Abstract
In this study we examined mechanisms that regulate T-helper lymphocyte 1 (Th1) commitment in Helicobacter pylori-infected human gastric mucosa. The levels of gamma interferon (IFN-γ), interleukin-4 (IL-4), and IL-12 in total extracts of gastric biopsies taken from H. pylori-infected and uninfected patients were determined by an enzyme-linked immunosorbent assay. The levels of signal transducer and activator of transcription 4 (STAT4), STAT6, and T-box expressed in T cells (T-bet) in total proteins extracted from gastric biopsies were determined by Western blotting. Finally, the effect of a neutralizing IL-12 antibody on expression of Th1 transcription factors and the levels of IFN-γ in organ cultures of H. pylori-infected biopsies was examined. Increased levels of IFN-γ and IL-12 were found in gastric biopsy samples of H. pylori-infected patients compared to the levels in uninfected patients. In addition, H. pylori-infected biopsies exhibited high levels of expression of phosphorylated STAT4 and T-bet. Higher levels of IFN-γ and expression of Th1 transcription factors were associated with greater infiltration of mononuclear cells in the gastric mucosa. By contrast, production of IL-4 and expression of phosphorylated STAT6 were not associated with the intensity of mononuclear cell infiltration. In ex vivo organ cultures of H. pylori-infected biopsies, neutralization of endogenous IL-12 down-regulated the expression of phosphorylated STAT4 and T-bet and reduced IFN-γ production. Our data indicated that IL-12 contributes to the Th1 cell commitment in H. pylori-infected human gastric mucosa.
Helicobacter pylori is a gram-negative extracellular organism that colonizes the gastric mucosa of humans and causes chronic gastric inflammation, peptic ulcers, gastric adenocarcinoma, and lymphoma. Despite the development of immune responses against H. pylori infection, the bacteria are rarely eliminated, and colonization is generally persistent. Factors that contribute to the failure of the immune response to clear the organism have not been fully elucidated yet (3).
Chronic gastric inflammation induced by H. pylori is considered a T-helper lymphocyte 1 (Th1)-mediated process characterized by increased production of gamma interferon (IFN-γ), which is implicated in perpetuating the inflammatory changes that lead to disease (7, 10). Several studies have clearly demonstrated that distinct cytokine-activated signaling and transcription factors regulate the commitment of naïve T cells with the Th1 or Th2 phenotype, as well as maintenance of the polarized phenotype (16). Transcription factors, such as signal transducer and activator of transcription 4 (STAT4), are associated with IFN-γ production and play a major role in Th1-specific cytokine production (22). Molecular analysis of transcription factors expressed in polarized lymphocytes has led to the identification of the novel transcription factor T-box expressed in T cells (T-bet). T-bet has been shown to be required for Th1 differentiation, and its expression is sufficient to induce IFN-γ production in Th cells (24). On the other hand, Th2 commitment is characterized by activation of STAT6 and interleukin-4 (IL-4) production (28).
Cytokines involved in inducing and regulating Th1 responses have been extensively investigated and have often been shown to be redundant due to several signaling pathways that are potentially implicated in their production. Nevertheless, factors that regulate Th1 transcription factors in human gastric mucosa have not been investigated.
IL-12 is a heterodimeric cytokine produced by antigen-presenting cells that promotes the development of Th1 cells and stimulates proliferation, cytolytic activity, and IFN-γ production by T and natural killer cells (5, 14). The effects of IL-12 are mediated through an IL-12 receptor (IL-12R) consisting of two subunits, IL-12Rβ1 and IL-12Rβ2 (19). IL-12Rβ2, which transmits the signals inside the cell, is selectively expressed on Th1 cells (21, 20). Binding of IL-12 to its receptor leads to phosphorylation of STAT4 (2) and IFN-γ production.
The aim of this study was to examine mechanisms which regulate Th1 commitment in human gastric mucosa infected by H. pylori.
MATERIALS AND METHODS
Patients and samples.
