Abstract
The early influx of neutrophils to the site of infection may be an important step in host resistance against Mycobacterium tuberculosis. In this study, we investigated the effect of M. tuberculosis infection on the ability of guinea pig neutrophils to produce interleukin-8 (IL-8; CXCL8) and tumor necrosis factor alpha (TNF-α) and to activate alveolar macrophages. Neutrophils and alveolar macrophages were isolated from naïve guinea pigs, cultured together or alone, and infected with virulent M. tuberculosis for 3, 12, and 24 h. IL-8 protein production in cocultures, as measured by using an enzyme-linked immunosorbent assay, was found to be additive at 24 h and significantly greater in M. tuberculosis-infected cocultures than in uninfected cocultures and in cultures of the infected neutrophils or macrophages alone. The IL-8 mRNA levels, determined by real-time reverse transcription-PCR, were elevated at 24 h in infected cocultures and infected cells cultured alone. In order to elucidate the contributions of neutrophils and their soluble mediators to the activation of alveolar macrophages, neutrophils and alveolar macrophages were cultured in a contact-independent manner by using a Transwell insert system. Neutrophils were infected with virulent M. tuberculosis in the upper wells, and alveolar macrophages were cultured in the lower wells. The release of hydrogen peroxide from alveolar macrophages exposed to soluble products from infected neutrophils was significantly increased compared to that from unexposed alveolar macrophages. Significant up-regulation of IL-1β and TNF-α mRNA levels in alveolar macrophages was observed at 24 and 30 h, respectively, compared to those in cells not exposed to soluble neutrophil products. Treatment with anti-guinea pig TNF-α polyclonal antibody completely abolished the response of alveolar macrophages to neutrophil products. This finding suggests that TNF-α produced by infected neutrophils may be involved in the activation of alveolar macrophages and hence may contribute to the containment of M. tuberculosis infection during the early period of infection.
Tuberculosis (TB) is a global health threat, killing 2 to 3 million people annually and developing in one-third of the world's population (13). Infection with Mycobacterium tuberculosis in humans results in outcomes which range from the development of protective immunity and containment of the infection to clinical tuberculosis (31). The control of M. tuberculosis infection requires the coordinated interaction of macrophages, dendritic cells, and T cells. Polymorphonuclear neutrophils are also known to be recruited to the site of mycobacterial infection (4). The accumulation of these inflammatory cells, along with their interactions, activation, and specific cell-trafficking patterns at the site of disease, is mediated by cytokines and chemokines, resulting in a successful immune response (32). The study of the role of these cytokines and chemokines in the early immune response against mycobacterial infection in guinea pigs may contribute to the elucidation of mechanisms used by the host immune system to control M. tuberculosis infection.
Alveolar macrophages (AM) are the first cells to encounter M. tuberculosis during an infection (40, 45, 47). Upon stimulation with M. tuberculosis, these macrophages produce cytokines and chemokines which cause an influx of inflammatory neutrophils followed by monocytes and T cells (37). Neutrophil infiltration is the earliest response to invasion by mycobacteria (2, 28). Depleting mice of neutrophils resulted in increased susceptibility to bacterial growth in an intravenous model of M. avium infection (3). The presence of neutrophils in inflammatory infiltrates has been correlated with elevated levels of interleukin-12 (IL-12), tumor necrosis factor alpha (TNF-α), IL-1β, gamma interferon (IFN-γ) (6, 32), and chemokines (28). Human neutrophils can also actively phagocytose bacilli in lesions in the presence of TNF-α (22).
The recruitment of neutrophils to bronchoalveolar spaces during active human tuberculosis has also been described and is known to be associated with local chemokine expression (35). This finding suggests an immunomodulatory role for neutrophils which corresponds with the production of chemokines such as IL-8 (CXCL8) that will attract monocytes and T cells. This early influx of neutrophils to the site of infection may be a vital initial step in the mechanism of host defense against pulmonary infection with M. tuberculosis (25). The exact role of neutrophils in mycobacterial infections is not known (30, 32), but it has been suggested that neutrophils play an indirect, nonphagocytic role (30).
IL-8 has been found in the bronchoalveolar lavage fluids of patients with tuberculosis (47). Pretreatment of guinea pig neutrophils with recombinant guinea pig IL-8 induces significant levels of IL-8 and TNF-α mRNA and protein after stimulation with M. tuberculosis H37Ra (25). TNF-α also plays an important role in granuloma formation and the control of mycobacterial infection (15, 21). Anti-TNF antibody treatment increases mycobacterial replication and reactivates disease in mice (21, 36). Recombinant TNF-α injections have resulted in significantly decreased numbers of viable bacteria in the lungs and spleens of mice (12). TNF-α enhances the respiratory burst capacity of mouse resident peritoneal macrophages (39). Previous studies in our lab have shown that M. bovis BCG vaccination increases bioactive TNF-α production in guinea pig leukocyte populations (24). IL-12p40 and TNF-α mRNA levels are up-regulated in guinea pig resident peritoneal macrophages and AM treated with recombinant guinea pig TNF-α (10). The treatment of AM with anti-TNF-α antibody results in the increased intracellular growth of virulent mycobacteria (10). Splenomegaly has been observed in BCG-vaccinated M. tuberculosis-infected pigs treated with polyclonal anti-recombinant guinea pig TNF-α antiserum (23). The treatment of splenocyte cultures with recombinant guinea pig TNF-α enhances lymphoproliferation and IFN-γ mRNA production in cells from BCG-vaccinated guinea pigs (8). Conversely, the neutralization of endogenous guinea pig TNF-α with anti-recombinant guinea pig TNF-α antiserum suppresses purified protein derivative-induced splenocyte proliferation and IFN-γ mRNA levels (9). These results indicate the importance of adequate local concentrations of TNF-α for maintaining an effective immune response to mycobacteria in guinea pigs (23).
During an M. tuberculosis infection, AM are stimulated by M. tuberculosis first to produce IL-8, which may then attract and stimulate neutrophils to produce more IL-8 and TNF-α. We hypothesize that TNF-α produced by infected neutrophils may be modulating the activation of alveolar macrophages, thus helping the AM to combat the M. tuberculosis infection in the early stage.
