity.
To eliminate assay endpoint inaccuracy caused by agglutination, we used a flow cytometry binding and uptake assay, which relies on bacterial fluorescence and cell association. This fluorescent method provides a more directly proportional measurement of the number of bacteria bound and taken up by the phagocyte. Binding and uptake of fluorescent bacteria was not affected by agglutination and showed dose-related responses with increasing amounts of IgA and complement in combination. No activity was seen with the flow-based assay in the absence of complement, and complement was not required for agglutination. Thus IgA, and particularly pIgA and SIgA, supports two distinct mechanisms of antibody-mediated bacterial clearance, complement-independent agglutination and complement-dependent phagocytosis.
Although IgA at mucosal sites is exposed to potential cleavage and inactivation by host- and bacterium-derived proteases, SIgA is more resistant than serum IgA to a metalloproteinase from Proteus mirabilis (1), type VI protease (56), trypsin (47), and neutrophil elastase (45) and to intestinal proteases (3, 12). IgA1 protease produced by H. influenzae mediates degradation of SIgA (1, 10) equal to that seen with nonsecretory IgA, as did the enzyme from both H. influenzae and S. pneumoniae in our hands. Whether persistently bound Fab fragments would inhibit subsequent antibody-mediated phagocytosis by capsule-specific IgA or IgG is under investigation. Inhibitors of IgA1 protease, including antienzyme antibodies, may be present in serum and secretions (16, 33), but the antiphagocytic activity of IgA1 protease may pose an obstacle to the effective development of mucosal vaccines against S. pneumoniae and other invasive mucosal pathogens.
In summary, SIgA supports opsonophagocytosis as well as pIgA, and both molecular forms are more efficient than mIgA in the presence of complement. These results relate to their increased avidity and ability to lower the complement levels required for phagocytosis. In addition to phagocytosis, these multimeric molecular forms of IgA mediate agglutination, with mediation by SIgA being complement dependent and that by pIgA complement independent. In concert, these two activities may enhance our ability to clear S. pneumoniae at mucosal sites.
Acknowledgments
This work was supported by National Institutes of Health grants AI-48796, HD-41361, and DE-015072 (E.N.J.), AI-44231 and AI-38446 (J.N.W.), and RO1 DE-15844 and GRASP Digestive Disease Research Center grant NIH P30DK34928 (A.G.P.), Swiss Science Research Foundation grant 3200-109545 (B.C.), the Colorado Center for AIDS Research, the Mucosal and Vaccine Research Center, and the Veterans Affairs Research Service.
We thank Ann Emery for excellent secretarial assistance.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Almogren, A., B. W. Senior, L. M. Loomes, and M. A. Kerr. 2003. Structural and functional consequences of cleavage of human secretory and human serum immunoglobulin A1 by proteinases from Proteus mirabilis and Neisseria meningitidis. Infect. Immun. 71:3349-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann, M. F., A. Kalinke, A. Althage, G. Freer, C. Burkhart, H.-P. Roost, H. Aguet, H. Hengartner, and R. M. Zinkernagel. 1997. The role of antibody concentration and avidity in antiviral protection. Science 276:2024-2027. [DOI] [PubMed] [Google Scholar]