Twenty patients (12 men and 8 women; age range, 19 to 68 years; median age, 36 years) who underwent esophagogastroduodenoscopy for dyspeptic symptoms were studied. No patient had previously undergone anti-H. pylori treatment or had received antibiotics within the previous 2 months. Patients were classified as H. pylori infected (n = 10) when at least two of the following three tests were positive: urease quick test (Yamanouchi Pharma, Milan, Italy), histology, and [13C]urea breath test (Cortex, Milan, Italy). All three tests had to be negative in order to classify a patient as uninfected (n = 10). During endoscopy, 5 to 11 biopsies were taken in the antral area 2 cm below the incisura angularis; 1 of the biopsies was used for the urease quick test, 2 were used for histologic examination, and 2 were used for protein extraction, and an additional 6 biopsies from the 10 H. pylori-positive patients were used for culturing. Sections of biopsy specimens were embedded in paraffin and stained with Giemsa stain in order to detect H. pylori and with hematoxylin and eosin in order to evaluate gastric inflammation (mononuclear cell and polymorphonuclear cell [PMN] infiltration) according to the Sydney scoring system (8) using a four-point scale, as follows: 0, none; 1, mild; 2, moderate; and 3, severe. Six of the H. pylori-negative subjects had a nonspecific nonsteroid anti-inflammatory drug (NSAID)-related gastritis, while in the remaining H. pylori-negative subjects the gastric mucosa was normal. The median weekly NSAID intake in the previous 2 months was 400 mg nimesulide (range, 200 to 700 mg) per os.
Informed consent was obtained from all patients, and the study protocol was approved by the local ethics committee.
Gastric biopsy culture.
Antral biopsies from 10 H. pylori-positive patients were placed on steel grids in an organ culture chamber at 37°C in 5% CO2-95% O2 with RPMI 1640 containing 5% fetal bovine serum, 10 mM l-glutamine, 100 U/ml penicillin, and 100 U/ml streptomycin for 24 h (all obtained from Invitrogen, Carlsbad, CA) in the presence or absence of a neutralizing IL-12p70 antibody (5 μg/ml; R&D Systems, Minneapolis, MN) or control immunoglobulin G (IgG) antibody (1 μg/ml; R&D Systems). After this, organ culture supernatants were collected and used to measure IFN-γ and IL-4 production with an enzyme-linked immunosorbent assay (ELISA), while tissues were used to extract total proteins.
Extraction of total proteins.
Total proteins were extracted from freshly obtained and cultured gastric biopsies using a lysis buffer containing 50 mM HEPES (pH 7.6), 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 10 mM NaF, 30 mM Na4P2O7, 10% glycerol, 1 mM benzamidine, 1 mM dithiothreitol, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride (all obtained from Sigma, Milan, Italy). After incubation for 30 min on ice, the membranes were sedimented by centrifugation for 30 min at 14,000 rpm and 4°C, and the supernatant was stored at −80°C for later use in Western blot analysis.
Western blotting.
Total proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and electrophoretically transferred onto an Immobilon-P membrane (Amersham, Life Sciences Inc., Buckinghamshire, United Kingdom). Ponceau S staining was performed to confirm equal loading and transfer of proteins. The membranes were blocked overnight at 4°C in Tris-buffered saline with 0.05% Tween 20 (5% nonfat dry milk in 10 mM Tris-HCl, 100 mM NaCl, 0.1% Tween 20; pH 7.6). This was followed by incubation at room temperature with phosphorylated STAT6 (pSTAT6) and pSTAT4 antibodies (obtained from Invitrogen, Carlsbad, CA) diluted 1/2,000 in blocking buffer for 2 h and then with horseradish peroxide-conjugated goat anti-rabbit IgG monoclonal antibody (MAb) diluted 1/3,000 for 1 h. T-bet antibody was diluted 1/1,000, and the secondary antibody used was horseradish peroxide-conjugated goat anti-mouse IgG MAb diluted 1/3,000. The blots containing pSTAT4 and pSTAT6 were stripped and reprobed with antibodies directed to STAT4 and STAT6, and the blots containing T-bet were stripped and reprobed with an antibody directed to β-actin diluted 1/2,000 (all antibodies were obtained from Santa Cruz Biotechnology). Luminol chemiluminescence reagent (Santa Cruz Biotechnology) was used for detection.
Enzyme-linked immunosorbent assay.
The levels of IL-4, IL-12, and IFN-γ in total protein extracts from freshly gastric biopsies and in 24-h culture biopsies were determined using lysis buffer (50 mM HEPES [pH 7.6], 150 mM NaCl, 1% Triton X-100, 1 mM Na3VO4, 10 mM NaF, 30 mM Na4P2O7, 10% glycerol, 1 mM benzamidine, 1 mM dithiothreitol, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride). The total proteins were harvested, and the cytokine levels were determined using a sensitive ELISA (EuroClone Ltd., Westyorks, United Kingdom). One hundred micrograms of total protein extract was then analyzed to determine the levels of active IL-4, IL-12, and IFN-γ according to the manufacturer's instructions. The results for active IL-4, IL-12, and IFN-γ were expressed in pg/100 μg of total proteins.
