Abstract
Indonesian thin-tail (ITT) sheep resist infection by Fasciola gigantica by an immunological mechanism within 2 to 4 weeks of infection yet are susceptible to F. hepatica infection. Studies of ITT sheep show that little liver damage occurs following F. gigantica infection, suggesting that the invading parasites are killed within the peritoneum or shortly after reaching the liver. We investigated whether cells isolated from the peritoneums of ITT sheep could kill newly excysted juvenile F. gigantica in vitro and act as a potential mechanism of resistance against F. gigantica infection. Peritoneal cells from F. gigantica-infected sheep, rich in macrophages and eosinophils, mediated antibody-dependent cytotoxicity against juvenile F. gigantica in vitro. Cytotoxicity was dependent on contact between the parasite and effector cells. Isolated mammary gland eosinophils of F. gigantica-infected sheep, or resident peritoneal monocytes/macrophages from uninfected sheep, also killed the juvenile parasites in vitro. By using inhibitors, we show that the molecular mechanism of killing in these assays was dependent on the production of superoxide radicals by macrophages and eosinophils. In contrast, this cytotoxic mechanism was ineffective against juvenile F. hepatica parasites in vitro. Analysis of superoxide dismutase activity and mRNA levels showed that activity and gene expression were higher in F. hepatica than in F. gigantica, suggesting a possible role for this enzyme in the resistance of F. hepatica to superoxide-mediated killing. We suggest that ovine macrophages and eosinophils, acting in concert with a specific antibody, may be important effector cells involved in the resistance of ITT sheep to F. gigantica.
Fasciola gigantica is the most common Fasciola species infecting ruminants in Asia and Africa and is estimated to cause worldwide losses to the livestock industry of more than 3 billion U.S. dollars per annum (44). Human infection with liver flukes is also recognized by the World Health Organization as an emerging human health problem, with more than 500 million people at risk of infection with Fasciola, Opisthorchis, or Clonorchis (11, 23, 44). There are at least 2.4 million people infected with Fasciola, and infection rates in children of up to 72% have been observed in Bolivia (23). Chemotherapy is not a sustainable method of control because of the development of parasites resistant to available flukicides and the cost of treatment impedes its application within the rural areas of developing countries (7, 12, 23, 28, 43). Thus, other cost-effective control mechanisms such as vaccines need to be developed for control of fasciolosis (12, 43). However, the development of vaccines requires knowledge of the immune mechanisms involved in host resistance against Fasciola parasites since such knowledge may lead to the rational design of delivery methods for a vaccine.
There is no practical rodent model for studying immune responses to F. gigantica, since rodents are not permissive to infection (8, 15, 22, 34, 44). Consequently, little is known about the humoral or cell-mediated responses important for host immunity against F. gigantica (34, 42). However, studies of the natural hosts (sheep and cattle) provide evidence that ruminants do acquire resistance to F. gigantica infection (1, 34, 37, 38, 39, 44). When the susceptibilities of sheep breeds to F. gigantica are compared, the Indonesian thin-tail (ITT) sheep exhibits a high degree of resistance to infection relative to other breeds such as St. Croix and merino (34, 42). For example, ITT sheep express high resistance to a primary infection with F. gigantica compared to Merino sheep and acquire further resistance to infection after exposure (34, 37, 38, 39, 49). Analysis of fluke burdens in sheep at various times following infection showed that significant killing of parasites occurs between 2 and 4 weeks of challenge, with little liver damage detected following infection, suggesting that many migrating flukes may not survive long enough to establish themselves in the liver (39). Importantly, resistance to F. gigantica infection by ITT sheep is suppressed by the administration of dexamethasone, suggesting that the acquired resistance is immunologically based (39).
Taken together, these observations suggest that the peritoneal cavity may be an important site of immunological killing of migrating F. gigantica parasites in ITT sheep. They also suggest that the immature newly excysted juvenile (NEJ) parasite could be the primary target of the effective immune response expressed in ITT sheep. These observations are analogous to those obtained with rats (a resistant host) during F. hepatica infection, where resistance is immunologically based and occurs at both the gut wall and peritoneal cavity (13, 34, 46, 47). In the rat model, NEJ F. hepatica parasites are susceptible to antibody-dependent cell-mediated killing by reactive nitrogen intermediates released by peritoneal macrophages (33). Another recent study with rats confirmed that macrophage-mediated killing of F. hepatica was NO dependent although an antibody dependence was not confirmed (41). Here, we have evaluated the possibility that a cell-mediated cytotoxicity mechanism is also expressed in the peritoneums of ITT sheep against the juvenile F. gigantica parasite. We show that juvenile F. gigantica parasites are susceptible to killing in vitro by superoxide radicals produced by macrophages isolated from the peritoneum of ITT sheep and by mammary gland eosinophils; we suggest that this killing mechanism may be involved in determining the resistance of ITT sheep to F. gigantica infection.
MATERIALS AND METHODS
Animals, parasites, parasite extracts, and reagents.
F. gigantica-naive male ITT sheep 6 to 8 months old were bred and raised in pens at Balitvet, Bogor, Indonesia. The naivety of the animals was confirmed by a negative reaction in an enzyme-linked immunosorbent assay and a Western blot assay with F. gigantica whole worm extract (WWE) as the antigen (6). Throughout the experiments, the sheep were maintained in pens on a diet of freshly cut Pennisetum purpureum and dairy concentrate (38, 39). Metacercariae for infections and parasite excystment were obtained from infected Lymnaea rubiginosa snails collected at Surade, West Java, Indonesia (for F. gigantica) or from Lymnaea tomentosa snails collected from laboratory snail cultures at the Elizabeth Macarthur Agricultural Institute, Menangle, New South Wales, Australia (for F. hepatica). Sheep were infected with 250 metacercariae of F. gigantica or F. hepatica by loading the required metacercariae onto filter paper, which was placed inside gelatin capsules (Torpac Inc., Fairfield, NJ) and delivered orally with a dosing gun. Sheep experiments in Indonesia were performed with approval by the Research Institute for Veterinary Science (Bogor, Indonesia) according to local guidelines and custom (38, 39). Adult F. gigantica and F. hepatica parasites were obtained from the livers of infected ITT sheep, and somatic fluke extracts were prepared as previously described (6).
Catalase, cytochrome c, gentamicin, mannitol, RPMI, superoxide dismutase (SOD), toluidine blue, trypan blue, and the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) were purchased from Sigma Chemical Co. Amphotericin B was purchased from Life Technologies (Rockville, MD). Enzyme-linked immunosorbent assay plates and 24-well tissue culture plates were purchased from Flow Laboratories Inc. and Greiner Labortechnik, Frickenhausen, Austria, respectively.
Cell populations.
Resident monocyte/macrophage-rich cell populations from naive sheep or cell populations from ITT sheep infected for 4 weeks with F. gigantica or F. hepatica were collected from the peritoneal cavity with sterile phosphate-buffered saline (PBS) containing 6 mM EDTA. The recovered lavage fluid was collected and centrifuged at 1,500 rpm for 10 min, and the cell pellet was resuspended in sterile RPMI containing 10% heat-inactivated fetal calf serum, 2 μg/ml amphotericin, and 10 μg/ml gentamicin.