- 3.Berdoz, J., C. T. Blanc, M. Reinhardt, J. P. Kraehenbuhl, and B. Corthésy. 1999. In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells. Proc. Natl. Acad. Sci. USA 96:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berdoz, J., and B. Corthésy. 2004. Human polymeric IgA is superior to IgG and single-chain Fv of the same monoclonal specificity to inhibit urease activity associated with Helicobacter pylori. Mol. Immunol. 41:1013-1022. [DOI] [PubMed] [Google Scholar]
- 5.Bogers, W. M. J. M., R.-K. Stad, L. A. van Es, and M. R. Daha. 1991. Immunoglobulin A: interaction with complement, phagocytic cells and endothelial cells. Complement Inflamm. 8:347-358. [DOI] [PubMed] [Google Scholar]
- 6.Burastero, S. E., P. Casali, R. L. Wilder, and A. L. Notkins. 1988. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J. Exp. Med. 168:1979-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.Carroll, W. L., E. Mendel, and S. Levy. 1988. Hybridoma fusion cell lines contain an aberrant kappa transcript. Mol. Immunol. 25:991-995. [DOI] [PubMed] [Google Scholar]
- 9.Carroll, W. L., K. Thielemans, J. Dilley, and R. Levy. 1986. Mouse x human heterohybridomas as fusion partners with human B cell tumors. J. Immunol. Methods 89:61-72. [DOI] [PubMed] [Google Scholar]
- 10.Chintalacharuvu, K. R., P. D. Chuang, A. Dragoman, C. Z. Fernandez, J. Qiu, A. G. Plaut, K. R. Trinh, F. A. Gala, and S. L. Morrison. 2003. Cleavage of the human immunoglobulin A1 (IgA1) hinge region by IgA1 proteases requires structures in the Fc region of IgA. Infect. Immun. 71:2563-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crottet, P., and B. Corthésy. 1998. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab′)2: a possible implication for mucosal defense. J. Immunol. 161:5445-5453. [PubMed] [Google Scholar]
- 13.Fauci, A., M. M. Frank, and J. S. Johnson. 1970. The relationship between antibody affinity and the efficiency of complement fixation. J. Immunol. 105:215-220. [PubMed] [Google Scholar]
- 14.Finn, A., Q. Zhang, L. Seymour, C. Fasching, E. Pettitt, and E. N. Janoff. 2002. Induction of functional secretory IgA responses in breast milk by pneumococcal capsular polysaccharides. J. Infect. Dis. 186:1422-1429. [DOI] [PubMed] [Google Scholar]
- 15.Friguet, B., A. F. Chaffotte, L. Djavadi-Ohaniance, and M. E. Goldberg. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77:305-319. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert, J. V., A. G. Plaut, B. Longmaid, and M. E. Lamm. 1983. Inhibition of bacterial IgA proteases by human secretory IgA and serum. Ann. N. Y. Acad. Sci. USA 409:625-636. [DOI] [PubMed] [Google Scholar]
- 17.Gordon, D. L., G. M. Johnson, and M. K. Hostetter. 1986. Ligand-receptor interactions in the phagocytosis of virulent Streptococcus pneumoniae by polymorphonuclear leukocytes. J. Infect. Dis. 154:619-626. [DOI] [PubMed] [Google Scholar]
- 18.Gorter, A., P. S. Hiemstra, P. C. J. Leijh, M. E. van der Sluys, M. R. van der Barselaar, L. A. van Es, and M. R. Daha. 1987. IgA and secretory IgA-opsonized S. aureus induce a respiratory burst and phagocytosis by polymorphonuclear leukocytes. Immunology 61:303-309. [PMC free article] [PubMed] [Google Scholar]
- 19.Gray, B. M. 1990. Opsonophagocidal activity in sera from infants and children immunized with Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate). Pediatrics 85:694-697. [PubMed] [Google Scholar]
- 20.Gray, B. M., and D. R. Shaw. 1993. Artifacts with the thiocyanate elution method for estimating relative antibody avidity. J. Immunol. Methods 157:269-271. [DOI] [PubMed] [Google Scholar]
- 21.Reference deleted.
- 22.Herr, A. B., E. R. Ballister, and P. J. Bjorkman. 2003. Insights into IgA-mediated immune responses from the crystal structures of human FcαRI and its complex with IgA1-Fc. Nature 423:614-620. [DOI] [PubMed] [Google Scholar]
- 23.Reference deleted.