Statistical analysis.
The significance of differences was assessed by one-way analysis of variance (ANOVA) and, when the F value was significant, by Tukey's multiple-comparison test. Differences were considered significant if the P value was <0.05. Differences of 30 to 50% between samples were considered biologically significant. Thus, to provide 80% power to detect 30 and 50% differences with an α value of 0.05, samples from six and four patients per group, respectively, were needed.
RESULTS
Th1- and Th2-type cytokines are produced differently.
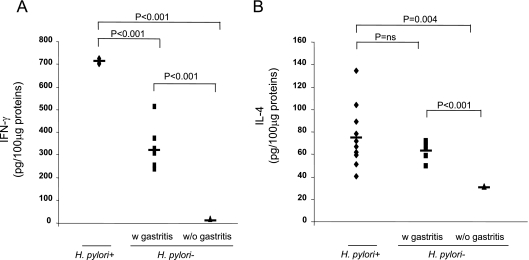
The median scores for gastric mononuclear cell infiltration were 2.5 ± 0.26, 1.5 ± 0.22, and 0 for the 10 H. pylori-infected patients, for six uninfected patients, and for four uninfected patients (P = 0.01 and P < 0.01 for comparisons of the H. pylori-infected patients with the two groups of uninfected patients, respectively). Subgrouping samples according to gastric mononuclear cell infiltration showed that the higher the inflammation score, the higher the level of IFN-γ. Thus, the levels of IFN-γ were significantly higher in the H. pylori-infected patients, who had a median inflammation score of 2.5 ± 0.26 (n = 10), than in the uninfected patients who had a median inflammation score of 1.5 ± 0.22 (n = 6) (713 ± 10 pg/100 μg versus 333 ± 100 pg/100 μg; P < 0.001); in turn, the levels of IFN-γ in the latter patients were higher than the levels in the uninfected patients without evidence of gastric mononuclear cell infiltration (n = 4; inflammation score, 0) (333 ± 100 pg/100 μg versus 15 ± 1 pg/100 μg; P < 0.001) (Fig. 1A). The levels of IL-4 were similar in the H. pylori-infected patients (n = 10) and in the six uninfected patients who exhibited gastric mononuclear cell infiltration (median inflammation scores, 2.5 ± 0.26 and 1.5 ± 0.22, respectively; 75 ± 27 pg/100 μg and 64 ± 8 pg/100 μg, respectively; difference not significant), while the levels of IL-4 were higher in these two groups of patients than in the patients without evidence of gastric mononuclear cell infiltration (n = 4; inflammation score, 0) (75 ± 27 pg/100 μg versus 31 ± 1 pg/100 μg [P < 0.004] and 64 ± 8 pg/100 μg versus 31 ± 1 pg/100 μg [P < 0.001]) (Fig. 1B).
FIG. 1.
Levels of the IFN-γ (A) and IL-4 (B) proteins were determined by ELISA using 100-μg portions of total protein extracts obtained from fresh gastric biopsies from H. pylori-infected patients (n = 10; median inflammation score, 2.5 ± 0.26) and from uninfected patients with NSAID-related gastritis (n = 6; median inflammation score, 1.5 ± 0.22) (w gastritis) and without gastritis (n = 4; inflammation score, 0) (w/o gastritis). Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests. ns, not significant.
The median score for gastric PMN cell infiltration was significantly higher for H. pylori-infected patients (n = 10) than for uninfected patients (n = 10) (2 ± 0.23 versus 0 ± 13; P < 0.001). Nevertheless, there was not a significant difference between the median PMN score for patients with evidence of gastric mononuclear cell infiltration (n = 6) and the median PMN score for patients without evidence of gastric mononuclear cell infiltration (n = 4) (0 ± 0.21 and 0, respectively).
Increased activation of Th1 transcription factors.