In this study, we have demonstrated that guinea pig neutrophils infected with virulent mycobacteria can activate alveolar macrophages. Using a Transwell insert culture system in which the neutrophils and AM were cocultured in a contact-independent manner, we have shown that M. tuberculosis-infected neutrophils produce soluble mediators which can activate alveolar macrophages. TNF-α appears to be one of the important effector molecules produced by infected neutrophils.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free outbred Hartley strain guinea pigs (Charles River Breeding Laboratory Inc., Wilmington, MA) were individually housed in an air-filtered environment within polycarbonate cages with stainless steel grid floors. They were provided with commercial food (Ralston Purina, St. Louis, MO) in stainless steel feeders and tap water ad libitum. All the procedures were reviewed and approved by the Texas A&M University Laboratory Animal Care Committee.
Bacteria.
The M. tuberculosis H37Rv strain (ATCC 27294; American Type Culture Collection, Manassas, VA) was cultured in Middlebrook 7H9 medium, and stocks were prepared and stored at −80°C according to an established procedure (18). Before use, mycobacterial preparations were rapidly thawed, vortexed, and sonicated with an Ultrasonics sonicator (Heat Systems-Ultrasonics, Inc., Plainview, NY) for 45 to 60 s at an output setting of 8.0 to disrupt bacterial clumps.
Isolation of alveolar macrophages.
The isolation of alveolar macrophages was based on established protocols (24, 26). Guinea pigs were euthanized by intramuscular injection with sodium pentobarbital (100 mg/kg of body weight; Sleepaway [Fort Dodge Laboratories, Inc., Fort Dodge, IA]). The thoracic cavity of each animal was opened aseptically, and the trachea was separated from the surrounding tissue. Bronchoalveolar lavage was then performed according to our published protocol (26) by instilling 10 to 15 ml of ice-cold 12 mM lidocaine (Sigma, St. Louis, MO) in phosphate-buffered saline (PBS) with 3% fetal bovine serum (FBS; Atlanta Biologicals, GA) into the exposed trachea through an 18-gauge cannula fixed to a 20-ml syringe. The solution was left in the lungs for 1 min for the adherent cells to loosen up, and then the fluid was drawn back into the syringe and collected in sterile 50-ml centrifuge tubes (Beckton Dickinson Labware, Franklin Lakes, NJ). Three lavages were performed on each animal. The lavage cells were then washed once in PBS, and the cell viability was determined by a trypan blue exclusion assay (Gibco Life Technologies, NY). The cells were diluted in RPMI 1640 medium (without phenol red; Gibco Life Technologies, NY) supplemented with 2% heat-inactivated FBS and 2-mercaptoethanol (2-ME), and the cell concentration was adjusted to 2 × 106/ml (24).
Isolation of neutrophils.
The guinea pigs were injected with 20 ml of 9% casein intraperitoneally 16 to 18 h before being given an intramuscular injection of sodium pentobarbital (100 mg/kg; Sleepaway [Fort Dodge Laboratories, Inc., Fort Dodge, IA]). The peritoneal cavity was then opened aseptically and washed three times with 10 to 15 ml of ice-cold harvesting solution (RPMI 1640 medium-l-glutamine without phenol red or antibiotics but with 2% heat-inactivated FBS and 10 U/ml heparin). The wash fluids were collected in two 50-ml Falcon tubes (Beckton Dickinson Labware, Franklin Lakes, NJ). The peritoneal exudate cells were pelleted by centrifugation for 10 min at 300 × g at 37°C and washed three times with media containing 2% heat-inactivated FBS and 2-ME. The pellet was finally resuspended in 15 ml of RPMI 1640 medium (without phenol red) containing 2% FBS and 2-ME. The cells were then purified by using Opti-prep density gradients (Axis-Shield, Norway) according to the manufacturer's instructions. The percentage of neutrophils was determined by using 3% acetic acid and counting cells on a hemocytometer. A differential cell count was also done by using Diff-Quick staining (Dade Behring Inc., Newark, DE). The cell viability was assessed by using the trypan blue exclusion assay (Gibco Life Technologies, NY). The cell suspension was found to contain >95% neutrophils, and the final cell concentration was adjusted to 2 × 106/ml (25).
Infection of neutrophils or alveolar macrophages.
Bronchoalveolar lavage cells (5 × 105/well) were allowed to adhere to 48-well, flat-bottom tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) for 2 h. The nonadherent cells were poured off, and the adherent cells were washed once with warm PBS. The adherent AM were then infected with live, virulent M. tuberculosis H37Rv (multiplicity of infection [MOI] of 0.1) or cultured in medium alone. At 3, 12, and 24 h after infection, supernatants were obtained from each of the culture wells, centrifuged at 12,000 × g, and stored at −80°C until analyzed for IL-8 by using an enzyme-linked immunosorbent assay (ELISA) (26). The cells were lysed with buffer RLT (QIAGEN), frozen at −80°C, and then processed to obtain total RNA.
Neutrophils were also cultured in 48-well plates (5 × 105/well) and infected with live, virulent M. tuberculosis H37Rv (MOI of 0.1) or cultured in medium alone (25). The supernatants and total RNA were harvested at the same time intervals as those from the AM, and the cell viability following in vitro infection was estimated using the lactate dehydrogenase assay (Promega).
Cocultures of AM and neutrophils with cell-cell contact.
AM were first allowed to adhere to the 48-well, flat-bottom tissue culture plates (Becton Dickinson Labware) for 2 h. The nonadherent cells were poured off, and the adherent cells were washed once with warm PBS. The isolated neutrophils were then added to the AM at a ratio of 1:1, and the cocultures were infected with M. tuberculosis at an MOI of 0.1. Supernatants and cells for RNA isolation were harvested at 3, 12, and 24 h and frozen at −80°C.
Contact-independent cocultures of AM and neutrophils.
The neutrophils were cultured in the upper wells (insert) of a 24-well Transwell system (0.1-μm pore size; Corning Costar), with the lower wells holding RPMI 1640 medium (without phenol red) containing 2% FBS and 2-ME (Gibco Life Technologies). The neutrophils were infected with M. tuberculosis (H37Rv) at an MOI of 5, and the supernatants from the lower wells were harvested at 12 and 24 h. The supernatants were centrifuged at 8,000 × g for 5 min by using a microcentrifuge and stored at −80°C until analyzed for IL-8 and TNF-α protein by ELISA and an L929 bioassay, respectively. The uninfected neutrophils were used as controls. Some of the supernatants from the lower wells were also plated onto 7H9 agar plates and incubated at 37°C in a CO2 incubator. No mycobacterial colonies were found on these plates, indicating that the transfer of bacteria from the insert to the lower chamber did not take place. The viability of the neutrophils infected at an MOI of 5 was measured using the lactate dehydrogenase assay (Promega), and >80% of the cells were found to be viable. The viability of neutrophils infected at an MOI of 10 was found to be >70%, and hence this MOI was not used in any further experiments (data not shown).
