Abstract
Anopheles gambiae is the major African vector of Plasmodium falciparum, the most deadly species of human malaria parasite and the most prevalent in Africa. Several strategies are being developed to limit the global impact of malaria via reducing transmission rates, among which are transmission-blocking vaccines (TBVs), which induce in the vertebrate host the production of antibodies that inhibit parasite development in the mosquito midgut. So far, the most promising components of a TBV are parasite-derived antigens, although targeting critical mosquito components might also successfully block development of the parasite in its vector. We previously identified A. gambiae genes whose expression was modified in P. falciparum-infected mosquitoes, including one midgut carboxypeptidase gene, cpbAg1. Here we show that P. falciparum up-regulates the expression of cpbAg1 and of a second midgut carboxypeptidase gene, cpbAg2, and that this up-regulation correlates with an increased carboxypeptidase B (CPB) activity at a time when parasites establish infection in the mosquito midgut. The addition of antibodies directed against CPBAg1 to a P. falciparum-containing blood meal inhibited CPB activity and blocked parasite development in the mosquito midgut. Furthermore, the development of the rodent parasite Plasmodium berghei was significantly reduced in mosquitoes fed on infected mice that had been immunized with recombinant CPBAg1. Lastly, mosquitoes fed on anti-CPBAg1 antibodies exhibited reduced reproductive capacity, a secondary effect of a CPB-based TBV that could likely contribute to reducing Plasmodium transmission. These results indicate that A. gambiae CPBs could constitute targets for a TBV that is based upon mosquito molecules.
Malaria remains a leading cause of morbidity and mortality in human populations, with over 3 billion people living in areas at risk for malaria transmission and an estimated 350 to 500 million clinical episodes occurring annually (29). Plasmodium falciparum malaria causes more than a million deaths each year, mainly in young children in sub-Saharan Africa. Moreover, the malaria burden has increased over the last 10 to 15 years, and this situation has been associated in part with parasite resistance to commonly used antimalarial drugs and resistance of mosquito vectors to insecticides (29). Several strategies are being developed which target either the disease or its transmission. Owing to the complexity of the parasite life cycle, with both human stages that result in disease and mosquito stages that ensure transmission, an effective vaccine might combine pre-erythrocytic (sporozoite and liver stage), asexual erythrocytic, and transmission-blocking components. Although modeling of vaccine effects on malaria transmission dynamics indicates that a transmission-blocking vaccine (TBV) will be most effective in regions where the initial basic reproductive rate of malaria (R0) is low (3, 4, 8, 9), a TBV offers the advantage of blocking the spread of escape mutants that are resistant to asexual-stage vaccine components or to antimalarial medications. In contrast to pre-erythrocytic- and erythrocytic-stage vaccine components, TBV target antigens are not under selection pressure in the human host (5, 13, 14, 25). As malaria case numbers increase in major African cities, the implementation of a TBV strategy should be beneficial in these urban areas for which the R0 remains low (4, 10).
Most of the work on TBV development has focused on parasite antigens expressed in the mosquito midgut (for reviews, see references 11, 13, 14, 28, and 32), whereas malaria transmission blocking could also be achieved by targeting mosquito components that are required for the successful development of the parasite in its vector. The feasibility of such an approach has been documented (7, 15, 22, 24); however, no specific target has yet been identified as a component for a TBV in Anopheles mosquitoes, the sole vectors of human malaria parasites.
To identify such components, we previously designed a molecular screen using Anopheles gambiae, the main vector of P. falciparum in Africa, infected under field conditions (2). From this screen, we identified CPBAg1 and CPBAg2, two carboxypeptidases B (CPB) that are expressed in the mosquito midgut (17). Here, we show that ingestion of the invasive stages (gametocytes) of P. falciparum up-regulates expression of both cbpAg1 and cpbAg2 and triggers an increase of midgut CPB activity, suggesting that CPB are involved in P. falciparum development and could therefore constitute candidate molecules for a TBV. We further investigate the ability of anti-CPBAg1 antibodies to block P. falciparum development, using membrane feeding assays, as well as the development of the rodent malaria parasite Plasmodium berghei using CBPAg1-immunized mice. Our data show that Plasmodium development is drastically reduced in both systems, indicating that CPB constitute candidate mosquito molecule components for a TBV. In addition, mosquitoes fed on anti-CPBAg1 serum have reduced reproductive capacity, a secondary effect that adds value to a TBV based on CPB for limiting Plasmodium transmission by further decreasing its R0.
MATERIALS AND METHODS
Mosquitoes.
