Abstract
We tested the hypothesis that helminth parasite coinfection would intensify viremia and accelerate disease progression in monkeys chronically infected with an R5 simian-human immunodeficiency virus (SHIV) encoding a human immunodeficiency virus type 1 (HIV-1) clade C envelope. Fifteen rhesus monkeys with stable SHIV-1157ip infection were enrolled into a prospective, randomized trial. These seropositive animals had undetectable viral RNA and no signs of immunodeficiency. Seven animals served as virus-only controls; eight animals were exposed to Schistosoma mansoni cercariae. From week 5 after parasite exposure onward, coinfected animals shed eggs in their feces, developed eosinophilia, and had significantly higher mRNA expression of the T-helper type 2 cytokine interleukin-4 (P = 0.001) than animals without schistosomiasis. Compared to virus-only controls, viral replication was significantly increased in coinfected monkeys (P = 0.012), and the percentage of their CD4+ CD29+ memory cells decreased over time (P = 0.05). Thus, S. mansoni coinfection significantly increased viral replication and induced T-cell subset alterations in monkeys with chronic SHIV clade C infection.
Since human immunodeficiency virus type 1 (HIV-1) is widespread in most developing countries where parasitic infections are highly prevalent, the likelihood of coinfection is high, and the interaction between these infections in the same host is an important clinical consideration. The host's ability to mount an immune response to a new pathogen and the nature of that response are greatly determined by the preexisting state of the immune system. For example, it has been suggested that the T helper type 2 (Th2)-skewed immune profile and anergy that are associated with helminth parasite infections jeopardize the host's ability to generate protective immunity to other infectious agents, including HIV-1 (6-10, 28, 29). Data to support this hypothesis come from studies of mice exposed to vaccinia virus expressing HIV envelope antigens. Compared to control animals, mice with schistosomiasis displayed a shift towards a Th2 response, with down-regulation of Th1 cytokine production and impaired cytotoxic T-lymphocyte activity to the coinfecting virus (1). Similarly, humans infected with Schistosoma mansoni have an impaired antigen-specific Th1-type response following immunization with tetanus toxoid (35).
Alteration of immune responses can also be caused by other helminthic infections. For example, signal transduction following in vitro stimulation of lymphocytes obtained from individuals with chronic helminth parasite infection is downregulated, as are proliferative responses to purified protein derivative and delayed-type hypersensitivity reactions to Mycobacterium bovis bacillus Calmette-Guerin (BCG) (11). Elimination or reduction of intestinal worm infections in individuals subsequently vaccinated with BCG resulted in a significant improvement in Th1-type purified protein derivative-specific immune responses (17). Recently, these in vitro observations were supported by the in vivo observation that schistosome infections are a risk factor for progression to active tuberculosis in Ugandan HIV-1 patients (14).
Despite the observations that parasitic worms shift immune responses to coinfecting agents, the influence of schistosomiasis or other helminth infections on the course of HIV-1 infection and disease progression remains controversial. Some studies support the hypothesis that parasitic infections associated with Th2-type immune responses increase the host's susceptibility to HIV-1 and promote viral replication in coinfected hosts. For example, peripheral blood mononuclear cells (PBMCs) from persons infected with helminths were shown to be more susceptible to HIV-1 infection in vitro than cells from uninfected persons (19, 37). In addition, expression of the HIV coreceptors CXCR4 and CCR5 on CD4+ lymphocytes was significantly higher in patients with active schistosomiasis than in individuals who had been treated for their schistosomiasis (36). Furthermore, plasma HIV-1 viral loads were reduced significantly in coinfected patients following successful treatment with antihelminthics (40). However, a drop in circulating viral load in HIV-1-positive individuals following treatment for schistosomiasis or other helminths was not observed in a number of other studies (12, 13, 18, 25, 32). A recent comparison of early versus delayed (by 3 months) treatment for schistosomiasis also found no immediate drop in circulating viral concentration (24). However, the progressive increase in viral load and CD4+ T-cell loss was halted in the persons who received early treatment compared to those who were treated later, confirming the benefit of schistosome clearance in persons with HIV-1 (24).
Due to the variability of results in the human studies, the uncertainty of infectious agent doses and duration of viral or parasite infections in patients, and the limited immune response data for these individuals, we have begun evaluating schistosome-primate immunodeficiency virus coinfections in rhesus macaques. Our initial work demonstrated that schistosome-infected macaques intravenously inoculated with simian-human immunodeficiency virus (SHIV) clade C had significantly higher acute viral RNA loads compared to parasite-free animals challenged with the same dose of virus (15). In addition, animals with previous SHIV infections that were subsequently infected with schistosomes demonstrated an increased viral load coincident with the acute phase of the schistosome infection. However, these findings were limited by the small number of animals studied and the use of each animal as its own control. We have now extended these findings with a prospective, randomized study that involved another, larger group of animals with preexisting chronic, stable R5 SHIV clade C infection but no previous exposure to schistosomes. We also examined alterations in the immune response profiles of coinfected and control animals to assess effects of schistosomiasis on SHIV disease progression.