In a separate experiment, the neutrophils (2 × 105/well) were cultured in the upper wells (insert) while the AM (5 × 105/well) were cultured in the lower wells. The neutrophils were then infected with M. tuberculosis (H37Rv) at an MOI of 5, with the uninfected cells acting as controls. The insert containing neutrophils was then removed at 24 h, and the AM were harvested at 24 and 30 h. The total RNA was isolated and analyzed by real-time PCR for TNF-α and IL-1β mRNA as previously described (1, 41, 46).
To study the effect of TNF-α neutralization on the activation of AM by the soluble products of infected neutrophils, the AM were treated with rabbit anti-recombinant guinea pig TNF-α polyclonal antibody (1:600), obtained as previously described (10, 23). The anti-recombinant guinea pig TNF-α antibody was added to AM at the time of neutrophil infection. AM were treated with normal rabbit serum as a negative control.
Total RNA isolation and real-time PCR.
The collection of total RNA from the cells was done by using the RNeasy kit (QIAGEN, Valencia, CA) with the addition of RNase-free DNase according to the manufacturer's instructions (25, 26). Total RNA (approximately 1 to 5 μg) was then reverse transcribed by using MultiScribe reverse transcriptase, random hexamers, and reverse transcriptase reagents (Applied Biosystems, Branchburg, NJ). The real-time primers for guinea pig IL-8, TNF-α, IL-1β, and hypoxanthine ribose transferase (HPRT) mRNA were designed by Primer Express software (Applied Biosystems) and have been described previously (1, 26, 46). Reverse-transcribed cDNA was then amplified using SYBR green PCR core reagents and an Applied Biosystems Prism 7700 sequence detector according to the manufacturer's instructions. The levels of induction as determined from the cycle threshold (CT) values were normalized for HPRT expression and also to values for cells cultured in medium alone at each time point (1, 26).
ELISA for IL-8 protein.
The guinea pig IL-8 protein in the experimental supernatants was measured by using the DuoSet ELISA development system for human IL-8 (R&D Systems, Minneapolis, MN), which has been shown to detect guinea pig IL-8 (26). The supernatants from the stimulated AM and neutrophil cultures were added to the plates according to the manufacturer's instructions. The plates were then read on a Dynatech MR5000 automated plate reader and analyzed using Biolinx Software, version 2.10 (Dynatech Laboratories, Inc., Chantilly, VA) (26).
L929 bioassay for TNF protein.
Guinea pig TNF-α protein concentrations in the supernatants were measured by using the L929 bioassay as described previously (10, 23, 25). Supernatants from infected and uninfected cell cultures along with a human TNF-α standard were added to the consecutive wells in a round-bottomed 96-well plate (Sarstedt) and then serially diluted 1:2. The serially diluted samples were then transferred to the L929 cell-containing 96-well plates in duplicate. Actinomycin D was then added to each well, and the plates were incubated at 37°C for approximately 20 h. A tetrazolium compound 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfonyl)-2H-tetrazolium monosodium salt (WST-1; Dojindo, Kumamoto, Japan) and 1-methoxymethyl phenazium methyl sulfate (Dojindo) at 6 and 0.4 mM, respectively, were mixed at a ratio of 1:1; 20 μl of this solution was added to each well; and the cells were incubated for 2 h at 37°C. The color development was stopped by the addition of 20 μl of 1N H2SO4, and the optical densities of the test and control samples at 450 and 630 nM were measured (10, 24). A standard curve was generated using human TNF-α (R&D Systems Inc., MN). All of the experimental data were expressed at the 50% cytotoxicity value based on the standard curve.
H2O2 production by AM.
AM were cultured separately in a 96-well tissue culture plate. The supernatants from both infected and uninfected neutrophils were harvested at 24 h and then added to the AM in separate wells after the removal of the existing medium. Some of the wells containing AM were then infected with M. tuberculosis (H37Rv) at an MOI of 0.1. Some of the wells also contained a stimulant (phorbol myristate acetate [PMA] at 100 ng/ml) of H2O2 production or a scavenger (100 to 200 μM catalase [Sigma, St. Louis, Mo]) of H2O2. To study the effect of TNF-α neutralization on the activation of AM by the soluble products of infected neutrophils, some of the wells containing AM with supernatants from infected neutrophils were also treated with rabbit anti-recombinant guinea pig TNF-α polyclonal antibody (1:600), obtained as previously described (10, 23). Twenty-four hours later, all the medium from the wells was removed and the H2O2 production by the alveolar macrophages was measured by using the horseradish peroxide-dependent oxidation of phenol red by H2O2 as reported previously by us and others (19, 33). Briefly, 100 μl of the assay solution (28 mM phenol red [Sigma], 100 U/ml horseradish peroxidase type II [Sigma, St. Louis, Mo]) in Hanks balanced salt solution was added to the test wells. The plates were then incubated for 1 h at 37°C in the presence of 5% CO2, and the reaction was stopped by the addition of 10 μl of 1 N NaOH. The standards were prepared by making twofold serial dilutions of 100 μM H2O2 (Sigma) in the assay solution and adding 10 μl of 1N NaOH. The mixtures on the plates were allowed to equilibrate for 3 min, the absorbance was measured in a plate reader at 630 nm, and the amount of H2O2 was expressed as nanomoles per well.
Statistics.
Analysis of variance (ANOVA) was used to determine the statistical significance of differences between the infected and uninfected cells by using Duncan's post hoc analysis. The statistical tests were performed using SAS software (release 8.01; SAS Institute Inc, NC).
RESULTS
Response of AM and neutrophils, alone and together in contact, to infection with M. tuberculosis.
