Abstract
Epidemiologic studies indicated that some infertile men who were infected with Ureaplasma urealyticum displayed positive antisperm antibodies in their serum and/or semen. The purpose of this study was to investigate the possible mechanism of antisperm antibodies production after infection with U. urealyticum and to analyze the relationship between U. urealyticum and infertility. The existence of cross-reactive antigens (61, 50, and 25 kDa) between U. urealyticum and human sperm membrane proteins was confirmed. Among the cross-reactive antigens, the urease complex component UreG of U. urealyticum was determined. By searching the Swiss-Prot protein database, a pentapeptide identity (IERLT) between UreG and human nuclear autoantigenic sperm protein (NASP) was found. Furthermore, using Western blot analysis and enzyme-linked immunosorbent assay, the cross-reaction between the NASP and UreG was verified. Both anti-rUreG antibody and the antiserum against the synthetic peptide NASP393-408 containing the pentapeptide inhibited mouse sperm egg binding and fusion. After immunization by rUreG or the synthetic peptide, 81.2 and 75% female mice became sterile, respectively. The effect on fertility in mice immunized with the synthetic peptide was reversible. These findings proved for the first time that it was feasible to screen antigens for immunocontraceptives from cross-reactive antigens between sperm and microorganisms which induce infertility.
Ureaplasma urealyticum lacks cell wall, belongs to the taxonomic class Mollicutes, and is one of the smallest self-replicating prokaryotes (33). Among Mollicutes, U. urealyticum is unique in its ability to hydrolyze urea and requires urea to support growth (16). In 1954, Shepard first isolated U. urealyticum from the urethras of patients with nongonococcal urethritis (29). Since then, there has been intense interest in determining what role U. urealyticum might play in human diseases, particularly in the pathogenesis of genital tract infectious diseases.
Because U. urealyticum is an inhabitant of the human lower genital tract, infections are considered to be sexually transmitted and occur more frequently during fertile ages. Although the contributory role of U. urealyticum in infertility has yet to be conclusively established, considerable data have been compiled to support the theory that U. urealyticum can cause infertility (10, 11, 40, 41). Early epidemiologic studies have indicated that U. urealyticum was linked to human reproductive failure on the basis of higher frequencies of isolation from infertile versus fertile couples (11). Friberg and Gnarpe (10) found that U. urealyticum existed in the semen of 76% of the husbands from 50 couples with unexplained infertility. Xu et al. (40) also observed significantly higher frequency of U. urealyticum infection among infertile males (549 of 1,416) compared to fertile controls (34 of 375). Furthermore, they demonstrated that U. urealyticum not only could adhere to the membrane of spermatozoa but also could interfere with sperm motility and sperm-egg interaction as well (41). On the other hand, other investigators have questioned these findings (1, 18) because the mechanism of U. urealyticum-induced infertility remains unclear.
In addition, Witkin and Toth (37) explored the possible relationships between U. urealyticum infection, antisperm antibodies, and infertility. They found that the incidence of antisperm antibodies in men with U. urealyticum infection was significantly higher than that in the control subjects. Similar findings were reported by Quesada et al. (28), who also noted that fertility in men with U. urealyticum infection was improved only after antibiotic therapy, whereas little improvement was seen in a group of men with infection plus antisperm antibodies.
Although U. urealyticum infection has been implicated either directly or indirectly in antisperm antibody production, the mechanism is controversial. Hass (12) and Witkin (36) showed that U. urealyticum could breakdown the blood-testis or blood-excurrent ductal barrier, resulting in inoculation with spermatozoal antigens. Mestecky (23) considered that U. urealyticum and its products could cause an intense local mucosal inflammation and the production of a variety of cytokines. The inflammatory cytokines activate antigen-processing cells and T-helper lymphocytes and stimulate the division and maturation of sensitized B cells. Sensitized B cells circulate through the lymphatic and circulatory systems, finally homing back to the site of the genital tract. Ye et al. (42) first reported the existence of cross-reactive antigens between U. urealyticum and human sperm. These authors demonstrated, by enzyme-linked immunosorbent assay (ELISA) and Western blotting, that anti-U. urealyticum immunoglobulin G (IgG) may react to human sperm membrane proteins (hSMP). Furthermore, it was confirmed that human serum with antisperm antibodies could react with several U. urealyticum proteins, including a 61-kDa protein. Wu et al. (39) reported similar results, which provided some new clues as to the potential mechanism(s) of how U. urealyticum caused the production of antisperm antibodies leading to infertility. However, that study did not definitively prove the association between U. urealyticum and infertility nor the mechanism.
In the present study, we investigated the possible mechanism of antisperm antibody production after infection with U. urealyticum and analyzed the relationship between U. urealyticum and infertility, aiming at providing useful information for the development of new contraceptive antigens.
MATERIALS AND METHODS
Subjects.
This study was approved by the Ethical Review Board at Shanghai Jiao Tong University School of Medicine. All subjects signed informed consent before participation in the study. Healthy donors and infertile patients who infected with U. urealyticum were recruited from Ren Ji Hospital, which is affiliated with Shanghai Jiao Tong University School of Medicine.
Animals.
BALB/c mice and New Zealand White rabbits were obtained from the Animal Center of the Chinese Academy of Sciences. Animals were housed under specific-pathogen-free conditions at Shanghai Jiao Tong University School of Medicine. All animal work was conducted in accordance with Shanghai Jiao Tong University School of Medicine Animal Studies Committee.
Culture, identification, and purification of U. urealyticum.
The strains of U. urealyticum in the present study were isolated from 40 infertile men who were infected with U. urealyticum and displayed positive antisperm antibodies in serum and semen. U. urealyticum was isolated, cultured, and purified as described previously (34). U. urealyticum strains were identified by colonial morphology. We picked the purified strains to expand into 10,000 ml of culture. U. urealyticum was then harvested, and U. urealyticum proteins were extracted according to the method of Thirkell et al. (34).
Preparation of hSMP.
Human semen samples were obtained by masturbation from healthy donors with normal semen parameters (38). All semen samples positive for U. urealyticum and anti-sperm antibodies were excluded. The spermatozoa were prepared, and membrane proteins were extracted according to the method of Snow (31). Samples were then subjected to electrophoretic analysis and preparation for anti-SMP antiserum.
Production of anti-SMP antiserum.