MATERIALS AND METHODS
Animals.
Rhesus monkeys (Macaca mulatta) of Indian origin were housed at the Yerkes National Primate Research Center (YNPRC) at Emory University, a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Protocols were approved and animals were maintained in accordance with the guidelines of the Institution Animal Care and Use Committees for Emory University and the Dana-Farber Cancer Institute. All procedures employed were consistent with the Guide for Care and Use of Laboratory Animals (23a). All animals were negative for SIV, simian T-lymphotropic virus type 1, and simian retrovirus type D and free of helminth infection prior to our study. Fifteen monkeys that had been infected with R5 SHIV clade C strain SHIV-1157ip 1.5 to 4 years earlier were enrolled into a controlled, prospective trial. These seropositive animals had no signs of immunodeficiency and had undetectable plasma viral RNA for at least 3 months; they were 1.5 to 4 years of age (mean age, 2.5 years) at enrollment. Seven animals served as controls; the other eight were percutaneously exposed to S. mansoni cercariae. Animals were monitored prospectively for clinical parameters (weight, physical exams, complete blood cell counts, liver and renal function tests, and T-cell subsets), plasma viral RNA, expression of cytokines and chemokines, expression of markers of immune activation and viral coreceptors, and specific immune responses. Virus-only controls were monitored for 14 weeks, and coinfected animals were monitored for 20 weeks.
R5 SHIV clade C.
SHIV-1157ip (39) consists of an SHIV-vpu+ backbone (27) and encodes most of gp120 and all of the extracellular and transmembrane regions of gp41 of the primary HIV clade C strain HIV1157i, which had been isolated from a 6-month-old Zambian infant with in utero infection (43). The initial SHIV construct, the infectious molecular clone SHIV-1157i, was adapted by serial blood transfer through a cohort of five rhesus monkeys that consisted of two infants, two neonates, and one juvenile. Three of these five monkeys had progressed to AIDS, and two remained long-term nonprogressors. AIDS, as defined by an absolute peripheral CD4+ T-cell count of <200 cells/μl, developed at approximately week 140 in the first animal; the other two progressed to AIDS within approximately 300 weeks postinoculation. SHIV-1157ip has also caused thrombocytopenia. The passaged virus, SHIV-1157ip, was isolated from PBMCs of the fifth animal. The monkeys enrolled in the current study were infected with a stock of SHIV1157ip that had been grown in rhesus monkey PBMCs (39).
Schistosoma mansoni inoculations.
Animals were anesthetized with ketamine and percutaneously exposed to 500 cercariae of a Puerto Rican strain of S. mansoni. An area on the abdomen was shaved, and cercariae were placed on the skin within a metal ring for 30 min to allow penetration. To monitor infection, fresh stool was obtained and processed by formalin-ethyl acetate sedimentation and concentration. Schistosome eggs were counted by microscopic examination.
Quantitation of plasma viral RNA load and simian cytokine mRNAs.
Peripheral blood samples were obtained by venipuncture and collected into Vacutainer cell preparation tubes containing sodium citrate (Becton Dickinson, Rutherford, NJ). Plasma and mononuclear cells were separated by centrifugation according to the manufacturer's instructions. Plasma samples from infected monkeys were stored at −80°C until viral RNA loads were assessed by real-time reverse transcription-PCR (20). For preparation of mRNA, cells were washed once in RPMI 1640 (Invitrogen, Carlsbad, CA), lysed with RLT lysis buffer (QIAGEN, Valencia, CA) containing 1% β-mercaptoethanol (Sigma Chemical Company, St. Louis, MO), and stored at −80°C. Cytokine mRNA levels were assessed by a quantitative real-time reverse transcription-PCR assay based on TaqMan chemistry (23). Variability in cytokine expression resulting from any disparities in cell numbers or RNA isolation efficiency was corrected by normalization against mRNA levels of the housekeeping gene coding for pyruvate dehydrogenase (PDH).
Enumeration of T-cell subsets and phenotypic characterization of lymphocytes by flow cytometry.
