Abstract
Polymorphoneutrophils (PMNs) are important effector cells in host defense against pneumonia. However, PMNs can also induce inflammation and tissue damage. To investigate the contribution of PMNs to host defense against pneumococcal pneumonia, we determined the effect of the PMN-depleting rat monoclonal antibody RB6-8C5 (RB6) on survival and inflammatory and cellular response in the lungs to a lethal intranasal infection with a serotype 8 pneumococcus in BALB/c mice. Control mice received rat immunoglobulin G (rIgG). Strikingly, the survival of RB6-treated mice was significantly prolonged compared to that of rIgG-treated mice. Although the numbers of CFU in the lungs were statistically similar in both groups 4, 24, and 32 h after infection, rIgG-treated mice developed higher levels of bacteremia, and histopathological examination of the lungs of infected mice revealed marked differences between RB6- and rIgG-treated mice. RB6-treated mice had focal, perivascular lesions without accompanying parenchymal inflammation, and rIgG-treated mice had diffuse, interstitial parenchymal inflammation. Lung homogenates from the rIgG-treated mice had more leukocytes and significantly more total and apoptotic PMNs as determined by fluorescence-activated cell sorter analysis with Annexin V and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling staining of lung tissue samples. Studies with a pneumolysin-deficient mutant of the serotype 8 strain we used also demonstrated the prolonged survival of RB6- compared to rIgG-treated mice. Taken together, our findings suggest that PMNs enhance the likelihood of early death and alter the pathological response to pneumococcal lung infection in BALB/c mice with serotype 8 pneumonia without significantly affecting bacterial clearance or the cytokine response.
Polymorphoneutrophils (PMNs) are major effector cells in host defense against pneumococcal pneumonia. As such, PMN recruitment is considered essential for microbial clearance from the lungs. Stimuli for PMN recruitment in experimental pneumonia models include inflammatory mediators, such as tumor necrosis factor alpha (TNF-α) and interleukin 1 (IL-1) (78). These mediators, along with the chemokines mouse keratinocyte-derived chemokine (KC) and macrophage inflammatory protein 2 (MIP-2), contribute to PMN emigration within the lung. Pneumolysin (PLY), a pneumococcal virulence factor, has also been implicated in early PMN recruitment and bacterial dissemination in experimental pneumococcal pneumonia (35, 63). PMNs appear in the lungs of mice 2 to 4 h after pulmonary challenge with pneumococci and continue to increase in number up to 24 to 48 h after infection (18, 77), although a decrease in lung PMNs was observed in some models (7, 33). When death is used as an endpoint of infection, these and other studies suggest an association between survival and an earlier increase and disappearance of lung PMNs. Death has also been associated with continued PMN recruitment and production of inflammatory mediators (12, 18). However, a requirement for PMNs in resistance to murine pneumococcal pneumonia has not been established.
Peripheral neutropenia is not generally considered to be a risk factor for adult pneumococcal disease. However, neutropenic patients are at increased risk for pneumonia (61), and neutropenia is a poor prognostic finding in patients with established pneumococcal disease (42). Studies of several mouse models have provided insight into the role that PMNs could play in pneumococcal pneumonia, but most of this information is indirect. Cyclophosphamide-treated mice with pneumococcal lung infection had similar blood CFU and lung cytokine profiles but slightly higher numbers of lung CFU than mice not treated with cyclophosphamide (77). A recent study demonstrated that pneumococci were cleared from the lungs of mice treated with cyclophosphamide, whereas Klebsiella pneumoniae and Staphylococcus aureus were not cleared (82). However, cyclophosphamide has pleiotropic effects on cells other than PMNs (70). In another model, mice treated with anti-granulocyte colony-stimulating factor had lower numbers of peripheral PMNs, but their rates of survival and numbers of lung PMNs and lung and blood CFU were similar to those of control mice (37). TLR2-deficient and Fas/FasL-deficient mice had less tissue inflammation and lower numbers of lung PMNs than wild-type controls but similar survival after pulmonary challenge with pneumococci (39, 50). In contrast, CD11b-deficient mice showed more bacterial dissemination and inflammation and more lung PMNs than wild-type controls (59). These observations suggest an association between lung inflammation, a higher number of lung PMNs, and reduced survival in murine pneumococcal pneumonia.
Burns et al. previously observed that pulmonary challenge with serotype 8 pneumococcus was associated with the delayed appearance of PMNs in mice that died, whereas mice that survived after antibody therapy had an earlier appearance of PMNs, which then disappeared (12). Serotype 8 is an important pneumococcal serotype that is notable for causing disease in older children and adults (65). In view of the fact that it is not included in the current vaccine used in infants and young children, there is a risk of an increase in infection with serotype 8 and other nonvaccine serotypes as a result of serotype replacement (26, 57). This study was undertaken to determine the effect of peripheral neutropenia on the pathogenesis of serotype 8 pneumococcal pneumonia in mice. We used the rat monoclonal antibody (MAb) RB6-8C5 (RB6) (23, 29) to induce peripheral PMN depletion in BALB/c mice and determined their susceptibility to intranasal (i.n.) infection with serotype 8 pneumococci. Our results show that RB6-treated mice had less dissemination of bacteria in the bloodstream, longer survival, unique histopathological findings, and less lung apoptosis than control mice.
MATERIALS AND METHODS
Bacteria.
Type 8 Streptococcus pneumoniae (pneumococcus) (ATCC 6308) was obtained from American Type Culture Collection (Rockville, MD). The organisms were grown in tryptic soy broth (TSB) (DIFCO, Sparks, MD) to mid-log phase at 37°C in 5% CO2, frozen in TSB with 10% glycerol, and stored at −80°C until being used, as previously described (12). A PLY-deficient serotype 8 strain (PLY−) was generated in S. pneumoniae ATCC 6308 by gene splicing by the overlap extension method. An erythromycin (Erm) resistance cassette (1,096 bp) was amplified from the ermB gene using primers ermF (5′-GGAAATAAGACTTAGAAGCAAAC-3′) and ermR (5′-CCAAATTTACAAAAGCGACTC-3′). Regions of approximately 1 kb flanking the ply gene were amplified with two primer pairs, PlyAF/PlyAR and PlyBF/PlyBR. Primer sequences were as follows: PlyAF, 5′-CTCAATCCAGCTACCTGTCGC-3′; PlyAR, 5′-GTTTGCTTCTAAGTCTTATTTCCCTTCTACCTCCTAATAAG-3′; PlyBF, 5′-GAGTCGCTTTTGTAAATTTGGGAGAGGAGAATGCTTGCG-3′; and PlyBR, 5′-GCTTGTTTAGCACGGTCG-3′. PlyAR and PlyBF served as fusion primers and consisted of primers ermF and ermR, respectively, and the target gene-specific sequence. A mixture of gel-purified preparations of the two flanking products and the Erm cassette was used as template for the PCR with gene splicing by the overlap extension method, using the outer PlyAF and PlyBR primers. The full-length PCR product consisting of the Erm cassette flanked by the ply-flanking sequences served as the knockout construct. The PCR fusion product was introduced into the chromosome of S. pneumoniae ATCC 6308 by transformation and homologous recombination based on standard methods (30). Deletion of the ply gene was verified by PCR sequencing. In addition, the hemolytic activity of all clones was determined using the protocol of Paton et al., with some modifications (55). Briefly, wild-type serotype 8 (ATCC 6308), PLY− serotype 8, strain D39, and strain TIGR4 pneumococci were grown in culture to an optical density at 620 nm of 0.5, and a 2-ml aliquot of each of the cultures was pelleted and lysed for 20 min in lysis buffer (0.1% sodium deoxycholate, 10% sodium dodecyl sulfate, 0.15 M sodium citrate). Prior to being serially diluted on the microtiter plates, the bacterial lysates were diluted 1:2, and 50 μl of each of the diluted bacterial lysates was serially diluted 1:3 across the microtiter plate into phosphate-buffered saline (PBS) containing 0.1% dithiothreitol (Sigma, St. Louis, MO). Washed 1% sheep red blood cells in PBS were added to the wells and incubated at 37°C for 30 min. After incubation, the sheep erythrocytes were pelleted, and 100 μl of the supernatant was read in a microtiter plate reader to determine the optical density at 410 nm. Negative controls consisted of the lysis buffer diluted 1:2 in PBS. Sheep erythrocytes in water served as the positive control or 100% lysis control. The hemolytic activities of both the wild-type and PLY− strains of serotype 8 were below the lower limit of detection, <3 hemolytic units (HU)/ml of bacteria, whereas D39 and TIGR4 were calculated to have hemolytic activities of 243 to 750 HU/ml of bacteria (data not shown). HU were defined as the reciprocal of the dilution that causes 50% lysis of sheep red blood cells. The nucleic acid sequence of the PLY from the wild-type serotype 8 strain (ATCC 6308) was determined. Briefly, primers PlyAF and PlyBR were used to amplify the full-length ply gene plus 1 kb of flanking DNA on each end from serotype 8 pneumococcus (ATCC 6308) chromosomal DNA. This product was used as template for sequencing reactions using primers JAT115 (5′-GGATTACTTAGTCCAACCAC-3′), JAT117 (5′-CATCACGAGCATACTCCAATG-3′), and JAT124 (5′-GCACCACTATGATCCAGCAG-3′), which gave the complete sequence for the ply gene. All sequencing was done at the Hartwell Center for Bioinformatics and Biotechnology, St. Jude Children's Research Hospital. Amino acid analysis revealed that it had the deletions V270 and K271 and amino acid substitutions T172 to I, L224 to R, and A265 to S. These mutations are the same as those reported for other serotype 8 strains (45). The T172 mutation is the only mutation that affects PLY function (45). Another amino acid substitution, Y150 to H, was also found. This mutation was recently identified in a clinical isolate of nonhemolytic serotype 1 pneumococcus (36).