Two healthy 6-month-old New Zealand White rabbits (body weight, ∼2.5 kg) were immunized with hSMP as described previously (14). On the first and third days, a mixture of the antigen and complete Freund adjuvant (Sigma, St. Louis, MO) was injected subcutaneously into the back and proximal limbs of the rabbit. On day 28, a mixture of the antigen and incomplete Freund adjuvant (Sigma) was injected the same way. Approximately 200 to 300 μg of antigen was injected into each rabbit per time. On day 35, the titer of the antiserum was checked by ELISA. The control sera were obtained from animals immunized with only the adjuvant.
Indirect immunofluorescence.
Spermatozoa were purified by the swim-up method as described by Mandal et al. (22), and indirect immunofluorescence technique was performed according to the method of Zhou et al. (43). The adjuvant control serum replacing the primary antibody served as the negative control. Digital images of fluorescent slides were taken by using a laser scanning confocal microscope (Carl Zeiss LSM-510) and attached software.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
Protein electrophoresis was performed on discontinuous polyacrylamide gels (5% stacking and 12% separating gels) on a Bio-Rad minigel apparatus. Gels were stained with Coomassie blue or blotted. For Western blots, proteins were transferred to nitrocellulose membrane and processed as previously described (30).
Purification of the cross-reactive antigens between U. urealyticum and hSMP and determination of the N-terminal amino acid sequence.
To prepare an affinity column, rabbit anti-hSMP antiserum was partially purified by precipitation with ammonium sulfate (40%) and coupled to CNBr-activated Sepharose 4B (Amersham Biosciences, Uppsala, Sweden) according to the manual. The cross-reactive antigens between U. urealyticum and hSMP were purified with immunoaffinity chromatography and fast protein liquid chromatography (FPLC)-MonoQ (Amersham) as described previously (15, 21). The purity and specificity of the protein was verified by SDS-PAGE and Western blotting with the same rabbit anti-hSMP antiserum used for the affinity column. The purified protein was electrophoresed by SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane. The protein on the membrane was stained with Coomassie blue, excised, and subjected to gas-phase microsequencing with an Applied Biosystems ABI-491A Protein sequencer (Genecore, Shanghai, China).
Synthetic peptide.
The peptide NASP393-408 (EPQTSIERLTETKDGS), corresponding to amino acids 393 to 408 of human testis NASP, was synthesized. The peptide was coupled to keyhole limpet hemocyanin (KLH) by the manufacturer (Genecore).
Cloning of UreG.
The full nucleotide sequence of urease complex component UreG was found according to the N-terminal sequence in U. urealyticum complete genome in PubMed. PCR was performed, and the cycle conditions were set up as described previously (20). The PCR products were cloned into pMD18-T vector (TaKaRa, Kanazawa, Japan) and transformed in Escherichia coli (strain DH16B). The nucleotide sequences were confirmed by sequencing.
Expression of recombinant protein and production of antiserum.
The target fragment of the cross-reactive antigen was inserted into the BamHI/HindIII site of the His tag pET-28a(+) expression vector (Novagen, Inc., Madison, Wis.). Subclones that included nucleotides (nt) 1 to 621, nt 43 to 621, nt 103 to 621, and nt 301 to 621 were amplified by PCR and inserted into pET-28a(+), respectively. The reading frame and identity of the insert in each construct were validated with plasmid sequencing. They were expected to produce progressively truncated proteins spanning: amino acids (aa) 1 to 206, aa 15 to 206, aa 35 to 206, and aa 101 to 206. Recombinant pET-28a(+) plasmids were propagated in E. coli BL21(DE3) host cells, and the encoded proteins were expressed as IPTG (isopropyl-β-d-thiogalactopyranoside)-induced His6-tagged fusion proteins. The molecular size of the expressed proteins was verified by SDS-PAGE. The rUreG (aa 1 to 206) was purified on a Ni2+ affinity column under denaturing conditions (22). Six New Zealand White rabbits were immunized with rUreG (aa 1 to 206) or the synthetic peptide NASP393-408-coupled KLH to produce antiserum as described above. The control sera were produced from the immunized animals with Freund adjuvant or KLH-emulsified Freund adjuvant. The specificity of the antisera for UreG or the synthetic peptide was tested by Western blotting against rUreG, as well as U. urealyticum or human sperm protein extracts. The antisera were purified with protein A affinity chromatography according to the manual (Millipore Corp., Bedford, MA).
Competitive ELISA.
Antiserum to rUreG was incubated with various concentrations (0.1 to 1 mM) of the synthesized peptides NASP393-408 at room temperature for 2 h (35). This mixture of peptide and antiserum against rUreG was then transferred to a microtiter plate coated with rUreG and further incubated for 2 h at room temperature to measure the antibody unabsorbed by the synthesized peptide with ELISA (19). Residual antibody is expressed as the percent ratio between NASP393-408 peptide preabsorbed antisera and unabsorbed antisera.
Determination of the relationship of U. urealyticum infection with rUreG or NASP393-408.
Sera were obtained from 22 infertile men infected with U. urealyticum who had antisperm antibodies, and 25 men with normal fertility. The study groups were matched in age (31 ± 3.5 years and 29.4 ± 3.4 years, respectively). All of the infertile men whose wives (without any detectable female factors) had not been pregnant for a period more than 1 year had no other known clinical disorders. Polystyrene 96-well microtiter plates (Nunc) were coated with rUreG or NASP393-408-KLH at a volume of 100 μl/well (0.5 μg per well). Using the infertile male sera as the primary antibodies, an ELISA was performed on the microtiter plates as described previously (3). The fertile male sera were used as control.
Sperm motility analysis.
BALB/c mouse spermatozoa were collected in prewarmed M16 medium (Sigma) by stripping the vasa deferentia and by applying mild pressure to caudae epididymides. The cells were incubated with 100 μg of purified adjuvant control IgG or purified anti-rUreG IgG or anti-NASP393-408 IgG/ml at 37°C for 1 h (6). Computer-assisted sperm motility analysis was performed by using a semen autoanalyzer (Hamilton-Thorne). Motile percentage, curvilinear velocity (VCL), and straight line velocity (VSL) values were measured.
Treatment of gametes.