Complete blood cell counts, differential, and enumeration of T-cell subsets in whole blood of animals collected at different time points were measured at YNPRC as described previously (2). Immunophenotyping of PBMCs from the animals was performed at Dana-Farber Cancer Institute by three-color flow cytometry analysis, using a fluorescence-activated cell sorter (Becton Dickinson Immunocytometry Systems) and CELLQuest software (Becton Dickinson). For surface staining, the cells were resuspended in staining buffer (phosphate-buffered saline [PBS] containing 15% fetal calf serum and 0.1% NaN3) and incubated for 30 min at 4οC with combinations of the following antihuman monoclonal antibodies: CD3 conjugated with fluorescein isothiocyanate (FITC), CD4 conjugated with peridinin-chlorophyll-protein complex (PerCP), CD8-FITC, and CD29 conjugated with phycoerythrin, which cross-react with rhesus monkey surface markers (Becton Dickinson and BD Biosciences PharMingen). The cell samples were then washed with PBS and fixed for 30 min at 4οC with 1 ml of PBS containing 4% paraformaldehyde (Sigma-Aldrich). A total of 106 events were acquired. Appropriate isotype-matched control FITC-, phycoerythrin-, or PerCP-conjugated mouse or rat immunoglobulin G1/immunoglobulin G2a monoclonal antibodies were used to obtain the settings (Becton Dickinson and BD Biosciences PharMingen).
Cellular immune responses.
PBMCs were tested for virus-specific responses against overlapping SIV of macaques 239 (SIVmac239) Gag peptide pools (AIDS Research & Reference Reagent Program, NIAID, NIH). To perform short-term enzyme-linked immunospot (ELISPOT) assays, cells were cultured at a final concentration of 2 × 106 cells per ml in RPMI 1640 that had been supplemented with 15% heat-inactivated FCS, 2 mM l-glutamine, 200 μg/ml streptomycin, 200 U/ml penicillin, and 5 × 10−5 β-mercaptoethanol. Cells were cultured in 96-well ELISPOT plates (100 μl per well) for 16 to 18 h without stimulation, with overlapping SIVmac239 Gag peptide pools at a final concentration of 2 μg/ml of each peptide, or with 25 ng/ml phytohemagglutinin and 1 μg/ml ionomycin at 37°C in a 5% CO2 environment. Gamma interferon (IFN-γ) ELISPOT assays were developed using the BD human IFN-γ ELISPOT kit (Becton Dickinson and BD Biosciences PharMingen) according to the manufacturer's instructions. For long-term ELISPOT assays, cells were cultured at a final concentration of 5 × 106 cells per ml in 24-well plates for 10 days with overlapping SIVmac239 Gag peptide pools at a final concentration of 2 μg/ml of each peptide. Interleukin-2 (IL-2) was added to the cultures every 3 days at a final concentration of 10 U/ml. On day 10, cells were washed, counted, and restimulated as described above for short-term ELISPOT assays.
Statistical analyses.
Generalized estimation equations were used to model the data collected over time and to compare parasite-negative and S. mansoni-coinfected groups (41, 42). The Wilcoxon rank-sum test was used to compare single-time-point data (26). All P values reported are for two-sided significance tests. P values of ≤0.05 were considered significant, and P values between 0.05 and 0.1 were considered marginally significant.
RESULTS
Parasite coinfection of rhesus monkeys with chronic SHIV-1157ip infection.
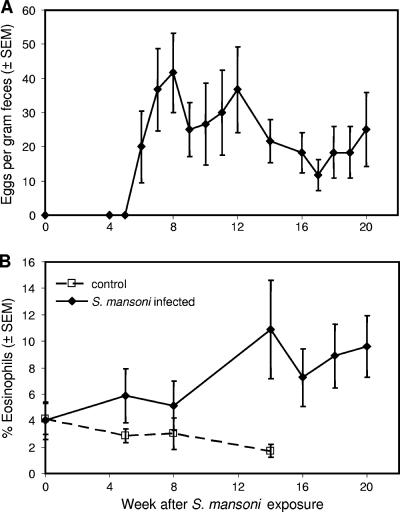
To test whether S. mansoni challenge would reactivate viral replication in monkeys with chronic stable SHIV clade C infection, a cohort of 15 rhesus monkeys that had been inoculated with SHIV-1157ip 1.5 to 4 years earlier was enrolled into a prospective, randomized trial. These chronically infected animals were seropositive but had no signs of immunodeficiency and had shown no detectable plasma viral RNA for at least 3 months prior to enrollment. Seven of these SHIV-1157ip-infected animals served as the parasite-negative control group. The other eight animals were percutaneously exposed to 500 S. mansoni cercariae. Beginning at week 5 after coinfection, parasite-challenged animals demonstrated signs indicative of S. mansoni infection: parasite eggs in stools (Fig. 1A) and eosinophilia (Fig. 1B). S. mansoni infection was established in all parasite-challenged monkeys.