All experiments were performed with the Anopheles gambiae Yaoundé strain (27), either at the Pasteur Institute (Paris, France) or at the IRD (Yaoundé, Cameroon, and Dakar, Senegal). Mosquitoes were reared at 26 to 28°C and 80% relative humidity, with a 12-h light/dark cycle. Dissections were performed in phosphate-buffered saline (PBS) at 4°C. Midguts and carcasses (whole mosquito minus midgut) were stored at −80°C until RNA or protein extraction.
Field infection of A. gambiae with P. falciparum.
Asymptomatic village residents (Senegal) or schoolchildren (Cameroon) were screened to detect parasite carriers as described elsewhere (26). All participants were volunteers, and the consent of the children's parents was obtained. The experimental protocols were approved by the Cameroonian and the Senegalese National Ethics Committees. Infections and control experiments were performed with blood from gametocyte carrier volunteers and with blood from parasite-free donors. Venous blood (10 ml) was collected in a heparin-coated and prewarmed tube and centrifuged at 37°C for 5 min at 5,000 rpm, and the plasma of the patient was immediately replaced with a prewarmed pool of AB serum collected from donors not living in a country where malaria is endemic. For each experiment, batches of 60 nulliparous female mosquitoes (5 days old) starved of sugar for 12 h were fed for 15 min, using the artificial membrane feeding technique (27). Fully engorged females were maintained at the insectarium until dissection. For each experiment a batch of control mosquitoes (n = 30) was used to determine the rate (number of infected mosquitoes/number of dissected mosquitoes) and intensity (mean number of oocysts per positive midgut) of infection by oocyst detection on day 7 post-blood meal (PBM).
Gene expression analysis.
Mosquitoes were fed on the blood of volunteers. The blood contained gametocytes but no asexual stages, as assessed by microscopic examination of thick blood smears. The gametocyte loads varied from 50 to 2,600 gametocytes per μl of blood. The proportion of infected mosquitoes fed on the blood from these gametocyte carriers was greater than 42% on day 7 PBM, with an intensity of infection varying from 1 to 80 oocysts per positive midgut.
Midguts were isolated from at least 10 females at 14 h, 24 h, and 48 h PBM. Total RNA was extracted from midgut pools using a Tri Reagent kit (M.R.C. Inc.) according to the manufacturer's instructions, and RNA was treated with the DNA-free kit (Ambion). The absence of contaminating genomic DNA in each RNA sample was determined by specific amplification of the cpbAg1 gene. Each reverse transcription (RT) experiment was performed with 100 ng of RNA plus random hexamer primer mixture and Moloney murine leukemia virus reverse transcriptase (400 units per reaction; Invitrogen) in a final volume of 40 μl. To minimize variations during the reverse transcription step, RT reactions were performed in triplicate and RT products were pooled. Real-time PCR was performed using the double-stranded DNA dye SybrGreen (MasterMix; Perkin Elmer) and an iCycler apparatus (Bio-Rad). PCR experiments were performed in quintuplicate in 25-μl final volumes containing 900 nM concentrations of each forward and reverse primer and 5 μl of a 1:5 dilution of the RT product. Relative quantification of cpbAg1 or cpbAg2 mRNA was performed by using the standard curve method (ABI), with ribosomal protein S7 mRNA as an endogenous reference. The relative amounts of cpbAg1 mRNA, cpbAg2 mRNA, and s7 mRNA were determined from a standard curve constructed using an mRNA sample of known concentration. Three independent infection experiments were analyzed, and significant differences in gene expression ratios were evaluated with the Wilcoxon test. The primers used for amplification were s7U (5′-CACCGCCGTGTACGATGCCA-3′), s7L (5′-ATGGTGGTCTGCTGGTTCTT-3′), cpbAg1U (5′-GGCGGCTGAGGCGTGACT-3′), cpbAg1L (5′-GACGGGTCTGATCGACTG-3′), cpbAg2U (5′-TCCGGCACAATTGGACTACT-3′), and cpbAg2L (5′-TACCGCAGGTACTTGTTGAG-3′).
Midgut CPB activity.
