Abstract
Bacillus anthracis edema toxin (ET), composed of protective antigen and an adenylate cyclase edema factor (EF), elicits edema in host tissues, but the target cells and events leading from EF-mediated cyclic-AMP production to edema are unknown. We evaluated the direct effect of ET on several cell types in vitro and tested the possibility that mediators of vascular leakage, such as histamine, contribute to edema in rabbits given intradermal ET. ET increased the transendothelial electrical resistance of endothelial monolayers, a response that is mechanistically inconsistent with the in vivo vascular leakage induced by ET. Screening of several drugs by intradermal treatment prior to toxin injection demonstrated reduced ET-induced vascular leakage with a cyclo-oxygenase inhibitor (indomethacin), agents that interfere with histamine (pyrilamine or cromolyn), or a neurokinin antagonist (spantide). Systemic administration of indomethacin or celecoxib (cyclo-oxygenase inhibitors), pyrilamine, aprepitant (a neurokinin 1 receptor antagonist), or indomethacin with pyrilamine significantly reduced vascular leakage associated with ET. Although the effects of pyrilamine, cromolyn, or aprepitant on ET-induced vascular leakage suggest a possible role for mast cells (MC) and sensory neurons in ET-induced edema, ET did not elicit degranulation of human skin MC or substance P release from NT2N cells in vitro. Our results indicate that ET, acting indirectly or directly on a target yet to be identified, stimulates the production/release of multiple inflammatory mediators, specifically neurokinins, prostanoids, and histamine. These mediators, individually and through complex interactions, increase vascular permeability, and interventions directed at these mediators may benefit hosts infected with B. anthracis.
Bacillus anthracis is a gram-positive, spore-forming bacillus that causes the disease known as anthrax. The vegetative form of B. anthracis results from spore inoculation of the host animal via cutaneous, gastrointestinal, or inhalational routes (57). Subsequently, spores germinate in a complex process that involves phagocytosis of spores by macrophages and transport of germinating spores within macrophages to regional lymphatics. The vegetative bacilli may then gain entry to the systemic circulation and spread to other sites in the host. This process is facilitated by virulence factors expressed by vegetative bacilli: (i) an antiphagocytic capsule and (ii) lethal toxin (LT) and edema toxin (ET). LT consists of protective antigen (PA) and lethal factor (LF), while ET consists of PA and edema factor (EF) (57).
PA undergoes proteolytic cleavage to a 63-kDa monomer, with subsequent formation of a heptamer capable of binding up to three molecules of EF or LF (15). PA heptamers bind to two different widely expressed cell surface receptors, anthrax toxin receptor/tumor endothelial marker 8 and/or capillary morphogenesis protein 2, and undergo receptor-mediated endocytosis with trafficking to an acidic endosomal compartment (5, 6, 54). Acidification of the endosome triggers a conformational change in the PA heptamer, leading to formation of a pore that allows translocation of EF or LF into the cytosol of target cells (26). EF is a Ca2+/calmodulin-dependent adenylate cyclase (AC) that rapidly converts host ATP into cyclic AMP (cAMP) (36). The downstream effects of this increase in host cell cAMP have been characterized among immune cells, such as neutrophils (polymorphonuclear leukocytes [PMNs]), T lymphocytes (T cells), bone marrow-derived dendritic cells, and monocytes. PMNs intoxicated with ET demonstrate increased chemotaxis but reduced phagocytosis and chemiluminescence (12, 43, 63). ET induces interleukin-6 production in human monocytes and significantly decreases lipopolysaccharide-induced monocyte tumor necrosis factor alpha production (28). ET also impairs antigen receptor activation of T cells and cytokine production in bone marrow-derived dendritic cells (45, 62). Despite these investigations, the mechanisms underlying the clinical finding of ET-induced edema have not been elucidated. Histopathological examination of infection sites from autopsies of humans and in vivo animal studies reveal protein-rich edema surrounding areas with bacilli (13, 25, 67). These clinical and histopathological changes are thought to result from the actions of toxins, specifically ET, but mechanistic evidence is lacking. A better understanding of the biological events leading to vascular leakage and edema formation induced by ET will suggest methods for counteracting these processes during infection with B. anthracis.
In order to better understand the mechanisms by which ET elicits edema, we began by investigating the time course of edema formation and the underlying histopathology, following intradermal (i.d.) injection of ET in rabbits. The relatively rapid onset of edema suggested that release of preformed or rapidly generated mediators of vascular leakage, such as histamine or prostaglandins, might play a role. This hypothesis was tested with the use of inhibitors, which are commercially available for use in humans. Pharmacological inhibitors of prostanoid synthesis, and antagonists of histamine and neurokinins, reduced ET-induced vascular leakage, implicating these inflammatory mediators in this process.
MATERIALS AND METHODS
Toxins and pharmacological agents.
The anthrax toxin components PA and EF were obtained from List Biological Laboratories (Campbell, CA) as recombinant (wild-type) lyophilized proteins from B. anthracis; these components were reconstituted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin for a stock concentration of 1 μg/μl and stored at −80°C. Reconstituted stock PA or EF had undetectable amounts of endotoxin based on the Limulus amebocyte lysate Endochrome assay (Charles River Endosafe, Charleston, SC). EF (K346R) was a gift from Wei-Jen Tang (University of Chicago, Chicago, IL), and LFN-DTA was kindly provided by R. John Collier (Harvard University, Cambridge, MA). Indomethacin, cromolyn sodium, pyrilamine, ranitidine, and vinblastine were all obtained from MP Biomedicals, LLC (Aurora, OH). AA-861, capsaicin, and spantide were purchased from Sigma Chemical Co. (St. Louis, MO). Celecoxib (Celebrex; Pfizer, Inc. New York, NY) 100-mg capsules and aprepitant (Emend; Merck & Co., Inc. Whitehouse Station, NJ) 80-mg capsules were both obtained from the University of Virginia pharmacy. The contents of these capsules were removed and weighed prior to being mixed with 2 ml of Nutri-Cal (Evsco Pharmaceuticals, Buena, NJ) for oral administration.
Cell culture.
Pooled human umbilical vein endothelial cells (HUVEC) were purchased from Cambrex (Walkersville, MD) and were maintained in Ham's F-12K medium (ATCC, Manassas, VA) with 2 mM l-glutamine, 1.5 g/liter sodium bicarbonate, supplemented with 10% heat-inactivated fetal bovine serum (Cellgro/Mediatech, Inc., Herndon, VA), 0.1 mg/ml porcine heparin (Sigma, St. Louis, MO), and 0.05 mg/ml endothelial cell growth supplement (BD Biosciences, Franklin Lakes, NJ). Cells were cultured in bovine collagen type I (BD Biosciences, Franklin Lakes, NJ)-coated flasks and were used for transendothelial electrical resistance (TER) experiments at passages 2 to 7. Primary human skin mast cells (MCs) were isolated and maintained in tissue culture in the Schwartz laboratory at Virginia Commonwealth University according to an institutional review board-approved protocol as previously described (32). NT2N cells were differentiated from the NT2 (Ntera2/D1) human embryonal carcinoma cell line (ATCC, Manassas, VA) as previously described (37). Briefly, NT2 cells were induced to differentiate into NT2N postmitotic neuronal cells via exposure to 10 μM all-trans retinoic acid (Sigma, St. Louis, MO) in Dulbecco's modified Eagle's medium with l-glutamine, 4.5-g/liter glucose (Cellgro/Mediatech, Inc., Herndon, VA) plus 10% heat-inactivated fetal bovine serum (Cellgro/Mediatech, Inc., Herndon, VA) over 6 weeks. Differentiated cells subsequently underwent enrichment via three cycles of trypsin harvesting and replating, with the second harvesting/replating medium containing mitotic inhibitors (10 μM 5-fluoro-2′-deoxyuridine, 10 μM uridine, 1 μM cytosine arabinoside; all from Sigma, St. Louis, MO) to inhibit the overgrowth of dividing NT2 precursor cells. After the third and final harvest, the NT2N cells were replated on poly-d-lysine and laminin (both from Sigma, St. Louis, MO)-coated plates prior to use in substance P (SP) and cytotoxicity assays.