Eight-week-old female BALB/c mice were primed with 10 IU of pregnant mare serum gonadotropin (Sigma) and then 48 to 56 h later induced to ovulate with 10 IU of human chorionic gonadotropin (Sigma). Cumulus enclosed-egg complexes were collected from the oviductal ampullae 15 h after human chorionic gonadotropin administration and treated with 0.1% hyaluronidase (Sigma) in M16 medium (Gibco-BRL, Carlsbad, CA) containing 10% fetal calf serum to disperse the cumulus cells (9). The zona pellucida (ZP) was removed with acidic Tyrode solution (pH 2.5; Sigma) (8). The eggs were washed five times with M16 medium. Spermatozoa were obtained from male BALB/c mice (8 to 10 weeks old) by placing the cauda epididymides and vasa deferentia in 900 μl of Tyrode solution (containing 3 mg of bovine serum albumin/ml). Spermatozoa were allowed to swim out for 10 min; the tissue was then removed from the solution, and in vitro sperm capacitation was performed according to the method of Howes et al. (13). Capacitated spermatozoa were pretreated for 1 h with a different diluted antiserum to rUreG or anti-NASP393-408 antibody. For controls, sperm were pretreated with adjuvant control serum.
Sperm-egg binding and fusion assays.
In vitro fertilization of ZP-free eggs was performed and assayed as described elsewhere (7). The average number of spermatozoa bound per egg and the number of eggs penetrated by sperm were counted. Eggs were considered penetrated if a decondensing sperm head or two pronuclei and at least a sperm tail were present in the ooplasm. The fusion rate (i.e., the percentage of eggs fertilized) was determined (25).
The peptide competitively inhibits anti-sperm reactivity.
For preabsorption, sera from infertile men, antiserum to rUreG, and the anti-NASP393-408 antibody were, respectively, incubated with a 1 mM concentration of the synthesized peptide NASP393-408 at room temperature for 2 h. Capacitated spermatozoa were treated for 1 h with 1:100 diluted sera from infertile men, antisera to rUreG, and anti-NASP393-408 antibody or with the preabsorbed sera or antisera, respectively. For controls, sperm cells were treated with adjuvant control serum or sera from fertile men. An in vitro fertilization assay was performed as described previously.
Fertility tests.
Female BALB/c mice of proven fertility were immunized against purified rUreG or the synthetic peptide NASP393-408 by intramuscular and subcutaneous routes as previously described (27). Each animal received a total of three injections. Each injection consisted of 100 μl of phosphate-buffered saline containing 25 μg of rUreG protein or the peptide-KLH emulsified with 100 μl of Freund adjuvant. The control animals were injected with KLH emulsified with 100 μl of phosphate-buffered saline and 100 μl of Freund adjuvant. On day 35 after the first injection, the animals were bled by retro-orbital puncture to collect serum for examination of the antibody titer and then housed with males of proven fertility (one male and two females in each cage). After 3 weeks, the number of pups delivered by each mated animal was counted. The animals were kept for a longer time, up to 180 days, to examine the regain of fertility (24).
Statistical analysis.
Experimental and control group averages were reported as means ± the standard deviations (SD). The results were analyzed by the Student t test. Results were considered statistically different at a P value of <0.05.
RESULTS
Cross-reactive antigens between U. urealyticum proteins and hSMP.
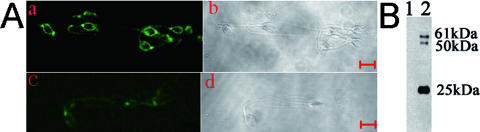
To ascertain the existence of cross-reactive antigens between U. urealyticum and proteins in hSMP and to purify the putative cross-reactive antigens by immunoaffinity chromatography, proteins from human sperm membrane were extracted and used as antigens to immunize rabbits. Antiserum against hSMP was prepared. Its specificity was confirmed by indirect immunofluorescent technique. In mature human spermatozoa, intense immunoreactivity was shown to localize on the membrane of the spermatozoa (Fig. 1A). When rabbit anti-hSMP antiserum was replaced by control serum (Fig. 1B), spermatozoa were negative for immunofluorescent labeling.
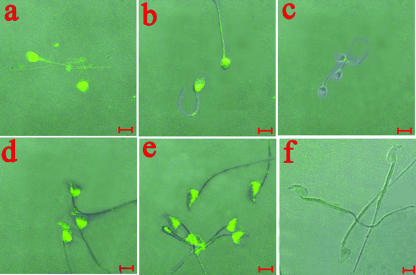
FIG. 1.
(A) Verification of specificity of rabbit anti-hSMP antiserum. Smeared spermatozoa were labeled with rabbit anti-hSMP antiserum at a 1:400 dilution (a) and with diluted rabbit control serum at the same dilution (c). Corresponding phase-contrast images are shown in panels b and d. Bar, 5 μm. (B) Western blot analysis of cross-reactive antigens between U. urealyticum proteins and hSMP. U. urealyticum proteins were run on a 12% gel, blotted, and probed with either adjuvant control serum (lane 1) or rabbit anti-hSMP antiserum (1:4,000) (lane 2).
We performed Western blot, using U. urealyticum proteins as the target antigen, to determine whether anti-hSMP IgG also reacted in response to U. urealyticum. These results indicated that there exist cross-reactive antigens (61-, 50-, and 25-kDa proteins) between U. urealyticum and hSMP (Fig. 1B).
Protein sequence identities between UreG and human nuclear autoantigenic sperm protein (NASP).