Eosinophil-enriched cell populations were obtained from the mammary glands of infected ewes with F. gigantica parasite extract as previously described (4). Briefly, ITT ewes were infected with 100 metacercariae of F. gigantica and 14 to 16 weeks later, eosinophil recruitment into the teat canal was achieved with F. gigantica soluble adult fluke somatic extract. Briefly, 200 μg of somatic fluke extract was suspended in 5 ml of sterile saline and infused via a sterile, smooth-end 22-gauge needle into the teat canals of sensitized sheep as previously described (35). Following isolation, a 5-μl sample of cells was diluted 10-fold with PBS, 50 μl of trypan blue (0.4% [wt/vol] in PBS) was then added, and the total number of viable white blood cells was determined with a Neubauer hemocytometer. For differential cell counts, Cytospin preparations were made by centrifuging samples of lavage cells at 400 rpm for 5 min at 4°C in a Beckman TJ-6 bench-top centrifuge prior to differential staining (Diff-Quik). Two hundred to 300 cells were identified microscopically, and the relative percentages of lymphocytes, monocytes/macrophages, eosinophils, neutrophils, basophils, and mast cells were determined.
Sheep serum.
Sheep were infected with 250 metacercariae of F. hepatica or F. gigantica, and blood was collected 8 weeks later by jugular venipuncture, with EDTA Vacutainer tubes. The blood was allowed to clot at room temperature for 1 h and centrifuged in a Beckman CS-6R centrifuge at 3,000 × g for 20 min. The serum was then removed, and complement activity was inactivated by heating at 56°C for 30 min prior to storage at −20°C.
Incubation of juvenile liver flukes with lavage cells.
Metacercariae were excysted, and NEJ flukes were separated from empty cysts and debris by incubation overnight at 37°C as previously described (50). Cytotoxicity assays were carried out with 24- or 96-well tissue culture plates with up to 50 NEJ liver flukes per well. Because of the lower yield of peritoneal lavage cells (PLCs) from uninfected sheep, the incubation volume was adjusted to 0.2 ml with this cell source. Plates were incubated for 3 days at 37°C in 5% CO2 in 0.2 ml (cells from uninfected sheep) or 1 ml (cells from Fasciola-infected sheep) of RPMI medium containing 10% heat-inactivated fetal calf serum, 2 μg/ml amphotericin B, and 10 μg/ml gentamicin, with or without the addition of 10% sheep serum, 10 μg/ml SOD, 10 μg/ml catalase, 10 μM mannitol, or combinations of these reagents and lavage cells at an effector-to-target (E/T) ratio of 0.25 × 105 to 2 × 105 cells per NEJ liver fluke. NEJ liver flukes were defined as viable when they were determined microscopically as motile and having a defined intestinal cecum and associated structures (lack of these structures results in an opaque appearance) and a defined parasite shape with no tegumental damage (as determined by exclusion of the dye toluidine blue). Following completion of the incubation period, NEJ liver flukes were incubated for 4 h in a solution of 2 mg/ml MTT and viability was assessed as the ability to reduce the tetrazolium salt MTT as previously described (32). Comparative incubations with F. hepatica and F. gigantica parasites were performed on the same day with the same communal reagents in the same culture plates.
Assay of enzyme protein activity.
Approximately 5,000 NEJ liver flukes or 20 to 50 adult parasites were manually homogenized in 100 mM Tris-HCl (pH 7.4) in an ice-cooled ground glass homogenizer for 5 min, and the homogenate was centrifuged at 4°C and 1,000 × g. The specific activities of SOD and glutathione S-transferase (GST) were measured in triplicate in the resultant supernatant. All reactions were carried out at 25°C in a Shimadzu UV-160 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) in a total reaction mixture of 1 ml, and the change in absorbance was monitored continuously for 2 min. The specific activity of SOD was determined by the cytochrome c reduction method with bovine erythrocyte SOD as the standard (32). Cytochrome c reduction was monitored at 550 nm. One unit of SOD activity was defined as the amount of enzyme necessary to inhibit the rate of reduction of cytochrome c by 50%. SOD specific enzyme activity is expressed as units per milligram of protein. The GST assay measured the conversion of glutathione from the oxidized form to the reduced form and was monitored spectrophotometrically as an increase in absorbance at 340 nm (32, 40). The specific activity of GST was defined as the amount of 1-chloro-2,4-dinitrobenzene conjugated per minute per milligram of protein. Protein concentration was determined with the Bio-Rad DC colorimetric assay for protein concentration following detergent solubilization.
Assay of enzyme mRNA expression.
RNA was extracted in duplicate with the Lipid RNeasy Mini Kit (QIAGEN) according to the manufacturer's guidelines. Total RNA (2 μg) was reversed transcribed with an Omniscript reverse transcription kit (QIAGEN) according to the manufacturer's guidelines in a total volume of 20 μl. The final reaction mixture was incubated at 37°C for 70 min and stored at −20°C. Oligonucleotides used to amplify Fasciola β-actin, GST, and SOD were designed from sequences obtained from the GenBank database with the text search program and the Primer3 software program (BioNavigator; www.entigen.com) under default parameters. Products from each primer set were sequence verified to ensure correct amplification. Real-time PCR conditions for each primer set were optimized with pooled cDNA. Efficiencies were calculated from the average of three standard curves (coefficient of determination, >0.9) generated from separate experiments and serially diluted cDNA (standard deviation, <5%). The primers for β-actin were sense primer 5′ATCACTGCCACCCAGAAGACT and antisense primer 5′CATGCCAGTGAGCTTCCCGTT, the primers for GST were sense primer 5′AGAAATGGTTGGGCGATAAA and antisense primer 5′AACACGAACAAAACCCATCC, and the primers for SOD were sense primer 5′GCGGGACCTCATTTCAACCC and antisense primer 5′CACAAGCCAAACGGCCTCCAG. The primer sets used here were 100% identical between the F. gigantica and F. hepatica cDNA sequences for each target product and ensured that the binding sites of the primers used in the real-time PCRs were identical for both F. gigantica and F. hepatica target genes and, hence, validate the real-time PCR efficiencies. Relative quantitation of SOD and GST mRNAs was done with an MX3000P real-time PCR machine (Stratagene) with the SYBR green (with dissociation curve) experiment type. Each 20-μl reaction mixture included 10 μl of 2× SYBR green master mix (QIAGEN) which incorporated the internal Rox Dye control, 0 to 1 μl of 50 mM MgCl2 (Invitrogen), 0.3 μM each primer, and 4 μl of cDNA (diluted 1:10). Real-time conditions began with a 15-min denaturation step at 95°C, followed by 40 cycles of 94°C for 15 s, the primer-specific temperature for 30 s, and 72°C for 30 s. A melting curve analysis (55 to 95°C at a heating rate of 0.01°C/s) was performed to ensure that only the required PCR product at a specific melting temperature was measured. Each experiment was repeated three times, and in each a designated calibrator was run in triplicate. Following amplification, the experiment was converted to a comparative quantification (calibrator) experiment type and analyzed with the MX3000P software. SOD and GST expression was normalized for each cDNA preparation with the respective β-actin housekeeping gene value, and final values represent the expression relative to the calibrator. Averages represent the results of two RNA extractions and six real-time PCR experiments for each sample.