Peripheral PMN depletion.
PMN depletion was induced by the intraperitoneal (i.p.) administration of RB6 to female, 6- to 8-week-old BALB/c mice (NCI, Bethesda, MD) as described previously (70). RB6 purified from ascites was provided by Marta Feldmesser (Albert Einstein College of Medicine). Rat IgG (rIgG; Sigma) was used as an isotype control. Twenty-five micrograms of RB6 or rIgG was administered in 100 μl of PBS (Cambrex, Walkersville, MD). The dose of RB6 used in this study was chosen because it induces neutropenia, without affecting the population of Ly6G+ dendritic cells or other cell types (70, 73). Control mice were given 25 μg of rIgG i.p. The extent of PMN depletion was determined 18 h after RB6 administration by counting of total white blood cells (WBC) on blood diluted 1:20 in Turk's solution using a hemacytometer. WBC differential counts were obtained from blood smears stained with the Hema3 system (Fischer Scientific, Middletown, VA) in accordance with the manufacturer's protocol.
Pneumococcal infection model.
Pneumococcal infection was induced 18 h after PMN depletion or control treatments. A PBS control group was studied in pilot experiments, which revealed that the outcomes of infection in PBS- and rIgG-treated mice were similar. Hence, further studies were performed with RB6 and rIgG only. For the induction of pneumonia, an i.n. model was employed. Pneumococci were thawed immediately before use, placed on ice, and diluted to the desired concentration in TSB. Mice were minimally anesthetized with isoflurane (Henry Schein, Inc. Melville, NY) and inoculated with 5 ×103 CFU of either wild-type or PLY− pneumococci in 40 μl of TSB by applying 20 μl to the opening of each nare by pipette. To determine the amount of bacteria actually administered, the inoculum was plated on Trypticase soy agar plates with 5% sheep's blood (Becton Dickinson, Sparks, MD) both before and after infecting the mice. The mice were held upright to ensure adequate involuntary inhalation into the alveolar spaces. Mice were placed back in their cages once aspiration was complete and they were regaining consciousness. Additionally, a systemic model was used to examine the applicability of our findings in the i.n. model to a systemic infection. In this model, mice were infected i.p. with 500 CFU of serotype 8 pneumococci. The 50% lethal dose (LD50) for i.p. infection was determined to be <10 CFU 48 h after infection, as described previously (11), and a preliminary study showed the LD90 to be 500 CFU (data not shown). The survival of infected mice was monitored and recorded daily. Separate experiments, in which mice were killed and samples were obtained to determine lung cytokines and chemokines, bacterial burden, and pathology, were performed. All animal experiments were carried out in accordance with the Institute for Animal Studies of the Albert Einstein College of Medicine.
Measurement of lung and blood bacterial loads.
Mice were bled from the retroorbital sinus using heparinized hematocrit capillary tubes (Fisher Scientific, Fairlawn, NJ) 4, 24, and 32 h after infection. Mice were then anesthetized with isofluorane and killed by cervical dislocation. Their lungs were removed aseptically and homogenized in Hanks balanced salt solution (HBSS) without calcium, magnesium, or phenol red (Cambrex). Both the blood and lung homogenate samples were serially diluted in TSB and plated onto Trypticase soy agar with 5% sheep's blood plates (Becton Dickinson) as described previously (12). The plates were incubated for 18 h at 37°C in 5% CO2, and then the number of CFU was determined.
Cytokine and chemokine levels in the lungs.
Lung samples were examined for levels of MIP-1α, MIP-2, IL-6, KC, IL-1β, IL-10, IL-12, IL-17, gamma interferon (IFN-γ), and TNF-α by enzyme-linked immunosorbent assay (ELISA) as described previously (13). Lungs were homogenized as previously described (12) and centrifuged at 1800 × g for 30 min. The supernatants were extracted and immediately frozen at −80°C. Before being used, the supernatants were spun at 3,000 × g for 30 min to remove any further cellular debris. ELISA Duoset kits (R&D Systems, Minneapolis, MN) were used for chemokine and cytokine determinations according to supplied protocols. Purified cytokines supplied in the kits were used as standards and positive controls. Concentrations of each cytokine were determined using the standard curves developed on each plate according to the manufacturer's protocol. The lower limit of detection for MIP-2, KC, IL-6, IL-17, and IL-1β was 15.6 pg/ml; for IFN-γ, IL-10, and TNF-α it was 31.25 pg/ml; and for IL-12 it was 39.1 pg/ml. The cytokine determinations were performed on samples from three separate experiments.
Histopathological and immunohistochemical analysis.
Groups of 4 BALB/c mice were treated with rIgG or RB6 and then killed 4, 24, or 32 h after i.n. infection with pneumococci as described above. The lungs were inflated with 4% formalin (Fisher Scientific, Fairlawn, NJ), fixed for 48 h in situ, removed, embedded in paraffin (Blue Ribbon, Surgipath, Richmond, IL), cut into 5-μm-thick sections, and adhered to Superfrost/Plus Slides (Fisher Scientific). Staining was then performed with hematoxylin and eosin (H&E) (Surgipath). This experiment was performed two times.
Pneumococci were detected in tissue by immunostaining with the serotype 8-specific human MAb D11 (81). Briefly, slides were deparaffinized, rehydrated in a series of graded alcohols, and washed in distilled water. Endogenous peroxidase activity was blocked for 10 min at room temperature (RT) with 3% H2O2. Nonspecific binding was blocked with 1% bovine serum albumin in PBS for 1 h at RT. Slides were incubated overnight at 4°C with 5 μg/ml of human MAb D11 or control IgM (Calbiochem, San Diego, CA). Then they were washed and incubated with biotinylated goat anti-human IgM for 30 min at RT. Slides were washed, incubated with streptavidin horseradish peroxidase (Zymed, South San Francisco, CA) at a 1:200 dilution for 30 min, developed with Fast 3,3′-diaminobenzidine tablets (Sigma Aldrich, Minneapolis, MN), counterstained with hematoxylin and Gill's formulation no. 2 (Santa Cruz Biotechonology, Santa Cruz, CA), dehydrated, and placed on coverslips.
All tissue sections were viewed and all still images were taken using an Axioskop 2 microscope with an attached MRC camera and Axiovision software (Carl Zeiss, Oberkochen, Germany) in the Analytical Imaging Facility of the Albert Einstein College of Medicine.
Flow cytometry analysis of lung cell populations.
Lungs were excised from infected mice and treated with rIgG or RB6, and cells were isolated as described previously (60). Briefly, lungs were rinsed in sterile HBSS and minced with clean razor blades in sterile petri dishes containing 10 ml of digestion buffer. The digestion buffer consisted of 10% fetal calf serum (HyClone, Logan, UT), 1 mg/ml collagenase A (Roche Diagnostics, Inc, Indianapolis, IN), and 30 μg/ml DNase I (Roche) in RPMI (MediaTech, Herndon, VA). Using the plunger from a 3-ml syringe (Becton Dickinson, Mountainview, CA), the minced lungs were put through 70-μm-pore-size mesh strainers. The undigested tissue was discarded, and the 10 ml of digestion buffer and strained lung cells were incubated for 1 h in a 37°C water bath and vortexed briefly every 15 min. Digested lung material was then centrifuged at 470 × g at 4°C for 10 min. The supernatant was removed, the cells were resuspended in 5 ml of 0.17 M NH4Cl (Sigma) buffer (pH 7.2), and the suspension was placed on ice for 10 min to lyse the red blood cells. RPMI was added to bring the volume to 15 ml; the cells were pelleted at 470 × g at 4°C for 10 min, washed in HBSS without phenol red, and centrifuged again, and then the pellets were resuspended in staining buffer (1% fetal calf serum in sterile PBS). Cells were counted in a hemacytometer using trypan blue (Sigma) to exclude dead cells, and the correct volume of each cell suspension was calculated to give 2 × 105 to 5 × 105 cells for each lung sample according to the method of Rivera et al. (60). The cells were pelleted and resuspended in 100 μl of staining buffer. Then the cells were stained with combinations of the following: fluorescein isothiocyanate (FITC)-labeled CD19 to detect B cells (clone 1D3) (41), R-phycoerythrin-labeled CD45 as a panleukocytic marker (clone 30-F11) (72), CD4-FITC for helper T cells (clone RM 4-5) (66), CD8-FITC for cytotoxic T cells (clone 53-6.7) (54), anti-Ly6G-FITC for neutrophils (clone 1A8) (43), and F4/80-FITC for macrophages (clone CI:A3-1) (5). All antibodies were purchased from BD PharMingen (San Diego, CA) and diluted 1:100 except for F4/80, which was purchased from Serotec (Raleigh, NC) and diluted 1:50. All fluorescence-activated cell sorting (FACS) analyses were performed using a FACSCalibur flow cytometer in the FACS facility of the Albert Einstein College of Medicine using four color analyses with adequate single color controls.
In situ staining for cell death in lung tissues.
In situ cell death was detected using the POD in situ cell death detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's protocol. Briefly, formalin-fixed, paraffin-embedded lung tissue slides were deparaffinized in xylene and rehydrated through a series of alcohols to distilled water. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min at RT, and the tissue was permeabilized by immersion in 0.1% Triton X-100 (EM Science, Gibbstown, NJ) in a 0.1% sodium citrate (Fisher) solution for 8 min at RT. Slides were washed in PBS, and nonspecific binding was blocked with 10% goat serum in PBS for 30 min at RT. Fifty microliters of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) reaction mixture was incubated on the slides for 1 h at 37°C in a dark humidified chamber. Slides were washed and incubated with 50 μl of Converter-POD for 30 min at 37°C in a humidified chamber. Slides were washed, developed using 3,3′-diaminobenzidine (Sigma-Aldrich), counterstained with hematoxylin (Santa Cruz Biotechnology), dehydrated, and placed on coverslips as described above.