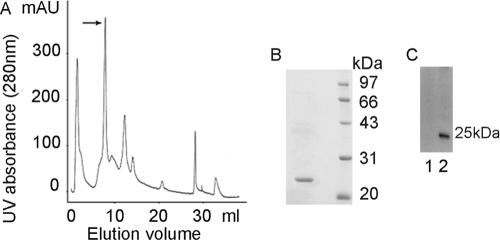
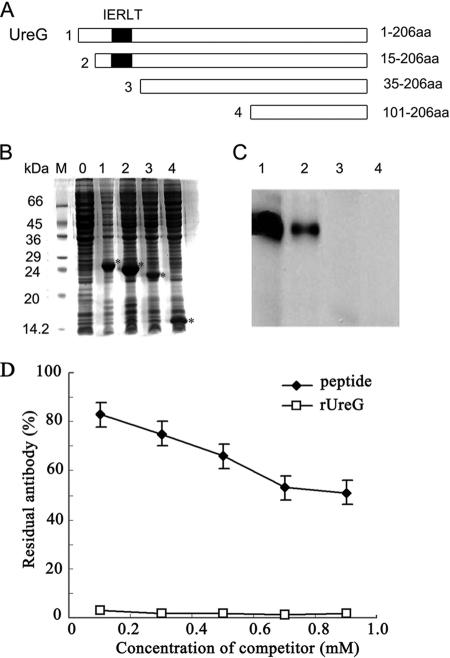
The 25-kDa U. urealyticum protein was purified by using immunoaffinity chromatography and FPLC-MonoQ. The effluent was monitored for UV absorbance (Fig. 2A), and a gradient of NaCl was applied after nonadsorbing material percolated through the column. One-milliliter fractions were collected and tested by SDS-PAGE (Fig. 2B) and Western blotting (Fig. 2C). However, 61- and 50-kDa proteins may not have been purified because of limited amounts. The N-terminal amino acids of the U. urealyticum 25-kDa protein were sequenced to be MKRPLIIGVG. By searching the NCBI database, we identified UreG (gene ID 876215; accession number NP_078266) of U. urealyticum had 10 amino acids of the N-terminal sequence identical to those of the N-terminal sequence of the U. urealyticum 25-kDa protein. The finding that infertile men infected with U. urealyticum displayed positive anti-sperm antibodies in their sera and/or semen led us to ask whether there existed any protein similarities between U. urealyticum proteins and hSMP. We searched the Swiss-Prot protein database and found a common pentapeptide sequence between UreG and human testis- and sperm-specific NASPs. This pentapeptide, IERLT, corresponded to published aa 21 to 25 in UreG and aa 398 to 402 in human NASP. A search of the Swiss-Prot database for IERLT containing human proteins revealed no other hits among 11,856 published human proteins. There were two fragments containing the pentapeptide among the four recombinant UreG fragments (Fig. 3A and B), and Western blot analysis (Fig. 3C) showed that these two fragments (1 and 2) containing the pentapeptide reacted strongly with rabbit anti-hSMP antiserum, while the other two fragments (3 and 4) without the pentapeptide did not. We also confirmed that there existed cross-reacting between anti-UreG antiserum and the synthetic peptide NASP393-408 by competitive ELISA (Fig. 3D).
FIG. 2.
Purification of the cross-reactive antigens between U. urealyticum proteins and hSMP. After U. urealyticum 25-kDa protein was partially purified by using immunoaffinity chromatography, positive fractions were further purified with FPLC-MonoQ. (A) The effluent was monitored for UV absorbance. A 1-ml fraction in peak 2 (arrow) was collected, and then the purity and specificity of U. urealyticum 25-kDa protein were tested by SDS-PAGE (B) and Western blotting (C).
FIG. 3.
Analysis of the rUreG fragments. (A) The rUreG fragments were introduced into a pET-28a(+) expression vector to produce the following subclones spanning aa 1 to 206, aa 15 to 206, aa 35 to 206, and aa 101 to 206. (B) SDS-PAGE (15% gel) was performed to test rUreG fragments at 5 μg of protein/lane. Coomassie brilliant blue staining was used (asterisks indicate rUreG fragments). Lane: 0, aa 1 to 206, IPTG−; 1, aa 1 to 206, IPTG+; 2, aa 15 to 206, IPTG+; 3, aa 35 to 206, IPTG+; 4, aa 101 to 206, IPTG+. (C) The same samples were probed with rabbit anti-hSMP antiserum (1:2,000) in a Western blot. (D) Competitive ELISA was used to measure anti-rUreG antibody unabsorbed by the synthesized peptide. Residual antibody is expressed as the percent ratio between NASP393-408 peptide-preabsorbed antisera and unabsorbed antisera.
Characterization of NASP.
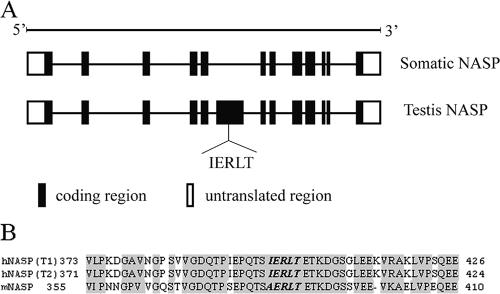
By comparing the somatic NASP (sNASP) and the testis NASP (tNASP) (Fig. 4A), we found an exon that is included in tNASP but excluded in sNASP. Interestingly, the exon included the pentapeptide, IERLT, indicating that the pentapeptide would be selectively expressed in tNASP but not in sNASP. This may explain why infertile men infected with U. urealyticum displayed positive anti-sperm antibodies in their serum and/or semen but did not show any other symptoms.
FIG. 4.
Characterization of NASP. (A) Schematic diagram of the gene structure of somatic and testis NASP showing the testis-selective exon that includes the pentapeptide, IERLT, corresponding to published amino acid positions 21 to 25 in UreG and aa 398 to 402 in NASP. (B) Sequence alignment of hNASP (including transcript 1 [T1] and transcript 2 [T2] encoding proteins) and mNASP. The pentapeptide, IERLT, is approximately the same in hNASP and mNASP protein sequences, except that the isoleucine is replaced by alanine.
We compared the sequence of human NASP (hNASP) (including transcript 1 and transcript 2) and mouse NASP (mNASP) (Fig. 4B) and found that hNASP and mNASP encoded proteins were 81% identical and that the conservation of sequence extended to the 3′ untranslated regions, which were 88% identical. The pentapeptide, IERLT, was similar in sequence between hNASP and mNASP, with the isoleucine replaced by an alanine. The other four amino acids were identical.
Cross-reacting between anti-rUreG antiserum or anti-NASP393-408 antibody and spermatozoa.
To examine the cross-reaction between anti-rUreG antiserum or anti-NASP393-408 and spermatozoa, an indirect immunofluorescence technique was performed. In the mature spermatozoa, strong immunoreactivity was observed in the posterior head area of the spermatozoa (Fig. 5). No signal was detected in control specimens, in which primary antibody was replaced by control serum. Because sperm-egg binding and fusion take place at the posterior head area of the spermatozoa, the results imply that anti-rUreG antibody and anti-NASP393-408 antisera may affect sperm-egg interaction.
FIG. 5.