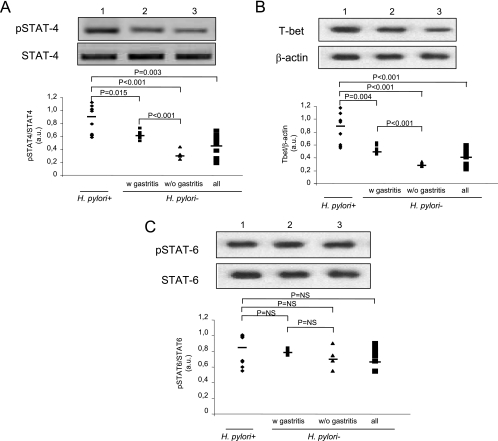
Along with the increased release of IFN-γ, the activation of STAT4 (Fig. 2A) and the levels of T-bet (Fig. 2B) were found to be significantly greater in H. pylori-infected patients (n = 10) than in uninfected patients (n = 10) (0.87 ± 0.22 versus 0.51 ± 0.18 arbitrary units [AU] [P = 0.003] and 0.90 ± 0.26 versus 0.46 ± 0.14 AU [P < 0.001], respectively). Furthermore, subgrouping samples on the basis of gastric mononuclear cell infiltration showed that the higher the inflammation score, the higher the levels of STAT4 and T-bet. Thus, the levels of STAT4 were significantly higher in H. pylori-infected patients having a median inflammation score of 2.5 ± 0.26 (n = 10) than in uninfected patients having a median inflammation score of 1.5 ± 0.22 (n = 6) (0.87 ± 0.22 versus 0.61 ± 0.07 AU; P = 0.015), as were the levels of T-bet (0.90 ± 0.26 versus 0.56 ± 0.07 AU; P = 0.004); and the levels of STAT4 were significantly higher in uninfected patients having a median inflammation score of 1.5 ± 0.22 (n = 6) than in uninfected patients without evidence of gastric mononuclear cell infiltration (n = 4; inflammation score, 0) (0.64 ± 0.07 versus 33 ± 0.08 AU; P < 0.001), as were the levels of T-bet (0.56 ± 0.07 versus 0.31 ± 0.03 AU; P < 0.001) (Fig. 2A and B). In addition, no significant differences in the level of activation of STAT6 (Fig. 3C) were found for H. pylori-infected (n = 10) and uninfected patients (n = 10) (0.85 ± 0.19 versus 0.76 ± 0.10 AU) or in the subgroups based on the gastric mononuclear cell infiltration scores.
FIG. 2.
Activation of Th1 and Th2 transcription factors in gastric mucosa in H. pylori-infected patients (n = 10; median inflammation score, 2.5 ± 0.26) and in uninfected patients with NSAIDS-related gastritis (n = 6; median inflammation score, 1.5 ± 0.22) (w gastritis) and without gastritis (n = 4; inflammation score, 0) (w/o gastritis). Western blot analyses of phosphorylated STAT4 and STAT4 (A), T-bet and β-actin (B), and pSTAT6 and STAT6 (C) were performed by using total proteins extracted from gastric biopsies from 20 patients. In each panel a polyacrylamide gel shows the results of one representative experiment for 10 (lane 1), 6 (lane 2), and 4 (lane 3) patients. The results are expressed in arbitrary units (a.u.). Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests. NS, not significant.
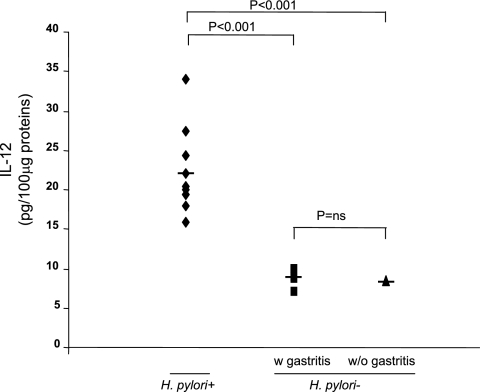
FIG. 3.
Levels of IL-12 protein as determined by ELISA in 100-μg portions of total protein extracts obtained from freshly gastric biopsies from H. pylori-infected patients (n = 10; median inflammation score, 2.5 ± 0.26) and from uninfected patients with NSAID-related gastritis (n = 6; median inflammation score, 1.5 ± 0.22) (w gastritis) and without gastritis (n = 4; inflammation score, 0) (w/o gastritis). Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests. ns, not significant.
Increased production of IL-12.