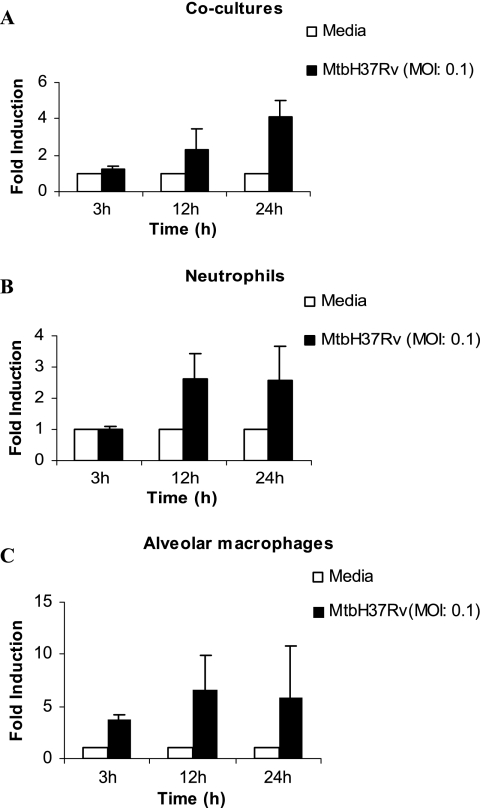
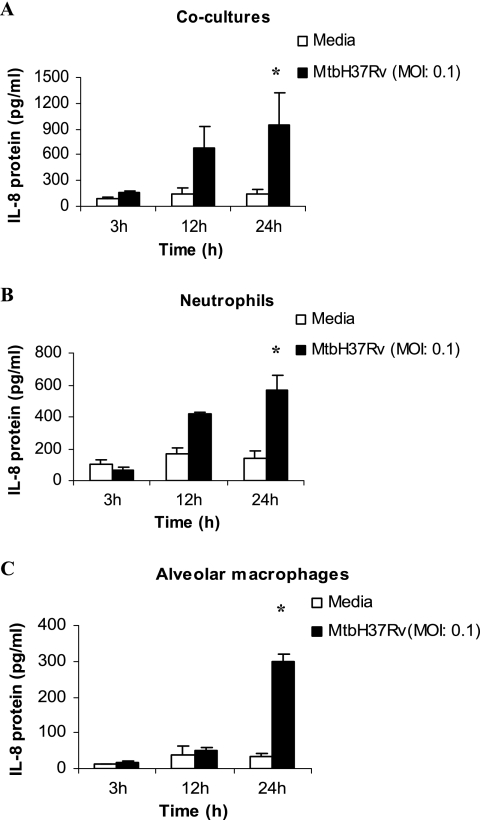
Guinea pig neutrophils and alveolar macrophages were cultured alone or together in cocultures at a ratio of 1:1 and infected with M. tuberculosis (MOI of 0.1). The expression of IL-8 mRNA was examined by real-time reverse transcription-PCR at 3, 12, and 24 h after infection. IL-8 mRNA expression was found to be elevated but not significant at 24 h in neutrophils as well as in cocultures (Fig. 1A and B) compared to that in the uninfected cells. The alveolar macrophages produced more IL-8 mRNA than the cocultures or the neutrophils alone (Fig. 1C). The supernatants were also collected at the same time points, and IL-8 protein was measured by ELISA (Fig. 2). The production of IL-8 protein increased considerably at 12 h and was found to be significantly elevated at 24 h in cocultures, neutrophils, and alveolar macrophages infected with M. tuberculosis compared to that in the infected cells at 3 h and the uninfected cells at other time points (P < 0.05) (Fig. 2). The production of IL-8 protein by the cocultures was found to be additive compared to the production of IL-8 protein by either neutrophils or alveolar macrophages alone.
FIG. 1.
Expression of IL-8 mRNA in cultures of neutrophils and alveolar macrophages, alone or together, following infection with M. tuberculosis H37Rv. The levels of expression of IL-8 mRNA in cocultures of alveolar macrophages (5 × 105/well) and neutrophils (5 × 105/well) in a ratio of 1:1 (A) and in cultures of neutrophils (B) and alveolar macrophages (C) alone at 3, 12, and 24 h after infection with M. tuberculosis H37Rv (MtbH37Rv) at an MOI of 0.1 are indicated. The level of induction (n-fold) was determined from the CT values normalized for HPRT expression and then normalized to values for uninfected cells for each respective time point. Results are expressed as the means ± standard errors of the means of results for three animals. The IL-8 mRNA levels in infected cells were not found to be statistically significant compared to those in the uninfected cells in the cocultures or the individual cultures.
FIG. 2.
Concentrations of IL-8 protein in cultures of neutrophils and alveolar macrophages, either together or alone, following infection with M. tuberculosis H37Rv. Shown are the concentrations of IL-8 protein in cocultures of alveolar macrophages and neutrophils in a ratio of 1:1 (A) and cultures of neutrophils (B) and alveolar macrophages (C) alone at 3, 12, and 24 h after infection with M. tuberculosis H37Rv (MtbH37Rv) at an MOI of 0.1. Protein concentrations were determined by ELISA. Results are expressed as the means ± standard errors of the means of results for three animals. Differences between the infected and uninfected cultures at different time points were compared by using ANOVA followed by Duncan's post hoc analysis. *, P < 0.05.
Characterization of soluble mediators from infected neutrophils in the Transwell insert culture system.
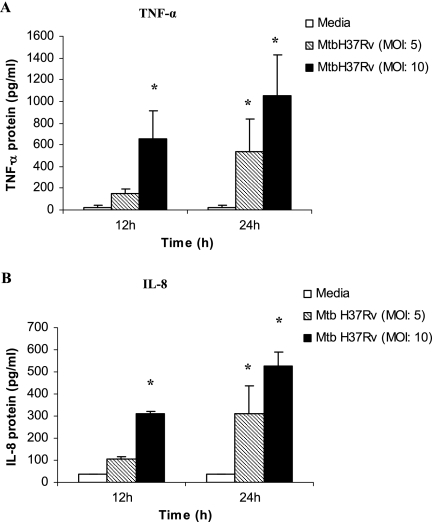
The Transwell insert culture system was set up as described in Materials and Methods. The neutrophils in the upper wells (insert) were infected with M. tuberculosis H37Rv (MOI of 5 or 10), and the supernatants in the lower wells were collected at 12 and 24 h and examined for the presence of IL-8 and TNF-α protein. Figure 3A shows that the production of bioactive TNF-α protein by the infected neutrophils was significantly elevated at 12 h in cultures infected at an MOI of 10 and also at 24 h in cultures infected at either MOI (5 or 10) compared to that in the uninfected controls (P < 0.05). Similarly, the concentration of IL-8 protein was significantly enhanced at 12 h in cultures infected at the higher MOI of 10 and also at 24 h in cultures infected at either MOI compared to that in the uninfected controls (Fig. 3B).
FIG. 3.