Statistical analysis.
Significant differences between treatment groups were determined by the nonparametric Dunnett multiple-comparison test. Significant differences for specific antioxidant defense enzyme activities between adult F. hepatica and F. gigantica WWEs were calculated with the unpaired alternative t test. To analyze real-time PCR results, crossing point values were used in the REST (Relative Expression Software Tool) version 2 software program (29). Statistical analysis of gene regulation between groups was performed under the default parameters of the program with pairwise fixed reallocation randomization tests.
RESULTS
Killing of NEJ F. gigantica parasites in vitro by PLCs and immune serum from ITT sheep.
PLCs collected from ITT sheep infected for 4 weeks with F. gigantica consisted of 40 to 50% monocytes/macrophages, 40 to 50% eosinophils, 2 to 20% lymphocytes, and less than 5% neutrophils. When NEJ F. gigantica was incubated with these cells and serum from F. gigantica-infected ITT sheep, large numbers of cells adhered to the NEJ liver fluke tegument, as previously described for F. hepatica (33); this resulted in a reduction in the mean viability of NEJ F. gigantica to 40% (Fig. 1). Dead NEJ F. gigantica parasites were characterized by extensive cell attachment, immotility, loss of a defined intestinal cecum, loss of parasite shape, extensive tegumental damage (as determined by toluidine blue staining), and an inability to reduce the tetrazolium salt MTT, as observed in the rat-F. hepatica model (33). Killing of NEJ F. gigantica parasites in vitro required the presence of both immune serum and PLCs. When NEJ F. gigantica parasites were incubated in the absence of serum, or with serum from F. gigantica-naive ITT sheep, a mean of 85% of the parasites were viable (Fig. 1). The viability of NEJ liver flukes was also not affected by incubation with serum from F. gigantica-infected ITT sheep in the absence of PLCs (90% viable NEJ liver flukes; Fig. 1).
FIG. 1.
Effect of increasing numbers of PLCs from F. gigantica-infected ITT sheep on the killing of NEJ F. gigantica. Each of three replicate wells containing 20 to 30 NEJ parasites and PLCs (E/T ratio of 0.25 × 105 to 2 × 105 cells to 1 NEJ parasite) in 1 ml of medium was incubated for 3 days. The viability of the NEJ parasites was then assessed as the ability to reduce the tetrazolium salt MTT. Results are the mean ± the standard deviation of three experiments. Asterisks indicate mean values that are significantly different by the Dunnett's multiple comparison test at P < 0.05, relative to control incubations (cells plus NEJ parasites alone). NS, serum from F. gigantica-naive ITT sheep; IS, immune serum from ITT sheep infected for 8 weeks with F. gigantica.
Killing of NEJ F. gigantica parasites in vitro by PLCs requires direct cell contact.
The mechanism of cytotoxicity to NEJ liver flukes by ITT sheep PLCs observed above required the inclusion of serum from F. gigantica-infected ITT sheep, which resulted in extensive cell attachment, suggesting that intimate contact between the effector cells and the NEJ liver fluke tegument was required for parasite killing. In order to test this possibility, NEJ F. gigantica parasites were incubated with serum from F. gigantica-infected ITT sheep in wells with tissue culture inserts in which the PLCs were separated from the NEJ liver flukes by a 0.45-μm-pore-size filter to inhibit direct contact between NEJ liver flukes and PLCs. Separation of NEJ liver flukes from PLCs in the presence of serum from F. gigantica-infected ITT sheep resulted in a mean parasite viability of 80%, compared with a mean of only 20% viable parasites in incubations performed in the absence of tissue culture inserts (Fig. 2). This result suggests that killing of NEJ F. gigantica by ITT PLCs requires intimate contact between the PLCs and the fluke tegument.
FIG. 2.
Effect of physical separation of NEJ F. gigantica and PLCs of F. gigantica-infected ITT sheep on the subsequent killing of the NEJ parasites. Each of three replicate wells containing 25 to 50 NEJ parasites was placed in 24-well tissue culture plates with immune serum from F. gigantica-infected ITT sheep. In those incubation wells containing the insert, the peritoneal cells were placed inside the insert at an E/T ratio of 2 × 105 cells to one NEJ parasite; the sheep peritoneal cells were thus separated from the NEJ parasites by a 0.45-μm-pore-size membrane. Following incubation for 3 days, the viability of the NEJ parasites was assessed as the ability to reduce the tetrazolium salt MTT. Results are representative of three experiments.
Identification of the cytotoxic mediator of parasite killing in vitro produced by PLCs.
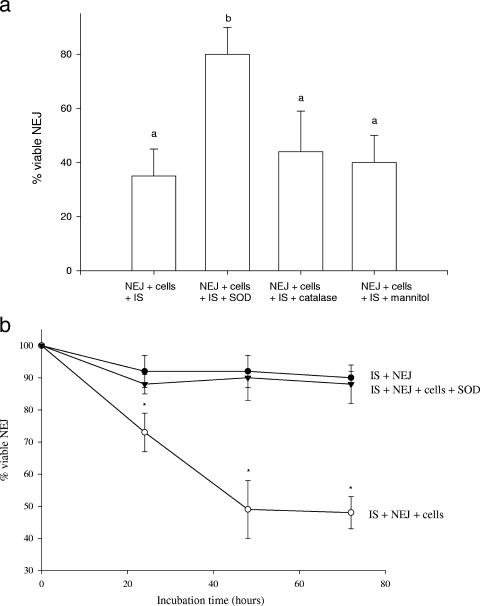
We attempted to identify which cytotoxic molecule(s) produced by ITT sheep PLCs was mediating the killing of NEJ F. gigantica. Cytotoxic molecules released by macrophages and eosinophils include reactive nitrogen (nitric oxide) and oxygen (superoxide, hydrogen peroxide) intermediates (3, 31). Inhibition of nitric oxide production by NG-monomethyl-l-arginine did not reverse the cytotoxic effects of ITT sheep PLCs as expected (data not shown), since we have previously shown that lavage cells of ITT sheep (and other sheep breeds) do not generate detectable levels of nitric oxide under our incubation conditions in vitro (33). However, addition of the superoxide radical inhibitor SOD significantly reversed the killing of NEJ F. gigantica parasites from a mean of 35% viable parasites in the absence of SOD to a mean of 80% viability with SOD present (Fig. 3a). Superoxide radicals can give rise to other reactive oxygen species, including hydrogen peroxide and hydroxyl radicals. However, inhibitors of hydrogen peroxide (catalase) and the hydroxyl radical scavenger (mannitol) had no significant effect on the mean killing of NEJ F. gigantica incubated with ITT sheep PLCs and serum from F. gigantica-infected ITT sheep alone (Fig. 3a). This superoxide-mediated cytotoxicity against NEJ F. gigantica appeared to exert its effects early in the incubation period with ITT PLCs and immune serum, as indicated by a reduction in mean parasite motility within 24 h compared to incubations with the addition of SOD (Fig. 3b).
FIG. 3.