Transwell TER experiments.
Polyester Transwell inserts (6.5 mm, 0.4-μm pore size) were purchased from Costar (Corning, NY). Transwell inserts were coated with bovine collagen type I (BD Biosciences, Franklin Lakes, NJ) before being seeded with HUVEC to confluence. Monolayers were allowed to mature for 7 to 10 days at 37°C and 5% CO2 prior to use in TER experiments. TER was measured for control and experimental monolayers using an EndOhm-6 chamber and an EVOM voltohmmeter, both from World Precision Instruments (Sarasota, FL). Toxin and control solutions were made using Hanks' balanced salt solution (HBSS; Cellgro/Mediatech, Inc., Herndon, VA) at 37°C and freshly thawed toxin stocks. Culture maintenance media were removed from both the luminal and the abluminal compartments of the HUVEC wells and replaced with 600 μl (abluminal) and 150 μl (luminal) of toxin or control solution. The time of luminal solution addition was considered time zero, and subsequent TER measurements were made relative to this point. A blank Transwell insert coated with collagen was used to assess the contribution of the insert porous membrane to TER; this value was then subtracted from all monolayer TER values to obtain the monolayer-specific TER contribution. Monolayer Transwell inserts were transferred to the EndOhm-6 chamber, which contained 1 ml of 37°C HBSS containing no toxins; this volume was used to equalize luminal and abluminal menisci to prevent alterations in TER secondary to hydrostatic shifts. Three separate TER measurements were made for each monolayer insert, and the mean was used to record TER values for each time point. The EndOhm-6 HBSS was replaced between inserts, and the chamber and upper electrode were washed twice with HBSS to minimize cross-contamination between experimental conditions. Monolayer inserts were maintained in a tissue culture incubator (37°C, 5% CO2) between measurements. TER values were expressed as ohms·cm2, with the insert surface area (SA) equal to 0.33 cm2.
Time course of toxin-induced edema in rabbits.
All animal experiments were performed according to a protocol approved by the University of Virginia Institutional Animal Care and Use Committee. New Zealand White rabbits (male or female, 3 to 4 kg) were purchased from Burleson Enterprises (Unionville, VA) and were allowed to accommodate to vivarium conditions for at least 2 days before use in experiments. Rabbits had access to food pellets and water ad libitum. The dorsum of each rabbit was shaved down to the epidermis using electric clippers prior to i.d. injection of toxins or control agents. Ten i.d. injections were performed on each rabbit, with 5 injections evenly distributed over each half of the dorsal skin, allowing for a minimum of 2 cm of space between each site. The volume of each injection was 100 μl, administered i.d. using a 25-gauge tuberculin syringe (Becton-Dickinson, Franklin Lakes, NJ). Time points were designated relative to the time of euthanasia. A 5% (wt/vol) solution of pontamine sky blue dye (Sigma-Aldrich, St. Louis, MO) in 0.45% sodium chloride (11) was injected intravenously (i.v.; 1.2 ml per kg of body weight) through a marginal ear vein over a 2-minute span 60 min prior to euthanasia via i.v. pentobarbital overdose (Euthasol; Delmarva Labs, Midlothian, VA). The entire area of shaved skin was then dissected from the rabbit postmortem and transilluminated using a horizontally positioned transilluminator (Fisher Scientific, Hampton, NH). Blue lesion measurements were made by recording the cranio-caudal length and lateral width (each in cm) of each blue lesion. The SA, an indirect measure of solute (dye) extravasation from the vasculature, was calculated based on the equation for the area of an ellipse (SA [cm2] = πab, where the longest dimension of the ellipse is 2a and the shortest dimension is 2b). A subset of excised skin lesions at different ET doses (n = 27; dose range, 10 ng to 10 μg) was dried at 45°C until a constant dry weight was achieved and the tissue wet-weight/dry-weight ratio was calculated as a measure of tissue water content (31). Skin wet-weight/dry-weight ratios correlated well (Spearman r = 0.76, P < 0.0001) with blue lesion SAs (BLSAs), indicating that BLSA is a reflection of edema formation.
Tissue processing for histopathology.
Immediately after measurement of transilluminated BLSAs for each injection site, 1-cm2 full-thickness skin sections were dissected from the central portion of each injection site. Skin sections were fixed in 10% formalin (Formalde-Fresh; Fisher Scientific, Hampton, NH) and submitted to the Research Histology Core Laboratory at the University of Virginia for paraffin embedding, cutting, and staining with hematoxylin and eosin (H+E). Stained sections were examined by a pathologist who was blinded to the experimental conditions; descriptive observations regarding the presence or absence of an inflammatory cellular infiltrate were recorded.
Pretreatment of toxin injection sites with pharmacological agents.
Rabbits pretreated with pharmacological agents at the sites of toxin injection were treated in the same manner as rabbits used for toxin time courses, using the same procedures for shaving, toxin injection, dye injection, and euthanasia. Indomethacin was prepared as a 1,000× stock solution in 50 mM Tris (pH 7.2), while cromolyn, pyrilamine, and ranitidine were prepared as 100× stock solutions in sterile water. All of these stocks were freshly made on the day of each experiment and were diluted to their final concentrations using sterile water to allow for injection of each dose in a 100-μl total volume (indomethacin dose = 2 × 10−10 mol [71.58 ng]/site; cromolyn dose = 20 μg/site; pyrilamine dose = 10−6 mol [401.5 μg]/site; ranitidine dose = 2 × 10−8 mol [7.018 μg]/site) (35). AA-861 was dissolved in 100% ethanol and kept at 4°C until dilutions in sterile water were made for use at a final ethanol concentration of 10%. Spantide was dissolved in sterile water for a stock concentration of 10 μg/μl. Indomethacin, pyrilamine, and ranitidine were injected at designated sites 60 min prior to the injection of ET (10 μg in a 100-μl volume), while cromolyn was injected 30 min prior to the same ET dose and volume. Spantide was injected 5 min prior to ET. Control sites for PA (10 μg) and EF (10 μg), as well as comparator sites with ET (10 μg), were preinjected with 100 μl PBS 30 min prior to the respective toxin or toxin component to equalize total site injection volumes at 200 μl. Measurements of BLSA were made as described above.
Neutropenic rabbit experiments.
Rabbits were given vinblastine (0.75 mg/kg) i.v. through an ear vein as a bolus injection on day 1 (22, 50). Blood smears were made for each vinblastine and control rabbit on day 3 using blood from an ear vein and were Giemsa stained to confirm the absence of PMNs. Neutropenic rabbits underwent toxin injection on day 4 after vinblastine injection.