Concentrations of TNF-α and IL-8 protein in supernatants of neutrophils infected with M. tuberculosis H37Rv (MtbH37Rv) in a Transwell insert. Neutrophils (2 × 105/well) were infected with M. tuberculosis at an MOI of 5 or 10, and the supernatants were collected at 12 and 24 h. The supernatants were then assayed for TNF-α protein by the L929 bioassay and for IL-8 protein by ELISA. Cell viability at MOIs of 5 and 10 was found to be >80% and 70%, respectively. Results are expressed as the means ± standard errors of the means of results from three experiments. There were three animals per group, and the differences between infected and uninfected cultures were compared by using ANOVA followed by Duncan's post hoc analysis. *, P < 0.05.
Production of H2O2 by alveolar macrophages exposed to the soluble mediators of infected neutrophils.
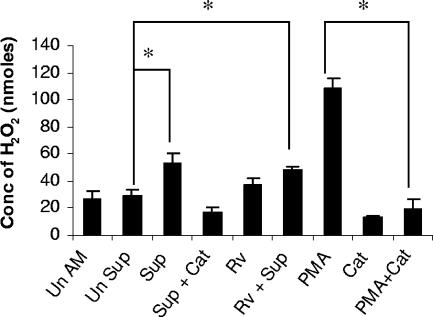
To investigate the effects of the soluble products of infected neutrophils on the activation of alveolar macrophages, the supernatants from infected and uninfected neutrophils harvested at 24 h postinfection were added to AM cultured in a 96-well plate. After 24 h, the release of H2O2 by the alveolar macrophages exposed to supernatants of infected neutrophils was measured. Figure 4 illustrates that the production of hydrogen peroxide by AM treated with supernatants from infected neutrophils was significantly elevated compared to that by macrophages treated with supernatants from uninfected neutrophils or untreated AM (P < 0.05). The macrophages treated with both M. tuberculosis H37Rv (MOI of 0.1) and supernatant from infected neutrophils also produced a significant amount of hydrogen peroxide compared to the macrophages treated with supernatants from uninfected neutrophils (P < 0.05). Incubation with PMA also induced large amounts of H2O2 in AM while catalase significantly inhibited the production of H2O2 by AM stimulated with either supernatants from infected neutrophils or PMA (P < 0.05).
FIG. 4.
Production of hydrogen peroxide by alveolar macrophages treated with supernatants from infected neutrophils. Alveolar macrophages (2 × 105/well) were treated with supernatants from neutrophils infected with M. tuberculosis H37Rv (MOI of 5) for 24 h (Sup), and H2O2 production was measured by using horseradish peroxide-dependent oxidation of phenol red. Alveolar macrophages treated with supernatants from uninfected neutrophils were used as controls (Un Sup). Some of the alveolar macrophages were treated with anti-recombinant guinea pig TNF-α antibody (1:600 dilution; data not shown). PMA and catalase (Cat) were used by macrophages as a stimulant and an inhibitor of H2O2 production, respectively. The other controls used were uninfected, untreated macrophages (Un AM); macrophages infected with M. tuberculosis H37Rv at an MOI of 0.1 (Rv); and alveolar macrophages treated with supernatants from infected neutrophils and also infected with M. tuberculosis at an MOI of 0.1 (Rv + Sup). H2O2 concentrations (Conc) were expressed in nanomoles. The levels of production of H2O2 by different treatment groups (three animals per group) were compared with that by the untreated control by using ANOVA followed by Duncan's post hoc analysis. *, P < 0.05.
Expression of IL-1β and TNF-α mRNA by alveolar macrophages in noncontact cocultures with infected neutrophils.
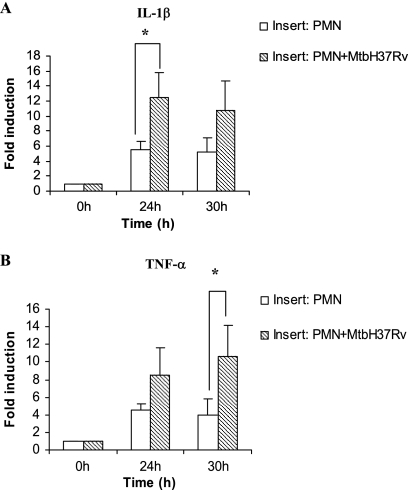
The Transwell insert culture system was set up with neutrophils infected with M. tuberculosis (MOI of 5) in the upper wells and AM in the lower wells. The insert containing the infected neutrophils was removed after 24 h. The AM in the lower wells were harvested at 24 and 30 h from the time of neutrophil infection. RNA was isolated and reverse transcribed, and the levels of IL-1β and TNF-α mRNA were measured by real-time PCR. As seen in Fig. 5, there was significant up-regulation of IL-1β mRNA at 24 h and TNF-α mRNA at 30 h in AM exposed to soluble products of infected neutrophils compared to AM exposed to uninfected neutrophils (P < 0.05).
FIG. 5.
Expression of IL-1β and TNF-α mRNA in alveolar macrophages cocultured with M. tuberculosis-infected or uninfected neutrophils in a contact-independent manner. Alveolar macrophages (5 × 105/well) were exposed to supernatants from infected neutrophils (2 × 105/well; MOI of 5; PMN+MtbH37Rv) or uninfected neutrophils (PMN) in a Transwell insert coculture system in a contact-independent manner for 24 h. The expression of IL-1β and TNF-α mRNA in alveolar macrophages was measured at 24 and 30 h, and the level of induction (n-fold) was determined from the CT values normalized for HPRT expression and then normalized to values for treated alveolar macrophages at 0 h. The results are expressed as the means ± standard errors of the means of results for four animals per group. Differences between the infected and uninfected cocultures were compared by using ANOVA followed by Duncan's post hoc analysis. *, P < 0.05.
Effect of neutralizing endogenous guinea pig TNF-α on the production of H2O2 and cytokine mRNA expression by alveolar macrophages in noncontact cultures.