Analysis of apoptosis by flow cytometry.
Cell death was assayed using Annexin V staining (40). Annexin V binds to the phospholipid phosphatidylserine. As cells undergo apoptosis, phosphatidylserine is translocated from the internal plasma membrane out to the extracellular environment (76). 7-AAD is a dye used for cell viability staining that is excluded by intact, viable cells and is found intracellularly in dead or dying cells. Cells that stained positive for Annexin V-antigen-presenting cell (APC) and negative for 7-AAD were taken to be apoptotic. Lungs were excised and processed for flow cytometry as described above. Cell populations were stained as described above and then stained with Annexin V-allophycocyanin (BD Pharmingen, San Jose, CA) and 7-AAD (BD Pharmingen) according to the manufacturer's protocols. Briefly, 2 × 105 to 5 × 105 stained cells were washed in cold PBS and resuspended in 100 μl of 1× binding buffer (BD Pharmingen). Then, 5 μl each of Annexin V-APC, and 7-AAD was added to each tube. After gentle vortexing, the cells were incubated at RT in the dark for 15 min. The volume of each tube was brought up to 500 μl with 1× binding buffer.
Evan's blue vascular permeability.
Evan's blue permeability was determined as described previously (25). Briefly, mice were treated with RB6 or rIgG 18 h prior to infection with serotype 8 pneumococci as described above. Six RB6-treated mice and six rIgG-treated mice were studied. One hour prior to killing the mice, 23 h after infection, mice were injected intravenously with 160 mg/kg of body weight of Evan's blue dye (dissolved in PBS). Mice were killed 24 h after infection, and the lungs were perfused with 10 ml of PBS (Cambrex), removed, rinsed in PBS, and homogenized in 1 ml of PBS. After homogenization, 2 ml of formamide (Sigma) was added, and the homogenates were incubated in a 60°C water bath for 18 h. After incubation, the homogenates were centrifuged at 5,000 × g for 30 min, the supernatants were removed, and the Evan's blue dye concentration was determined spectrophotometrically using the corrected absorbance at 620 nm (A620 corrected), which was calculated using the following formula: A620 corrected = A620 − (1.436 × A740 + 0.03). No difference in vascular permeability between the rIgG- and RB6-treated mice was detected 24 h after infection (data not shown). However, both treatment groups had at least two times more vascular permeability than uninfected mice, but this difference was only statistically significant in the rIgG-treated group (P = 0.02, unpaired t test) (data not shown).
Statistical analysis.
The percentages of peripheral PMNs and the results from FACS analysis of cell populations and cell death were analyzed using unpaired t testing and analysis of variance with Bonferroni's multiple comparison test to compare the treatment groups at different time points. The numbers of blood and lung CFU and cytokines and chemokines in the lung homogenates were analyzed using Mann-Whitney U testing to compare treatment groups and Kruskal-Wallis testing with Dunn's multiple comparison test to compare the treatment groups at different times. These and all other statistical analyses, including the Kaplan-Meier log rank survival test, were performed using Prism (version 4.03 for Windows) from GraphPad Software (San Diego, CA). P values of <0.05 were used to determine statistical significance.
Nucleotide sequence accession number.
The nucleic acid sequence of PLY has been submitted to GenBank under the accession number EF368014.
RESULTS
PMN depletion.
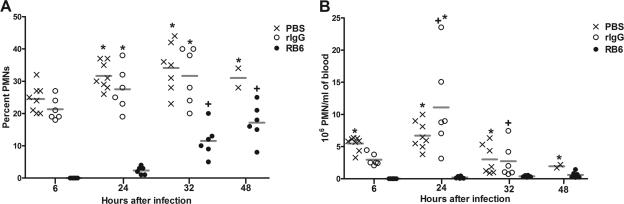
PMN depletion was assessed using blood smears stained with the Hema3 stain. The total number and percentage of blood PMNs relative to total WBC 6, 24, 32, and 48 h after infection corresponded to 24, 42, 50, and 66 h after RB6 administration, respectively (Fig. 1). There were no peripheral PMNs detected 6 h after infection (24 h after RB6 administration) (Fig. 1). Peripheral PMNs increased to detectable levels in RB6-treated mice 24 h after infection, and PMN levels increased significantly to 12% and 17% of total leukocytes 32 and 48 h after infection (P < 0.0001, 48 h) (Fig. 1A) but did not approach the levels found for rIgG-treated mice, which increased significantly from 21% of total leukocytes at 6 h after infection to 32% at 32 h; however, this difference was not significant (Fig. 1A). The percentages of PMNs in PBS- and rIgG-treated mice at 24 h (32% and 28%, respectively) and 32 h (34% and 32%, respectively) after infection were significantly greater than those in the RB6-treated mice (2% and 12%, respectively) (P < 0.0007) (Fig. 1A). PMNs could not be determined for rIgG-treated mice 48 h after infection, because the mice had already died. However, the remaining PBS-treated mice had a significantly increased percentage of peripheral PMNs 48 h (31%) after infection compared to RB6-treated mice (17%) (P = 0.02). The number of PMNs was significantly increased in the PBS- and rIgG-treated mice compared to RB6-treated mice 24 h after infection (P values of 0.01 and <0.001, respectively) (Fig. 1B), and PBS-treated mice had increased numbers of PMNs at 32 and 48 h compared to RB6-treated mice (P values of 0.02 and 0.01, respectively) (Fig. 1B). PBS-treated mice had increased numbers of PMNs 6 h after infection compared to rIgG-treated mice (P = 0.0007) (Fig. 1B). However, the total WBC counts (data not shown) and numbers of PMNs were significantly decreased at 32 h compared to 24 h after infection in the PBS and rIgG-treated mice (P < 0.01) (Fig. 1B).
FIG. 1.
PMNs in peripheral blood of PBS-, rIgG-, and RB6-treated mice. The percentages (A) and numbers (B) of PMNs in peripheral blood smears 6, 24, 32, and 48 h after i.n. infection with wild-type serotype 8 pneumococci, corresponding to 24, 42, 50, and 66 h after rIgG or RB6 treatment, are shown. The lines depict the geometric mean of the designated group. *, P value of <0.05 between groups at the indicated time, as determined by unpaired t testing; +, P value of <0.05 between times for the designated group, as determined by unpaired t testing. n is equal to six mice per group.
Survival studies.
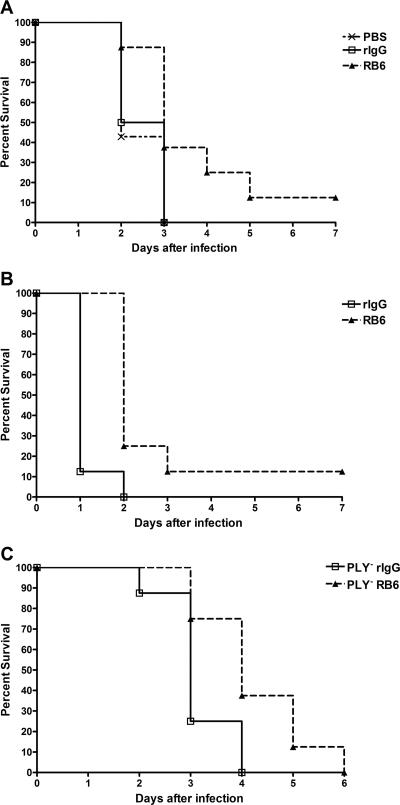
The inoculum used in this study, 5 × 103 CFU, was five times the LD50 for naïve BALB/c mice 48 h after i.n. infection with serotype 8 pneumococci determined by Reed and Muench (58a) LD50 calculations (data not shown). Mice in both treatment groups exhibited minor signs of sickness, such as ruffled fur and slight lethargy, 24 h after infection. By 48 h after infection with the wild-type strain, 50 to 55% of PBS- and rIgG-treated mice had died, whereas 85% of the RB6-treated mice were still alive. The median survival of the RB6-treated mice (3 days) was significantly longer than that of the PBS- and rIgG-treated mice (2 and 2.5 days, respectively) (P = 0.03, Kaplan-Meier log rank survival test) (Fig. 2A). The PBS- and rIgG-treated mice were all dead 72 h after infection. The RB6-treated mice died gradually, with 60% dead on day 3, until day 7 when there was one surviving mouse. A separate experiment showed that in the PBS- and rIgG-treated mice, a level of bacteremia corresponding to >2 × 105 CFU/ml of blood 32 h after infection was associated with death of the mice by 48 h after infection. Mice with <2 × 105 CFU/ml died at 54 and 72 h after infection. The median survival of the RB6-treated mice that received the PLY− strain (4 days) was significantly longer than that of the rIgG-treated mice (3 days) (P = 0.02) (Fig. 2C). In a separate experiment, it was found that mice receiving rIgG and infected with the wild-type serotype 8 strain survived statistically longer than mice receiving rIgG and infected with PLY− pneumococci (P = 0.004) (data not shown). RB6-treated mice also had significantly prolonged survival in the systemic model; RB6-treated mice had a median survival of 2 days, whereas that of rIgG-treated mice was 1 day (P = 0.0008) (Fig. 2B).
FIG. 2.
Survival of PBS-, rIgG-, and RB6-treated BALB/c mice after i.n. or i.p. infection with wild-type serotype 8 pneumococci and i.n. infection with PLY− serotype 8 pneumococci. Survival after i.n. infection with wild-type pneumococci (A), i.p. infection with wild-type pneumococci (B), and i.n. infection with PLY− strain (C). Survival of RB6-treated mice was greater than that of PBS- and rIgG-treated mice (P = 0.03, Kaplan-Meier log rank survival test) for i.n. infection, and survival of RB6-treated mice was greater than that of rIgG-treated mice for i.p. infection (P = 0.002, Kaplan-Meier log rank survival test). For i.n. infection with the PLY− mutant strain, the survival of RB6-treated mice was greater than that of rIgG-treated mice (P = 0.02, Kaplan-Meier log rank survival test). n is equal to eight mice per group.