FIG. 1.
Infection of rhesus macaques with S. mansoni. Eight animals were percutaneously exposed to 500 cercariae. (A) Egg counts in stool samples collected from the S. mansoni-inoculated animals. Data represent the running average of three consecutive values. SEM, standard error of the mean. (B) Percentage of eosinophils in SHIV-1157ip-infected rhesus monkeys with and without concurrent schistosomiasis. In coinfected animals, the percentage of eosinophils rose as a function of time of infection.
Cytokine profiles and cellular activation markers.
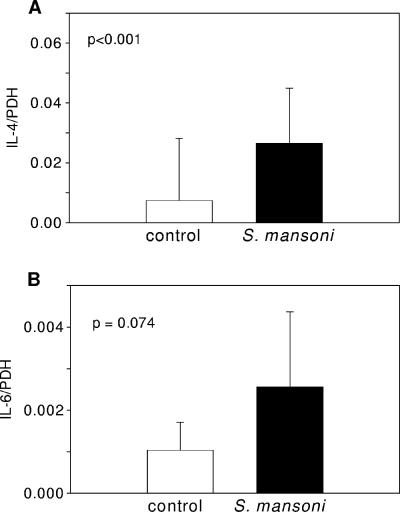
The levels of mRNA expression of the Th2 cytokines IL-4 (Fig. 2A) and IL-6 (Fig. 2B) were higher in the ex vivo PBMCs of virus-infected monkeys with concurrent schistosomiasis than in virus-only controls (P < 0.001, Fig. 2A, and P = 0.074, Fig. 2B, respectively) at week 9 after S. mansoni infection. No differences were observed in mRNA expression levels for IL-2, IFN-γ, tumor necrosis factor alpha, and RANTES (data not shown).
FIG. 2.
Ex vivo PBMC cytokine mRNA levels in S. mansoni-infected or control rhesus macaques. Shown is IL-4 (A) and IL-6 (B) mRNA expression relative to the expression of the housekeeping gene coding for PDH in PBMCs from control or schistosome-infected animals at 9 weeks after exposure to cercariae. Data represent column means ± standard deviations. Groups were compared by Wilcoxon's rank-sum test.
Influence of S. mansoni infection on plasma viral RNA loads.
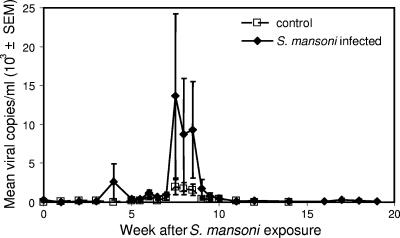
To test whether schistosome coinfection would reactivate clinically latent SHIV-1157ip replication, we prospectively monitored both groups of animals for viral RNA loads at weekly or semiweekly intervals. Whereas viral RNA loads in the control group remained at very low levels, coinfected animals developed viral RNA spikes of >104 copies/ml (Fig. 3). Compared to control animals, this increase of viral replication in coinfected monkeys was significant (P = 0.012), albeit transient, and coincided with the acute stage of S. mansoni infection. We tested for associations between egg counts and viral loads but found no significant correlations.
FIG. 3.
Acute schistosome infection reactivates latent viral infection. Plasma viral RNA loads in SHIV-1157ip-infected animals with loads that had been low to undetectable (<50 copies/ml) were elevated concurrent with acute S. mansoni infection. Generalized estimation equations were used to compare viral loads between the two groups over time (P = 0.012).
Influence of S. mansoni infection on T-cell subset profiles and antiviral immune parameters.
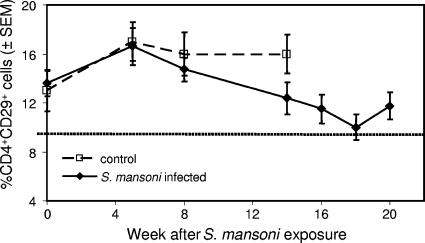
To test whether monkeys with schistosomiasis would demonstrate accelerated development of immunodeficiency compared to those free of parasites, we prospectively monitored the SHIV-1157ip-positive animals with or without parasites for absolute numbers of CD4+ T cells, CD4+/CD8+ ratios, and the percentages of CD4+ CD29+ memory T cells.