- 24.Hiemstra, P. S., J. Biewenga, A. Gorter, M. E. Stuurman, A. Faber, L. A. van Es, and M. R. Daha. 1988. Activation of complement by human serum IgA, secretory IgA and IgA1 fragments. Mol. Immunol. 25:527-533. [DOI] [PubMed] [Google Scholar]
- 25.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153:682-693. [DOI] [PubMed] [Google Scholar]
- 26.Hu, B. T., X. Yu, T. R. Jones, C. Kirch, S. Harris, S. W. Hildreth, D. V. Madore, and S. A. Quataert. 2005. Approach to validating an opsonophagocytic assay for Streptococcus pneumoniae. Clin. Diagn. Lab. Immunol. 12:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janoff, E. N., C. Fasching, J. C. Ojoo, J. O'Brien, and C. F. Gilks. 1997. Responsiveness of human immunodeficiency virus type 1-infected Kenyan women with or without prior pneumococcal disease to pneumococcal vaccine. J. Infect. Dis. 175:975-978. [DOI] [PubMed] [Google Scholar]
- 28.Janoff, E. N., C. Fasching, J. M. Orenstein, J. B. Rubins, N. L. Opstad, and A. P. Dalmasso. 1999. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J. Clin. Investig. 104:1139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janoff, E. N., W. D. Hardy, P. D. Smith, and S. M. Wahl. 1991. Humoral recall responses in HIV infection. Levels, specificity, and affinity of antigen-specific IgG. J. Immunol. 147:2130-2135. [PubMed] [Google Scholar]
- 30.Johnson, S., N. L. Opstad, J. M. Douglas, Jr., and E. N. Janoff. 1996. Prolonged and preferential production of polymeric immunoglobulin A in response to Streptococcus pneumoniae capsular polysaccharides. Infect. Immun. 64:4339-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karush, F. 1985. The affinity of antibody: range, variability, and role of multivalence, p. 85-101. In G. W. Litman and R. A. Good (ed.), Comprehensive immunology, vol. 5. Plenum Medical Book Co., New York, NY. [Google Scholar]
- 32.Kim, J. O., S. Romero-Steiner, U. B. Sorensen, J. Blom, M. Carvalho, S. Barnard, G. Carlone, and J. N. Weiser. 1999. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect. Immun. 67:2327-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkeby, L., T. T. Rasmussen, J. Reinholdt, and M. Kilian. 2000. Immunoglobulins in nasal secretions of healthy humans: structural integrity of secretory immunoglobulin A1 (IgA1) and occurrence of neutralizing antibodies to IgA1 proteases of nasal bacteria. Clin. Diagn. Lab. Immunol. 7:31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krug, N., T. Tschernig, V. J. Erpenbeck, J. M. Hohlfeld, and J. Kohl. 2001. Complement factors C3a and C5a are increased in bronchoalveolar lavage fluid after segmental allergen provocation in subjects with asthma. Am. J. Respir. Crit. Care Med. 164:1841-1843. [DOI] [PubMed] [Google Scholar]
- 35.Lefkovits, I., and H. Waldmann. 1984. Limiting dilution analysis of the cells of immune system. I. The clonal basis of the immune response. Immunol. Today 5:265-268. [DOI] [PubMed] [Google Scholar]
- 36.Lexau, C. A., R. Lynfield, R. Danila, T. Pilishivili, R. Facklam, M. M. Farley, L. H. Harrison, W. Schaffer, A. Reingold, N. M. Bennett, J. Hadler, P. R. Cieslak, C. G. Whitney, and the Active Bacterial Core Surveillance Team. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043-2051. [DOI] [PubMed] [Google Scholar]
- 37.Lindh, E. 1975. Increased resistance of immunoglobulin A dimers to proteolytic degradation after binding secretory component. J. Immunol. 114:284-286. [PubMed] [Google Scholar]
- 38.Lüllau, E., S. Heyse, H. Vogel, I. Marison, U. von Stockar, J. P. Kraehenbuhl, and B. Corthésy. 1996. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J. Biol. Chem. 271:16300-16309. [DOI] [PubMed] [Google Scholar]
- 39.Martinez, J. E., S. Romero-Steiner, T. Pilishvili, S. Barnard, J. Schinsky, D. Goldblatt, and G. M. Carlone. 1999. A flow cytometric opsonophagocytic assay for measurement of functional antibodies elicited after vaccination with the 23-valent pneumococcal polysaccharide vaccine. Clin. Diagn. Lab. Immunol. 6:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mestecky, J., M. W. Russell, S. Jackson, and T. A. Brown. 1986. The human IgA system: a reassessment. Clin. Immunol. Immunopathol. 40:105-114. [DOI] [PubMed] [Google Scholar]
- 41.Motegi, Y., and H. Kita. 1998. Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J. Immunol. 161:4340-4346. [PubMed] [Google Scholar]
- 42.Nikolova, E. B., M. Tomana, and M. W. Russell. 1994. The role of the carbohydrate chains in complement (C3) fixation by solid-phase-bound human IgA. Immunology 82:321-327. [PMC free article] [PubMed] [Google Scholar]