To assess midgut CPB activity upon ingestion of P. falciparum, pools of 20 midguts were dissected from unfed mosquitoes and from mosquitoes fed on noninfected and P. falciparum gametocyte-containing blood at different times PBM (14 h, 24 h, and 48 h). Three independent infection experiments were performed, and the proportion of P. falciparum infected mosquitoes on day 7 ranged from 30% to 70%. To assess inhibition of midgut CPB activity upon ingestion of anti-CPBAg1 antibodies, mosquitoes were fed on noninfected human red blood cells mixed with rabbit serum directed against a recombinant CPBAg1 protein (17) or with a pool of naive rabbit serum. For each condition, three independent feeding experiments were performed, and pools of 10 midguts were dissected at different times PBM (14 h, 24 h, and 48 h). CPB activity was determined with isolated midguts homogenized in E buffer (100 mM NaCl-50 mM HEPES-100 μM ZnCl2 [pH 7.2]) using a hand-held plastic pestle and centrifuged at 10,000 × g at 4°C for 20 min. Activities were assayed in 20 μl of E buffer containing 1 mM hippuryl-arginine dipeptide (Sigma) as the CPB substrate. The reaction was initiated by the addition of 20 μl of midgut extract (equivalent to one midgut), the mixture was incubated at 25°C for 5 to 40 min, and the reaction was stopped by the addition of 20 μl of ninhydrin reagent (Sigma). The rate of the reaction was measured by estimating the amount of released amino acids by the ninhydrin procedure (18). One unit of enzyme activity was defined as μmol of amino acids released/min. For all assays, triplicate reactions were performed.
P. falciparum transmission-blocking assay.
In a series of transmission-blocking experiments, the serum of the patient was replaced either with rabbit serum directed against a recombinant CPBAg1 protein (from two rabbits) or with rabbit immune serum diluted with human AB serum (1:1 ratio). As a control, the serum of the patient was replaced either with a pool of naive rabbit serum or with a pool of naive rabbit serum diluted in human AB serum at the same ratio. Each infection experiment was performed with gametocytes from a single carrier. Rates and intensities of infections were scored on day 7 after blood feeding. Significant differences in the rates and intensities of infections were determined by the chi-square test and by analysis of variance (ANOVA), respectively.
Mouse immunization.
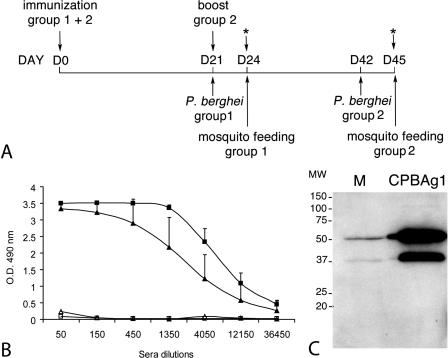
Mice were immunized with a recombinant CPBAg1 that was produced using the baculovirus expression system (17) (the immunization protocol is presented in Fig. 5A). Eleven 3- to 4-week-old female Swiss outbred mice (CERJ, Le Genest St-Isle, France) were injected subcutaneously with 20 μg of recombinant CPBAg1 in sterile PBS emulsified with complete Freund's adjuvant (Sigma). Eleven control mice were injected with sterile PBS-adjuvant following the same protocol on the same day. On day 21 postimmunization, eight immunized mice and eight control mice (group 1) were challenged with P. berghei for transmission-blocking assays (see below). The three remaining immunized mice were given boosters of 10 μg of recombinant CPBAg1 formulated in incomplete Freund's adjuvant (Sigma), and the three remaining control mice were injected simultaneously with sterile PBS-incomplete adjuvant. These six mice, which constituted group 2, were challenged with P. berghei 21 days after the booster immunization. Sera from immunized and control mice of both groups were prepared from 100-μl blood samples collected the same day as mosquito feeding (24 days after the first and booster immunizations).
FIG. 5.
Mouse immunization with recombinant CPBAg1. (A) Immunization protocol. Mice in group 1 and group 2 were immunized with recombinant CPBAg1 protein on day 0. Mice in group 1 (eight immunized and eight control mice) were inoculated with P. berghei on day 21 and were used for mosquito feeding and ELISA experiments on day 24. Mice in group 2 (three immunized and three control mice) were given a booster on day 21, inoculated with P. berghei on day 42, and used for mosquito feeding and ELISA experiments on day 45. The blood (100 μl per mouse) for the ELISA experiments was collected after mosquito feeding. (B) CPBAg1-specific ELISA titers in sera from immunized and control mice. The titer of specific antibodies against CPBAg1 was measured in duplicate for each mouse 3 weeks after the first (group 1) and the booster (group 2) immunization. For each group, the average optical density reading at 490 nm was plotted against the reciprocal serum dilution. ▴ and ▪, antibody titers in sera from group 1 and 2 immunized mice, respectively; ▵ and □, antibody titers in sera from group 1 and 2 control mice, respectively. Endpoints were defined as the highest dilution yielding an absorbance reading at 490 nm greater than 0.5. The error bars represent standard deviations of the mean antibody titers for all mice within a group. (C) Analysis of antibody recognition of mosquito midgut proteins. M, 10 μg of protein from sugar-fed mosquito midgut; CPBAg1, 10 ng of CPBAg1 recombinant protein. The blot was probed with either serum from CPBAg1-immunized mice or pooled serum from control mice at a dilution of 1:200. The serum from CPBAg1-immunized mice, unlike the pool of serum from control mice (data not shown), specifically recognizes two bands corresponding to the zymogen (48.2-kDa) and mature (37-kDa) forms of CPBAg1 protein in mosquito midguts.