Systemic treatment of rabbits with indomethacin, celecoxib, pyrilamine, or aprepitant prior to toxin injections.
Indomethacin (10 mg/kg in 50 mM Tris, pH 7.2), pyrilamine (6 mg/kg in sterile water), or both were given i.v. via marginal ear vein to rabbits 60 min prior to i.d. toxin injections as described above (33, 35). Celecoxib (75 mg/kg/day) was mixed with 2 ml of Nutri-Cal (Evsco Pharmaceuticals, Buena, NJ), a veterinary nutritional supplement containing 34.5% fat, to facilitate oral administration (64). Each dose of celecoxib was administered once daily for a total of four doses, and i.d. toxin injections were performed 60 min after the last dose. Aprepitant (5 mg/kg/dose) was given to rabbits in the same exact manner as celecoxib, but the i.d. toxin injections were performed 120 min after the fourth aprepitant dose.
β-Hexosaminidase assay for MC degranulation.
The β-hexosaminidase assay was performed as previously described (53). Briefly, cultured human skin MCs (0.5 × 105 cells/well, 96-well plates) were treated with toxins or controls as indicated, with two separate experiments performed in triplicate. Toxin components were added to wells for 60 min at 37°C prior to addition of the positive-control monoclonal antibody (MAb) (anti-FcɛRIα MAb 22E7, a gift from J. Kochan, Hoffman-LaRoche, Nutley, NJ) (48) to allow time for toxin endocytosis, given the short time frame (<5 min) available for the positive control to induce degranulation. Degranulation was stopped using ice-cold PBS, cells were centrifuged at 4,000 rpm for 10 min at 4°C, and supernatants were transferred to new tubes to allow measurement of β-hexosaminidase activities in both the supernatants (releasates) and the cell pellets (retentates). Cell pellets were resuspended in PBS adjusted to 1 M NaCl and sonicated (68). β-Hexosaminidase activities for the cell pellet and supernatant fractions were then assayed by measuring the release of p-nitrophenol from p-nitrophenyl N-acetyl-β-d-glucosaminide at an absorbance of 405 nm (68). The percentage of degranulation was then calculated as follows: percent release = (A405 of supernatant/A405 of supernatant + A405 of pellet) × 100.
MC or NT2N cytotoxicity assays.
Human skin MCs (0.5 × 105 cells/well, 96-well plates) were exposed to toxin components (PA [1 μg/ml], LFN-DTA [1 μg/ml], PA [1 μg/ml] plus LFN-DTA [1 μg/ml]) for 24 h (two separate experiments, each done in triplicate). Cytotoxicity was then determined using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer's instructions. Briefly, the assay detects the conversion of a tetrazolium salt to a formazan dye by mitochondrial cytochromes via measurement of cell medium absorbance at 450 nm. Cytotoxicity is then calculated as follows: percent cell survival = (A450 of treated cells/A450 of untreated cells) × 100.
NT2N cells (1 × 104 cells/well, 24-well plates) were exposed to the same toxin components for 24 h, with cytotoxicity measured in the same manner as with MCs.
SP measurements from NT2N cells.
SP release from cultured NT2N cells was measured using a commercially available immunoassay kit (Parameter SP immunoassay; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Briefly, NT2N cells (104 cells/well, 24-well plates) were plated on poly-d-lysine and laminin (Sigma, St. Louis, MO)-coated plates and maintained at 37°C, 5% CO2 in a tissue culture incubator for 7 days to allow neurite outgrowth. The medium was replaced on the day of each experiment with fresh medium containing toxin components (PA [1 μg/ml], EF [1 μg/ml], ET [1 μg/ml]) or capsaicin (50 nM) (60). Culture medium was then removed after 60 min and microcentrifuged (104 rpm for 5 min) to remove cellular debris. The medium was then assayed for SP using the SP immunoassay noted above.
Statistics.
All statistics were computed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). Data are displayed using bars to indicate standard errors of the means (SEM). A P value of <0.05 was used to determine statistical significance. Toxin dose and time responses were compared using one-way analysis of variance and the Bonferroni posttest. TER experimental groups were compared to the control group using two-way analysis of variance for repeated measures and the Bonferroni posttest. Unpaired two-tailed Student t tests were used to compare both local treatment groups and systemically treated rabbits with respective controls. Unpaired two-tailed Student t tests were also used for comparisons among β-hexosaminidase groups and cytotoxicity groups.
RESULTS
ET rapidly induces vascular leakage in rabbit skin.
Doses of ET ranging from 10 ng to 10 μg were injected i.d. in rabbits at different times prior to i.v. injection of pontamine sky blue dye (11). Solute (dye) leakage, measured using the SA of the extravasated blue dye as a surrogate marker, was dose dependent (Fig. 1A and C), and the negative controls (PBS, PA alone, or EF alone) produced no vascular leakage (data not shown). Blue lesions appeared within 30 min of ET (PA [10 μg] plus EF [10 μg] per site) administration, and BLSA increased linearly over the 3-hour observation period (Fig. 1B). A mutated EF (K346R, kindly provided by Wei-Jen Tang, University of Chicago) lacking AC enzymatic activity due to a point mutation in the ATP-binding site (18) did not produce edema when injected i.d. with PA, demonstrating that AC activity is essential.
FIG. 1.
ET-induced dye leakage is dose/time dependent. The mean BLSA (▪) for each ET dose is shown; error bars represent SEM. (A) ET dose response. Three rabbits were used, with six sites per dose. P was <0.05 for comparisons between all ET doses except with the 10-ng and 100-ng responses. (B) ET-induced dye leakage increases over time. The ET dose at each time point was 10 μg. Numbers above data points represent the number of independent observations at each time point. P was <0.05 for comparisons between all time points. (C) Transilluminated skin demonstrating the appearance of ET-induced dye leakage 180 min after toxin injection. Top lesion, ET (1 μg); bottom lesion, ET (10 μg). The white bar represents 1 cm.
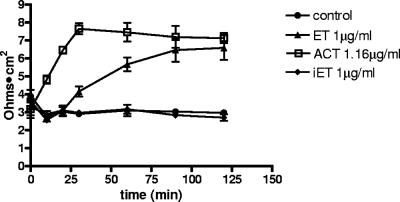
ET increases TER.
The most direct mechanism for ET-induced alteration of vascular permeability is intoxication of endothelial cells, and that possibility was investigated using HUVEC grown in monolayers on collagen-coated Costar Transwell inserts (51). In this system, ET induces a gradual increase in TER over 120 min and the mutated ET (K346R) has no effect (Fig. 2). That this increase in TER is due to toxin-produced cAMP was confirmed using a different bacterial AC toxin, namely, AC toxin from Bordetella pertussis, which rapidly enters eukaryotic cells through the plasma membrane and increases cAMP levels (23). As shown in Fig. 2, AC toxin elicits an increase in TER comparable to that produced by ET, but with a more rapid time course, consistent with its entry kinetics. ET and AC toxin also produce similar increases in the TER of human dermal microvascular endothelial monolayers, indicating that the effects observed with HUVEC are not specific for or limited to macrovascular endothelium (data not shown). The increase in TER induced by ET represents decreased paracellular permeability, an effect which would be counter to vascular leakage and edema formation (41). Thus, not only do the direct effects of ET on endothelium not account for edema formation in vivo, they create conditions that are antagonistic and must be overcome by other factors in order to yield net increases in endothelial permeability.