Blood and lung bacterial burden.
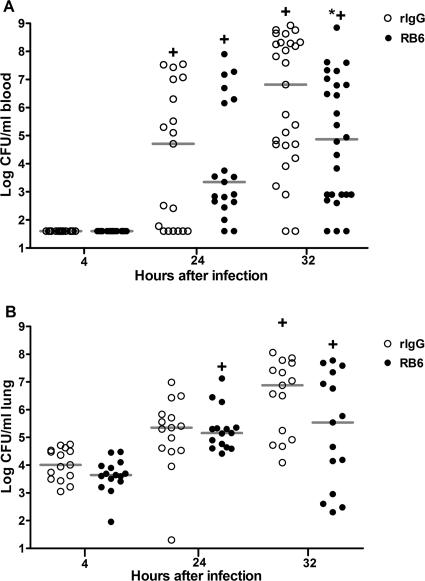
The number of bacteria in blood was below the level of detection in our assay (40 CFU/ml) 4 h after infection of both rIgG- and RB6-treated mice (Fig. 3A). There was no significant difference in numbers of blood CFU between the rIgG- and RB6-treated mice 24 h after infection. There were significantly fewer CFU in RB6-treated than rIgG-treated mice 32 h after infection (P = 0.03). The number of CFU in blood increased for each group between 4 and 24 h after infection (P < 0.01) and 4 and 32 h after infection (P < 0.001). The number of CFU in the lungs (Fig. 3B) was similar in rIgG- and RB6-treated mice 4 and 24 h after infection; RB6-treated mice had nearly 2 logs fewer CFU 32 h after infection, but the difference did not reach statistical significance (P = 0.11). Numbers of CFU in the lungs increased significantly in both groups between 4 and 32 h after infection (P < 0.05) and between 4 and 24 h after infection in the RB6-treated mice (P < 0.01). The data presented represent four independent experiments in which the data were combined.
FIG. 3.
CFU in blood and lungs of rIgG- and RB6-treated mice after infection with wild-type serotype 8 pneumococci. CFU enumerated in the blood (A) and lung homogenates (B) 4, 24, and 32 h after i.n. infection with wild-type serotype 8 pneumococci are shown. The line in the scatter plot denotes the median of the designated group. *, P value of <0.05 for comparison of rIgG- and RB6-treated mice; +, P value of <0.05 between times for the designated groups, as determined by Kruskal-Wallis testing with Dunn's multiple comparison test. n is equal to 19 mice per group at 24 h, 27 mice per group at 32 h (blood CFU), and 14 mice per group (lung CFU).
For the PLY− strain, there were no significant differences in the number of CFU in the blood or lungs between the RB6- and rIgG-treated mice, although the number of CFU increased significantly within each group from 4 to 32 h (P < 0.05) and between 24 and 32 h in the rIgG-treated mice (P < 0.05) (Table 1).
TABLE 1.
Numbers of CFU in blood and lungs of mice infected with PLY− serotype 8 pneumococcia
| Site | MAb | No. of CFUb detected at:
|
||
|---|---|---|---|---|
| 4 h | 24 h | 32 h | ||
| Blood | rIgG | ≤40 (40-40) | ≤40 (40-40) | 2.2 × 105 (800-3.1 × 108)c |
| RB6 | ≤40 (40-40) | 40 (40-2.7 × 106) | 8.0 × 105 (1.8 × 104-1.1 × 107)c | |
| Lungs | rIgG | 1,315 (215-2,700) | 1.7 × 104 (200-7.8 × 104) | 2.4 × 105 (8.9 × 104-3.8 × 107)c |
| RB6 | 5,800 (655-1.6 × 104) | 2.8 × 104 (6,350-1.2 × 105) | 1.6 × 105 (1.2 × 104-8.6 × 105) | |
The lower limit of detection for this assay is 40 CFU.
All data represent median values with minimum and maximum values in parentheses (n = 5).
P value of <0.05 as calculated by Kruskal-Wallis testing for comparison within treatment group to earlier time points.
Lung cytokine/chemokine levels.
Soluble lung cytokines and chemokines were quantified by ELISA (Table 2). The only significant difference between the RB6- and rIgG-treated groups was that RB6-treated mice had significantly higher levels of IL-6 in the lungs than rIgG-treated mice 24 h after infection with both the wild-type and PLY− strains (Table 3) (P values of 0.04 and 0.008, respectively). For the wild-type strain, there was a trend toward higher KC levels 24 h after infection in RB6-treated mice, but these differences did not reach statistical significance (P = 0.06) (Table 2). The levels of MIP-2, TNF-α, IL-10, IL-1β, and IFN-γ were similar between the treatment groups at the times examined (Table 2). Increases in IL-6 and KC within groups were noted between 4 and 32 h (P < 0.05), and increases in IL-6 were noted between 4 and 24 h in the rIgG-treated mice (P < 0.01). The cytokine levels in mice that received the PLY− mutant strain reflected the same trends seen in mice that received the wild-type strain. There was also a trend towards higher levels of KC in RB6-treated mice 32 h after infection, which was not statistically significant (P = 0.06) (Table 3).
TABLE 2.
Chemokines and cytokines in lung lysates from RB6- and rIgG-treated mice infected with wild-type serotype 8 pneumococci
| Cytokine | Concn in mice treated with indicated MAba
|
|||
|---|---|---|---|---|
| 24 h
|
32 h
|
|||
| rIgG | RB6 | rIgG | RB6 | |
| IL-6 | 1.2 (0.7-8.9) | 2.3 (1.2-8.7)b | 2.3 (0.3-19) | 2.2 (0.16-17) |
| KC | 1.9 (0.3-7.7) | 4.1 (1.6-7.3) | 4.0 (0.3-14) | 8.6 (0.3-11) |
| MIP-2 | 0.6 (0.2-3.2) | 0.8 (0.5-2.1) | 1.7 (0.2-13) | 2.2 (0.2-7.6) |
| TNF-α | 1.5 (1.1-2.9) | 1.6 (1.1-2.1) | 2.2 (0.7-9.1) | 1.6 (0.9-3.0) |
| IFN-γ | 140 (90-340) | 110 (75-230) | 330 (75-740) | 190 (110-480) |
| IL-1β | 0.43 (0.04-1.3) | 0.08 (0.06-0.5) | 4.1 (0.05-13) | 2.8 (0.06-8.4) |
| IL-10 | 3.1 (1.3-6.2) | 3.2 (2.7-3.9) | 1.7 (0.08-6.2) | 3.0 (0.07-7.6) |
All data represent median values with minimum and maximum values in parentheses. All values are expressed in nanograms per milliliter except those corresponding to IFN-γ, which are expressed in picograms per milliliter. For IL-6, KC, MIP-2, TNF-α, and IL-10, groups contained 10 to 14 mice each. For IFN-γ and IL-1β, groups contained 5 to 9 mice each. The lower limits of detection for these assays were as follows: 15.6 pg/ml for MIP-2, KC, IL-6, and IL-1β; and 31.25 pg/ml for IFN-γ, IL-10, and TNF-α.
P value of 0.04 as calculated by Mann-Whitney U testing.
TABLE 3.
Chemokines and cytokines in lung lysates for RB6- and rIgG-treated mice infected with PLY− serotype 8 pneumococci
| Cytokine | Concn (ng/ml) in mice treated with indicated MAba
|
|||
|---|---|---|---|---|
| 24 h
|
32 h
|
|||
| rIgG | RB6 | rIgG | RB6 | |
| IL-6 | 0.2 (0.2-0.3) | 0.4 (0.3-0.6)b | 0.4 (0.2-2.1) | 0.3 (0.2-1.2) |
| KC | 0.5 (0.1-1.8) | 0.6 (0.3-3.5) | 0.6 (0.3-1.7) | 2.1 (0.9-5.0) |
All data represent median values with minimum and maximum values in parentheses (n = 5).
P value of 0.008 as calculated by Mann-Whitney U testing.
Histopathological analysis.
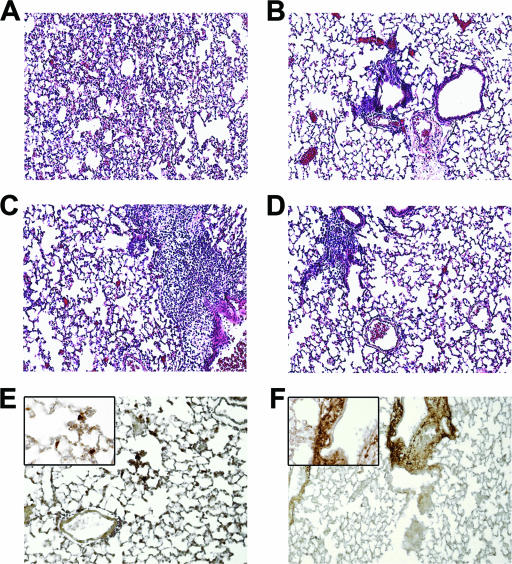
H&E-stained tissue sections from the lungs of RB6- and rIgG-treated mice that were infected with the wild-type strain were examined by light microscopy. PMN infiltration and inflammation of blood vessels and alveolar walls was observed in sections from rIgG-, but not RB6-treated mice 4 h after infection (data not shown). PMNs constituted a major portion of the cellular population in both treatment groups 24 h after infection and were often seen undergoing cell death (keratinitis). The predominant finding in rIgG-treated mice was diffuse interstitial inflammation without alveolar involvement (Fig. 4A and C). The inflammation was evident in all lung fields. The predominant finding in RB6-treated mice was perivascular lesions without parenchymal or alveolar involvement (Fig. 4B and D). The lesions were discrete and focal; they were observed only in a minority of vessels and lung fields (20% at 24 h after infection and 29% at 32 h after infection). Staining of the pneumococcal capsular polysaccharide confirmed the presence of bacteria in the interstitial infiltrates of rIgG-treated mice (Fig. 4E) and in the perivascular lesions of RB6-treated mice (Fig. 4F).