Immunofluorescence localization of spermatozoa incubated with labeled rabbit antiserum against rUreG or NASP393-408. Smeared spermatozoa were labeled with either antiserum to rUreG (1:50) (a and d), anti-NASP393-408 antiserum (1:50) (b and e), or adjuvant control serum (c and f). Human spermatozoa (a, b, and c) and mouse spermatozoa (d, e, and f) are shown. Bar, 5 μm.
Determination of the relationship of U. urealyticum infection with rUreG or NASP393-408.
To confirm the fact that U. ureaplasma infection results in the production of anti-UreG and anti-peptide antibodies that lead to infertility, we collected sera from infertile men infected with U. urealyticum and assessed their reactivity against rUreG and NASP393-408 by ELISA. The absorbances of the rUreG group and the NASP393-408 group in infertile men (mean ± SD, 0.14 ± 0.04 and 0.16 ± 0.06) were significantly higher (P < 0.01) than in fertile men (0.028 ± 0.026 and 0.029 ± 0.027) (Fig. 6). The results showed that sera from infertile men infected with U. urealyticum who had antisperm antibodies could react with rUreG and the synthesized peptide NASP393-408.
FIG. 6.
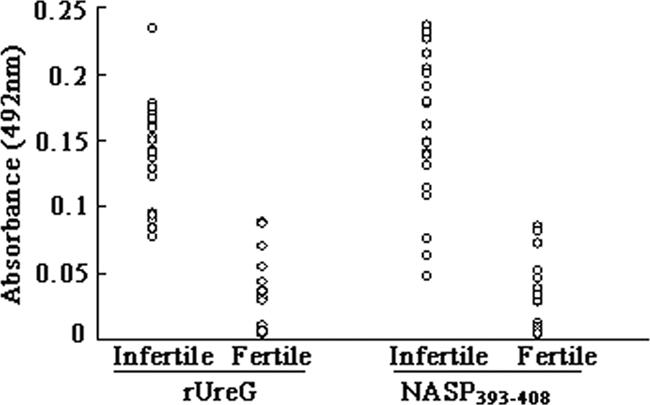
Determination of the relationship of U. urealyticum infection with rUreG or NASP393-408. Microtiter plates were coated with rUreG or NASP393-408-KLH, and ELISA was performed on the microtiter plates using sera from infertile men as the primary antibodies. Sera from fertile men were used as controls. The absorbance was measured at 492 nm.
Effect of antibody against rUreG and anti-NASP393-408 antibody on sperm motility.
To assess whether antibody to rUreG and anti-NASP393-408 antibody inhibits sperm motility, we performed a computer-assisted sperm motility analysis. In the experimental group, the motile percentage, the VCL, and the VSL were not significantly changed (Table 1). Thus, sperm motility appeared to be unaffected by antibody against rUreG and anti-NASP393-408 IgG at concentrations of 100 μg/ml.
TABLE 1.
Effect of antibody against rUreG and anti-NASP393-408 antibody on sperm motilitya
| Group | Mean ± SD
|
||
|---|---|---|---|
| % Motile | VCL (μm/s) | VSL (μm/s) | |
| Control | 64 ± 11 | 266.8 ± 72 | 136.6 ± 68 |
| Anti-rUreG IgG | 55 ± 16 | 268.0 ± 89 | 117.6 ± 66 |
| Anti-NASP393-408 IgG | 60 ± 24 | 243.0 ± 62 | 142 ± 85 |
BALB/c mouse spermatozoa were incubated with 100 μg of purified adjuvant control IgG or the same concentration of purified anti-rUreG IgG or anti-NASP393-408 IgG/ml at 37°C for 1 h.
Fertility assay.
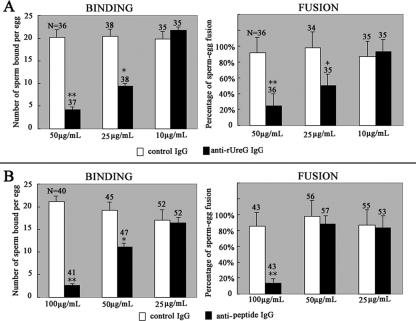
To assess the effect of anti-rUreG antibody and anti-NASP393-408 antisera on sperm-egg interaction, in vitro fertilization assay was performed. The data (Fig. 7) showed a significant inhibition of binding and fusion of mouse sperm to the eggs when sperm had been pretreated with higher concentrations of anti-rUreG antiserum or anti-NASP393-408 antibody (P < 0.05). The inhibition of binding and fusion was completely abolished when the antibodies were diluted to a lower level.
FIG. 7.
Sperm-egg binding and fusion assay. Capacitated mouse spermatozoa were preincubated with different dilutions of purified adjuvant control IgG or purified anti-rUreG IgG (A) and anti-NASP393-408 IgG (B) and then were coincubated with ZP-free eggs. The numbers of sperm bound per egg were counted, and the fusion rates were determined under a laser scanning confocal microscope. The concentrations are give as the amount of purified antibodies (IgG) per milliliter of medium. Histograms represent means ± the SD. N, total number of oocytes per group. The asterisk and double asterisk denote a significant difference between the experimental groups and the control groups. *, P < 0.05; **, P < 0.01.
We went on to perform fa ertility test in vivo to observe the effect of rUreG and the synthetic peptide NASP393-408 immunization on the fertility of female mice. The result of the fertility test is shown in Table 2. Active immunization with rUreG antigen or the synthetic peptide NASP393-408 caused a significant reduction in the fertility, as shown by the fact that 81.2 and 75% of the female mice, respectively, became sterile. Moreover, the immunocontraception effect observed with the synthetic peptide NASP393-408 antigen was reversible.
TABLE 2.
Effect of rUreG and peptide NASP393-408 immunization on fertility of female micea
| Group | No. of animals | No. of pups born (mean ± SEM) housed for:
|
|
|---|---|---|---|
| 21 to 30 days with males | 150 to 180 days with males | ||
| Control | 16 | 8.63 ± 0.26 | 7.43 ± 0.35 |
| rUreG immunized | 16 | 1.63 ± 1.31* | 2.54 ± 0.26* |
| htNASP393-408 immunized | 16 | 1.75 ± 1.34* | 8.72 ± 0.93 |
Female BALB/c mice of proven fertility were immunized with purified rUreG or the synthesized peptide NASP393-408 coupled to KLH. The control animals were immunized with KLH emulsified in PBS and Freund adjuvant. The animals were then housed with males of proven fertility. After 3 weeks, the number of babies delivered by each mated animal was counted. *, P < 0.01.