FIG. 2.
ET and ACT both increase TER in HUVEC monolayers. Data points, means; error bars, SEM. Five experiments were performed. iET, PA plus EF (K346R); ACT, AC toxin from Bordetella pertussis. P was <0.05 for the following comparisons: ET versus the control at 90 and 120 min; ACT versus the control at 20, 30, 60, 90, and 120 min; and ET versus ACT at 20 and 30 min only. Results for iET versus the control were not significant at any time point.
Neutrophilic infiltration associated with ET-induced edema is not required for increased vascular permeability.
Examination of H+E-stained skin sections following i.d. ET injection revealed margination of neutrophils (PMNs) and infiltration into the deep and superficial dermis at later (120- and 180-min) but not earlier (30- to 120-min) time points (Fig. 3A). To test the hypothesis that these PMNs are involved in and contribute to ET-induced edema, neutropenia was induced in rabbits by use of i.v. vinblastine, and the absence of PMNs was confirmed prior to ET administration (22). Unexpectedly, ET-induced BLSAs in neutropenic rabbits were not significantly different from those in nonneutropenic rabbits, indicating that the neutrophilic infiltration is not necessary for edema caused by ET (Fig. 3B).
FIG. 3.
ET-induced dye leakage is associated with, but not dependent on, perivascular neutrophil infiltration. (A) Photomicrograph of dermis (H+E; magnification, ×20) 180 min after i.d. injection of ET (10 μg). Arrows highlight PMN margination and infiltration in the perivascular space. (B) Graph of BLSAs over time in neutropenic (▴) (n = 3) and nonneutropenic (□) (n = 5 at 120 min, n = 31 at 180 min) rabbits. Data points, means; error bars, SEM; *, P was not significant.
Indomethacin and celecoxib attenuate ET-induced vascular leakage.
The rapidity of onset (≤30 min) of ET-induced vascular leakage suggests that edema formation is mediated by the production/release of preformed and/or rapidly synthesized mediators, such as arachidonic acid derivatives. To examine this hypothesis, skin sites were prepared for ET administration by i.d. injection with indomethacin, a nonselective cyclo-oxygenase (COX) inhibitor. This prior treatment with indomethacin reduced 180-min BLSA by 38% (Fig. 4A) compared to ET-induced BLSA in control sites (difference in means = 1.24 cm2; 95% confidence interval [CI], 0.25 to 2.23; P = 0.0157). Similarly, systemic administration of indomethacin (10 mg/kg i.v. 60 min prior to toxin injection) reduced ET-induced vascular leakage by 41% (difference in means = 1.24 cm2; 95% CI, 0.45 to 2.23; P = 0.004) (Fig. 4B). Indomethacin, however, may induce vasoconstriction due to its suppression of the synthesis of vasodilatory prostanoids, such as prostacyclin and prostaglandin E2 (PGE2), by inhibition of the constitutively expressed COX-1 isoform (56). In order to ensure that local vasoconstriction was not responsible for the indomethacin-induced reduction in ET-induced BLSA, oral celecoxib, a selective inhibitor of the inducible isoform COX-2, was tested. Systemic celecoxib inhibited ET-induced BLSA by 61% (difference in means = 2.00 cm2; 95% CI, 0.65 to 3.35; P = 0.005) (Fig. 4B), indicating that the effects of prostaglandin synthesis inhibitors on ET-induced vascular leakage are not solely due to altered homeostatic prostanoid production. Celecoxib also inhibited dye leakage elicited by i.d. arachidonic acid (10 μg, with bradykinin [1 ng]) but not PGE2 (1 μg, with bradykinin [1 ng]); these controls support COX inhibition by celecoxib in these experiments (data not shown).
FIG. 4.
Effects of local (i.d.) antagonists (A) and systemic (oral or i.v.) antagonists (B) on ET-induced BLSA. IND, indomethacin; PYR, pyrilamine; CROM, cromolyn; RAN, ranitidine. See Results for specific drug doses/routes. Numbers above bars represent the number of independent observations for each condition. P values refer to each individual condition in comparison to ET alone (control). See Results for specific P values. All ET doses were 10 μg/site.
Arachidonic acid, in addition to being converted to prostanoids by the COX isoforms, may also be converted into leukotrienes, a family of proinflammatory lipid mediators, via the 5-lipoxygenase (5-LO) pathway (27). The possible role of leukotrienes as mediators of ET-induced edema was tested using AA-861, a potent inhibitor of 5-lipoxygenase (66). Treatment of skin sites with i.d. doses of AA-861 ranging from 1 to 100 μg had no significant effect on ET-induced vascular leakage (data not shown).
Pyrilamine and cromolyn attenuate ET-induced vascular leakage.
Histamine is synthesized/stored predominantly in MCs and basophils and exhibits a diverse range of biological effects, including increased vascular permeability (19). These properties make histamine an excellent candidate for the observed in vivo effects of ET, and this possibility was tested by i.d. administration of three reagents that affect the release or biological effects of histamine: pyrilamine, a histamine receptor type 1 (H1) antagonist; ranitidine, an H2 antagonist; and cromolyn, an inhibitor of MC degranulation (1, 17, 38). Pyrilamine (10−6 mol/site [401.5 μg/site]) decreased ET-induced BLSA by 51% (difference in means = 1.68 cm2; 95% CI, 0.45 to 2.91; P = 0.009), while ranitidine had no significant effect (Fig. 4A). Cromolyn reduced ET-induced BLSA by 36% (difference in means = 1.16 cm2; 95% CI, 0.13 to 2.20; P = 0.028) (Fig. 4A). Systemic pyrilamine (6 mg/kg i.v. 60 min prior to toxin injection) also significantly reduced ET-induced BLSA by 57% (difference in means = 1.86 cm2; 95% CI, 0.49 to 3.22; P = 0.009) (Fig. 4B). Both local and systemic administration of pyrilamine completely inhibited the dye leakage elicited by i.d. histamine (1 μg) but did not inhibit that elicited by bradykinin (1 ng; data not shown). Together, these data on the effects of pyrilamine and cromolyn support a contribution from histamine in ET-induced vascular leakage.
Since our data demonstrate that ET-induced edema involves at least two different types of inflammatory mediators, prostanoids and histamine, we hypothesized that interference with the effects of both types could result in additive or synergistic reduction in ET-induced vascular leakage. This hypothesis was tested by giving rabbits single i.v. doses of indomethacin (10 mg/kg) and pyrilamine (6 mg/kg) 1 hour prior to i.d. toxin injections. Figure 4B shows the substantial reduction in ET-induced BLSA (P = 0.002) by the indomethacin-pyrilamine combination, indicating at least an additive effect of this combination on ET-induced vascular leakage.