No significant changes were observed in absolute CD4+ T-cell numbers, CD4+/CD8+ ratios, or the percentages of CD4+ CD29+ memory T cells in the controls during the 14-week period of observation. In the coinfected group, the absolute numbers of CD4+ T cells and the CD4+/CD8+ ratios similarly remained unchanged. However, the percentage of CD4+ CD29+ memory T cells in coinfected monkeys decreased from 5 weeks of infection onward (P = 0.05, Fig. 4). By 18 weeks after inoculation with schistosomes, the mean percentage in coinfected animals fell to 10%, the lower limit for normal CD4+ CD29+ memory T cells (4, 16, 22, 31).
FIG. 4.
Memory T-cell analysis in SHIV-1157ip-infected monkeys. The percentage of CD4+ CD29+ memory T cells was stable in animals infected with SHIV-1157ip alone but decreased over time in coinfected macaques. Generalized estimation equations were used to model the data collected over time (P = 0.05). The dotted line represents the lower limit of normal for the percentage of CD4+ CD29+ memory T cells. SEM, standard error of the mean.
We also evaluated whether coinfected animals responded differently to viral antigens compared to parasite-free, SHIV-infected monkeys. PBMCs were tested for specific responses to overlapping SIVmac239 Gag peptide pools by IFN-γ ELISPOT assays. All animals from both groups produced IFN-γ in response to the peptides, and there were no statistically significant differences in the numbers of IFN-γ-secreting T cells between animals infected with SHIV only and coinfected monkeys in either short-term (1,198 ± 1,656 [standard deviation] spot-forming units [SFU] per 106 cells versus 1,667 ± 1,956 SFU per 106 cells; P = 0.627) or long-term assays (1,138 ± 1,520 SFU per 106 cells versus 2,737 ± 2,622 SFU per 106 cells; P = 0.181). Finally, we tested whether the levels of anti-SHIV antibodies changed in parasite-positive versus parasite-free monkeys; no statistically significant differences were seen over the course of the experiment as measured by anti-SIV Gag enzyme-linked immunosorbent assay.
DISCUSSION
Coinfection with S. mansoni significantly increased viral replication and induced alterations in the T-cell subsets in monkeys with chronic, clinically stable SHIV clade C infection. From week 5 after S. mansoni exposure onward, coinfected animals shed parasite eggs in the stool, developed eosinophilia, and expressed mRNA of the Th2 cytokine IL-4 at significantly higher levels than animals without schistosomiasis. Compared to virus-only controls, viral replication was significantly increased in the coinfected monkeys. Although the spikes in plasma viral RNA were transient, they coincided with the acute stage of schistosomiasis, when Th2 responses are strongest. In contrast to virus-only controls, the percentage of CD4+ CD29+ memory T cells decreased in the coinfected group starting from week 5 after coinfection until the end of follow-up.
The results of the present study support our previous observation that parasite infection upregulates R5 SHIV clade C replication (15). In the earlier pilot study, no parasite-free, virus-only control group was enrolled in parallel to the coinfected group. Data from the present prospective, controlled trial support the hypothesis that S. mansoni infection and the ensuing establishment of the Th2-skewed cytokine milieu reactivate latent virus and may promote immunologic deterioration.
Previous studies have demonstrated that a decrease in the percentage of CD4+ CD29+ memory T cells is an early sign of immune dysfunction and a prognostic parameter for the development of subsequent immunodeficiency (4, 16, 22, 31). In our primate studies (4, 5, 21; unpublished data), depletion of CD4+ CD29+ memory T cells preceded the loss of absolute numbers of CD4+ T cells by several months to years. Within the time of follow-up of the current two groups of monkeys, we did not observe significant changes in either absolute CD4+ T-cell counts or CD4+/CD8+ ratios.
The transient character of the viral reactivation could possibly be ascribed to the transient nature of schistosome infections in rhesus macaques. Although these animals are permissive hosts for schistosomiasis in that they allow establishment of patent infections, most monkeys clear the parasites following the acute phase of infection without treatment intervention (30, 38). In humans, where schistosome infections are more persistent, the elevation of plasma viremia may similarly be prolonged. From these data, we cannot predict whether persons with chronic schistosomiasis would similarly experience increased viral replication or if only persons who have acute schistosomiasis would be affected. However, the observation that coinfected persons who received early treatment for their schistosomiasis demonstrated significantly lower increases in viral loads and CD4+ T-cell declines compared to persons whose treatment was delayed for as little as 3 months argues strongly that any schistosome infection in HIV-positive individuals promotes immunologic deterioration (24).