(a) Effect of adding exogenous SOD, catalase, or mannitol to culture incubations on the viability of NEJ F. gigantica following exposure to immune serum and PLCs from F. gigantica-infected ITT sheep. Each of three replicate wells containing 20 to 30 NEJ parasites, cells (E/T ratio of 2 × 105 cells to 1 NEJ parasite), and immune serum in 1 ml of medium was incubated for 3 days with or without exogenous SOD (10 μg/ml), catalase (10 μg/ml), or mannitol (10 μM). The viability of the NEJ parasites was then assessed as the ability to reduce the tetrazolium salt MTT. Results are the means ± standard deviations of three experiments. For each incubation, mean values with the same superscript (a or b) could not be significantly differentiated by the Dunnett multiple-comparison test at P < 0.05. IS, immune serum from ITT sheep infected for 8 weeks with F. gigantica. (b) Effect of adding exogenous SOD to culture incubations on the motility of NEJ F. gigantica in the presence of immune serum and PLCs from F. gigantica-infected ITT sheep. Each of three replicate wells containing 20 to 30 NEJ liver flukes, cells (E/T ratio of 2 × 105 cells to 1 NEJ parasite), and immune serum in 1 ml of medium was incubated for 3 days with or without exogenous SOD (10 μg/ml). At 24 h and 48 h, viability was assessed as the motility and structural integrity of NEJ; at 72 h, viability was assessed as the ability to reduce the tetrazolium salt MTT. Results are the means ± standard deviations of three experiments. Asterisks indicate means that were significantly different by the Dunnett multiple-comparison test at P < 0.05 from incubations with cells plus NEJ parasites.
Identification of effector cells in ITT PLCs mediating cytotoxicity in vitro.
The in vitro cytotoxicity assays used PLCs that consisted of two major immune cell types, monocytes/macrophages and eosinophils, which have been shown to have important roles in helminth parasite killing in other animal models (21, 35). We therefore obtained cell populations highly enriched for macrophages or eosinophils to test whether each cell type was capable of mediating superoxide-dependent cytotoxicity to NEJ F. gigantica. Resident peritoneal cell populations from F. gigantica-naive ITT sheep contained greater than 90% monocytes/macrophages with no eosinophils present. Cell populations collected from ITT mammary glands infused with a soluble somatic F. gigantica lysate contained a mean of 90% eosinophils with about 8 to 10% monocytes/macrophages (representing an E/T ratio of 2 × 104 monocytes/macrophages to one NEJ parasite [Fig. 4b]). As shown in Fig. 1, this level of monocytes/macrophages would only reduce NEJ viability by about 15%. When NEJ F. gigantica parasites were incubated with either of these enriched cell populations and serum from F. gigantica-infected ITT sheep, a significant portion of the parasites was killed, with the mean viability reduced to 30% in each case (Fig. 4); this is comparable to the level of killing of NEJ parasites seen with the whole peritoneal cell population from F. gigantica-infected ITT sheep described above. The cytotoxicity to NEJ F. gigantica mediated by the monocyte/macrophage-rich or eosinophil-rich population was also abrogated by the addition of SOD to inhibit superoxide radical formation (Fig. 4).
FIG. 4.
Effect of adding exogenous SOD to incubations of (a) PLCs of F. gigantica-naive ITT sheep and (b) mammary elicited (ME) lavage cells from F. gigantica-infected ITT sheep on the subsequent killing of NEJ F. gigantica in the presence of ITT immune serum. Each of three replicate wells containing 20 to 30 NEJ parasites, cells (E/T ratio of 2 × 105 cells to 1 NEJ parasite), and immune serum in 1 ml of medium were incubated for 3 days with or without exogenous SOD (10 μg/ml). The viability of the NEJ parasites was then assessed as the ability to reduce the tetrazolium salt MTT. Results are the means ± standard deviations of three experiments. Asterisks indicate means that were significantly different (P < 0.05) from incubations with NEJ parasites and lavage cells only (data not shown). The immune serum used was from ITT sheep infected for 8 weeks with F. gigantica.
ITT PLCs do not kill NEJ F. hepatica parasites in vitro.
Our previous work showed that ITT sheep are susceptible to primary and secondary infections with the temperate liver fluke F. hepatica (38, 39). Interestingly, we have also shown that NEJ F. hepatica parasites are highly resistant to oxygen free-radical-mediated killing in vitro (32, 33). These observations suggested that F. hepatica could be resistant to the ITT effector mechanism(s) that is active against F. gigantica. In order to determine whether there are inherent differences between the susceptibilities of F. hepatica and F. gigantica to killing by ITT effector mechanisms, we directly compared the killing of NEJ F. hepatica and that of NEJ F. gigantica in vitro by incubating PLCs isolated from the same F. gigantica-naive ITT sheep with each parasite in the presence of homologous immune serum. These incubations were carried out on the same day with cells from the same donor sheep. Only NEJ F. gigantica parasites were susceptible to killing by PLCs of F. gigantica-naive ITT sheep (mean, 25% viability); no cytotoxic effect against NEJ F. hepatica was observed (Fig. 5). Extended incubations (10 days) of PLCs with NEJ F. hepatica did not result in an increase in parasite killing (data not shown).
FIG. 5.
Comparative susceptibility of NEJ F. gigantica (FgNEJ) and F. hepatica (FhNEJ) to killing by PLCs of F. gigantica-naive ITT sheep following incubation with homologous Fasciola-immune serum (IS). Each of 10 replicate wells containing four NEJ parasites and cells (E/T ratio of 2 × 105 cells to one NEJ parasite) and homologous immune serum in 0.2 ml of medium was incubated for 3 days. At 24 h and 48 h, viability was assessed as the motility and structural integrity of NEJ; at 72 h, viability was assessed as the ability to reduce the tetrazolium salt MTT. Results are the means ± standard deviations of five experiments. Asterisks indicate means that were significantly different by the Dunnett multiple-comparison test at P < 0.05 from incubations with immune serum plus FgNEJ parasites alone. FgIS, immune serum from ITT sheep infected for 8 weeks with F. gigantica; FhIS, immune serum from ITT sheep infected for 8 weeks with F. hepatica.
A second question we addressed was whether the ability to kill NEJ F. hepatica is influenced by the source of immune serum or cells, i.e., whether homologous or heterologous immune serum and PLCs from exposed sheep mediate in vitro cytoxicity against NEJ F. hepatica. Accordingly, NEJ F. hepatica or F. gigantica were incubated with homologous or heterologous immune serum or PLCs from F. gigantica-infected or F. hepatica-infected animals, respectively. As shown in Table 1, incubations of NEJ F. gigantica with homologous or heterologous serum or with PLCs from F. gigantica-infected animals or F. hepatica-infected animals also resulted in killing of NEJ F. gigantica (mean, 43 to 58% viable parasites). In contrast, the viability of NEJ F. hepatica was unaffected by incubation with either homologous or heterologous serum or PLCs (Table 1). These incubations were performed on the same day with communal sources of cells and serum.
TABLE 1.