The abilities of pyrilamine and cromolyn to interfere with ET-induced vascular leakage in skin suggest that skin MCs could be targets of ET. Histamine can be released from MCs via a complex signaling cascade that involves antigen-induced aggregation of immunoglobulin E-bound Fcɛ I receptors (FcɛRI), leading to MC degranulation (49). We tested the hypothesis that ET directly triggers MC degranulation, using primary human skin MCs exposed to toxin components and/or an FcɛRI-cross-linking MAb (positive control for degranulation) and subsequently measuring β-hexosaminidase release as a marker of degranulation (68). Figure 5 displays the percentages of β-hexosaminidase activity released from human skin MCs in response to these different conditions. ET does not directly stimulate MCs to degranulate, nor does it enhance degranulation in response to FcɛRI cross-linking via MAb. To determine if the lack of an ET effect on MCs could result from the absence of toxin receptors on these cells, we tested the susceptibilities of these MCs to a cytolethal protein construct bearing the PA-binding amino terminus of LF covalently linked to the active domain of diphtheria toxin (LFN-DTA, a kind gift from R. John Collier, Harvard University) (42). LFN-DTA is cytotoxic for human MCs in the presence, but not the absence, of PA (data not shown). This finding is consistent with the presence of a functional receptor for B. anthracis toxins on human MCs and indicates that the lack of an ET effect on human skin MCs is not due to the lack of toxin receptors.
FIG. 5.
ET does not elicit human skin MC degranulation. The y axis represents the percentage of β-hexosaminidase release from primary human skin MCs for each condition. 22E7 mAb, MAb for FcɛRI; 22E7 mAb+ET, MCs exposed to ET (1 μg/ml) for 45 min prior to addition of 22E7 antibody. *, P was 0.0007 for comparison to vehicle control; #, P was not significant for comparison to vehicle control; @, P was not significant for comparison to 22E7 MAb alone. Two experiments were performed, each in triplicate.
Neurokinin antagonists attenuate ET-induced vascular leakage.
The efficacies of agents influencing histamine in reducing ET-induced vascular leakage and the lack of a direct effect of ET on MC degranulation together implicate an indirect mechanism for ET-induced elicitation of histamine release. SP, a peptide neurokinin synthesized/released from afferent sensory neurons in peripheral tissues, is a potent mediator of inflammation, with a diversity of effects predominantly mediated through the neurokinin 1 receptor (NK1R) (7). These effects include increased vascular permeability, direct induction of histamine release from MCs, leukocyte chemoattraction, and increased proinflammatory cytokine production by macrophages (4, 10, 16). SP release from sensory fibers occurs in response to a wide variety of stimuli, including bacterial toxins, neuronal depolarization, capsaicin, and bradykinin (7, 60). We hypothesized that ET directly triggers or augments SP release by increasing cAMP levels in sensory-neuronal projections innervating skin. This hypothesis was tested using i.d. spantide, an 11-amino-acid peptide antagonist of SP at the neurokinin receptors (2). Spantide (500 μg i.d.) reduced ET-induced BLSA by 65% (difference in means = 2.12 cm2; 95% CI, 1.12 to 3.12; P = 0.0001) at 180 minutes compared to ET injected alone at the same dose (Fig. 4A).
In order to determine whether ET directly triggers SP release from sensory neurons, we used an SP immunoassay to quantify in vitro SP release from NT2N cells, a sensory-neuron-like cell line. Capsaicin, used as a positive control for SP release (60), increased SP in culture supernatant after 60 min, but ET failed to release SP or augment SP levels released by capsaicin when NT2N cells were pretreated with ET (data not shown). This lack of SP release was not likely due to the absence of a cognate toxin receptor, because NT2N cells were susceptible to diphtheria toxin-induced cytotoxicity (data not shown) via a PA-dependent delivery mechanism using the same LFN-DTA construct mentioned for our MC experiments (42).
The reduction in ET-induced BLSA requires a large dose of spantide, perhaps due to proteolytic inactivation of spantide in skin (52). To examine this possibility, we systemically administered a nonpeptide NK1R antagonist, aprepitant, to rabbits prior to i.d. toxin injection (47). Aprepitant was given orally (5 mg/kg/day for four doses) at a dose that was chosen to ensure high NK1R occupancy based on pharmacodynamic data obtained from healthy human volunteers (47), and i.d. injections (ET and controls) were performed 120 min after the last dose. Aprepitant reduced the dye leakage induced by i.d. SP (1.25 ng, with bradykinin [1 ng]) but had minimal effect on dye leakage elicited by bradykinin (1 ng) alone (data not shown). Figure 4B shows the reduction in ET-induced vascular leakage by aprepitant (32%; difference in means = 1.06 cm2; 95% CI, 0.282 to 1.842; P = 0.014), which implicates neurokinins such as SP in the edema induced by i.d. injection of ET in rabbit skin.
DISCUSSION
Our findings regarding the involvement of several important mediators in ET-induced vascular leakage indicate that ET-induced edema is not a simple, linear process but rather is a complex one resulting from a network of inflammatory mediators. A fascinating aspect of these findings is the role of ET as a proinflammatory toxin, given that cAMP, the major product resulting from ET intoxication, is thought to serve as a largely anti-inflammatory modulator (9). In addition to the effects of cAMP on PMN phagocytosis, chemotaxis, and oxidative burst, cAMP can also stimulate production of some cytokines and chemokines from various cell types, including endothelial cells, macrophages, monocytes, and MCs (21, 29, 44, 55). Our in vitro finding of increased TER in response to ET intoxication is consistent with prior studies demonstrating that cAMP-elevating agents (e.g., β-adrenergic agonists, forskolin) increase the barrier function of endothelial monolayers (29, 41, 58, 59). The inability of an EF mutant lacking AC activity to induce increased TER supports the concept that ET-induced TER is due to endothelial cell cAMP.
Since vascular leakage is not the result of a direct effect of ET on endothelium, we studied ET-induced vascular leakage in a rabbit skin model to identify specific mediators involved in edema formation. The initial 90 min after toxin injection is characterized by vascular leakage without an inflammatory infiltrate. Subsequent progression of vascular leakage is associated with margination and deep dermal perivascular infiltration of neutrophils, leading to the hypothesis that PMNs contribute to edema formation through multiple mechanisms (30). However, in this study, induction of neutropenia did not alter ET-induced vascular leakage, indicating that PMNs are not required for the initiation or maintenance of ET-induced edema. This observation does not preclude a role for PMNs at later time points not included in this study. Wade et al. found that ET enhanced directed PMN chemotaxis toward formyl peptides or zymosan-activated serum (63). However, ET has also been shown to inhibit human PMN NADPH oxidase activity, thus reducing production of reactive oxygen species (12). Wright and Mandell demonstrated an ET-induced blockade of PMN priming by lipopolysaccharide or muramyl dipeptide (65). O'Brien et al. showed that ET decreased PMN chemiluminescence to particulate and phorbol ester stimulants and impaired PMN phagocytosis of opsonized Sterne strain B. anthracis (43). These earlier studies and our observations regarding PMN infiltration suggest that PMN chemoattraction and chemotaxis may be enhanced by ET but that infiltrating PMNs may subsequently become intoxicated and impaired in their abilities to generate edema-propagating molecules, such as reactive oxygen species.
Since PMNs are not present during the early phase of ET-induced dye leakage, we propose that this phase may be initiated and/or propagated by mediators released by resident cells directly or indirectly affected by ET. We tested several drugs for their abilities to interfere with the effects of these candidate mediators. Indomethacin is a nonselective COX inhibitor and reduces production of prostanoids (e.g., PGE2, prostacyclin). Both PGE2 and prostacyclin can function as vasodilators in systemic microvascular beds and augment vascular leakage indirectly by increasing local blood flow (34, 61). To determine if the observed attenuation of ET-induced vascular leakage by indomethacin is secondary to loss of this prostanoid effect at the injection site and not due to a direct effect of indomethacin on ET-liberated prostanoids, we used celecoxib, a selective COX-2 inhibitor. Celecoxib reduced ET-induced vascular leakage, indicating that COX-2-derived prostanoids play a role in ET-induced edema.