Increased release of IL-12 was also observed in human gastric biopsy cultures from H. pylori-infected patients compared to the release in human gastric biopsy cultures from uninfected patients (Fig. 3). Subgrouping samples based on gastric mononuclear cell infiltration showed that the highest levels of IL-12 (22.1 ± 5.3 pg/100 μg) were found in H. pylori-infected patients (n = 10), who had the highest inflammation scores (2.5 ± 0.26). The levels of IL-12 were similar for uninfected patients with (n = 6; median inflammation score, 1.5 ± 0.22) and without (n = 4; inflammation score, 0) evidence of gastric mononuclear cell infiltration (8.9 ± 1.1 pg/100 μg and 8.43 ± 0.1 pg/100 μg, respectively; difference not significant).
Neutralization of IL-12 down-regulates Th1 transcription factors and IFN-γ production.
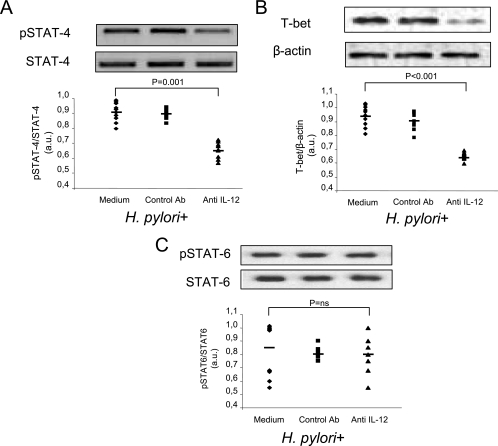
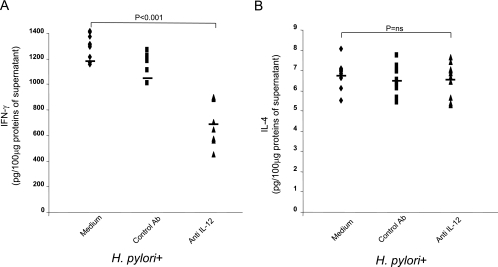
To examine the role of IL-12 in regulating the production of IFN-γ, gastric biopsies taken from patients with H. pylori infections (n = 10) were cultured in the presence or absence of a neutralizing IL-12 antibody or a control antibody. We found that culturing human gastric biopsies with the neutralizing IL-12 antibody significantly decreased the activation of STAT4 (0.91 ± 0.03 AU versus 0.66 ± 0.05 AU; P = 0.001) (Fig. 4A) and T-bet (Fig. 4B) (0.97 ± 0.01 AU versus 0.62 ± 0.01 AU; P < 0.001). No significant differences in the levels of phosphorylated STAT4 and T-bet were found when the control antibody was used. In addition, no significant differences in the level of activation of STAT6 (Fig. 4C) were found in gastric biopsies treated with neutralizing IL-12 antibody compared to biopsies treated with medium (0.85 ± 0.20 AU versus 0.80 ± 0.12 AU) or control antibody. At the same time, the presence of neutralizing IL-12 antibody significantly decreased the production of IFN-γ (1,197 ± 98 pg/100 μg versus 718 ± 173 pg/100 μg; P < 0.001) (Fig. 5A), while it did not modify IL-4 secretion (6.8 ± 0.7 pg/100 μg versus 6.5 ± 0.8 pg/100 μg; difference not significant) (Fig. 5B). No significant differences in the levels of IFN-γ and IL-4 were found when the control antibody was used.
FIG. 4.
Neutralization of endogenous IL-12 results in decreased activation of Th1 transcription factors. Western blot analyses of STAT4 (A), T-bet (B), and STAT6 (C) were performed by using total protein extracts from gastric biopsy cultures from 10 H. pylori-positive patients treated without or with a control IgG antibody (1 μg/ml) or anti-IL-12 antibody (5 μg/ml). In each panel a polyacrylamide gel shows the results of one representative experiment for the 10 patients. The results are expressed in arbitrary units (a.u.). Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests. Ab, antibody; ns, not significant.
FIG. 5.
Neutralization of endogenous IL-12 results in decreased IFN-γ production but not decreased IL-4 production. IFN-γ (A) and IL-4 (B) levels were determined by ELISA by using 100 μg of total protein from the supernatant after 24 h of culture of gastric biopsies from the 10 H. pylori-infected patients treated without or with a control IgG antibody (1 μg/ml) or an anti-IL-12 antibody (5 μg/ml). Statistical significance was assessed by ANOVA and Tukey's multiple-comparison tests. Ab, antibody; ns, not significant.