Nonhuman primate studies, while imperfect models of human infection for schistosomes or immunodeficiency viruses, may provide important insights into disease progression and extend the ability to address questions that are difficult to evaluate in human populations. For example, our previous rhesus coinfection study (15) showing that acute immunodeficiency virus replication is elevated in schistosome-infected monkeys and our present study showing reactivation of viral replication during the acute phase of schistosomiasis would be impossible to assess in humans. Another area in which the primate coinfection model may prove useful is the assessment of HIV-1 vaccine efficacy in the presence of helminths. The effects of schistosomiasis and other helminthic infections on the modulation of immune responses are well established (3, 10, 33, 34), and alterations in response to vaccination as a consequence of schistosome infection have been demonstrated (35). It is likely that human field trials testing the efficacy of HIV candidate vaccines will take place in Africa and Asia, areas with a high incidence of HIV infections. However, potentially effective vaccines may show suboptimal results if such clinical trials are confounded by the immune activation and/or cytokine profiles that may be shifted by helminth infections that predominate in the developing world. Well-planned studies utilizing the nonhuman primate model for parasite-immunodeficiency virus coinfection as described here, in which pathogens, timing, route, and dose can be controlled, may yield critical adjunctive information for the successful implementation of HIV-1 and other vaccination programs.
Acknowledgments
We thank Pei-Lin Li for assistance with sample preparation, Stephanie Ehnert for coordinating blood collections, and Susan Sharp for preparation of the manuscript.
This work was supported by NIH grants PO1 AI48240 and R56 AI062515 to R.M.R. and RR00165, which provided base grant support to the Yerkes National Primate Research Center. R.H.-L. is the recipient of a professorship by the Swiss National Science Foundation (PP00B 102866).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Actor, J. K., M. Shirai, M. C. Kullberg, R. M. Buller, A. Sher, and J. A. Berzofsky. 1993. Helminth infection results in decreased virus-specific CD8+ cytotoxic T-cell and Th1 cytokine responses as well as delayed virus clearance. Proc. Natl. Acad. Sci. USA 90:948-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed-Ansari, A., J. D. Powell, P. E. Jensen, T. Yehuda-Cohen, H. M. McClure, D. Anderson, P. N. Fultz, and K. W. Sell. 1990. Requirements for simian immunodeficiency virus antigen-specific in vitro proliferation of T cells from infected rhesus macaques and sooty mangabeys. AIDS 4:399-407. [DOI] [PubMed] [Google Scholar]
- 3.Ayash-Rashkovsky, M., Z. Bentwich, and G. Borkow. 2005. TLR9 expression is related to immune activation but is impaired in individuals with chronic immune activation. Int. J. Biochem. Cell Biol. 37:2380-2394. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 6.Bentwich, Z. 2003. Concurrent infections that rise the HIV viral load. J. HIV Ther. 8:72-75. [PubMed] [Google Scholar]
- 7.Bentwich, Z., A. Kalinkovich, and Z. Weisman. 1995. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol. Today 16:187-191. [DOI] [PubMed] [Google Scholar]
- 8.Bentwich, Z., A. Kalinkovich, Z. Weisman, G. Borkow, N. Beyers, and A. D. Beyers. 1999. Can eradication of helminthic infections change the face of AIDS and tuberculosis? Immunol. Today 20:485-487. [DOI] [PubMed] [Google Scholar]
- 9.Bentwich, Z., G. Maartens, D. Torten, A. A. Lal, and R. B. Lal. 2000. Concurrent infections and HIV pathogenesis. AIDS 14:2071-2081. [DOI] [PubMed] [Google Scholar]
- 10.Borkow, G., and Z. Bentwich. 2004. Chronic immune activation associated with chronic helminthic and human immunodeficiency virus infections: role of hyporesponsiveness and anergy. Clin. Microbiol. Rev. 17:1012-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borkow, G., Q. Leng, Z. Weisman, M. Stein, N. Galai, A. Kalinkovich, and Z. Bentwich. 2000. Chronic immune activation associated with intestinal helminth infections results in impaired signal transduction and anergy. J. Clin. Investig. 106:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, M., M. Kizza, C. Watera, M. A. Quigley, S. Rowland, P. Hughes, J. A. Whitworth, and A. M. Elliott. 2004. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J. Infect. Dis. 190:1869-1879. [DOI] [PubMed] [Google Scholar]