ELISAs.
Microtiter plates (Maxisorp immunoplate; Nunc) were coated overnight at 4°C with 50 ng/well of CPBAg1 in PBS and saturated with 100 μl/well 0.5% gelatin (Sigma) in PBS for 1 h. Plates were then incubated for 90 min with serial dilutions (1:50 to 1:36,450) of serum from immunized mice diluted in 0.5% gelatin-0.1% Tween 20 (Merck)-PBS. Plates were washed extensively and incubated with a 1:1,000 dilution of goat anti-mouse immunoglobulin G conjugated to horseradish peroxidase (Bio-Rad). After washing, the plates were developed with 100 μl/well of 0.4 mg/ml o-phenylenediamine (Sigma) in 0.05 M phosphate-citrate buffer (pH 5) and 0.1% H2O2. After 10 min incubation, the reaction was stopped with 50 μl 3 N HCl, and absorbance was measured at 490 nm using a microplate reader (Molecular Devices). Serum dilutions at an absorbance value of 0.5 were designated as the endpoints of enzyme-linked immunosorbent assay (ELISA) titers.
Analysis of antibody recognition of mosquito midgut proteins by Western blotting.
Proteins were prepared from mosquito midguts by tissue homogenization in 25 mM Tris-HCl (pH 8) using a plastic pestle and further centrifugation at 20,000 × g at 4°C for 15 min. Soluble midgut proteins, recombinant CPBAg1 protein and recombinant CPBAg2 protein (B. Boisson, C. Lavazec, and R. Tahar, unpublished data) were separated on a 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel under reducing conditions and blotted onto a polyvinylidene difluoride membrane (Hybond-P; Amersham). After saturation in PBS containing 5% skim milk and 0.1% Tween 20, the membrane was incubated with serum from rabbits or from each immunized mouse (dilution, 1:500). Antibody binding was detected by incubating the membrane with mouse anti-rabbit immunoglobulin or goat anti-mouse immunoglobulin peroxidase-conjugated antibody (Santa Cruz; dilution, 1:10,000) and subsequent treatment with a peroxidase chemiluminescent substrate (SuperSignal Ultra; Pierce).
P. berghei transmission-blocking assays.
On day 21 after the first and booster immunizations, mice were inoculated peritoneally with P. berghei ANKA strain 2.34. On day 3 after inoculation, parasitemias and gametocytemias were determined using Giemsa-stained thin blood smears, and the mice were used for mosquito feeding. Parasitemias and gametocytemias ranged from 3.2% to 6.2% and from 6,500 to 11,500 gametocytes/μl for control mice and from 3% to 7.4% and from 7,500 to 13,500 gametocytes/μl for immunized mice in group 1. In group 2, values ranged from 2% to 2.9% and from 2,000 to 4,000 gametocytes/μl for control mice and from 1.5% to 3.6% and from 1,000 to 2,000 gametocytes/μl for immunized mice. Each mouse was placed onto a netted cage containing 50 starved 5-day-old A. gambiae females, and feeding was carried out for 15 min at 21°C. After mosquito feeding, mice were bled to collect sera for ELISA experiments, and fully engorged mosquito females were maintained at 21°C and 80% humidity until dissection. The rates and intensities of infections were scored on day 9 to 11 after blood feeding.
Effect of anti-CPBAg1 on mosquito reproductive capacity.
Groups of at least 50 5-day-old females were fed on noninfected human red blood cells mixed with serial dilutions of rabbit anti-CPBAg1 or of naive rabbit serum (Sigma) in human AB serum using the membrane feeding system. Unfed females were removed. From day 2 to day 7 PBM, eggs from each group were collected daily and transfer into labeled larval containers. Hatching larvae were scored daily for 10 days. On day 7 PBM, females were given a normal blood meal composed of human red blood cells mixed with human AB serum. As before, unfed females were removed and eggs produced from this blood meal were collected over 5 days, and the number of hatching larvae was scored daily. The survival rate in each group was measured by counting dead mosquitoes every other day after the first blood meal. For each group and treatment, the number of hatching larvae per female was determined, and data from four independent replicates were analyzed. Analyses were performed in Genstat version 7 by fitting a generalized linear model with normal distribution and an identity link function with larvae/female as the response variate and both dilution and treatment type as factors, and their interactions were considered as explanatory variables. Model simplification sequentially removed nonsignificant explanatory variables. The data were overdispersed, and so a dispersion parameter was estimated.