DISCUSSION
In this study we confirmed that a Th1 signaling pathway is activated in human gastric mucosa infected by H. pylori. In particular, we focused on the functional role of IL-12. It was found previously that the level of the IL-12p70 heterodimer was greater in the gastric mucosa from H. pylori-infected patients than in the gastric mucosa from controls. It is well known that IL-12 binding is rapidly followed by tyrosine phosphorylation of STAT4 and translocation of STAT4 to the nucleus. Indeed, the levels of active (phosphorylated) STAT4 were greater in H. pylori-infected gastric mucosa than in uninfected controls. Also, the expression of T-bet and the levels of IFN-γ were greater in H. pylori-infected samples. In particular, subgrouping of patients showed that up-regulation of the Th1 signaling pathway was associated with a high level of infiltration of the gastric mucosa by mononuclear cells (i.e., the level in patients with H. pylori gastritis was higher than the level in patients with H. pylori-negative gastritis, which was higher than the level in normal gastric mucosa). These findings suggest that an important source of mediators of the Th1 response (i.e., cytokines and transcription factors) is mononuclear cells, which are a hallmark of H. pylori-related chronic inflammation in the gastric mucosa and are a signal that an adaptive immune response has been induced by the bacterium. Even if the source of IL-12 was not specifically identified in experiments in this study, it is traditionally thought that macrophages, monocytes, and dendritic cells, which were included in our biopsy samples, are the major sites of IL-12 production. On the other hand, specific H. pylori virulence factors have been shown to elicit cells of the innate immune system (i.e., PMNs) to produce IL-12 (1). Surprisingly, H. pylori-negative NSAID-related gastritis was associated with significantly increased levels of IFN-γ, STAT4, and T-bet, suggesting that NSAID-related gastritis is Th1 mediated. However, no increase in IL-12 levels was found in this subgroup of patients. The low levels of PMN cell infiltration in NSAID-related gastritis found in our study may have contributed to the low levels of IL-12 in these patients. Furthermore, Bauditz et al. found previously that the level of IL-12 was significantly increased in patients with H. pylori-associated gastritis compared with patients with H. pylori-negative gastritis with similar gastritis scores (4). Thus, it seems that in this study the Th1 signaling could have been driven by mediators other than IL-12. IFN-α and IL-23 are the only cytokines known to induce phosphorylation of STAT4 (6, 18). While it has been shown that IFN-α is not up-regulated (27) in human gastric mucosa infected by H. pylori, no data regarding NSAID-related gastritis are available. Therefore, it can be argued that in NSAID-related gastritis the activation of STAT4 is induced predominantly by IFN-α and IL-23. Nevertheless, the possibility that the activation of STAT4 in H. pylori-related gastritis is partially induced by IL-23, which shares the p40 subunit with IL-12 and has similar biological effects, cannot be excluded.
No significant differences in activation of STAT6 were found between H. pylori-infected human gastric mucosa and uninfected human gastric mucosa, irrespective of the intensity of mononuclear cell infiltration. Accordingly, the production of IL-4 in H. pylori-infected human gastric mucosa and the production of IL-4 in uninfected human gastric mucosa, two tissues which displayed different degrees of mononuclear cell infiltration, were similar, while the production of IL-4 was negligible in uninfected samples without evidence of mononuclear cell infiltration. These data are not surprising since the same numbers of IL-4-secreting cells have been found in human gastric mucosa with H. pylori and human gastric mucosa without H. pylori (10). Thus, it appears that IL-4 may play a role in modulating the inflammatory process that occurs in both H. pylori-negative and -positive gastritis, contributing to down-regulation of IL-12 production. Furthermore, data indicate that a Th2 signaling pathway is not induced by H. pylori. Nonetheless, to our knowledge this is the first report concerning the expression of STAT6 in human gastric mucosa.