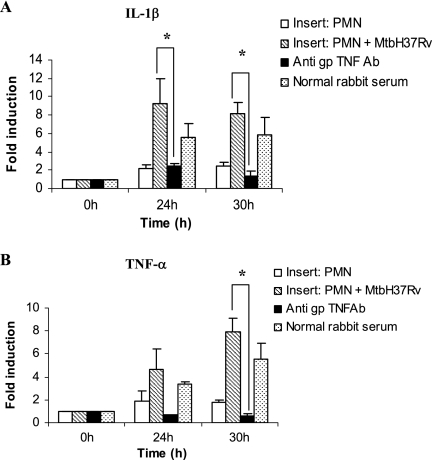
The Transwell insert culture system was set up with uninfected neutrophils or neutrophils infected with M. tuberculosis (MOI of 5) in the upper wells and lower wells containing AM, with or without rabbit anti-guinea pig TNF-α polyclonal antibody (1:600 dilution). Control wells were treated with normal rabbit serum. The AM exposed to soluble products from infected or uninfected neutrophils were harvested at 24 and 30 h. RNA was isolated, transcribed, and amplified for analysis of IL-1β and TNF-α mRNA expression. Figure 6 shows that there was significant up-regulation of both IL-1β (Fig. 6A) and TNF-α (Fig. 6B) mRNA expression in AM exposed to the soluble products of infected neutrophils and that the mRNA levels corresponding to both cytokines were suppressed significantly in AM pretreated with anti-TNF-α antibody (P < 0.05). The AM in noncontact cocultures with infected neutrophils treated with anti-guinea pig TNF-α antibody also produced significantly less H2O2 (11.2 ± 4.6 nmol/well) than the AM in identical cocultures without neutralizing anti-guinea pig TNF-α antibody (38.9 ± 9.1 nmol/well; P < 0.05) (Fig. 4). These results show that TNF-α produced by infected neutrophils may play an important role in activating alveolar macrophages in our noncontact coculture system.
FIG. 6.
Effect of anti-guinea pig TNF-α on levels of TNF-α and IL-1β mRNA in alveolar macrophages cocultured with M. tuberculosis-infected neutrophils in a contact-independent manner. Alveolar macrophages were exposed to supernatants from infected neutrophils (MOI of 5; PMN + MtbH37Rv) or uninfected neutrophils (PMN) in a Transwell insert coculture system in a contact-independent manner for 24 h, and some cocultures were treated with anti-guinea pig TNF antibody (anti gp TNFAb). Levels of IL-1β (A) and TNF-α (B) mRNA in alveolar macrophages were quantified at 24 and 30 h, and the level of induction (n-fold) was determined from the CT values normalized for HPRT expression and then normalized to values for cells at 0 h. Results are expressed as the means ± standard errors of the means of results for four animals. Differences between the cultures receiving the various treatments were compared by using ANOVA followed by Duncan's post hoc analysis. *, P < 0.05.
DISCUSSION
The exact role of neutrophils in the control of M. tuberculosis infection is still not completely defined. Neutrophils are known to be recruited to the site of mycobacterial infection and are found in the granulomas of humans, mice, and guinea pigs (4, 28, 29). The protective role of neutrophils has been demonstrated by the in vivo depletion of these cells. Neutrophil-depleted mice have diminished capacities to form granulomas (32). Fulton et al. have reported that neutrophil depletion results in enhanced M. bovis BCG growth in the lungs which correlates with a decrease in neutrophil numbers, while Seiler et al. observed no influence of neutrophil depletion on mycobacterial loads in lungs and spleens of neutrophil-depleted mice, thus concluding that neutrophil depletion had no effect on the dissemination or systemic control of M. tuberculosis (17, 38). Conversely, Sugawara et al. reported the control of early mycobacterial infection in rats having lipopolysaccharide-induced neutrophilia (42). Also, several research groups have reported divergent results regarding the ability of neutrophils to kill M. tuberculosis (11, 27, 30). Hence, it is possible that neutrophils may play an immunomodulatory role which is indirect and mediated by their soluble products.
Even though neutrophils are terminally differentiated cells, recently, several groups have shown that neutrophils stimulated with bacteria, lipopolysaccharide, or cytokines can produce an array of cytokines and chemokines, including IL-1β, IL-8, TGF-β, TNF-α, macrophage inflammatory protein-1α/β (MIP-1α/β), and growth-related oncogene protein-alpha, in mice, humans, and guinea pigs (7, 20, 25, 34). BCG stimulation of human polymorphonuclear neutrophils results in the de novo synthesis of IL-1α, TNF-α, IL-1β, MIP-1α, MIP-1β, and macrophage chemotactic protein-1 mRNA (44). Of all the chemokines produced by stimulated neutrophils, IL-8 has been known to play an important role in the pathogenesis of TB. IL-8 has been reported to be present in patients with active TB and is also produced by various immune system cells, i.e., AM, neutrophils, and monocytes, upon infection with M. tuberculosis (16, 20, 25, 26, 47). Hence, we employed coculture experiments involving both neutrophils and AM in direct contact with each other and compared the levels of IL-8 mRNA and protein production in cells cultured together and those cultured alone in response to infection with virulent M. tuberculosis (H37Rv). The significant production of IL-8 protein by neutrophils and AM, either in coculture or alone (Fig. 2), suggests that neutrophils may assist alveolar macrophages in response to M. tuberculosis infection by contributing additional IL-8 (CXCL-8) which augments the accumulation of an inflammatory exudate.
Our preliminary investigations involving the infection of neutrophils with M. tuberculosis H37Rv at an MOI of 0.1 did not show any production of TNF-α protein and showed very little production of TNF-α mRNA. Hence, to obtain the maximum effect of M. tuberculosis infection on the neutrophils in a short period of time, a higher MOI of 5 was used for infection. Also, for the contact-dependent cultures involving AM, it was necessary to use a low MOI (0.1). Previous research in our lab (data not shown) has shown that a higher MOI may be cytotoxic to AM. In our study, the infection of neutrophils with M. tuberculosis (H37Rv) at an MOI of 5 induced significant amounts of TNF-α and IL-8 protein (Fig. 3A and B) at 24 h. Since neutrophils have a short life span, an assessment of the viability of neutrophils infected at MOIs of both 5 and 10 was also done. Neutrophil viability at an MOI of 10 was decreased, and hence an MOI of 10 was not considered for any other experiments. The presence of these cytokines in the supernatants of the infected neutrophils may suggest a role for neutrophils in the early transition to adaptive immunity against M. tuberculosis infection.
The role of alveolar macrophages in innate as well as adaptive immunity against M. tuberculosis infection is very well documented (40, 45, 47). An increase in the respiratory burst by AM after treatment with recombinant TNF-α has been reported by Cho et al. and Sharber et al. (39; H. Cho, A. Jeevan, and D. N. McMurray, submitted for publication). Our results show that AM exposed to supernatants from infected neutrophils produced significantly enhanced levels of H2O2, suggesting an activated state.