FIG. 4.
Histopathology and bacterial staining of lung sections from rIgG- and RB6-treated mice. Mouse lungs obtained from rIgG (A, C, and E)- and RB6 (B, D, and F)-treated mice 24 (A, B, E, and F) and 32 h (C and D) after infection with wild-type serotype 8 pneumococci. Sections from rIgG-treated mice showed diffuse interstitial inflammation 24 h after infection (A) and more intense diffuse interstitial inflammation with perivascular inflammation 32 h after infection (C). In RB6-treated mice, there were localized inflammatory lesions around blood vessels without involvement of the parenchyma 24 h after infection (B) and more cellular lesions remaining localized 32 h after infection (D). Bacterial staining of sections obtained 24 h after infection from rIgG (E)- and RB6 (F)-treated mice was performed with the human MAb D11 (81). Magnification is ×10 for all panels and ×63 in the insets of panels E and F. The images shown are representative of the sections examined from eight mice in each treatment group.
Cellular composition of lung homogenates: FACS analysis.
The cellular composition of whole lung homogenates from mice infected with the wild-type strain was evaluated by FACS. We chose this method because of the paucity of alveolar inflammation found in histopathological sections from both groups of mice. Lung homogenates have been used by other groups to assess the cellular response in mouse models of pneumonia (9, 35, 38). The number of total leukocytes (as enumerated by counting with a hemocytometer) in the lungs of RB6- and rIgG-treated mice was statistically similar 24 h after infection but was significantly lower in RB6-treated mice 32 h after infection (P = 0.05) (Table 4). RB6-treated mice had significantly fewer PMNs 24 and 32 h after infection (P values of 0.01 and 0.05, respectively) (Table 4). CD4 T cells were significantly higher at 24 than 32 h after infection in rIgG-treated mice (P = 0.02) and significantly higher in rIgG-treated than RB6-treated mice 32 h after infection (P = 0.03) (Table 4). RB6-treated mice had lower numbers of CD8 T cells 24 h after infection (P = 0.009), but their levels increased significantly from 24 to 32 h (P = 0.02) (Table 4). Numbers of B cells increased from 24 to 32 h after infection in both groups (P of 0.01 for both groups). Macrophage numbers were similar between the treatment groups but increased significantly in rIgG-treated mice from 24 to 32 h after infection (P = 0.03), whereas RB6-treated mice had an increase that did not reach significance (P = 0.06) (Table 4). Staining for Ly6G+ cells showed minimal to no overlap with other cell types, suggesting that it was highly specific for PMNs. This assay was performed in three separate experiments, and the data were pooled, corresponding to 12 mice per group 24 h after infection and 5 mice per group 32 h after infection.
TABLE 4.
Cellular infiltration into mouse lungs after infection
| Cell type | No. of cells/ml of lung lysate in mice treated with indicated MAba
|
|||
|---|---|---|---|---|
| 24 h
|
32 h
|
|||
| rIgG | RB6 | rIgG | RB6 | |
| Total leukocytes | 8.7 × 105 ± 6.2 × 104 | 7.2 × 105 ± 6.6 × 104 | 1.4 × 106 ± 2.4 × 105c | 6.6 × 105 ± 2.2 × 104b |
| PMNs | 1.9 × 105 ± 4.8 × 104b | 3.4 × 104 ± 1.3 × 104 | 4.8 × 105 ± 1.6 × 105b | 5.1 × 104 ± 1.6 × 104 |
| CD4 T cells | 3.1 × 105 ± 2.0 × 104 | 2.7 × 105 ± 3.0 × 104 | 4.4 × 105 ± 6.1 × 104bc | 2.3 × 105 ± 1.7 × 104 |
| CD8 T cells | 1.1 × 105 ± 6.7 × 103b | 7.8 × 104 ± 7.2 × 103 | 1.5 × 105 ± 2.2 × 104 | 1.1 × 105 ± 9.4 × 103c |
| B cells | 1.2 × 105 ± 1.1 × 104 | 1.1 × 105 ± 1.1 × 104 | 1.9 × 105 ± 2.6 × 104c | 1.9 × 105 ± 3.8 × 104c |
| Macrophages | 6.1 × 104 ± 7.5 × 103 | 5.9 × 104 ± 7.5 × 103 | 1.4 × 105 ± 2.4 × 104c | 2.1 × 105 ± 5.3 × 104 |
All values represent means ± SEM. There were 12 mice/group 24 h after infection and 5 mice/group 32 h after infection.
P value of 0.05, as determined by unpaired t testing, for comparison of rIgG-treated mice to RB6-treated mice.
P value of 0.05, as determined by unpaired t testing, for comparison of groups at 24 and 32 h.
In situ cell death assay.
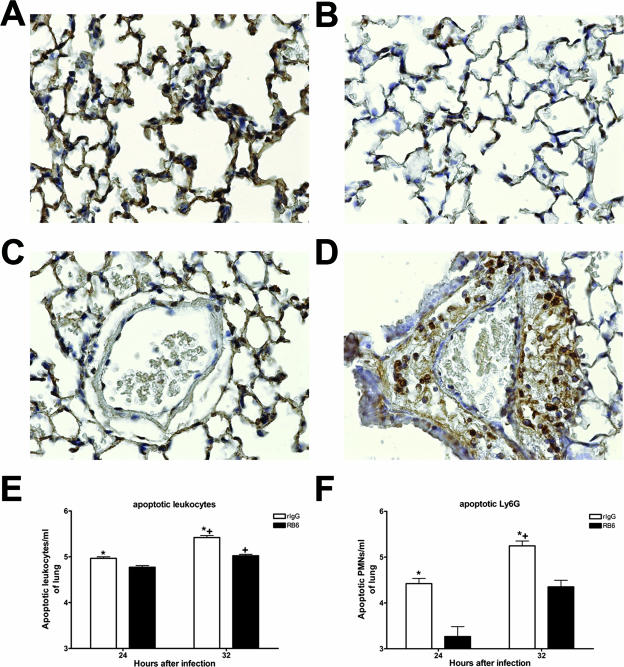
Light microscopy of TUNEL-stained lung sections from rIgG- and RB6-treated mice infected with the wild-type strain revealed evidence of cell death in both groups 24 and 32 h after infection. However, in rIgG-treated mice, interstitial staining was observed (Fig. 5A and C), paralleling the inflammatory pattern seen with H&E staining. In RB6-treated mice, staining was localized in perivascular lesions (Fig. 5B and D).
FIG. 5.
TUNEL staining and FACS analysis of apoptosis in lungs of rIgG- and RB6-treated mice. TUNEL staining was performed on sections from rIgG (A and C)- and RB6 (B and D)-treated mice 24 h after infection with wild-type serotype 8 pneumococci. Staining was observed in areas of interstitial inflammation in rIgG-treated mice (A) but was absent in RB6-treated mice (B). TUNEL staining was localized in perivascular lesions in RB6-treated mice (D) but not rIgG-treated mice (C). The images shown are representative of the sections examined from eight mice in each treatment group. Magnification, ×40 (A to D). The numbers of total apoptotic leukocytes (E) and apoptotic PMNs (F) were determined by FACS analysis as described in the text. Each bar represents the log of the mean of the designated group; the error bars show the standard errors of the means. *, P value of <0.05 for comparison between treatment groups; +, P value of <0.05 for comparison between time points in the same treatment group, as determined by unpaired t testing. n is equal to seven mice per group at 24 h and four and five for RB6- and rIgG-treated mice, respectively, at 32 h after infection.
Apoptosis of lung cells and FACS analysis.
Annexin V staining demonstrated significantly more apoptotic cells in rIgG- than RB6-treated mice both 24 and 32 h after infection with the wild-type strain (P values of 0.0003 and <0.0001, respectively). Apoptotic cells also increased significantly from 24 to 32 h in both groups (P of <0.0001 for both groups) (Fig. 5E). The numbers of apoptotic PMNs in the lungs of rIgG-treated mice were higher than those for RB6-treated mice 24 and 32 h after infection (P values of 0.02 and 0.04, respectively) and were higher for rIgG-treated mice at 32 h than 24 h after infection (P = 0.04) (Fig. 5F). This assay was performed in three separate experiments, and the data were pooled, corresponding to 12 mice per group 24 h after infection and 5 mice per group 32 h after infection.
DISCUSSION
The data presented herein demonstrate that depletion of peripheral PMNs with the MAb RB6 resulted in prolongation of the survival of BALB/c mice after i.n. or i.p. infection with serotype 8 pneumococci, as well as after i.n. infection with a PLY-deficient serotype 8 pneumococcal strain. PMN-depleted mice infected with the wild-type strain had a unique lung inflammatory response with focal perivascular infiltrates that differed markedly from the interstitial infiltrates and parenchymal involvement observed in rIgG-treated mice. PMN-depleted mice also had significantly less bacteremia 32 h after infection, with markedly fewer lung PMNs and less lung apoptosis. Since there were only limited differences in other cell types or cytokine levels in the lungs between the RB6- and rIgG-treated groups, our findings implicate lung PMNs as a causal factor in the earlier death of rIgG-treated mice in our model.