Factors which up-regulate Th1 transcription factors in human gastric mucosa infected by H. pylori have not been investigated. This study clearly shows, for the first time in ex vivo experiments, that IL-12 plays a role in the Th1 signaling pathway in the gastric mucosa of patients with H. pylori infection. In fact, addition of a neutralizing IL-12 antibody to the gastric biopsy cultures resulted in significant down-regulation of STAT4 and T-bet transcription factors along with a significant decrease in IFN-γ production. Nevertheless, preliminary experiments in our laboratory have shown that anti-IL-12 treatment of H. pylori-negative gastric samples resulted in significant down-regulation of STAT4, T-bet, and IFN-γ (data not shown). Thus, it appears that in H. pylori-negative patients, IL-12 drives the Th1 response, at a lower level. There could be various mechanisms by which blocking IL-12 results in this effect. It is possible that neutralization of IL-12 inhibits IFN-γ gene activation and/or enhances Th1 cell apoptosis (26, 13). Anti-IL-12 antibody could also negatively regulate the activation of STAT4 and the expression of T-bet, as was the case in this study. Another possibility is that anti-IL-12 treatment could be involved in increased production of transforming growth factor β1, which eventually leads to suppression of IFN-γ-secreting cell development (12). Other results support the results of this study showing that IFN-γ is the gene induced by IL-12 in a STAT4-dependent fashion and that the defect in Th1 polarization in STAT4 knockout mice can be restored by adding exogenous IFN-γ to the developing Th1 cells (11). Although STAT4 has been shown to be required for long-term Th1 differentiation and IFN-γ production (25), cells deficient for STAT4 can differentiate into functional IFN-γ-producing Th1 cells (9). Transcription factor T-bet may be involved in the STAT4-independent Th1 differentiation. Indeed, T-bet has been shown to be required for Th1 differentiation, and expression of T-bet was sufficient to induce IFN-γ production in Th cells (24, 23). Induction of T-bet during Th1 commitment leads to remodeling of the IFN-γ locus, induction of IFN-γ production, and IL-12Rβ2 expression, which is essential for STAT4-mediated IL-12 signaling. Recent evidence suggests that instead of being the primary factor inducing Th1 differentiation (17), STAT4 is instead involved in increasing initial IFN-γ production to optimal levels (15).
In conclusion, in this study we demonstrated that the transcription factors STAT4 and T-bet are activated in the human gastric mucosa of H. pylori-infected patients. This activation is driven, at least in part, by IL-12 and is associated with increased levels of IFN-γ. Specific targeting of this pathway could be a rational approach for controlling the H. pylori-related inflammatory processes in the gastric mucosa. However, it is still not known whether the modulation of the inflammatory response in the human gastric mucosa is effective for elimination of the bacterium and, more importantly, for prevention of H. pylori-related sequelae.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Amedei, A., A. Cappon, G. Codolo, A. Cabrelle, A. Polenghi, M. Benagiano, E. Tasca, A. Azzurri, M. M. D'Elios, G. Del Prete, and M. de Bernard. 2006. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J. Clin. Investig. 116:1092-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacon, C. M., E. F. Petricoin 3rd, J. R. Ortaldo, R. C. Rees, A. C. Larner, J. A. Johnston, and J. J. O'Shea. 1995. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc. Natl. Acad. Sci. USA 92:7307-7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldari, C. T., A. Lanzavecchia, and J. L. Telford. 2005. Immune subversion by Helicobacter pylori. Trends Immunol. 4:199-207. [DOI] [PubMed] [Google Scholar]
- 4.Bauditz, J., M. Ortner, M. Bierbaum, G. Niedobitek, H. Lochs, and S. Schreiber. 1999. Production of IL-12 in gastritis relates to infection with Helicobacter pylori. Clin. Exp. Immunol. 117:316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunda, M. J. 1994. Interleukin-12. J. Leukoc. Biol. 55:280-288. [DOI] [PubMed] [Google Scholar]
- 6.Cho, S. S., C. M. Bacon, C. Sudarshan, R. C. Rees, D. Finbloom, R. Pine, and J. J. O'Shea. 1996. Activation of STAT4 by IL-12 and IFN-alpha: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J. Immunol. 157:4781-4789. [PubMed] [Google Scholar]