Our observations on the effects of pyrilamine and cromolyn on ET-induced vascular leakage implicate histamine in ET-induced edema. The additive reduction in ET-induced BLSA by use of a combination of indomethacin and pyrilamine supports the concept that multiple inflammatory mediators are involved in ET-induced edema and may directly increase or augment the effects of other mediators on vascular permeability. The predominant cellular sources of histamine include MCs and basophils. Since MCs are resident in skin and the ET-induced edema is of rapid onset, MCs, rather than circulating basophils, were considered a more likely source of histamine. The lack of human skin MC degranulation after ET exposure may indicate an indirect effect of ET on MC histamine release. Interestingly, Gozes et al. found that i.d. LT, but not ET, elicited Evans blue dye leakage in some inbred murine strains, and this leakage was inhibited by ketotifen, an inhibitor of MC degranulation (24). The absence of ET-induced dye leakage in this study may relate to the short interval (60 min) between toxin injection and skin resection. Cromolyn, a potent MC stabilizer in rodents and inhibitor of MC degranulation in response to FcɛRI cross-linking by immunoglobulin E-specific antigens (1), also reduced ET-induced vascular leakage. This effect could reflect a direct influence on MCs or alternatively an antineurokinin effect. Crossman et al. found that cromolyn antagonized both SP- and neurokinin B-induced wheal responses in human skin and inhibited radiolabeled SP binding in rabbit aorta and rat central nervous system tissue in a dose-dependent manner (14). Spantide, a peptide antagonist of SP, and aprepitant, an NK1R antagonist, inhibit ET-induced edema, indicating a likely role for neurokinins in this process. SP exerts a broad range of proinflammatory effects, including increased plasma extravasation, enhanced leukocyte adhesion to endothelial cells, histamine release from MCs, and stimulation or augmentation of cytokine release from monocytes and macrophages (40). Blockade of SP at NK1R abrogates many of these effects, though SP is still able to elicit histamine release from MCs in a receptor-independent fashion involving direct interaction of the amino-terminal fragment of SP with the MC membrane (8). We were unable to demonstrate ET-induced release of SP from NT2N cells, a sensory-neuron-like cell line known to synthesize and release SP (37). An intriguing possibility that could explain the observed effects of neurokinin antagonists on ET-induced vascular leakage despite the absence of direct SP release by ET from NT2N cells is the direct intoxication of nonneuronal cells that express and release SP, such as endothelial cells, macrophages, and skin fibroblasts (3, 39, 46); future studies will examine the possibility that ET elicits SP from these nonneuronal sources.
Our studies have implicated several important proinflammatory mediators in ET-induced vascular leakage, including prostanoids, histamine, and neurokinins. The specific cell types involved in the production/release of these mediators have yet to be identified, but several candidates include macrophages (prostanoids, SP, cytokines), MCs (histamine, prostanoids, cytokines), endothelial cells (prostanoids, SP, chemokines), neurons (SP), and fibroblasts (SP, prostanoids, chemokines). The expression of anthrax toxin receptors by a wide variety of cell types (5, 6, 54) suggests that many target cells are simultaneously intoxicated by ET, resulting in the generation of many cell-specific responses that interact to produce the toxin phenotype of vascular leakage, chemoattraction of neutrophils, and tissue edema. We propose the hypothesis that, rather than a single cell type or mediator being solely responsible for ET-induced edema, multiple cells and mediators are involved in the increased vascular permeability and proinflammatory effects of ET. The observations that we report here regarding the abilities of several classes of anti-inflammatory drugs to inhibit ET-induced vascular leakage support this hypothesis and lay the groundwork for future studies targeting specific aspects of ET-induced edema formation and its role in the pathogenesis of anthrax. Firoved et al. have shown that ET causes significant morbidity and mortality in BALB/cJ mice at lower mortality-inducing doses than LT (20). The possibility that mediators such as histamine, prostanoids, and/or neurokinins contribute to ET-induced murine mortality deserves further investigation. Our observations also highlight potential therapeutic pharmaceuticals that are currently available for use in humans, and future studies need to explore the benefits or hazards that these agents offer for the B. anthracis-infected host.
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grants U54-AI057168 (Mid-Atlantic Regional Center of Excellence) and R01-AI18000.
We also thank the University of Virginia Research Histology Core laboratory for their help in processing rabbit skin tissue.
Editor: D. L. Burns
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Alton, E. W., and A. A. Norris. 1996. Chloride transport and the actions of nedocromil sodium and cromolyn sodium in asthma. J. Allergy Clin. Immunol. 98:S102-S105. [PubMed] [Google Scholar]
- 2.Annunziata, P., C. Cioni, R. Santonini, and E. Paccagnini. 2002. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J. Neuroimmunol. 131:41-49. [DOI] [PubMed] [Google Scholar]
- 3.Bae, S. J., Y. Matsunaga, M. Takenaka, Y. Tanaka, Y. Hamazaki, K. Shimizu, and I. Katayama. 2002. Substance P induced preprotachykinin-a mRNA, neutral endopeptidase mRNA and substance P in cultured normal fibroblasts. Int. Arch. Allergy Immunol. 127:316-321. [DOI] [PubMed] [Google Scholar]
- 4.Bardelli, C., G. Gunella, F. Varsaldi, P. Balbo, E. Del Boca, I. S. Bernardone, A. Amoruso, and S. Brunelleschi. 2005. Expression of functional NK1 receptors in human alveolar macrophages: superoxide anion production, cytokine release and involvement of NF-kappaB pathway. Br. J. Pharmacol. 145:385-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonuccelli, G., F. Sotgia, P. G. Frank, T. M. Williams, C. J. de Almeida, H. B. Tanowitz, P. E. Scherer, K. A. Hotchkiss, B. I. Terman, B. Rollman, A. Alileche, J. Brojatsch, and M. P. Lisanti. 2005. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis' three sites of entry: implications for the pathogenesis of anthrax infection. Am. J. Physiol. Cell Physiol. 288:C1402-C1410. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. [DOI] [PubMed] [Google Scholar]
- 7.Campos, M. M., and J. B. Calixto. 2000. Neurokinin mediation of edema and inflammation. Neuropeptides 34:314-322. [DOI] [PubMed] [Google Scholar]
- 8.Church, M. K., S. el Lati, and J. P. Caulfield. 1991. Neuropeptide-induced secretion from human skin mast cells. Int. Arch. Allergy Appl. Immunol. 94:310-318. [DOI] [PubMed] [Google Scholar]