- 13.Brown, M., P. A. Mawa, S. Joseph, J. Bukusuba, C. Watera, J. A. Whitworth, D. W. Dunne, and A. M. Elliott. 2005. Treatment of Schistosoma mansoni infection increases helminth-specific type 2 cytokine responses and HIV-1 loads in coinfected Ugandan adults. J. Infect. Dis. 191:1648-1657. [DOI] [PubMed] [Google Scholar]
- 14.Brown, M., G. Miiro, P. Nkurunziza, C. Watera, M. A. Quigley, D. W. Dunne, J. A. Whitworth, and A. M. Elliott. 2006. Schistosoma mansoni, nematode infections, and progression to active tuberculosis among HIV-1-infected Ugandans. Am. J. Trop. Med. Hyg. 74:819-825. [PubMed] [Google Scholar]
- 15.Chenine, A. L., K. A. Buckley, P. L. Li, R. A. Rasmussen, H. Ong, S. Jiang, T. Wang, P. Augostini, W. E. Secor, and R. M. Ruprecht. 2005. Schistosoma mansoni infection promotes SHIV clade C replication in rhesus macaques. AIDS 19:1793-1797. [DOI] [PubMed] [Google Scholar]
- 16.Crockard, A. D., N. A. Boyd, T. A. McNeill, and D. R. McCluskey. 1992. CD4 lymphocyte subset abnormalities associated with impaired delayed cutaneous hypersensitivity reactions in patients with X-linked agammaglobulinaemia. Clin. Exp. Immunol. 88:29-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elias, D., D. Wolday, H. Akuffo, B. Petros, U. Bronner, and S. Britton. 2001. Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin. Exp. Immunol. 123:219-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott, A. M., P. A. Mawa, S. Joseph, P. B. Namujju, M. Kizza, J. S. Nakiyingi, C. Watera, D. W. Dunne, and J. A. Whitworth. 2003. Associations between helminth infection and CD4+ T cell count, viral load and cytokine responses in HIV-1-infected Ugandan adults. Trans. R. Soc. Trop. Med. Hyg. 97:103-108. [DOI] [PubMed] [Google Scholar]
- 19.Gopinath, R., M. Ostrowski, S. J. Justement, A. S. Fauci, and T. B. Nutman. 2000. Filarial infections increase susceptibility to human immunodeficiency virus infection in peripheral blood mononuclear cells in vitro. J. Infect. Dis. 182:1804-1808. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 21.Hofmann-Lehmann, R., J. Vlasak, A.-L. Chenine, P.-L. Li, T. W. Baba, D. C. Montefiori, H. M. McClure, D. C. Anderson, and R. M. Ruprecht. 2002. Molecular evolution of human immunodeficiency virus env in humans and monkeys: similar patterns occur during natural disease progression or rapid virus passage. J. Virol. 76:5278-5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann-Lehmann, R., J. Vlasak, A. L. Williams, A. L. Chenine, H. M. McClure, D. C. Anderson, S. O'Neil, and R. M. Ruprecht. 2003. Live attenuated, nef-deleted SIV is pathogenic in most adult macaques after prolonged observation. AIDS 17:157-166. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann-Lehmann, R., A. L. Williams, R. K. Swenerton, P. L. Li, R. A. Rasmussen, A. L. Chenine, H. M. McClure, and R. M. Ruprecht. 2002. Quantitation of simian cytokine and beta-chemokine mRNAs, using real-time reverse transcriptase-polymerase chain reaction: variations in expression during chronic primate lentivirus infection. AIDS Res. Hum. Retrovir. 18:627-639. [DOI] [PubMed] [Google Scholar]
- 23a.Institute of Laboratory Animal Research. 1996. Guide for care and use of laboratory animals. National Academy Press, Washington, DC.
- 24.Kallestrup, P., R. Zinyama, E. Gomo, A. E. Butterworth, B. Mudenge, G. J. van Dam, J. Gerstoft, C. Erikstrup, and H. Ullum. 2005. Schistosomiasis and HIV-1 infection in rural Zimbabwe: effect of treatment of schistosomiasis on CD4 cell count and plasma HIV-1 RNA load. J. Infect. Dis. 192:1956-1961. [DOI] [PubMed] [Google Scholar]
- 25.Lawn, S. D., D. M. Karanja, P. Mwinzia, J. Andove, D. G. Colley, T. M. Folks, and W. E. Secor. 2000. The effect of treatment of schistosomiasis on blood plasma HIV-1 RNA concentration in coinfected individuals. AIDS 14:2437-2443. [DOI] [PubMed] [Google Scholar]
- 26.Lehmann, E. L. 1975. Nonparametrics: statistical methods based on ranks. Holden-Day, McGraw-Hill, San Francisco, CA.