The PLY expressed by the serotype 8 strain used in the current study was nonhemolytic, a characteristic that was previously reported for other serotype 8 strains, in addition to certain serotype 7F (45) and serotype 1 strains (36). The hemolytic activity of PLY has been implicated in lung injury (62, 79), and a reduction in PLY activity resulted in a reduction in the virulence of serotype 2 (2, 8). However, only 0.1% of PLY hemolytic activity was required for full virulence in one study (2), whereas strains that lack all hemolytic activity and PLY-deficient strains had markedly reduced virulence or were avirulent (2, 63). The nonhemolytic PLY of the strain we used could explain the lack of early lung injury and the ultimately similar lethality of the wild-type and PLY− strains in our model. However, the ability of a low inoculum of this strain to induce lethal disease suggests that factors other than the hemolytic activity of PLY contribute to its virulence. This is consistent with the observation that nonhemolytic serotype 1 strains were also highly invasive in patients (36). One factor that could influence virulence is enhanced dissemination. Bloodstream dissemination (bacteremia) has been attributed to the complement activity of PLY (1, 2), which, as previously described, is likely to be intact even when hemolytic activity is reduced (1, 33, 62). The complement-activating activity of PLY inhibits PMN-mediated killing of pneumococci (56) and lymphocyte function (22) and has been implicated in T-cell recruitment (33). We found a significantly higher level of bacteremia in rIgG-treated mice 32 h after infection, which was associated with death within 16 to 24 h in mice that received the wild-type strain. As expected, mice that received the PLY− strain survived longer and had no detectable bacteremia 4 and 24 h after infection with lower levels than the wild-type strain 32 h after infection. Although bacteremia may not correlate with disease for other serotypes (2, 6), it invariably led to death in our model, as reported for other models of pneumococcal pneumonia (12, 18, 36).
RB6-mediated PMN depletion was also associated with prolonged survival after infection with Staphylococcus aureus (27) and Cryptococcus neoformans (51). Doses of RB6 that exceed 25 μg deplete other cell types (51). As observed in our model and in other models in which the same dose of RB6 was used (27, 70), PMNs in the lungs (or tissue) were reduced, but not eliminated. Other groups investigating the impact of neutropenia on pneumococcal infection have used cyclophosphamide. Cyclophosphamide affects populations of cells other than PMNs (70). In a pneumonia model with serotype 3, cyclophosphamide-treated mice had similar numbers of CFU in the blood, but these were higher in the lungs and survival was slightly reduced compared to control mice (77). However, in another model, cyclophosphamide-treated mice receiving a clinical penicillin-resistant strain survived, although the study did not include a control group (82). In other models of mouse pneumococcal pneumonia in which PMN recruitment was reduced due to another defect, a lower number of lung PMNs was not associated with higher numbers of CFU in the lungs (37, 38, 50). This was also observed in other models (16, 17, 71), suggesting that PMNs are not required to control the bacterial burden. Alveolar macrophages reduced CFU in a resolving pneumococcal pneumonia model (19) but diminished the inflammatory response and prolonged survival without affecting CFU in a lethal model with serotype 3 (38). In our model, the number of lung macrophages was similar in both treatment groups, and a reduction in PMNs translated into an altered tissue inflammatory response but did not affect numbers of CFU in the lungs. Hence, our findings question the view that nonopsonic clearance of pneumococci is mediated by PMNs (80) and corroborate data indicating that cerebral spinal fluid leukocyte levels do not correlate with reduced CFU in pneumococcal meningitis (20).
The tissue inflammatory response to pneumococcal pneumonia is a function of the mouse (58) and the pneumococcal strain (52). In our study, the predominantly interstitial inflammatory response in the rIgG-treated mice resembled the bronchopneumonia previously observed in BALB/c mice with serotype 3 infection (58). In contrast, RB6-treated mice had discrete, focal lesions localized in perivascular areas, with little discernible parenchymal involvement. Since the distribution of organisms paralleled the histopathological findings in both treatment groups, our findings suggest that the pattern of bacterial localization and dissemination in the lungs depends on PMNs in our model. The mechanism by which PMNs could govern bacterial localization is unknown at this time. The complement-activating activity of PLY has been implicated in localization of inflammatory cells (33). As such, the fact that PMN-depleted mice had focal, rather than disseminated, lesions raises the possibility that PMNs may subvert bacterial localization that depends on PLY-mediated localization of inflammatory cells. Dissemination could also depend on surfactant (32). Further studies are required to investigate these hypotheses. Another mechanism by which PMNs could influence bacterial localization is that they could function as a means of dissemination. In this regard, other gram-positive organisms, such as Staphylococcus aureus and group A streptococcus, can persist and replicate in PMNs (27, 69). Therefore, we wonder whether pneumococci could disseminate in the lungs inside of PMNs. This question requires further investigation since, to our knowledge, uptake and persistence of pneumococci in PMNs in vivo has not been described.
Notably, the lesions we observed in the lungs of RB6-treated mice bore a resemblance to the lesions reported in mice with Fas/FasL deficiency and/or given an apoptosis inhibitor (50). In the aforementioned model, FasL-mediated apoptosis induced lung damage and earlier death in mice with pneumococcal pneumonia (50). Like Fas/Fas L-deficient mice (50), RB6-treated mice had significantly fewer lung and apoptotic PMNs than rIgG-treated mice. The higher number of apoptotic PMNs, widespread PMN apoptosis in the lungs, and higher level of bacteremia in rIgG-treated mice 32 h after infection suggest that PMN apoptosis is detrimental and could enhance dissemination in our model. Pneumococcus-induced apoptosis can be induced by PLY binding to TLR4 and non-PLY-dependent mechanisms (10, 68). The effect of apoptosis on pneumococcal pathogenesis is tissue and cell type specific (48) and is also likely to be serotype specific. However, data on the effect of apoptosis on the course of pulmonary infection are conflicting. Pneumococcus-induced apoptosis increased resistance to nasopharyngeal colonization and low-inoculum pulmonary infection (19, 68) but increased lung damage and inflammation in lethal, higher-inoculum models (9, 50). Macrophage apoptosis was beneficial in a resolving (nonlethal) model of pneumococcal pneumonia but was not beneficial in lethal infections (19, 49). Apoptotic PMNs have been shown to accumulate in the lungs of mice in the absence of alveolar macrophages (38), and PMN persistence has been associated with lung destruction and inflammation (18), most likely due to secondary necrosis of uncleared apoptotic PMNs, which subsequently release their granules (28). Our data demonstrate an association between apoptosis and earlier death and suggest that the survival advantage in PMN-depleted mice is a result of a reduction in PMN-mediated tissue damage stemming from apoptotic PMNs.
RB6-treated mice that were infected with either the wild-type or PLY− strain had higher levels of IL-6 in the lungs than rIgG-treated mice 24 h after infection. Pneumococcal infection induces IL-6 production in vitro and in vivo (14, 31). Although IL-6 is often viewed as a marker of sepsis and excessive inflammation, it was required for early host defense against pneumococcal pneumonia (74). Further, it was essential for effector cell function against C. neoformans, another encapsulated pathogen (67). Potentially protective properties of IL-6 include its ability to inhibit proinflammatory cytokine release (64) and PMN apoptosis (3, 44). These immunoregulatory properties, in particular a reduced amount of PMN apoptosis, could have had a beneficial effect in RB6-treated mice by controlling tissue inflammation. The cellular source of and possible beneficial role of IL-6 in our model deserves further investigation. It would be informative to examine the effect of PMN depletion in IL-6 knockout mice, but this was beyond the scope of the current study. In other mouse models of pneumococcal pneumonia, death was associated with increased levels of cytokines and chemokines (12, 18, 38). However, it is difficult to link cytokine/chemokine levels with the outcome of infection because of the redundant pathways and multiple cell types by which they are produced. In addition to PMNs, alveolar epithelial cells, pulmonary macrophages, and endothelial cells produce MIP-2 and KC (15, 47, 53). The numbers of macrophages were similar in both groups, but the number of CD4 T lymphocytes was higher in rIgG-treated mice 32 h after infection. CD4 T lymphocytes have been implicated in PMN recruitment and innate immunity to pneumococcal pneumonia (34, 46, 75).
In summary, our data show that depletion of peripheral PMNs from BALB/c mice conferred a survival benefit over PMN-sufficient mice during lethal pulmonary infection with serotype 8 pneumococci. This survival difference was accompanied by a unique lung tissue inflammatory response, lower numbers of lung and apoptotic PMNs despite similar lung CFU, and similar levels of most cytokines, chemokines and effector cells in the lung. Further studies are needed to ascertain whether our findings are specific to the pneumococcal strain we used or another aspect of our model. Nonetheless, our model has important features that raise confidence that it could provide insight into human disease: namely, the relatively low inoculum required to induce pneumonia, the fact that it uses a serotype that causes disease in adults, and the fact that the strain lacks PLY hemolytic activity, a characteristic that was found in invasive clinical strains that caused an outbreak of invasive pneumococcal disease in patients (36). In our model, PMN recruitment to the lungs was associated with earlier death. Interestingly, rIgG-treated mice developed leukopenia, a phenomenon that underscores clinical data showing that leukopenia is a poor prognostic finding in pneumococcal pneumonia (4, 24). However, the relevance of our findings to human disease is uncertain. The major risk factor for pneumococcal disease is impaired humoral immunity (61), but the frequency of pneumococcal bloodstream infections in patients with neutropenia was higher for hospital- than community-acquired infections (42). Neutropenia is a risk factor for fungal, gram-positive bacterial, and gram-negative bacterial pneumonias (61), but pneumococcal infections can also occur (21, 42). Although more studies are needed to define the contribution of neutropenia to the pathogenesis of pneumococcal pneumonia in patients, our data suggest that the long-held view that PMNs are essential for host defense against pneumococcal pneumonia requires closer scrutiny.