The peptide competitively inhibited anti-sperm reactivity.
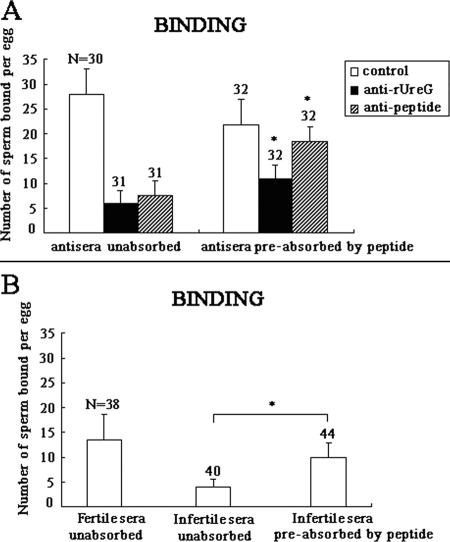
To determine whether NASP393-408 competitively inhibited anti-sperm reactivity by rUreG antiserum, anti-NASP393-408 antibody, as well as sera from infertile men, we performed a sperm-egg binding assay using capacitated mouse spermatozoa that had been pretreated with the described antisera or those preabsorbed with NASP393-408, respectively. The results showed that the peptide significantly and competitively inhibited anti-sperm reactivity by antiserum to rUreG, anti-NASP393-408 (Fig. 8A), and by sera from infertile men (Fig. 8B) (P < 0.05). In the anti-rUreG group, peptide preabsorption treatment significantly increased the average number of sperm bound per egg from 5.93 ± 2.61 (unabsorbed) to 11.06 ± 2.46 (P < 0.05). In the anti-NASP393-408 group, peptide preabsorption also significantly increased the number from 7.45 ± 3.02 (unabsorbed) to 18.4 ± 2.89 (P < 0.05), a level comparable to that of the adjuvant control (Fig. 8A). Similarly, peptide pretreatment of sera from infertile men increased the number of sperm bound from 4.17 ± 2.64 (the unabsorbed level) to 11.89 ± 3.74 (P < 0.05) (Fig. 8B). Therefore, NASP393-408 competitively inhibited anti-sperm reactivity exhibited by sera from infertile males.
FIG. 8.
The peptide competitively inhibited anti-sperm reactivity. Capacitated mouse spermatozoa were preincubated with 1:100 dilutions of antiserum to rUreG, anti-NASP393-408 antibody (A), and sera from infertile men (B), respectively, or the same antisera preabsorbed by the peptide NASP393-408. For controls, sperm were pretreated with adjuvant control sera (A) or sera from fertile men (B). The number of sperm bound per egg was counted. Histograms represent means ± the SD. N, total number of oocytes per group. The asterisk denotes a significant statistical difference between the respective preabsorption group and the unabsorbed group. *, P < 0.05.
DISCUSSION
Proteins on the sperm membrane play important roles in sperm-egg interaction. By using SMP as antigens to produce antiserum and showing its specificity via immunofluorescence staining, we have confirmed the existence of cross-reactive antigens (61, 50, 25 and kDa) between U. urealyticum and hSMP. This is the first systematic study on cross-reactive antigens between U. urealyticum and hSMP. We were able to purify one of the cross-reactive antigens and identify a common pentapeptide between UreG and NASP, one of the human sperm proteins. The result provides strong evidence for the underlying cause of infertility in men infected with U. urealyticum and displayed positive anti-sperm antibodies in their serum and/or semen. Although we have identified three cross-reactive antigens between hSMP and U. urealyticum, there may very well be more that escaped detection by Western blot, owing to their limited amounts and/or instability. We chose the 25-kDa protein that was purified in sufficient quantities for further study. We have yet to purify the 61- and 50kDa antigens.
UreG is a Ni2+-binding GTPase involved in the regulation of expression and the maturation of urease and hydrogenase. NASP encodes a H1 histone-binding protein (778 aa, 86 kDa) that is involved in transporting histones into the nucleus of dividing cells (4). Multiple isoforms are encoded by transcript variants of this gene. The somatic form is expressed in all mitotic cells, is localized to the nucleus, and is coupled to the cell cycle. The testicular form is expressed in embryonic tissues, tumor cells, and the testis. In male germ cells, this protein is localized to the cytoplasm of primary spermatocytes and the periacrosomal region of mature spermatozoa (17). Batova et al. (5) reported that most vasectomized men (86%) developed anti-NASP antibody. NASP is a highly conserved gene, and the overall genomic organization of human and mouse NASP genes is remarkably similar. So we used the mouse as an animal model to perform in vitro and in vivo experiments.
The hydrophilicity and antigenicity of the pentapeptide (IERLT) were predicted by DNAssist software. Excluding the isoleucine (I), ERLT exhibits high hydrophilicity and antigenicity. This suggests that isoleucine may not play a key role in the pentapeptide. The pentapeptide is included in the testicular form of NASP but not in the somatic form of NASP.
Although some authors recognized that U. urealyticum could interfere with sperm function by, for example, releasing ammonia (2), and that U. urealyticum may decrease prostatic function (32), anti-sperm antibodies due to U. urealyticum infection may still play an important role in infertility. Our results imply that only patients who can produce enough high-titer anti-sperm antibodies after U. urealyticum infection may be infertile. If the titer of the antibodies is still high, antibiotic therapy will not be effective to prevent infertility.
Our study suggests that the cross-reactive antigens between U. urealyticum and human spermatozoa play key roles in producing anti-sperm antibodies, which are involved in immune infertility. The results imply that patients who produce antibodies to U. urealyticum also produce antibodies to hSMP after U. urealyticum infection. The molecular mimicry may be an alternative mechanism for the generation of the immunodominant epitope of NASP.