- 9.Coffey, R. G. 1992. Effects of cyclic nucleotides on granulocytes. Immunol. Ser. 57:301-338. [PubMed] [Google Scholar]
- 10.Costa, S. K., L. M. Yshii, R. N. Poston, M. N. Muscara, and S. D. Brain. 2006. Pivotal role of endogenous tachykinins and the NK1 receptor in mediating leukocyte accumulation, in the absence of oedema formation, in response to TNFalpha in the cutaneous microvasculature. J. Neuroimmunol. 171:99-109. [DOI] [PubMed] [Google Scholar]
- 11.Craig, J. P. 1965. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature 207:614-616. [DOI] [PubMed] [Google Scholar]
- 12.Crawford, M. A., C. V. Aylott, R. W. Bourdeau, and G. M. Bokoch. 2006. Bacillus anthracis toxins inhibit human neutrophil NADPH oxidase activity. J. Immunol. 176:7557-7565. [DOI] [PubMed] [Google Scholar]
- 13.Cromartie, W. J., W. L. Bloom, and D. W. Watson. 1947. Studies on infection with Bacillus anthracis. I. A histopathological study of skin lesions produced by B. anthracis in susceptible and resistant animal species. J. Infect. Dis. 80:1-13. [DOI] [PubMed] [Google Scholar]
- 14.Crossman, D. C., M. R. Dashwood, G. W. Taylor, R. Wellings, and R. W. Fuller. 1993. Sodium cromoglycate: evidence of tachykinin antagonist activity in the human skin. J. Appl. Physiol. 75:167-172. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham, K., D. B. Lacy, J. Mogridge, and R. J. Collier. 2002. Mapping the lethal factor and edema factor binding sites on oligomeric anthrax protective antigen. Proc. Natl. Acad. Sci. USA 99:7049-7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dianzani, C., M. Collino, G. Lombardi, G. Garbarino, and R. Fantozzi. 2003. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br. J. Pharmacol. 139:1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driver, A. G., and S. J. Mustafa. 1987. Correlation of histamine H1 receptor function and [3H]mepyramine binding in porcine tracheal tissue. Eur. J. Pharmacol. 139:287-295. [DOI] [PubMed] [Google Scholar]
- 18.Drum, C. L., S. Z. Yan, J. Bard, Y. Q. Shen, D. Lu, S. Soelaiman, Z. Grabarek, A. Bohm, and W. J. Tang. 2002. Structural basis for the activation of anthrax adenylyl cyclase exotoxin by calmodulin. Nature 415:396-402. [DOI] [PubMed] [Google Scholar]
- 19.Ehringer, W. D., M. J. Edwards, and F. N. Miller. 1996. Mechanisms of alpha-thrombin, histamine, and bradykinin induced endothelial permeability. J. Cell. Physiol. 167:562-569. [DOI] [PubMed] [Google Scholar]
- 20.Firoved, A. M., G. F. Miller, M. Moayeri, R. Kakkar, Y. Shen, J. F. Wiggins, E. M. McNally, W. J. Tang, and S. H. Leppla. 2005. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am. J. Pathol. 167:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flamand, N., M. Surette, S. Picard, S. Bourgoin, and P. Borgeat. 2002. Cyclic AMP-mediated inhibition of 5-lipoxygenase translocation and leukotriene biosynthesis in human neutrophils. Mol. Pharmacol. 62:250-256. [DOI] [PubMed] [Google Scholar]
- 22.Folkesson, H. G., M. A. Matthay, C. A. Hebert, and V. C. Broaddus. 1995. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J. Clin. Investig. 96:107-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon, V. M., W. W. Young, Jr., S. M. Lechler, M. C. Gray, S. H. Leppla, and E. L. Hewlett. 1989. Adenylate cyclase toxins from Bacillus anthracis and Bordetella pertussis. Different processes for interaction with and entry into target cells. J. Biol. Chem. 264:14792-14796. [PubMed] [Google Scholar]
- 24.Gozes, Y., M. Moayeri, J. F. Wiggins, and S. H. Leppla. 2006. Anthrax lethal toxin induces ketotifen-sensitive intradermal vascular leakage in certain inbred mice. Infect. Immun. 74:1266-1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinberg, L. M., F. A. Abramova, O. V. Yampolskaya, D. H. Walker, and J. H. Smith. 2001. Quantitative pathology of inhalational anthrax I: quantitative microscopic findings. Mod. Pathol. 14:482-495. [DOI] [PubMed] [Google Scholar]
- 26.Guidi-Rontani, C., M. Weber-Levy, M. Mock, and V. Cabiaux. 2000. Translocation of Bacillus anthracis lethal and oedema factors across endosome membranes. Cell. Microbiol. 2:259-264. [DOI] [PubMed] [Google Scholar]
- 27.Henderson, W. R., Jr. 1994. The role of leukotrienes in inflammation. Ann. Intern. Med. 121:684-697. [DOI] [PubMed] [Google Scholar]
- 28.Hoover, D. L., A. M. Friedlander, L. C. Rogers, I.-K. Yoon, R. L. Warren, and A. S. Cross. 1994. Anthrax edema toxin differentially regulates lipopolysaccharide-induced monocyte production of tumor necrosis factor alpha and interleukin-6 by increasing intracellular cyclic AMP. Infect. Immun. 62:4432-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irie, K., E. Fujii, H. Ishida, K. Wada, T. Suganuma, T. Nishikori, T. Yoshioka, and T. Muraki. 2001. Inhibitory effects of cyclic AMP elevating agents on lipopolysaccharide (LPS)-induced microvascular permeability change in mouse skin. Br. J. Pharmacol. 133:237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Issekutz, A. 1981. Vascular responses during acute neutrophilic inflammation: their relationship to in vivo neutrophil emigration. Lab. Investig. 45:435-441. [PubMed] [Google Scholar]
- 31.Jelenko, C., III, W. I. Smulyan, and M. L. Wheeler. 1967. Studies in burns: IV. The relationship of eschar water content to eschar water transmissivity. Ann. Surg. 166:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kambe, N., M. Kambe, J. P. Kochan, and L. B. Schwartz. 2001. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood 97:2045-2052. [DOI] [PubMed] [Google Scholar]
- 33.Kasznicki, J., and A. Wiktorowska-Owczarek. 2001. Effects of indomethacin on hemodynamic parameters after intravenous administration of propranolol and enalaprilat in rabbits. Pol. J. Pharmacol. 53:487-493. [PubMed] [Google Scholar]
- 34.Kingston, W. P., and M. W. Greaves. 1985. Actions of prostaglandin E2 metabolites on skin microcirculation. Agents Actions 16:13-14. [DOI] [PubMed] [Google Scholar]
- 35.Le Filliatre, G., S. Sayah, V. Latournerie, J. Renaud, M. Finet, and R. Hanf. 2001. Cyclo-oxygenase and lipoxygenase pathways in mast cell dependent-neurogenic inflammation induced by electrical stimulation of the rat saphenous nerve. Br. J. Pharmacol. 132:1581-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations in eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Y., S. D. Douglas, D. E. Pleasure, J. Lai, C. Guo, P. Bannerman, M. Williams, and W. Ho. 2003. Human neuronal cells (NT2-N) express functional substance P and neurokinin-1 receptor coupled to MIP-1 beta expression. J. Neurosci. Res. 71:559-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin, J. H. 1991. Pharmacokinetic and pharmacodynamic properties of histamine H2-receptor antagonists. Relationship between intrinsic potency and effective plasma concentrations. Clin. Pharmacokinet. 20:218-236. [DOI] [PubMed] [Google Scholar]