To separate the contact and noncontact interactions between neutrophils and AM in the cocultures, a Transwell insert culture system was used to examine the effects of soluble mediators produced by infected neutrophils on the function of uninfected alveolar macrophages. We hypothesized that proinflammatory cytokines (e.g., TNF-α) produced by infected neutrophils might be responsible for the activation of AM in our coculture system. Our data (Fig. 5) confirm that the activation of AM by supernatants of infected neutrophils was accompanied by the up-regulation of mRNA levels corresponding to TNF-α and IL-1β. Furthermore, the neutralization of endogenous TNF-α by the treatment of cultures with polyclonal anti-guinea pig TNF-α resulted in the suppression of IL-1β and TNF-α mRNA (Fig. 6) and the inhibition of hydrogen peroxide production (Fig. 4). These results indicate that TNF-α may be an important effector cytokine which is responsible, at least in part, for activating the AM in our cocultures. Both neutrophils and alveolar macrophages could be producing TNF-α in the Transwell insert experiments. There is evidence of the production of TNF-α by neutrophils in mice (32) and humans (14) following infection with M. tuberculosis. The ability of AM to produce TNF-α has also been very well documented (24, 43). In our noncontact coculture system, the neutrophils responded to infection with M. tuberculosis by producing TNF-α, as shown in Fig. 3A. The TNF-α produced by neutrophils likely exerts a paracrine effect on AM. However, TNF-α can induce its own production in an endocrine fashion (5, 39). Thus, AM exposed to TNF-α and other neutrophil products could also produce TNF-α. In our Transwell insert culture system, the anti-guinea pig TNF-α antiserum was present in the medium which was in contact with both the cell types and could have blocked both the paracrine and autocrine effects of endogenous TNF-α. Future experiments will attempt to determine the relative contributions of neutrophils and AM to the total TNF-α available in noncontact cultures.
We have shown that guinea pig neutrophils infected with virulent M. tuberculosis produce cytokines, such as TNF-α, which may activate AM in noncontact cocultures. We believe that our results support the contention that neutrophils may play an important role in the early host response to M. tuberculosis infection in the lungs.
Acknowledgments
This work was supported by National Institutes of Health grant ROI AI 15495 to D.N.M.
We are indebted to Shannon Sedberry Allen and Hyosun Cho for their guidance and help in this study.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Allen, S. S., and D. N. McMurray. 2003. Coordinate cytokine gene expression in vivo following induction of tuberculous pleurisy in guinea pigs. Infect. Immun. 71:4271-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antony, V. B., S. A. Sahn, R. N. Harada, and J. E. Repine. 1983. Lung repair and granuloma formation. Tubercle bacilli stimulated neutrophils release chemotactic factors for monocytes. Chest 83:95S-96S. [DOI] [PubMed] [Google Scholar]
- 3.Appelberg, R., A. G. Castro, S. Gomes, J. Pedrosa, and M. T. Silva. 1995. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect. Immun. 63:3381-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelberg, R., and M. T. Silva. 1989. T cell-dependent chronic neutrophilia during mycobacterial infections. Clin. Exp. Immunol. 78:478-483. [PMC free article] [PubMed] [Google Scholar]
- 5.Baeuerle, P. A., and D. Baltimore. 1996. NF-kappa B: ten years after. Cell 87:13-20. [DOI] [PubMed] [Google Scholar]
- 6.Bliss, S. K., Y. Zhang, and E. Y. Denkers. 1999. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-gamma-independent IL-12. J. Immunol. 163:2081-2088. [PubMed] [Google Scholar]
- 7.Cassatella, M. A. 1999. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 73:369-509. [DOI] [PubMed] [Google Scholar]
- 8.Cho, H., and D. N. McMurray. 2007. Recombinant guinea pig TNF-alpha enhances antigen-specific type 1 T lymphocyte activation in guinea pig splenocytes. Tuberculosis 87:87-93. [DOI] [PubMed]
- 9.Cho, H., and D. N. McMurray. 2005. Neutralization of tumor necrosis factor alpha supresses antigen-specific type 1 cytokine responses and reverses the inhibition of mycobacterial survival in cocultures of immune guinea pig T lymphocytes and infected macrophages. Infect. Immun. 73:8437-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho, H., T. M. Lasco, S. S. Allen, T. Yoshimura, and D. N. McMurray. 2005. Recombinant guinea pig tumor necrosis factor alpha stimulates the expression of interleukin-12 and the inhibition of Mycobacterium tuberculosis growth in macrophages. Infect. Immun. 73:1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denis, M. 1991. Human neutrophils, when activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. Cell. Immunol. 132:150-157. [DOI] [PubMed] [Google Scholar]
- 12.Denis, M. 1991. Involvement of cytokines in determining resistance and acquired immunity in murine tuberculosis. J. Leukoc. Biol. 50:495-501. [DOI] [PubMed] [Google Scholar]
- 13.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 14.Faldt, J., C. Dahlgren, and M. Ridell. 2002. Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS 110:593-600. [DOI] [PubMed] [Google Scholar]
- 15.Flynn, J. L., M. M. Goldstein, J. Chan, K. J. Triebold, K. Pfeffer, C. J. Lowenstein, R. Schreiber, T. W. Mak, and B. R. Bloom. 1995. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561-572. [DOI] [PubMed] [Google Scholar]
- 16.Friedland, J. S., D. G. Remick, R. Shattock, and G. E. Griffin. 1992. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur. J. Immunol. 22:1373-1378. [DOI] [PubMed] [Google Scholar]
- 17.Fulton, S. A., S. M. Reba, T. D. Martin, and W. H. Boom. 2002. Neutrophil-mediated mycobacteriocidal immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect. Immun. 70:5322-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grover, A. A., H. K. Kim, E. H. Wiegeshaus, and D. W. Smith. 1967. Host-parasite relationships in experimental airborne tuberculosis. II. Reproducible infection by means of an inoculum preserved at −70°C. J. Bacteriol. 94:832-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeevan, A., C. T. McFarland, T. Yoshimura, T. Skwor, H. Cho, T. Lasco, and D. N. McMurray. 2006. Production and characterization of guinea pig recombinant gamma interferon and its effect on macrophage activation. Infect. Immun. 74:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasahara, K., I. Sato, K. Ogura, H. Takeuchi, K. Kobayashi, and M. Adachi. 1998. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J. Infect. Dis. 178:127-137. [DOI] [PubMed] [Google Scholar]