We observed that the antisera to rUreG or NASP393-408 did not affect sperm motility. However, the antisera reduced sperm binding to ZP-free eggs, suggesting that the antibodies interfere with sperm-egg receptor-binding sites interactions on the egg plasma membrane. Since binding occurs prior to fusion, the decrease in sperm binding may result in a decrease in the incidence of fusion, thus making it difficult to interpret whether the antisera affect sperm-egg fusion. The inhibition was completely abolished when the antibody was sufficiently diluted. This result implies that the inhibition effect is reversible. Our findings indicated that the immunocontraceptive effect through active immunization with the synthetic peptide NASP393-408 antigen can wane with the reduction in immune response. Animals immunized with rUreG remained infertile by the end of the study. It is likely that other immune reactions were activated.
Sperm antigens constitute the most promising and exciting targets for contraceptive vaccines. Several sperm-specific antigens have been delineated and are being actively explored for development (26). However, no single recombinant and/or synthetic sperm antigen has demonstrated a complete block of fertility in all of the vaccinated animals. Anti-sperm antibody-mediated immunoinfertility provides a naturally occurring model to indicate how an anti-sperm vaccine will work in humans. Since most infertile men and women are healthy individuals without any disease concomitant with infertility, we searched for immunocontraceptive antigens from immunoinfertile asymptomatic patients with U. urealyticum infection. These findings may help identify putative antigens as targets for the development of a new immunocontraceptive.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China 30470909 (to C.X.), the Deng Shan Project of Shanghai Municipal Commission of Science and Technology 06JC14046 (to C.X.), the “973” Basic Research Funding Scheme of China G199905501 (to C.X.), and the Science Research Project of Science and Technology Commission of Shanghai 06ZR14057 (to J.S.).
We gratefully acknowledge Yuankang Ye (Tongji University, China) for U. urealyticum culture and identification. We also thank Yiqing Wang (Chinese Academy of Sciences, China) for protein purification and Zuofeng Li (Fudan University, China) for bio-informatic analysis. We also gratefully acknowledge Barry T. Hinton (Virginia University) and Taylor Guo (Shanghai Jiao Tong University School of Medicine) for revision and critical suggestions on the manuscript.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Abdulrazzak, A. A., and S. S. Bakr. 2000. Role of mycoplasma in male infertility. East. Mediterr. Health J. 6:149-155. [PubMed] [Google Scholar]
- 2.Anderson, D. P., C. W. Beard, and R. P. Hanson. 1966. Influence of poultry house dust, ammonia, and carbon dioxide on the resistance of chickens to Newcastle disease virus. Avian. Dis. 10:177-188. [PubMed] [Google Scholar]
- 3.Baczynska, A., H. F. Svenstrup, J. Fedder, S. Birkelund, and G. Christiansen. 2005. The use of enzyme-linked immunosorbent assay for detection of Mycoplasma hominis antibodies in infertile women serum samples. Hum. Reprod. 20:1277-1285. [DOI] [PubMed] [Google Scholar]
- 4.Batova, I., and M. G. O'Rand. 1996. Histone-binding domains in a human nuclear autoantigenic sperm protein. Biol. Reprod. 54:1238-1244. [DOI] [PubMed] [Google Scholar]
- 5.Batova, I. N., R. T. Richardson, E. E. Widgren, and M. G. O'Rand. 2000. Analysis of the autoimmune epitopes on human testicular NASP using recombinant and synthetic peptides. Clin. Exp. Immunol. 121:201-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betancourt, M., A. Reséndiza, and E. C. Fierroa. 2006. Effect of two insecticides and two herbicides on the porcine sperm motility patterns using computer-assisted semen analysis (CASA) in vitro. Reprod. Toxicol. 22:508-512. [DOI] [PubMed] [Google Scholar]
- 7.Cohen, D. J., D. A. Ellerman, and P. S. Cuasnicu. 2000. Mammalian sperm-egg fusion: evidence that epididymal protein DE plays a role in mouse gamete fusion. Biol. Reprod. 63:462-468. [DOI] [PubMed] [Google Scholar]
- 8.Evans, J. P., R. M. Schultz, and G. S. Kopf. 1995. Identification and localization of integrin subunits in oocytes and eggs of the mouse. Mol. Reprod. Dev. 40:211-220. [DOI] [PubMed] [Google Scholar]
- 9.Evans, J. P., R. M. Schultz, and G. S. Kopf. 1995. Mouse sperm-egg plasma membrane interactions: analysis of roles of egg integrins and the mouse sperm homologue of PH-30 (fertilin) beta. J. Cell Sci. 108:3267-3278. [DOI] [PubMed] [Google Scholar]
- 10.Friberg, J., and H. Gnarpe. 1974. Mycoplasmas in semen from fertile and infertile men. Andrologia 6:45-52. [DOI] [PubMed] [Google Scholar]
- 11.Gnarpe, H., and J. Friberg. 1973. T-mycoplasmas as a possible cause for reproductive failure. Nature 242:120-121. [DOI] [PubMed] [Google Scholar]
- 12.Hass, G. G. 1987. Antibody-mediated causes of male infertility. Urol. Clin. N. Am. 14:539-550. [PubMed] [Google Scholar]
- 13.Howes, E., J. C. Pascall, W. Engel, and R. Jones. 2001. Interactions between mouse ZP2 glycoprotein and proacrosin; a mechanism for secondary binding of sperm to the zona pellucida during fertilization. J. Cell Sci. 114:4127-4136. [DOI] [PubMed] [Google Scholar]
- 14.Hu, Y. X., J. Y. Guo, L. Shen, Y. Chen, Z. C. Zhang, and Y. L. Zhang. 2002. Get effective polyclonal antisera in one month. Cell Res. 12:157-160. [DOI] [PubMed] [Google Scholar]
- 15.Hui, K.-S., M. Saito, and M. Hui. 1998. A novel neuron-specific aminopeptidase in rat brain synaptosomes: its identification, purification, and characterization. J. Biol. Chem. 273:31053-31060. [DOI] [PubMed] [Google Scholar]