- 7.D'Elios, M. M., M. Manghetti, M. De Carli, F. Costa, C. T. Baldari, D. Burroni, J. L. Telford, and S. Romagnani. 1997. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J. Immunol. 158:962-967. [PubMed] [Google Scholar]
- 8.Dixon, M. F., R. M. Genta, J. H. Yardley, and P. Correa. 1996. Classification and grading of gastritis. The updated Sydney System. International Workshop on the histopathology of gastritis, Houston. Am. J. Surg. Pathol. 20:1161-1181. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan, M. H., A. L. Wurster, and M. J. Grusby. 1998. A signal transducer and activator of transcription (Stat)4-independent pathway for the development of T helper type 1 cells. J. Exp. Med. 188:1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karttunen, R., T. Karttunen, H. P. Ekre, and T. T. MacDonald. 1995. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut 36:341-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund, R. J., Z. Chen, J. Scheinin, and R. Lahesmaa. 2004. Early target genes of IL-12 and STAT4 signaling in Th cells. J. Immunol. 172:6775-6782. [DOI] [PubMed] [Google Scholar]
- 12.Marth, T., W. Strober, R. A. Seder, and B. L. Kelsall. 1997. Regulation of transforming growth factor-beta production by interleukin-12. Eur. J. Immunol. 27:1213-1220. [DOI] [PubMed] [Google Scholar]
- 13.Marth, T., M. Zeitz, B. R. Ludviksson, W. Strober, and B. L. Kelsall. 1999. Extinction of IL-12 signaling promotes Fas-mediated apoptosis of antigen-specific T cells. J. Immunol. 162:7233-7240. [PubMed] [Google Scholar]
- 14.McKnight, A. J., G. J. Zimmer, I. Fogelman, S. F. Wolf, and A. K. Abba. 1994. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J. Immunol. 152:2172-2179. [PubMed] [Google Scholar]
- 15.Mullen, A. C., F. A. High, A. S. Hutchins, H. W. Lee, A. V. Villarino, D. M. Livingston, A. L. Kung, N. Cereb, T. P. Yao, S. Y. Yang, and S. L. Reiner. 2001. Role of T-bet in commitment of Th1 cells before IL-12-dependent selection. Science 292:1907-1910. [DOI] [PubMed] [Google Scholar]
- 16.Murphy, K. M., and S. L. Reiner. 2002. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2:933-944. [DOI] [PubMed] [Google Scholar]
- 17.Nishikomori, R., T. Usui, C. Y. Wu, A. Morinobu, J. J. O'Shea, and W. Strober. 2002. Activated STAT4 has an essential role in Th1 differentiation and proliferation that is independent of its role in the maintenance of IL-12R beta 2 chain expression and signaling. J. Immunol. 169:4388-4398. [DOI] [PubMed] [Google Scholar]
- 18.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 19.Presky, D. H., H. Yang, L. J. Minetti, A. O. Chua, N. Nabavi, C. Y. Wu, M. K. Gately, and U. Gubler. 1996. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 93:14002-14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogge, L., L. Barberis-Maino, M. Biffi, N. Passini, D. H. Presky, U. Gubler, and F. Sinigaglia. 1997. Selective expression of an interleukin-12 receptor component by human T helper 1 cells. J. Exp. Med. 185:825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szabo, S. J., A. S. Dighe, U. Gubler, and K. M. Murphy. 1997. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 185:817-824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szabo, S. J., B. M. Sullivan, S. L. Peng, and L. H. Glimcher. 2003. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 21:713-758. [DOI] [PubMed] [Google Scholar]
- 23.Szabo, S. J., B. M. Sullivan, C. Stemmann, A. R. Satoskar, B. P. Sleckman, and L. H. Glimcher. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295:338-342. [DOI] [PubMed] [Google Scholar]
- 24.Takeda, K., T. Kishimoto, and S. Akira. 1997. STAT6: its role in interleukin 4-mediated biological functions. J. Mol. Med. 75:317-326. [DOI] [PubMed] [Google Scholar]
- 25.Thierfelder, W. E., J. M. van Deursen, K. Yamamoto, R. A. Tripp, S. R. Sarawar, R. T. Carson, M. Y. Sangster, D. A. Vignali, P. C. Doherty, G. C. Grosveld, and J. N. Ihle. 1996. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature 382:171-174. [DOI] [PubMed] [Google Scholar]
- 26.Trinchieri, G. 1994. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 84:4008-4027. [PubMed] [Google Scholar]
- 27.Yamaoka, Y., M. Kita, T. Kodama, N. Sawai, K. Kashima, and J. Imanishi. 1995. Expression of cytokine mRNA in gastric mucosa with Helicobacter pylori infection. Scand. J. Gastroenterol. 30:1153-1159. [DOI] [PubMed] [Google Scholar]
- 28.Zheng, W., and R. A. Flavell. 1997. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89:587-596. [DOI] [PubMed] [Google Scholar]