Acknowledgments
This work was supported by grants RO1AI44374 and RO1AI45459 (L.P.) and R01AI34597 (E.T.) from the National Institutes of Health and by the Immunology and Immunooncology Training Grant (2T33CA009173-31) from the National Cancer Institute (T.B.).
We thank Marta Feldmesser for supplying MAb RB6, Dinah Carroll for her assistance in the pathological studies, and Eliseo Eugenin for his assistance with the TUNEL assay.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Alcantara, R. B., L. C. Preheim, and M. J. Gentry. 1999. Role of pneumolysin's complement-activating activity during pneumococcal bacteremia in cirrhotic rats. Infect. Immun. 67:2862-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J. E., A. M. Berry, J. C. Paton, J. B. Rubins, P. W. Andrew, and T. J. Mitchell. 1998. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb. Pathog. 24:167-174. [DOI] [PubMed] [Google Scholar]
- 3.Asensi, V., E. Valle, A. Meana, J. Fierer, A. Celada, V. Alvarez, J. Paz, E. Coto, J. A. Carton, J. A. Maradona, A. Dieguez, J. Sarasua, M. G. Ocana, and J. M. Arribas. 2004. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect. Immun. 72:3823-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austrian, R., and J. Gold. 1964. Pneumococcal bacteremia with especial reference to bacteremic pneumococcal pneumonia. Ann. Intern. Med. 60:759-776. [DOI] [PubMed] [Google Scholar]
- 5.Austyn, J. M., and S. Gordon. 1981. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 11:805-815. [DOI] [PubMed] [Google Scholar]
- 6.Benton, K. A., M. P. Everson, and D. E. Briles. 1995. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect. Immun. 63:448-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron, Y., N. Ouellet, A. M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branger, J., S. Knapp, S. Weijer, J. C. Leemans, J. M. Pater, P. Speelman, S. Florquin, and T. van der Poll. 2004. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 72:788-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braun, J. S., J. E. Sublett, D. Freyer, T. J. Mitchell, J. L. Cleveland, E. I. Tuomanen, and J. R. Weber. 2002. Pneumococcal pneumolysin and H(2)O(2) mediate brain cell apoptosis during meningitis. J. Clin. Investig. 109:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald, U. K., A. Lees, M. Steinitz, and L.-A. Pirofski. 2005. A peptide mimotope of type 8 pneumococcal capsular polysaccharide induces a protective immune response in mice. Infect. Immun. 73:325-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns, T., M. Abadi, and L.-A. Pirofski. 2005. Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin M monoclonal antibody to serotype 8 capsular polysaccharide. Infect. Immun. 73:4530-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns, T., Z. Zhong, and L. Pirofski. 2001. A human monoclonal IgM to PPs 8 prevents dissemination and downregulates inflammation, abstr. E-112, p. 353. Abstr. 101st Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, D.C.
- 14.Burns, T., Z. Zhong, M. Steinitz, and L.-A. Pirofski. 2003. Modulation of polymorphonuclear cell interleukin-8 secretion by human monoclonal antibodies to type 8 pneumococcal capsular polysaccharide. Infect. Immun. 71:6775-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cailhier, J. F., D. A. Sawatzky, T. Kipari, K. Houlberg, D. Walbaum, S. Watson, R. A. Lang, S. Clay, D. Kluth, J. Savill, and J. Hughes. 2006. Resident pleural macrophages are key orchestrators of neutrophil recruitment in pleural inflammation. Am. J. Respir. Crit. Care Med. 173:540-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen, L., T. Watanabe, H. Watanabe, and F. Sendo. 2001. Neutrophil depletion exacerbates experimental Chagas' disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur. J. Immunol. 31:265-275. [DOI] [PubMed] [Google Scholar]
- 17.Chen, L., Z. Zhang, and F. Sendo. 2000. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin. Exp. Immunol. 120:125-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dallaire, F., N. Ouellet, Y. Bergeron, V. Turmel, M. C. Gauthier, M. Simard, and M. G. Bergeron. 2001. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J. Infect. Dis. 184:292-300. [DOI] [PubMed] [Google Scholar]
- 19.Dockrell, D. H., H. M. Marriott, L. R. Prince, V. C. Ridger, P. G. Ince, P. G. Hellewell, and M. K. Whyte. 2003. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 171:5380-5388. [DOI] [PubMed] [Google Scholar]
- 20.Ernst, J. D., J. M. Decazes, and M. A. Sande. 1983. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect. Immun. 41:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewig, S., A. Glasmacher, B. Ulrich, K. Wilhelm, H. Schafer, and K. H. Nachtsheim. 1998. Pulmonary infiltrates in neutropenic patients with acute leukemia during chemotherapy: outcome and prognostic factors. Chest 114:444-451. [DOI] [PubMed] [Google Scholar]
- 22.Ferrante, A., B. Rowan-Kelly, and J. C. Paton. 1984. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect. Immun. 46:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleming, T. J., M. L. Fleming, and T. R. Malek. 1993. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J. Immunol. 151:2399-2408. [PubMed] [Google Scholar]
- 24.Fruchtman, S. M., M. E. Gombert, and H. A. Lyons. 1983. Adult respiratory distress syndrome as a cause of death in pneumococcal pneumonia. Report of ten cases. Chest 83:598-601. [DOI] [PubMed] [Google Scholar]
- 25.Gold, J. A., M. Parsey, Y. Hoshino, S. Hoshino, A. Nolan, H. Yee, D. B. Tse, and M. D. Weiden. 2003. CD40 contributes to lethality in acute sepsis: in vivo role for CD40 in innate immunity. Infect. Immun. 71:3521-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez, B. E., K. G. Hulten, L. Lamberth, S. L. Kaplan, and E. O. Mason, Jr. 2006. Streptococcus pneumoniae serogroups 15 and 33: an increasing cause of pneumococcal infections in children in the United States after the introduction of the pneumococcal 7-valent conjugate vaccine. Pediatr. Infect. Dis. J. 25:301-305. [DOI] [PubMed] [Google Scholar]
- 27.Gresham, H. D., J. H. Lowrance, T. E. Caver, B. S. Wilson, A. L. Cheung, and F. P. Lindberg. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713-3722. [DOI] [PubMed] [Google Scholar]
- 28.Haslett, C. 1999. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 160:S5-S11. [DOI] [PubMed] [Google Scholar]
- 29.Hestdal, K., F. W. Ruscetti, J. N. Ihle, S. E. Jacobsen, C. M. Dubois, W. C. Kopp, D. L. Longo, and J. R. Keller. 1991. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J. Immunol. 147:22-28. [PubMed] [Google Scholar]
- 30.Horton, R. M., H. D. Hunt, S. N. Ho, J. K. Pullen, and L. R. Pease. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61-68. [DOI] [PubMed] [Google Scholar]
- 31.Jagger, M. P., Z. Huo, and P. G. Riches. 2002. Inflammatory cytokine (interleukin 6 and tumour necrosis factor alpha) release in a human whole blood system in response to Streptococcus pneumoniae serotype 14 and its capsular polysaccharide. Clin. Exp. Immunol. 130:467-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jounblat, R., H. Clark, P. Eggleton, S. Hawgood, P. W. Andrew, and A. Kadioglu. 2005. The role of surfactant protein D in the colonisation of the respiratory tract and onset of bacteremia during pneumococcal pneumonia. Respir. Res. 6:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jounblat, R., A. Kadioglu, T. J. Mitchell, and P. W. Andrew. 2003. Pneumococcal behavior and host responses during bronchopneumonia are affected differently by the cytolytic and complement-activating activities of pneumolysin. Infect. Immun. 71:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadioglu, A., W. Coward, M. J. Colston, C. R. Hewitt, and P. W. Andrew. 2004. CD4-T-lymphocyte interactions with pneumolysin and pneumococci suggest a crucial protective role in the host response to pneumococcal infection. Infect. Immun. 72:2689-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirkham, L. A., J. M. Jefferies, A. R. Kerr, Y. Jing, S. C. Clarke, A. Smith, and T. J. Mitchell. 2006. Identification of invasive serotype 1 pneumococcal isolates that express nonhemolytic pneumolysin. J. Clin. Microbiol. 44:151-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knapp, S., L. Hareng, A. W. Rijneveld, P. Bresser, J. S. van der Zee, S. Florquin, T. Hartung, and P. T. van der. 2004. Activation of neutrophils and inhibition of the proinflammatory cytokine response by endogenous granulocyte colony-stimulating factor in murine pneumococcal pneumonia. J. Infect. Dis. 189:1506-1515. [DOI] [PubMed] [Google Scholar]
- 38.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 39.Knapp, S., C. W. Wieland, C. van ′t Veer, O. Takeuchi, S. Akira, S. Florquin, and T. van der Poll. 2004. Toll-like receptor 2 plays a role in the early inflammatory response to murine pneumococcal pneumonia but does not contribute to antibacterial defense. J. Immunol. 172:3132-3138. [DOI] [PubMed] [Google Scholar]
- 40.Koopman, G., C. P. Reutelingsperger, G. A. Kuijten, R. M. Keehnen, S. T. Pals, and M. H. van Oers. 1994. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415-1420. [PubMed] [Google Scholar]
- 41.Krop, I., A. L. Shaffer, D. T. Fearon, and M. S. Schlissel. 1996. The signaling activity of murine CD19 is regulated during cell development. J. Immunol. 157:48-56. [PubMed] [Google Scholar]