Comparative susceptibilities of NEJ F. gigantica and F. hepatica to killing by PLCs of F. hepatica-infected or F. gigantica-infected ITT sheep following incubation with homologous or heterologous Fasciola-immune seruma
| Incubation | % Viable NEJ parasites |
|---|---|
| Fh PLCs + Fh NEJ + NS | 95 ± 4† |
| Fh PLCs + Fh NEJ + Fh IS | 95 ± 4† |
| Fh PLCs + Fh NEJ + Fg IS | 93 ± 4† |
| Fg PLCs + Fh NEJ + NS | 92 ± 4† |
| Fg PLCs + Fh NEJ + Fh IS | 93 ± 4† |
| Fg PLCs + Fh NEJ + Fg IS | 94 ± 4† |
| Fg PLCs + Fg NEJ + NS | 88 ± 8† |
| Fg PLCs + Fg NEJ + Fh IS | 58 ± 15‡ |
| Fg PLCs + Fg NEJ + Fg IS | 43 ± 17‡ |
| Fh PLCs + Fg NEJ + NS | 92 ± 3† |
| Fh PLCs + Fg NEJ + Fh IS | 49 ± 13‡ |
| Fh PLCs + Fg NEJ + Fg IS | 48 ± 4‡ |
Each of three replicate wells containing 20 to 30 NEJ F. gigantica (Fg) or F. hepatica (Fh) parasites and PLCs (E/T ratio of 2 × 105 cells to 1 NEJ parasite) in 1 ml of medium was incubated for 3 days with either serum from Fasciola-naive ITT sheep (NS) or immune serum (IS) from Fasciola-infected ITT sheep. The viability of the NEJ parasites was then assessed at 72 h as the ability to reduce the tetrazolium salt MTT. Results are the mean ± standard deviation obtained with PLCs from five ITT sheep. For each incubation, mean values followed by the same symbol († or ‡) could not be significantly differentiated by the Dunnett multiple-comparison test at P < 0.05. No significant killing was observed with F. gigantica or F. hepatica NEJ liver flukes incubated with naive-sheep serum or immune serum alone (data not shown). F. gigantica PLCs were obtained from F. gigantica-infected ITT sheep; F. hepatica PLCs were obtained from F. hepatica-infected ITT sheep.
Antioxidant defense enzyme mRNA levels and protein activities in somatic extracts of NEJ and adult Fasciola parasites.
The significant difference in susceptibility to killing by superoxide exhibited by NEJ of F. gigantica and F. hepatica could result from differences in the levels of expression of the superoxide radical defense enzyme SOD in the two parasite species. We also wanted to determine whether any putative differences in defense enzymes were a general trend between the parasites by measuring levels of the antioxidant enzyme GST, a general defense enzyme against most tissue damage arising from free radicals. We compared the specific activities of the SOD and GST defense enzymes in somatic WWEs of NEJ and adult parasites of F. hepatica and F. gigantica; the relative gene expression levels in adult liver fluke mRNA were determined by reverse transcription-PCR. To ensure that meaningful comparisons could be obtained, WWEs of F. hepatica and F. gigantica were prepared from 5,000 NEJ parasites excysted on the same day or adult flukes collected on the same day, and the assays for activity were performed immediately under identical conditions. Because of the limited amount of sample, only specific enzyme activities of GST and SOD were measured in NEJ liver fluke WWEs.
GST and SOD specific enzyme activities were detected in WWEs from two separate batches of NEJ of each liver fluke species (Table 2). The mean GST specific activities in two preparations of NEJ were similar in the two species. The mean SOD specific activity was 33% greater in WWE of NEJ F. hepatica compared to NEJ F. gigantica. SOD and GST specific enzyme activity and gene expression levels were measured in adult F. hepatica and F. gigantica parasites (Table 2; Fig. 6). Adult WWE of F. hepatica had significantly higher specific enzyme activity (P < 0.001) of SOD (fivefold) compared to adult WWE of F. gigantica, whereas GST specific enzyme activities were similar in WWEs of both Fasciola spp. (Table 2). These findings were validated by significantly higher SOD-encoding gene expression levels in F. hepatica relative to F. gigantica adult parasites, while the GST-encoding gene expression levels were equivalent in the two parasite species (Fig. 6).
TABLE 2.
Antioxidant defense enzyme activities in WWEs of adult and NEJ F. hepatica and F. gigantica
| Enzyme | Sp acta
|
|||
|---|---|---|---|---|
| NEJ parasitesb of:
|
Adult parasitesc of:
|
|||
| F. gigantica | F. hepatica | F. gigantica | F. hepatica | |
| SOD | 33, 32 | 44, 46 | 21 ± 5 | 117 ± 44d |
| GST | 512, 491 | 486, 503 | 4,214 ± 1,084 | 6,508 ± 959 |
Five sheep were infected with F. gigantica parasites, and five sheep were infected with F. hepatica parasites. SOD specific enzyme activity is expressed as units per milligram of protein. GST specific enzyme activity is expressed as nanomoles of 1-chloro-2,4-dinitrobenzene conjugated per minute per milligram of protein. Significant differences (P < 0.01) for antioxidant defense enzyme activities between adult F. hepatica and F. gigantica WWEs were calculated with the unpaired alternative t test.
Values represent the mean enzyme activities of three determinations from two separate preparations of 5,000 NEJ parasites.
Values represent the mean ± standard deviation from separate preparations of 20 to 50 adult parasites collected from 10 age-matched ITT donor sheep infected with 250 metacercariae.
Statistically significantly different.
FIG. 6.
Relative expression levels of SOD and GST mRNAs in adult F. hepatica (Fh) and F. gigantica (Fg) parasites isolated from Fasciola-infected ITT sheep. Ten ITT sheep were infected with 250 metacercariae of either F. gigantica or F. hepatica (five animals per group) for 12 weeks, and whole adult parasites were recovered from their livers. Parasites were washed with 1× PBS, incubated for 1 h at 37°C, and preserved in RNAlater (QIAGEN). Parasites from each group were combined, and 50 parasites of each Fasciola species were homogenized in Qiazol (QIAGEN). Relative mRNA expression levels were determined by real-time PCR. Significant differences (P < 0.01) in antioxidant defense enzyme mRNA levels between adult F. hepatica and F. gigantica parasites were calculated with the unpaired alternative t test.
DISCUSSION
This study demonstrates, for the first time, a cytotoxic immune effector mechanism expressed by sheep against a major trematode parasite, F. gigantica, and has revealed fundamental differences between F. gigantica and F. hepatica parasites in their susceptibility to this effector mechanism in vitro. Our results show that PLCs from ITT sheep are able to kill NEJ F. gigantica in vitro by a dose-dependent cell-mediated mechanism that exhibits several features. This cell-mediated killing is antibody dependent since parasite death does not occur in the absence of immune serum, strongly suggesting that killing requires direct attachment of cells to the parasite's surface; this is similar to results obtained with rats, where antibody-dependent cytotoxicity against NEJ F. hepatica was observed (33). The cytotoxic mechanism expressed by ITT sheep appears to be mediated by superoxide radicals since killing is blocked by the addition of SOD and is unaffected by the addition of NG-monomethyl-l-arginine, catalase, or mannitol, known inhibitors of nitric oxide production, hydrogen peroxide, and hydroxyl radicals, respectively. Both monocytes/macrophages and eosinophils appear to be able to mediate this effector mechanism since cell populations enriched (>90%) for these cells are effective at killing NEJ F. gigantica parasites. Most importantly, NEJ parasites of the related species F. hepatica are resistant to this in vitro effector mechanism which is active against NEJ F. gigantica.