- 16.Kenny, G. E. 1983. Inhibition of the growth of Ureaplasma urealyticum by a new urease inhibitor, fluorofamide. Yale. J. Biol. Med. 56:717-722. [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, Y. H., and M. G. O'Rand. 1993. Ultrastructural localization of a nuclear autoantigenic sperm protein in spermatogenic cells and spermatozoa. Anat. Rec. 236:442-448. [DOI] [PubMed] [Google Scholar]
- 18.Levy, R., M. P. Layani-Milon, S. Giscard D'Estaing, F. Najioullah, J. Lornage, M. Aymard, and B. Lina. 1999. Screening for Chlamydia trachomatis and Ureaplasma urealyticum infection in semen from asymptomatic male partners of infertile couples prior to in vitro fertilization. Int. J. Androl. 22:113-118. [DOI] [PubMed] [Google Scholar]
- 19.Lou, Y. H., H. J. Ang, H. Thai, F. McElveen, and K. S. K. Tung. 1995. A zona pellucida 3 peptide vaccine induces antibodies and reversible infertility without ovarian pathology. J. Immunol. 155:2715-2720. [PubMed] [Google Scholar]
- 20.Lu, M. G., J. L. Shi, and C. Xu. 2005. Establishment and application of the approach to detecting two biovars of Ureaplasma urealyticum in human semen. Nat. J. Androl. 11:175-178. (In Chinese.) [PubMed] [Google Scholar]
- 21.Manchekar, M., P. E. Richardson, T. M. Forte, G. Datta, J. P. Segres, and N. Dashti. 2004. Apolipoprotein B-containing lipoprotein particle assembly: lipid capacity of the nascent lipoprotein particle. J. Biol. Chem. 279:39757-39766. [DOI] [PubMed] [Google Scholar]
- 22.Mandal, A., S. Naaby-Hansen, M. J. Wolkowicz, K. Klotz, J. Shetty, J. D. Retief, S. A. Coonrod, et al. 1999. FSP95, a testis-specific 95-kilodalton fibrous sheath antigen that undergoes tyrosine phosphorylation in capacitated human spermatozoa. Biol. Reprod. 61:1184-1197. [DOI] [PubMed] [Google Scholar]
- 23.Mestecky, J. 1987. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J. Clin. Immunol. 7:265-276. [DOI] [PubMed] [Google Scholar]
- 24.Millar, S. E., S. M. Chamow, A. W. Baur, C. Oliver, F. Robey, and J. Dean. 1989. Vaccination with a synthetic zona pellucida peptide produces long-term contraception in female mice. Science 246:935-938. [DOI] [PubMed] [Google Scholar]
- 25.Myles, D. G. 1993. Molecular mechanisms of sperm-egg membrane binding and fusion in mammals. Dev. Biol. 158:35-45. [DOI] [PubMed] [Google Scholar]
- 26.Naz, R. K. 2005. Contraceptive vaccines. Drugs 65:593-603. [DOI] [PubMed] [Google Scholar]
- 27.Naz, R. K., and X. Zhu. 1998. Recombinant fertilization antigen-1 causes a contraceptive effect in actively immunized mice. Biol. Reprod. 59:1095-1100. [DOI] [PubMed] [Google Scholar]
- 28.Quesada, E. M., C. D. Duke, G. H. Deen, and R. R. Franklin. 1968. Genital infection and sperm agglutinating antibodies in infertile men. J. Urol. 99:106. [DOI] [PubMed] [Google Scholar]
- 29.Shepard, M. C. 1954. The recovery of pleuropneumonia-like organisms from Negro men with or without nongonococcal urethritis. Am. J. Syphilis Gonorrhea Vener. Dis. 38:113-124. [PubMed] [Google Scholar]
- 30.Shetty, J., A. B. Diekman, F. C. Jayes, N. E. Sherman, S. Naaby-Hansen, C. J. Flickinger, and J. C. Herr. 2001. Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis 22:3053-3066. [DOI] [PubMed] [Google Scholar]
- 31.Snow, K. 1992. Characterization of human sperm antigens and antisperm antibodies in infertile patients. Fertil. Steril. 58:1011-1019. [DOI] [PubMed] [Google Scholar]
- 32.Soffer, Y., R. Ron-El, A. Golan, A. Herman, E. Caspi, and Z. Samra. 1990. Male genital mycoplasmas and Chlamydia trachomatis culture: its relationship with accessory gland function, sperm quality, and autoimmunity. Fertil. Steril. 53:331-336. [DOI] [PubMed] [Google Scholar]
- 33.Styler, M., and S. S. Shapiro. 1985. Mollicutes (Mycoplasma) in infertility. Fertil. Steril. 44:1-12. [DOI] [PubMed] [Google Scholar]
- 34.Thirkell, D., A. D. Myles, and W. C. Russell. 1989. Serotype 8- and serocluster-specific surface-expressed antigens of Ureaplasma urealyticum. Infect. Immun. 57:1697-1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson, E. D., H. C. Flick-Smith, C. Lebutt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, et al. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 73:3598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witkin, S. S. 1988. Mechanisms of active suppression of the immune response to spermatozoa. Am. J. Reprod. Immunol. Microbiol. 17:61-64. [DOI] [PubMed] [Google Scholar]
- 37.Witkin, S. S., and A. Toth. 1983. Relationship between genital tract infections, sperm antibodies in seminal fluid, and infertility. Fertil. Steril. 40:805-808. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization (ed.). 1999. WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction, 4th ed. Cambridge University Press, Cambridge, United Kingdom.
- 39.Wu, A. W., G. L. Huang, T. F. Lin, and J. L. Shen. 1996. Analyses of the common antigen between the membrane protein of sperm and Ureaplasma urealyticum. J. Androl. 10:203-205. (In Chinese.) [Google Scholar]
- 40.Xu, C., G. F. Sun, Y. F. Zhu, and Y. F. Wang. 1997. The correlation of Ureaplasma urealyticum infection with infertility. Andrologia 29:219-226. [DOI] [PubMed] [Google Scholar]
- 41.Xu, C., Y. F. Wang, Z. H. Zhang, and Q. X. Shi. 1994. Pathogenic mechanisms of male infertility cause by Ureaplasma urealyticum infection—the effect on sperm penetrating capability. Reprod. Contract 1994(Suppl.):67-71. [Google Scholar]
- 42.Ye, Y. K., D. Y. Lu, Y. F. Zhu, W. Peng, and Y. F. Wang. 1994. Study on the common antigen between human sperm and Ureaplasma urealyticum. J. Androl. 8:1-4. (In Chinese.) [Google Scholar]
- 43.Zhou, Z. Y., C. Xu, and Y. F. Wang. 2004. Spatial and temporal expression of germ cell nuclear factor in murine epididymis. Asian J. Androl. 6:23-28. [PubMed] [Google Scholar]