- 42.Kumashi, P., E. Girgawy, J. J. Tarrand, K. V. Rolston, I. I. Raad, and A. Safdar. 2005. Streptococcus pneumoniae bacteremia in patients with cancer: disease characteristics and outcomes in the era of escalating drug resistance (1998-2002). Medicine (Baltimore) 84:303-312. [DOI] [PubMed] [Google Scholar]
- 43.Lagasse, E., and I. L. Weissman. 1996. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197:139-150. [DOI] [PubMed] [Google Scholar]
- 44.Lindemans, C. A., P. J. Coffer, I. M. Schellens, P. M. de Graaff, J. L. Kimpen, and L. Koenderman. 2006. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J. Immunol. 176:5529-5537. [DOI] [PubMed] [Google Scholar]
- 45.Lock, R. A., Q. Y. Zhang, A. M. Berry, and J. C. Paton. 1996. Sequence variation in the Streptococcus pneumoniae pneumolysin gene affecting haemolytic activity and electrophoretic mobility of the toxin. Microb. Pathog. 21:71-83. [DOI] [PubMed] [Google Scholar]
- 46.Malley, R., K. Trzcinski, A. Srivastava, C. M. Thompson, P. W. Anderson, and M. Lipsitch. 2005. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc. Natl. Acad. Sci. USA 102:4848-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Manzer, R., J. Wang, K. Nishina, G. McConville, and R. J. Mason. 2006. Alveolar epithelial cells secrete chemokines in response to IL-1β and lipopolysaccharide but not to ozone. Am. J. Respir. Cell Mol. Biol. 34:158-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marriott, H. M., and D. H. Dockrell. 2006. Streptococcus pneumoniae: the role of apoptosis in host defense and pathogenesis. Int. J. Biochem. Cell Biol. 38:1848-1854. [DOI] [PubMed] [Google Scholar]
- 49.Marriott, H. M., P. G. Hellewell, S. S. Cross, P. G. Ince, M. K. Whyte, and D. H. Dockrell. 2006. Decreased alveolar macrophage apoptosis is associated with increased pulmonary inflammation in a murine model of pneumococcal pneumonia. J. Immunol. 177:6480-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matute-Bello, G., W. C. Liles, C. W. Frevert, S. Dhanireddy, K. Ballman, V. Wong, R. R. Green, H. Y. Song, D. R. Witcher, J. A. Jakubowski, and T. R. Martin. 2005. Blockade of the Fas/FasL system improves pneumococcal clearance from the lungs without preventing dissemination of bacteria to the spleen. J. Infect. Dis. 191:596-606. [DOI] [PubMed] [Google Scholar]
- 51.Mednick, A. J., M. Feldmesser, J. Rivera, and A. Casadevall. 2003. Neutropenia alters lung cytokine production in mice and reduces their susceptibility to pulmonary cryptococcosis. Eur. J. Immunol. 33:1744-1753. [DOI] [PubMed] [Google Scholar]
- 52.Mohler, J., E. Azoulay-Dupuis, C. Amory-Rivier, J. X. Mazoit, J. P. Bedos, V. Rieux, and P. Moine. 2003. Streptococcus pneumoniae strain-dependent lung inflammatory responses in a murine model of pneumococcal pneumonia. Intensive Care Med. 29:808-816. [DOI] [PubMed] [Google Scholar]
- 53.Neff, T. A., R. F. Guo, S. B. Neff, J. V. Sarma, C. L. Speyer, H. Gao, K. D. Bernacki, M. Huber-Lang, S. McGuire, L. M. Hoesel, N. C. Riedemann, B. Beck-Schimmer, F. S. Zetoune, and P. A. Ward. 2005. Relationship of acute lung inflammatory injury to Fas/FasL system. Am. J. Pathol. 166:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Rourke, A. M., and M. F. Mescher. 1993. The roles of CD8 in cytotoxic T lymphocyte function. Immunol. Today 14:183-188. [DOI] [PubMed] [Google Scholar]
- 55.Paton, J. C., R. A. Lock, and D. J. Hansman. 1983. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 40:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43:1085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pelton, S. I., A. M. Loughlin, and C. D. Marchant. 2004. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr. Infect. Dis. J. 23:1015-1022. [DOI] [PubMed] [Google Scholar]
- 58.Preston, J. A., K. W. Beagley, P. G. Gibson, and P. M. Hansbro. 2004. Genetic background affects susceptibility in nonfatal pneumococcal bronchopneumonia. Eur. Respir. J. 23:224-231. [DOI] [PubMed] [Google Scholar]
- 58a.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 59.Rijneveld, A. W., A. F. de Vos, S. Florquin, J. S. Verbeek, and T. van der Poll. 2005. CD11b limits bacterial outgrowth and dissemination during murine pneumococcal pneumonia. J. Infect. Dis. 191:1755-1760. [DOI] [PubMed] [Google Scholar]
- 60.Rivera, J., J. Mukherjee, L. M. Weiss, and A. Casadevall. 2002. Antibody efficacy in murine pulmonary Cryptococcus neoformans infection: a role for nitric oxide. J. Immunol. 168:3419-3427. [DOI] [PubMed] [Google Scholar]
- 61.Rolston, K. V. 2001. The spectrum of pulmonary infections in cancer patients. Curr. Opin. Oncol. 13:218-223. [DOI] [PubMed] [Google Scholar]
- 62.Rubins, J. B., D. Charboneau, C. Fasching, A. M. Berry, J. C. Paton, J. E. Alexander, P. W. Andrew, T. J. Mitchell, and E. N. Janoff. 1996. Distinct roles for pneumolysin's cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 153:1339-1346. [DOI] [PubMed] [Google Scholar]
- 63.Rubins, J. B., D. Charboneau, J. C. Paton, T. J. Mitchell, P. W. Andrew, and E. N. Janoff. 1995. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J. Clin. Investig. 95:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schindler, R., J. Mancilla, S. Endres, R. Ghorbani, S. C. Clark, and C. A. Dinarello. 1990. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood 75:40-47. [PubMed] [Google Scholar]
- 65.Scott, J. A. G., A. J. Hall, R. Dagan, J. M. S. Dixon, S. J. Eykyn, A. Fenoll, M. Hortal, L. P. Jette, J. H. Jorgensen, F. Lamothe, C. Latorre, J. T. Macfarlane, D. M. Shlaes, L. E. Smart, and A. Taunay. 1996. Serogroup-specific epidemiology of Streptococcus pneumoniae: associations with age, sex, and geography in 7,000 episodes of invasive disease. Clin. Infect. Dis. 22:973-981. [DOI] [PubMed] [Google Scholar]
- 66.Shevach, E. M. 2000. Regulatory T cells in autoimmmunity*. Annu. Rev. Immunol. 18:423-449. [DOI] [PubMed] [Google Scholar]
- 67.Siddiqui, A. A., R. J. Shattock, and T. S. Harrison. 2006. Role of capsule and interleukin-6 in long-term immune control of Cryptococcus neoformans infection by specifically activated human peripheral blood mononuclear cells. Infect. Immun. 74:5302-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 73:6479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Staali, L., M. Morgelin, L. Bjorck, and H. Tapper. 2003. Streptococcus pyogenes expressing M and M-like surface proteins are phagocytosed but survive inside human neutrophils. Cell Microbiol. 5:253-265. [DOI] [PubMed] [Google Scholar]
- 70.Stephens-Romero, S. D., A. J. Mednick, and M. Feldmesser. 2005. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect. Immun. 73:114-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tateda, K., T. A. Moore, J. C. Deng, M. W. Newstead, X. Zeng, A. Matsukawa, M. S. Swanson, K. Yamaguchi, and T. J. Standiford. 2001. Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J. Immunol. 166:3355-3361. [DOI] [PubMed] [Google Scholar]
- 72.Thomas, M. L. 1989. The leukocyte common antigen family. Annu. Rev. Immunol. 7:339-369. [DOI] [PubMed] [Google Scholar]
- 73.Tvinnereim, A. R., S. E. Hamilton, and J. T. Harty. 2004. Neutrophil involvement in cross-priming CD8+ T cell responses to bacterial antigens. J. Immunol. 173:1994-2002. [DOI] [PubMed] [Google Scholar]
- 74.van der Poll, T., C. V. Keogh, X. Guirao, W. A. Buurman, M. Kopf, and S. F. Lowry. 1997. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J. Infect. Dis. 176:439-444. [DOI] [PubMed] [Google Scholar]
- 75.van Rossum, A. M., E. S. Lysenko, and J. N. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vermes, I., C. Haanen, H. Steffens-Nakken, and C. Reutelingsperger. 1995. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 184:39-51. [DOI] [PubMed] [Google Scholar]
- 77.Wang, E., M. Simard, N. Ouellet, Y. Bergeron, D. Beauchamp, and M. G. Bergeron. 2002. Pathogenesis of pneumococcal pneumonia in cyclophosphamide-induced leukopenia in mice. Infect. Immun. 70:4226-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang, Q., C. M. Doerschuk, and J. P. Mizgerd. 2004. Neutrophils in innate immunity. Semin. Respir. Crit. Care Med. 25:33-41. [DOI] [PubMed] [Google Scholar]
- 79.Witzenrath, M., B. Gutbier, A. C. Hocke, B. Schmeck, S. Hippenstiel, K. Berger, T. J. Mitchell, J. R. de los Toyos, S. Rosseau, N. Suttorp, and H. Schutte. 2006. Role of pneumolysin for the development of acute lung injury in pneumococcal pneumonia. Crit. Care Med. 34:1947-1954. [DOI] [PubMed] [Google Scholar]
- 80.Wood, W. B., M. R. Smith, and B. Watson. 1946. Studies on the mechanism of recovery in pneumococcal pneumonia IV. The mechanism of phagocytosis in the absence of antibody. J. Exp. Med. 84:387-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhong, Z., T. Burns, Q. Chang, M. Carroll, and L. Pirofski. 1999. Molecular and functional characteristics of a protective human monoclonal antibody to serotype 8 Streptococcus pneumoniae capsular polysaccharide. Infect. Immun. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zuluaga, A. F., B. E. Salazar, C. A. Rodriguez, A. X. Zapata, M. Agudelo, and O. Vesga. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC. Infect. Dis. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]