The demonstration of an effector mechanism that is active in vitro against juvenile F. gigantica suggests the possibility that superoxide-mediated killing of migrating parasites by peritoneal cells could be occurring in vivo in ITT sheep and that this may be at least one mechanism of resistance expressed by this sheep breed against tropical fasciolosis. Indeed, the experimental data obtained in vivo support this hypothesis. ITT sheep exhibit a rapid induction of eosinophilia and immunoglobulins G and E within 8 to 14 days of infection with F. gigantica (9), and significant killing of the invading parasites in ITT sheep occurs within 2 to 4 weeks of infection and before significant damage to the liver occurs (38, 39, 44). This lack of damage to the liver observed within 2 to 4 weeks of infection suggests that many invading parasites are killed in the peritoneal cavity or shortly after reaching the liver. The fact that peritoneal cells (mainly monocytes/macrophages) from naive ITT sheep can also elicit killing of NEJ F. gigantica in vitro suggests that ITT sheep possess a resident population that is competent to act against the invading juvenile parasite. Our results suggest that such resident cells can be effective, provided there is sufficient specific antibody present to promote the attachment of the effector cells to the surface of the parasite, as observed within 8 to 14 days in infected ITT sheep (9). Interestingly, rats mediate effective immunity to F. hepatica and also have resident cells (monocytes/macrophages) which can kill parasites in vitro in the presence of a parasite-specific antibody, albeit the effector mechanism is nitric oxide and not superoxide radicals (33, 34, 41, 46, 47). Furthermore, intraperitoneal injection of this parasite-specific antibody into uninfected rats on the day of F. hepatica challenge results in parasite killing (10, 13, 34, 36). The requirement for a specific antibody in this killing mechanism in ITT sheep may reflect the need to focus a concentrated attack by free radicals at the tegumental surface of a relatively large target such as F. gigantica in order to achieve a lethal hit, as observed in the rat-F. hepatica model (33). Such a mechanism of rapidly induced killing is analogous to that proposed to act against F. hepatica in exposed rats, where killing occurs in the gut wall or peritoneum within 24 to 48 of challenge and before significant damage to the liver occurs (34, 45, 46, 47, 48).
A curious and important aspect of our observations is that ITT sheep are fully susceptible to infection with F. hepatica (38, 39) and, in parallel, NEJ F. hepatica parasites are resistant to the superoxide-mediated in vitro killing mechanism expressed by peritoneal cells of ITT sheep. Such a correlation suggests that the inability of ITT cells to kill NEJ F. hepatica in vitro and the inability of ITT sheep to resist F. hepatica infection are related phenomena. The resistance of NEJ F. hepatica to superoxide-mediated killing in vitro is clearly a property of the parasite, since our killing assays with both parasite species were conducted on the same day with the same sheep cell source, serum, and medium. This suggests that NEJ F. hepatica and F. gigantica differ in some fundamental biochemical parameter which renders F. hepatica resistant to superoxide radicals. We have previously reported that NEJ F. hepatica parasites are indeed relatively resistant to killing by chemically generated free radicals, compared with schistosomula of S. mansoni parasites, and this correlated with an up-to-10-fold higher specific enzyme activity of SOD and glutathione peroxidase in NEJ F. hepatica relative to schistosomula (25, 26, 27, 32). We therefore examined the possibility that NEJ F. hepatica and F. gigantica differ in their specific activities of the superoxide defense enzyme SOD or have higher general antioxidant enzyme defenses such as GST. With somatic extracts of NEJ from these two species, we found that the mean SOD specific activity in two NEJ preparations was 33% higher in F. hepatica relative to F. gigantica, whereas the GST specific activities were comparable. We also assayed specific defense enzyme activities in adult parasites exposed to the ITT sheep immune response. Adult parasite SOD gene expression and specific enzyme activity levels were significantly higher in F. hepatica. Thus, although our initial results are limited, the data reveal a trend toward higher SOD defense enzyme levels in NEJ and adult F. hepatica parasites relative to F. gigantica and suggest that F. hepatica has the potential to mount a more effective defense against a superoxide free-radical attack by host immune cells; whether this trend is sufficient to account for the difference in susceptibility to killing by the superoxide-mediated mechanisms expressed by PLCs from ITT sheep is unclear.
Another possibility is that the relative resistance of NEJ F. hepatica to superoxide-mediated killing in vitro results from the active suppression by the parasite of superoxide production by peritoneal cells in vivo or expression of a nonenzymatic mechanism for absorbing superoxide (e.g., via a molecule expressed in the tegument). Jefferies and colleagues (16) showed in vitro that increasing concentrations of excretory-secretory product (ESP) molecules released by adult F. hepatica correlated with increasing suppression of superoxide and hydrogen peroxide production from sheep neutrophils. Adult F. gigantica ESP was also shown to inhibit reactive oxygen radical production from sheep neutrophils in vitro (5). SOD activity and protein have been detected in adult F. hepatica ESP (17, 30), and a cDNA encoding F. hepatica SOD has been reported (20). Interestingly, in our study, PLCs isolated from F. hepatica-infected ITT sheep were still able to mediate killing of NEJ F. gigantica in vitro, suggesting that if suppression of superoxide production occurs in vivo during F. hepatica infection in sheep, it is transient or ineffective under our experimental conditions. We are currently examining the effect of NEJ flukes of the two Fasciola spp. on superoxide production by ITT PLCs.
Our results raise broader issues relating to the nature of the host and parasite factors that determine the host specificity of a parasite. If biochemical differences can occur between parasite species such that resistance to a host effector mechanism is expressed, then clearly the host specificity of a particular parasite is a dynamic interplay between the evolution, and/or level of expression, of a parasite's defenses and the evolution of a host's effector armory. NEJ F. hepatica parasites are susceptible to antibody-dependent NO-mediated killing by rat monocytes/macrophages, and rat monocytes/macrophages make a robust inducible NO response which is associated with resistance to F. hepatica (32, 33, 41). Rats express an even higher resistance to F. gigantica infection (15, 22, 44), and we have shown that NEJ F. gigantica parasites are highly susceptible to NO killing in the absence of antiparasite antibodies (unpublished data). In complete contrast, monocytes/macrophages from sheep, including ITT sheep, do not generate significant levels of inducible NO in vitro (2, 18, 19, 33); accordingly, sheep are fully susceptible to F. hepatica. Thus, rats and sheep represent two ends of the spectrum with respect to both inducible NO production and susceptibility to F. hepatica. From the parasite's perspective, F. hepatica appears to express higher levels of certain defense enzymes relative to F. gigantica. Interestingly, Miller et al. (24) demonstrated variations in isoenzyme expression and activity of GSTs in adult F. hepatica parasites recovered from different hosts. Lower GST activity levels were observed in flukes removed from resistant hosts (cattle and rats) as opposed to susceptible hosts (sheep and mice), confirming that defense enzyme levels can vary, depending on the host in which the parasite resides. Such observations show that the outcome of infection by Fasciola sp. is determined by both host and parasite factors. It should be noted that F. hepatica and F. gigantica diverged about 19 million years ago, which is sufficient time for variation in the level of expression of defense enzymes to evolve in these two parasite species (14).