- 27.Li, J., C. I. Lord, W. Haseltine, N. L. Letvin, and J. Sodroski. 1992. Infection of cynomolgus monkeys with a chimeric HIV-1/SIVmac virus that expresses the HIV-1 envelope glycoproteins. J. Acquir. Immune Defic. Syndr. 5:639-646. [PubMed] [Google Scholar]
- 28.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 29.Maizels, R. M., and M. J. Holland. 1998. Parasite immunology: pathways for expelling intestinal helminths. Curr. Biol. 8:R711-R714. [DOI] [PubMed] [Google Scholar]
- 30.McMullen, D. B., L. S. Ritchie, J. Oliver-Gonzalez, and W. B. Knight. 1967. Schistosoma mansoni in Macaca mulatta. Long-term studies on the course of primary and challenge infections. Am. J. Trop. Med. Hyg. 16:620-627. [PubMed] [Google Scholar]
- 31.Mihailov, C., A. Lamour, V. Beaudre-Bellein, N. Jezequel, M. Garre, D. Mottier, G. Guillet, and P. Youinou. 1993. Prognostic significance of cytotoxic T cells in individuals infected with human immunodeficiency virus. J. Clin. Immunol. 13:139-144. [DOI] [PubMed] [Google Scholar]
- 32.Modjarrad, K., I. Zulu, D. T. Redden, L. Njobvu, H. C. Lane, Z. Bentwich, and S. H. Vermund. 2005. Treatment of intestinal helminths does not reduce plasma concentrations of HIV-1 RNA in coinfected Zambian adults. J. Infect. Dis. 192:1277-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson, T. M., and J. D. Boyer. 2004. HIV-1 vaccines and co-infection. Expert Opin. Biol. Ther. 4:1483-1492. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, T. M., R. G. Nelson, and J. D. Boyer. 2003. Parasitic infection and the polarized Th2 immune response can alter a vaccine-induced immune response. DNA Cell Biol. 22:421-430. [DOI] [PubMed] [Google Scholar]
- 35.Sabin, E. A., M. I. Araujo, E. M. Carvalho, and E. J. Pearce. 1996. Impairment of tetanus toxoid-specific Th1-like immune responses in humans infected with Schistosoma mansoni. J. Infect. Dis. 173:269-272. [DOI] [PubMed] [Google Scholar]
- 36.Secor, W. E., A. Shah, P. M. N. Mwinzi, B. A. Ndenga, C. O. Watta, and D. M. S. Karanja. 2003. Increased density of human immunodeficiency virus type 1 coreceptors CCR5 and CXCR4 on the surfaces of CD4+ T cells and monocytes of patients with Schistosoma mansoni infection. Infect. Immun. 71:6668-6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapira-Nahor, O., A. Kalinkovich, Z. Weisman, Z. Greenberg, J. Nahmias, M. Shapiro, A. Panet, and Z. Bentwich. 1998. Increased susceptibility to HIV-1 infection of peripheral blood mononuclear cells from chronically immune-activated individuals. AIDS 12:1731-1733. [PubMed] [Google Scholar]
- 38.Smithers, S. R., and R. J. Terry. 1965. Naturally acquired resistance to experimental infections of Schistosoma mansoni in the rhesus monkey (Macaca mulatta). Parasitology 55:701-710. [PubMed] [Google Scholar]
- 39.Song, R. J., A.-L. Chenine, R. A. Rasmussen, C. R. Ruprecht, S. Mirshahidi, R. D. Grisson, W. Xu, J. B. Whitney, L. M. Goins, H. Ong, P.-L. Li, E. Shai-Kobiler, T. Wang, C. M. McCann, H. Zhang, C. Wood, C. Kankasa, W. E. Secor, H. M. McClure, E. Strobert, J. G. Else, and R. M. Ruprecht. 2006. Molecularly cloned SHIV-1157ipd3N4: a highly replication-competent, mucosally transmissible R5 simian-human immunodeficiency virus encoding HIV clade C env. J. Virol. 80:8729-8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolday, D., S. Mayaan, Z. G. Mariam, N. Berhe, T. Seboxa, S. Britton, N. Galai, A. Landay, and Z. Bentwich. 2002. Treatment of intestinal worms is associated with decreased HIV plasma viral load. J. Acquir. Immune Defic. Syndr. 31:56-62. [DOI] [PubMed] [Google Scholar]
- 41.Zeger, S. L., and K. Y. Liang. 1986. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42:121-130. [PubMed] [Google Scholar]
- 42.Zeger, S. L., K. Y. Liang, and P. S. Albert. 1988. Models for longitudinal data: a generalized estimating equation approach. Biometrics 44:1049-1060. [PubMed] [Google Scholar]
- 43.Zhang, H., G. Orti, Q. Du, J. He, C. Kankasa, G. Bhat, and C. Wood. 2002. Phylogenetic and phenotypic analysis of HIV type 1 env gp120 in cases of subtype C mother-to-child transmission. AIDS Res. Hum. Retrovir. 18:1415-1423. [DOI] [PubMed] [Google Scholar]