- 21.Kindler, V., A. P. Sappino, G. E. Grau, P. F. Piguet, and P. Vassalli. 1989. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56:731-740. [DOI] [PubMed] [Google Scholar]
- 22.Kisich, K. O., M. Higgins, G. Diamond, and L. Heifets. 2002. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect. Immun. 70:4591-4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lasco, T. M., L. Cassone, H. Kamohara, T. Yoshimura, and D. N. McMurray. 2005. Evaluating the role of tumor necrosis factor-alpha in experimental pulmonary tuberculosis in the guinea pig. Tuberculosis (Edinburgh) 85:245-258. [DOI] [PubMed] [Google Scholar]
- 24.Lasco, T. M., T. Yamamoto, T. Yoshimura, S. S. Allen, L. Cassone, and D. N. McMurray. 2003. Effect of Mycobacterium bovis BCG vaccination on Mycobacterium-specific cellular proliferation and tumor necrosis factor alpha production from distinct guinea pig leukocyte populations. Infect. Immun. 71:7035-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2004. Interleukin (IL)-8 (CXCL8) induces cytokine expression and superoxide formation by guinea pig neutrophils infected with Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 84:283-292. [DOI] [PubMed] [Google Scholar]
- 26.Lyons, M. J., T. Yoshimura, and D. N. McMurray. 2002. Mycobacterium bovis BCG vaccination augments interleukin-8 mRNA expression and protein production in guinea pig alveolar macrophages infected with Mycobacterium tuberculosis. Infect. Immun. 70:5471-5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majeed, M., N. Perksvist, J. D. Ernst, K. Orselius, and O. Stendahl. 1998. Roles of calcium and annexins in phagocytosis and elimination of an attenuated strain of Mycobacterium tuberculosis in human neutrophils. Microb. Pathog. 24:309-320. [DOI] [PubMed] [Google Scholar]
- 28.Montgomery, L. G., and W. S. Lemon. 1993. The cellular reaction of the pleura to infection with Mycobacterium tuberculosis. J. Thorac. Cardiovasc. Surg. 2:429. [Google Scholar]
- 29.Orme, I. M. 1998. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 6:94-97. [DOI] [PubMed] [Google Scholar]
- 30.Pedrosa, J., B. M. Saunders, R. Appelberg, I. M. Orme, M. T. Silva, and A. M. Cooper. 2000. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect. Immun. 68:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perskvist, N., M. Long, O. Stendahl, and L. Zheng. 2002. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J. Immunol. 168:6358-6365. [DOI] [PubMed] [Google Scholar]
- 32.Petrofsky, M., and L. E. Bermudez. 1999. Neutrophils from Mycobacterium avium-infected mice produce TNF-alpha, IL-12, and IL-1beta and have a putative role in early host response. Clin. Immunol. 91:354-358. [DOI] [PubMed] [Google Scholar]
- 33.Pick, E. 1986. Microassays for superoxide and hydrogen peroxide production and nitroblue tetrazolium reduction using an enzyme immunoassay microplate reader. Methods Enzymol. 132:407-421. [DOI] [PubMed] [Google Scholar]
- 34.Riedel, D. D., and S. H. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sadek, M. I., E. Sada, Z. Toossi, S. K. Schwander, and E. A. Rich. 1998. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 19:513-521. [DOI] [PubMed] [Google Scholar]
- 36.Scanga, C. A., V. P. Mohan, H. Joseph, K. Yu, J. Chan, and J. L. Flynn. 1999. Reactivation of latent tuberculosis: variations on the Cornell murine model. Infect. Immun. 67:4531-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scapini, P., J. A. Lapinet-Vera, S. Gasperini, F. Calzetti, F. Bazzoni, and M. A. Cassatella. 2000. The neutrophil as a cellular source of chemokines. Immunol. Rev. 177:195-203. [DOI] [PubMed] [Google Scholar]
- 38.Seiler, P., P. Aichele, S. Bandermann, A. E. Hauser, B. Lu, N. P. Gerard, C. Gerard, S. Ehlers, H. J. Mollenkopf, and S. H. Kaufmann. 2003. Early granuloma formation after aerosol Mycobacterium tuberculosis infection is regulated by neutrophils via CXCR3-signaling chemokines. Eur. J. Immunol. 33:2676-2686. [DOI] [PubMed] [Google Scholar]
- 39.Sharber, M., and C. F. Nathan. 1986. Autocrine activation of macrophages by recombinant tumor necrosis factor but not recombinant interleukin-1. Blood 68:86a-92a. [Google Scholar]
- 40.Sibille, Y., and H. Y. Reynolds. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am. Rev. Respir. Dis. 141:471-501. [DOI] [PubMed] [Google Scholar]
- 41.Skwor, T. A., H. Cho, C. Cassidy, T. Yoshimura, and D. N. McMurray. 2004. Recombinant guinea pig CCL5 (RANTES) differentially modulates cytokine production in alveolar and peritoneal macrophages. J. Leukoc. Biol. 76:1229-1239. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara, I., T. Udagawa, and H. Yamada. 2004. Rat neutrophils prevent the development of tuberculosis. Infect. Immun. 72:1804-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surewicz, K., H. Aung, R. A. Kanost, L. Jones, R. Hejal, and Z. Toossi. 2004. The differential interaction of p38 MAP kinase and tumor necrosis factor-alpha in human alveolar macrophages and monocytes induced by Mycobacterium tuberculosis. Cell. Immunol. 228:34-41. [DOI] [PubMed] [Google Scholar]
- 44.Suttmann, H., N. Lehan, A. Bohle, and S. Brandau. 2003. Stimulation of neutrophil granulocytes with Mycobacterium bovis bacillus Calmette-Guerin induces changes in phenotype and gene expression and inhibits spontaneous apoptosis. Infect. Immun. 71:4647-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallis, R. S., and J. J. Ellner. 1994. Cytokines and tuberculosis. J. Leukoc. Biol. 55:676-681. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura, T., and D. G. Johnson. 1993. cDNA cloning and expression of guinea pig neutrophil attractant protein-1 (NAP-1). NAP-1 is highly conserved in guinea pig. J. Immunol. 151:6225-6236. [PubMed] [Google Scholar]
- 47.Zhang, Y., M. Broser, H. Cohen, M. Bodkin, K. Law, J. Reibman, and W. N. Rom. 1995. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J. Clin. Investig. 95:586-592. [DOI] [PMC free article] [PubMed] [Google Scholar]