In conclusion, our results suggest that a mechanism of antibody-dependent cell-mediated cytotoxicity involving superoxide-mediated killing may play a role in the control of F. gigantica infection in ITT sheep. This killing appears to be mediated, at least in vitro, by monocytes/macrophages and eosinophils, and such cells are known to be present, or rapidly induced following infection, in the peritoneal cavities of ITT sheep. Studies are in progress to further define the effector mechanisms involved in determining the resistance of sheep to F. gigantica, as well as the parasite factors involved in subverting this resistance.
Acknowledgments
This work was supported by Monash University, the University of Sydney, the Australian Centre for International Agricultural Research (Canberra), the Cooperative Research Centre for Vaccine Technology, and the Research Institute for Veterinary Science (Bogor, Indonesia). T. Spithill holds a Canada Research Chair in Immunoparasitology.
We thank Kemperly Dynon for assistance in the preparation of the manuscript.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.A'Gadir, H., E. M. Haroun, and A. A. Gameel. 1987. The protective effect of irradiated metacercariae of Fasciola gigantica against homologous challenge in sheep. J. Helminthol. 61:137-142. [DOI] [PubMed] [Google Scholar]
- 2.Bogdan, C. 1997. Of microbes, macrophages and nitric oxide. Behring Inst. Mitt. 99:58-72. [PubMed] [Google Scholar]
- 3.Brophy, P. M., and D. I. Pritchard. 1992. Immunity to helminths: ready to tip the biochemical balance? Parasitol. Today 8:419-422. [DOI] [PubMed] [Google Scholar]
- 4.Duffus, W. P. H., and D. Franks. 1980. In vitro effect of immune serum and bovine granulocytes on juvenile Fasciola hepatica. Clin. Exp. Immunol. 41:430-440. [PMC free article] [PubMed] [Google Scholar]
- 5.El-Ghaysh, A., R. J. Turner, P. M. Brophy, and J. Barrett. 1999. Effect of Fasciola gigantica somatic extracts and excretory/secretory products on superoxide production by activated neutrophils. Vet. Parasitol. 84:91-100. [DOI] [PubMed] [Google Scholar]
- 6.Estuningsih, S. E., P. M. Smooker, E. Wiedosari, S. Widjajanti, S. Vaiano, S. Partoutomo, and T. W. Spithill. 1997. Evaluation of antigens of Fasciola gigantica as vaccines against tropical fasciolosis in cattle. Int. J. Parasitol. 27:1419-1428. [DOI] [PubMed] [Google Scholar]
- 7.Fairweather, I. 2005. Triclabendazole: new skills to unravel an old(ish) enigma. J. Helminthol. 79:227-234. [DOI] [PubMed] [Google Scholar]
- 8.Gupta, S. C., and R. Chandra. 1987. Susceptibility of some laboratory animals to infection with Fasciola gigantica. J. Vet. Parasitol. 1:19-21. [Google Scholar]
- 9.Hansen, D. S., D. G. Clery, S. E. Estuningsih, S. Widjajanti, S. Partoutomo, and T. W. Spithill. 1999. Immune responses in Indonesian thin tail and merino sheep during a primary infection with Fasciola gigantica: lack of a specific IgG2 antibody response is associated with increased resistance to infection in Indonesian sheep. Int. J. Parasitol. 29:1027-1035. [DOI] [PubMed] [Google Scholar]
- 10.Hayes, T. J., J. Bailer, and M. Mitrovic. 1974. Serum transfer of immunity to Fasciola hepatica in rats. J. Parasitol. 60:722-723. [PubMed] [Google Scholar]
- 11.Hillyer, G. V., and W. Apt. 1997. Food borne trematode infections in the Americas. Parasitol. Today 13:87-88. [Google Scholar]
- 12.Hillyer, G. V. 2005. Fasciola antigens as vaccines against fascioliasis and schistosomiasis. J. Helminthol. 79:241-247. [DOI] [PubMed] [Google Scholar]
- 13.Hughes, D. L. 1987. Fasciola and fascioloides, p. 91-114. In E. J. L. Soulsby (ed.), Immune responses in parasitic infections: immunology, immunopathology, and immunoprophylaxis. Volume II. Trematodes and cestodes. CRC Press, Inc., Boca Raton, FL. [Google Scholar]
- 14.Irving, J. A., T. W. Spithill, R. N. Pike, J. C. Whisstock, and P. M. Smooker. 2003. The evolution of enzyme specificity in Fasciola spp. J. Mol. Evol. 57:1-15. [DOI] [PubMed] [Google Scholar]
- 15.Itagaki, T., T. Sakamoto, Y. Tsutsumi, and H. Itagaki. 1994. Infectivity of three species of Fasciola to Wistar rats. J. Vet. Med. Sci. 56:977-979. [DOI] [PubMed] [Google Scholar]
- 16.Jefferies, J. R., R. J. Turner, and J. Barrett. 1997. Effect of Fasciola hepatica excretory-secretory products on the metabolic burst of sheep and human neutrophils. Int. J. Parasitol. 27:1025-1029. [DOI] [PubMed] [Google Scholar]
- 17.Jefferies, J. R., A. M. Campbell, A. J. van Rossum, J. Barrett, and P. M. Brophy. 2001. Proteomic analysis of Fasciola hepatica excretory-secretory products. Proteomics 1:1128-1132. [DOI] [PubMed] [Google Scholar]
- 18.Jungi, T. W., H. Pfister, H. Sager, R. Fatzer, M. Vandevelde, and A. Zurbriggen. 1997. Comparison of inducible nitric oxide synthase expression in the brains of Listeria monocytogenes-infected cattle, sheep, and goats and in macrophages stimulated in vitro. Infect. Immun. 65:5279-5288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jungi, T. W., M. Brcic, H. Sager, D. A. Dobbelaere, A. Furger, and I. Roditi. 1997. Antagonistic effects of IL-4 and interferon-gamma (IFN-γ) on inducible nitric oxide synthase expression in bovine macrophages exposed to gram-positive bacteria. Clin. Exp. Immunol. 109:431-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, T. S., Y. Jung, B. K. Na, K. S. Kim, and P. R. Chung. 2000. Molecular cloning and expression of CuZn-containing superoxide dismutase from Fasciola hepatica. Infect. Immun. 68:3941-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 22.Mango, A. M., C. K. Mango, and D. Esamal. 1972. A preliminary note on the susceptibility, prepatency and recovery of Fasciola gigantica in small laboratory animals. J. Helminthol. 46:381-386. [DOI] [PubMed] [Google Scholar]
- 23.Mas-Coma, S., M. D. Bargues, and M. A. Valero. 2005. Fascioliasis and other plant-borne trematode zoonoses. Int. J. Parasitol. 35:1255-1278. [DOI] [PubMed] [Google Scholar]
- 24.Miller, C. M. D., M. J. Howell, and J. C. Boray. 1993. Host effects on glutathione S-transferase activity in Fasciola hepatica. Int. J. Parasitol. 23:1073-1076. [DOI] [PubMed] [Google Scholar]