- 43.Pettersen, H. B., E. Johnson, T. E. Mollnes, and P. Garred. 1988. Synthesis of soluble C3 and C9 neopeptides by human alveloar macrophages in vitro. Scand. J. Immunol. 28:431-434. [DOI] [PubMed] [Google Scholar]
- 44.Phalipon, A., A. Cardona, J. P. Kraehenbuhl, L. Edelman, P. J. Sansonetti, and B. Corthésy. 2002. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17:107-115. [DOI] [PubMed] [Google Scholar]
- 45.Pilette, C., Y. Ouadrhiri, F. Dimanche, J.-P. Vaerman, and Y. Sibille. 2003. Secretory component is cleaved by neutrophil serine proteases but its epithelial production is increased by neutrophils through NF-κB- and p38 mitogen-activated protein kinase-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 28:485-498. [DOI] [PubMed] [Google Scholar]
- 46.Pullen, G. R., M. G. Fitzgerald, and C. S. Hosking. 1986. Antibody avidity determination by ELISA using thiocyanate elution. J. Immunol. Methods 86:83-87. [DOI] [PubMed] [Google Scholar]
- 47.Renegar, K. B., G. D. F. Jackson, and J. Mestecky. 1998. In vitro comparison of the biologic activities of monoclonal monomeric IgA, polymeric IgA, and secretory IgA. J. Immunol. 160:1219-1223. [PubMed] [Google Scholar]
- 48.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. E. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 50.Roos, A., L. H. Bouwman, D. J. van Gijlswijk-Janssen, M. C. Faber-Krol, G. L. Stahl, and M. R. Daha. 2001. Human IgA activates the complement system via the mannan-binding lectin pathway. J. Immunol. 167:2861-2868. [DOI] [PubMed] [Google Scholar]
- 51.Schneiderman, R. D., T. F. Lint, and K. L. Knight. 1990. Activation of the alternative pathway of complement by twelve different rabbit-mouse chimeric transfectoma IgA isotypes. J. Immunol. 145:233-237. [PubMed] [Google Scholar]
- 52.Strunk, R. C., D. M. Eidlen, and R. J. Mason. 1988. Pulmonary alveolar type II epithelial cells synthesize and secrete proteins of the classical and alternative complement pathways. J. Clin. Investig. 81:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stubbe, H., J. Berdoz, J.-P. Kraehenbuhl, and B. Corthésy. 2000. Polymeric IgA is superior to monomeric IgA and IgG carrying the same variable domain in preventing Clostridium difficile toxin A damaging of T84 monolayers. J. Immunol. 164:1952-1960. [DOI] [PubMed] [Google Scholar]
- 54.Sun, K., F.-E. Johansen, L. Eckmann, and D. W. Metzger. 2004. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J. Immunol. 173:4576-4581. [DOI] [PubMed] [Google Scholar]
- 55.Ueki, Y., I. Goldfarb, N. Harindranath, M. Gore, H. Koprowski, A. L. Notkins, and P. Casali. 1990. Clonal analysis of a human antibody response. J. Exp. Med. 171:19-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Underdown, B. J., and K. J. Dorrington. 1974. Studies on the structural and conformational basis for the relative resistance of serum and secretory immunoglobulin A to proteolysis. J. Immunol. 112:949-959. [PubMed] [Google Scholar]
- 57.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reference deleted.
- 59.Vidarsson, G., W. L. van Der Pol, J. M. van Den Elsen, H. Vile, M. Jansen, J. Duijs, H. C. Morton, E. Boel, M. R. Daha, B. Corthésy, and J. G. van De Winkel. 2001. Activity of human IgG and IgA subclasses in immune defense against Neisseria meningitidis serogroup B. J. Immunol. 166:6250-6256. [DOI] [PubMed] [Google Scholar]
- 60.Wani, J. H., J. V. Gilbert, A. G. Plaut, and J. N. Weiser. 1996. Identification, cloning, and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect. Immun. 64:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiser, J. N., D. Bae, C. Fasching, R. W. Scamurra, A. J. Ratner, and E. N. Janoff. 2003. Antibody-enhanced pneumococcal adherence requires IgA1 protease. Proc. Natl. Acad. Sci. USA 100:4215-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat for the Active Bacterial Core Surveillance of the Emerging Infections Program Network. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 63.Wijburg, O. L., T. K. Uren, K. Simpfendorfer, F.-E. Johansen, P. Brandtzaeg, and R. A. Strugnell. 2006. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 203:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Winkelstein, J. A., J. A. Bocchini, Jr., and G. Schiffman. 1976. The role of the capsular polysaccharide in the activation of the alternative pathway by the pneumococcus. J. Immunol. 116:367-370. [PubMed] [Google Scholar]
- 65.Reference deleted.