- 39.Linnik, M. D., and M. A. Moskowitz. 1989. Identification of immunoreactive substance P in human and other mammalian endothelial cells. Peptides 10:957-962. [DOI] [PubMed] [Google Scholar]
- 40.Maggi, C. A. 1997. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 70:75-90. [DOI] [PubMed] [Google Scholar]
- 41.Malik, A., J. Lynch, and J. Cooper. 1989. Endothelial barrier function. J. Investig. Dermatol. 93:62S-67S. [DOI] [PubMed] [Google Scholar]
- 42.Milne, J. C., S. R. Blanke, P. C. Hanna, and R. J. Collier. 1995. Protective antigen-binding domain of anthrax lethal factor mediates translocation of a heterologous protein fused to its amino- or carboxy-terminus. Mol. Microbiol. 15:661-666. [DOI] [PubMed] [Google Scholar]
- 43.O'Brien, J., A. Friedlander, T. Dreier, J. Ezzell, and S. Leppla. 1985. Effects of anthrax toxin components on human neutrophils. Infect. Immun. 47:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ollivier, V., G. Parry, R. Cobb, D. de Prost, and N. Mackman. 1996. Elevated cyclic AMP inhibits NF-κB-mediated transcription in human monocytic and endothelial cells. J. Biol. Chem. 271:20828-20835. [DOI] [PubMed] [Google Scholar]
- 45.Paccani, S. R., F. Tonello, R. Ghittoni, M. Natale, L. Muraro, M. M. D'Elios, W. J. Tang, C. Montecucco, and C. T. Baldari. 2005. Anthrax toxins suppress T lymphocyte activation by disrupting antigen receptor signaling. J. Exp. Med. 201:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pascual, D. W., and K. L. Bost. 1990. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology 71:52-56. [PMC free article] [PubMed] [Google Scholar]
- 47.Patel, L., and C. Lindley. 2003. Aprepitant—a novel NK1-receptor antagonist. Expert Opin. Pharmacother. 4:2279-2296. [DOI] [PubMed] [Google Scholar]
- 48.Riske, F., J. Hakimi, M. Mallamaci, M. Griffin, B. Pilson, N. Tobkes, P. Lin, W. Danho, J. Kochan, and R. Chizzonite. 1991. High affinity human IgE receptor (Fc epsilon RI). Analysis of functional domains of the alpha-subunit with monoclonal antibodies. J. Biol. Chem. 266:11245-11251. [PubMed] [Google Scholar]
- 49.Rivera, J., and A. M. Gilfillan. 2006. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 117:1214-1225. [DOI] [PubMed] [Google Scholar]
- 50.Rosenshein, M. S., T. H. Price, and D. C. Dale. 1979. Neutropenia, inflammation, and the kinetics of transfused neutrophils in rabbits. J. Clin. Investig. 64:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sandoval, R., A. B. Malik, T. Naqvi, D. Mehta, and C. Tiruppathi. 2001. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L239-L247. [DOI] [PubMed] [Google Scholar]
- 52.Scholzen, T. E., M. Steinhoff, P. Bonaccorsi, R. Klein, S. Amadesi, P. Geppetti, B. Lu, N. P. Gerard, J. E. Olerud, T. A. Luger, N. W. Bunnett, E. F. Grady, C. A. Armstrong, and J. C. Ansel. 2001. Neutral endopeptidase terminates substance P-induced inflammation in allergic contact dermatitis. J. Immunol. 166:1285-1291. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz, L. B., R. A. Lewis, D. Seldin, and K. F. Austen. 1981. Acid hydrolases and tryptase from secretory granules of dispersed human lung mast cells. J. Immunol. 126:1290-1294. [PubMed] [Google Scholar]
- 54.Scobie, H., J. Rainey, K. Bradley, and J. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shichijo, M., N. Inagaki, M. Kimata, I. Serizawa, H. Saito, and H. Nagai. 1999. Role of cyclic 3′,5′-adenosine monophosphate in the regulation of chemical mediator release and cytokine production from cultured mast cells. J. Allergy Clin. Immunol. 103:S421-S428. [DOI] [PubMed] [Google Scholar]
- 56.Simmons, D. L., R. M. Botting, and T. Hla. 2004. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 56:387-437. [DOI] [PubMed] [Google Scholar]
- 57.Spencer, R. 2003. Bacillus anthracis. J. Clin. Pathol. 56:182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staddon, J., and T. Hirase. 1999. Tight junctions and adherens junctions in endothelial cells: structure and regulation, p. 109-124. In J. Pearson (ed.), Vascular adhesion molecules and inflammation. Birkhäuser Verlag, Basel, Switzerland.
- 59.Sugio, K., and J. W. Daly. 1983. Effect of forskolin on alterations of vascular permeability induced with bradykinin, prostaglandin E1, adenosine, histamine and carrageenin in rats. Life Sci. 33:65-73. [DOI] [PubMed] [Google Scholar]
- 60.Tang, H. B., A. Inoue, M. Iwasa, I. Hide, and Y. Nakata. 2006. Substance P release evoked by capsaicin or potassium from rat cultured dorsal root ganglion neurons is conversely modulated with bradykinin. J. Neurochem. 97:1412-1418. [DOI] [PubMed] [Google Scholar]
- 61.Teixeira, M. M., T. J. Williams, and P. G. Hellewell. 1993. E-type prostaglandins enhance local oedema formation and neutrophil accumulation but suppress eosinophil accumulation in guinea-pig skin. Br. J. Pharmacol. 110:416-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tournier, J. N., A. Quesnel-Hellmann, J. Mathieu, C. Montecucco, W. J. Tang, M. Mock, D. R. Vidal, and P. L. Goossens. 2005. Anthrax edema toxin cooperates with lethal toxin to impair cytokine secretion during infection of dendritic cells. J. Immunol. 174:4934-4941. [DOI] [PubMed] [Google Scholar]
- 63.Wade, B. H., G. G. Wright, E. L. Hewlett, S. H. Leppla, and G. L. Mandell. 1985. Anthrax toxin components stimulate chemotaxis of human polymorphonuclear neutrophils (42078). Proc. Soc. Exp. Biol. Med. 179:159-162. [DOI] [PubMed] [Google Scholar]
- 64.Wang, K., K. Tarakji, Z. Zhou, M. Zhang, F. Forudi, X. Zhou, A. T. Koki, M. E. Smith, B. T. Keller, E. J. Topol, A. M. Lincoff, and M. S. Penn. 2005. Celecoxib, a selective cyclooxygenase-2 inhibitor, decreases monocyte chemoattractant protein-1 expression and neointimal hyperplasia in the rabbit atherosclerotic balloon injury model. J. Cardiovasc. Pharmacol. 45:61-67. [DOI] [PubMed] [Google Scholar]
- 65.Wright, G., and G. Mandell. 1986. Anthrax toxin blocks priming of neutrophils by lipopolysaccharide and by muramyl dipeptide. J. Exp. Med. 164:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshimoto, T., C. Yokoyama, K. Ochi, S. Yamamoto, Y. Maki, Y. Ashida, S. Terao, and M. Shiraishi. 1982. 2,3,5-Trimethyl-6-(12-hydroxy-5,10-dodecadiynyl)-1,4-benzoquinone (AA861), a selective inhibitor of the 5-lipoxygenase reaction and the biosynthesis of slow-reacting substance of anaphylaxis. Biochim. Biophys. Acta 713:470-473. [PubMed] [Google Scholar]
- 67.Zaucha, G., M. Pitt, J. Estep, B. Ivins, and A. M. Friedlander. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982-992. [PubMed] [Google Scholar]
- 68.Zhao, W., C. L. Kepley, P. A. Morel, L. M. Okumoto, Y. Fukuoka, and L. B. Schwartz. 2006. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J. Immunol. 177:694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]