- 25.Mkoji, G. M., J. M. Smith, and R. K. Prichard. 1988. Antioxidant systems in Schistosoma mansoni: correlation between susceptibility to oxidant killing and the levels of scavengers of hydrogen peroxide and oxygen free radicals. Int. J. Parasitol. 18:661-666. [DOI] [PubMed] [Google Scholar]
- 26.Mkoji, G. M., J. M. Smith, and R. K. Prichard. 1988. Antioxidant systems in Schistosoma mansoni: evidence for their role in protection of the adult worms against oxidant killing. Int. J. Parasitol. 18:667-673. [DOI] [PubMed] [Google Scholar]
- 27.Nare, B., J. M. Smith, and R. K. Prichard. 1990. Schistosoma mansoni: levels of antioxidants and resistance to oxidants increase during development. Exp. Parasitol. 70:389-397. [DOI] [PubMed] [Google Scholar]
- 28.Overend, D. J., and F. L. Bowen. 1995. Resistance of Fasciola hepatica to triclabendazole. Aust. Vet. J. 72:275-276. [DOI] [PubMed] [Google Scholar]
- 29.Pfaffl, M. W., G. W. Horgan, and L. Dempfle. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piacenza, L., R. Radi, F. Goni, and C. Carmona. 1998. CuZn superoxide dismutase activities from Fasciola hepatica. Parasitology 117:555-562. [DOI] [PubMed] [Google Scholar]
- 31.Piedrafita, D., and F. Y. Liew. 1998. Nitric oxide: a protective or pathogenic molecule? Rev. Med. Microbiol. 9:179-189. [Google Scholar]
- 32.Piedrafita, D., T. W. Spithill, J. P. Dalton, P. J. Brindley, M. R. Sandeman, P. R. Wood, and J. C. Parsons. 2000. Juvenile Fasciola hepatica are resistant to killing in vitro by free radicals compared with larvae of Schistosoma mansoni. Parasite Immunol. 22:287-295. [DOI] [PubMed] [Google Scholar]
- 33.Piedrafita, D., J. C. Parsons, R. M. Sandeman, P. R. Wood, S. E. Estuningsih, S. Partoutomo, and T. W. Spithill. 2001. Antibody-dependent cell-mediated cytotoxicity to newly excysted juvenile Fasciola hepatica in vitro is mediated by reactive nitrogen intermediates. Parasite Immunol. 23:473-482. [DOI] [PubMed] [Google Scholar]
- 34.Piedrafita, D., H. Raadsma, R. Prowse, and T. W. Spithill. 2004. Immunology of the host parasite relationship in fasciolosis (Fasciola hepatica and Fasciola gigantica). Can. J. Zool. 82:233-250. [Google Scholar]
- 35.Rainbird, M. A., D. Macmillan, and E. N. Meeusen. 1998. Eosinophil-mediated killing of Haemonchus contortus larvae: effect of eosinophil activation and role of antibody, complement and interleukin-5. Parasite Immunol. 20:93-103. [DOI] [PubMed] [Google Scholar]
- 36.Rajasekariah, G. R., and M. J. Howell. 1979. Fasciola hepatica in rats: transfer of immunity by serum and cells from infected to F. hepatica naive animals. J. Parasitol. 65:481-487. [PubMed] [Google Scholar]
- 37.Roberts, J. A., S. Widjajanti, and E. Estuningsih. 1996. Acquired resistance of merino sheep against Fasciola gigantica. Parasitol. Res. 82:743-746. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, J. A., E. Estuningsih, S. Widjayanti, E. Wiedosari, S. Partoutomo, and T. W. Spithill. 1997. Resistance of Indonesian thin tail sheep against Fasciola gigantica and F. hepatica. Vet. Parasitol. 68:69-78. [DOI] [PubMed] [Google Scholar]
- 39.Roberts, J. A., E. Estuningsih, E. Wiedosari, and T. W. Spithill. 1997. Acquisition of resistance against Fasciola gigantica by Indonesian thin tail sheep. Vet. Parasitol. 73:215-224. [DOI] [PubMed] [Google Scholar]
- 40.Salvatore, L., G. Wijffels, J. L. Sexton, M. Panaccio, S. Mailer, I. McCauley, and T. W. Spithill. 1995. Biochemical analysis of recombinant glutathione S-transferase of Fasciola hepatica. Mol. Biochem. Parasitol. 69:281-288. [DOI] [PubMed] [Google Scholar]
- 41.Sibille, P., O. Tliba, and C. Boulard. 2004. Early and transient cytotoxic response of peritoneal cells from Fasciola hepatica-infected rats. Vet. Res. 35:573-584. [DOI] [PubMed] [Google Scholar]
- 42.Spithill, T. W., D. Piedrafita, and P. M. Smooker. 1997. Immunological approaches for the control of fasciolosis. Int. J. Parasitol. 27:1221-1235. [DOI] [PubMed] [Google Scholar]
- 43.Spithill, T. W., and J. P. Dalton. 1998. Progress in development of liver fluke vaccines. Parasitol. Today 14:224-228. [DOI] [PubMed] [Google Scholar]
- 44.Spithill, T. W., P. M. Smooker, and D. B. Copeman. 1999. Fasciola gigantica: epidemiology, control, immunology and molecular biology, p. 377-410. In J. P. Dalton (ed.), Fasciolosis. CABI Publishing, Wallingford, United Kingdom.
- 45.Tliba, O., P. Sibille, C. Boulard, and A. Chauvin. 2000. Local hepatic immune response in rats during primary infection with Fasciola hepatica. Parasite 7:9-18. [DOI] [PubMed] [Google Scholar]
- 46.Van Milligen, F. J., J. B. Cornelissen, and B. A. Bokhout. 1998. Location of induction and expression of protective immunity against Fasciola hepatica at the gut level: a study using an ex vivo infection model with ligated gut segments. J. Parasitol. 84:771-777. [PubMed] [Google Scholar]
- 47.Van Milligen, F. J., J. B. Cornelissen, I. M. Hendriks, C. P. Gaasenbeek, and B. A. Bokhout. 1998. Protection of Fasciola hepatica in the gut mucosa of immune rats is associated with infiltrates of eosinophils, IgG1 and IgG2a antibodies around the parasites. Parasite Immunol. 20:285-292. [DOI] [PubMed] [Google Scholar]
- 48.Van Milligen, F. J., J. B. Cornelissen, and B. A. Bokhout. 1999. Protection against Fasciola hepatica in the intestine is highly correlated with eosinophil and immunoglobulin G1 responses against newly excysted juveniles. Parasite Immunol. 21:243-251. [DOI] [PubMed] [Google Scholar]
- 49.Wiedosari, E., and D. B. Copeman. 1990. High resistance to experimental infection with Fasciola gigantica in Javanese thin-tailed sheep. Vet. Parasitol. 37:101-111. [DOI] [PubMed] [Google Scholar]
- 50.Wilson, L. R., R. T. Good, M. Panaccio, G. L. Wijffels, R. M. Sandeman, and T. W. Spithill. 1998. Fasciola hepatica: characterization and cloning of the major cathepsin B protease secreted by newly excysted juvenile liver fluke. Exp. Parasitol. 88:85-94. [DOI] [PubMed] [Google Scholar]