RESULTS
P. falciparum gametocytes upregulate cpbAg1 and cpbAg2 expression and trigger an increase of CPB activity in A. gambiae midguts.
The expression patterns of cpbAg1 and cpbAg2 were monitored in the midguts of mosquitoes fed on noninfected and Plasmodium-infected blood at different times PBM, using real-time quantitative RT-PCR. These time points correspond to the initial transformation of ingested gametocytes from zygotes to ookinetes in the midgut lumen (14 h), to the peak of abundance of ookinetes in the midgut lumen (24 h), and to the migration and early differentiation of ookinetes into oocysts in the midgut epithelium (48 h) (21, 30).
As shown in Fig. 1, expression of cpbAg1 and cpbAg2 differed substantially in mosquitoes fed on P. falciparum gametocytes and mosquitoes fed on noninfected blood. Because the serum of the P. falciparum carriers was replaced with a pool of serum from individuals not living in a country where malaria is endemic, this effect appears to be due solely to the presence of P. falciparum gametocytes and not to serum-related factors. The strongest effect was observed at 14 h PBM, when cpbAg1 and cpbAg2 expression increased roughly six- and twofold, respectively, compared to the level detected in mosquitoes fed on noninfected blood (Fig. 1). Upregulation of cpbAg1 lasted over the next hours, while overexpression of cpbAg2 occurred at 48 h PBM in addition to 14 h PBM. These data indicate that ingestion of P. falciparum gametocytes triggers an upregulation of expression of both cpbAg1 and cpbAg2, with the strongest effect at 14 h PBM, a time that corresponds to the transformation of zygotes to ookinetes in the mosquito midgut lumen.
FIG. 1.
Quantitative expression of cpbAg1 and cpbAg2 in A. gambiae midguts after ingestion of P. falciparum. Expression of cpbAg1 and cpbAg2 was determined by real-time RT-PCR at different time points (14 h, 24 h, and 48 h) after ingestion of gametocyte-containing or noninfected blood. Expression was normalized to the expression of the ribosomal protein gene s7. Bars indicate standard deviations from three independent infection experiments. Asterisks indicate statistically significant differences compared to the noninfected-blood control, based on the P value from the Wilcoxon test (<0.05).
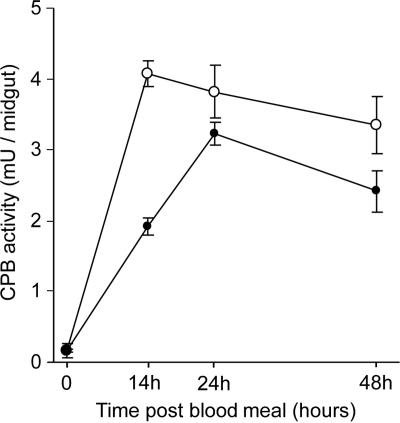
To determine if cpbAg1 and cpbAg2 upregulation in P. falciparum-infected mosquitoes correlates with increased CPB activity in the mosquito midgut, CPB activity was assessed in mosquitoes fed on blood containing P. falciparum gametocytes and on noninfected blood as a control. As depicted in Fig. 2, CPB activity in midguts of mosquitoes fed on P. falciparum gametocytes was greater than that detected in midguts of noninfected mosquitoes. The greatest increase was observed at 14 h PBM, with a 113% increase in CPB activity in mosquitoes fed on P. falciparum. The enzymatic activity in infected mosquitoes remained 18% and 39% above the control level at 24 h and 48 h PBM, respectively. In addition, the maximal CPB activity occurred at 14 h PBM in P. falciparum-infected mosquitoes, whereas it occurred at 24 h PBM in noninfected mosquitoes. The peak of enzymatic activity at 14 h PBM overlaps the peak of expression observed for cpbAg1 and cpbAg2 in gametocyte-infected mosquitoes.
FIG. 2.
Carboxypeptidase activity in noninfected and P. falciparum-infected A. gambiae midguts. CPB activity was determined in midguts isolated before and at 14 h, 24 h, and 48 h after ingestion of a noninfected human blood meal (•) or P. falciparum-infected human blood meal (○). One unit of enzyme activity is defined as 1 μmol of amino acids released/min. CPB activity in midguts isolated from three independent infection experiments and controls was analyzed. Bars indicate standard deviations for each set of three independent experiments.
Anti-CPBAg1 serum reduces CPB activity and inhibits P. falciparum development.
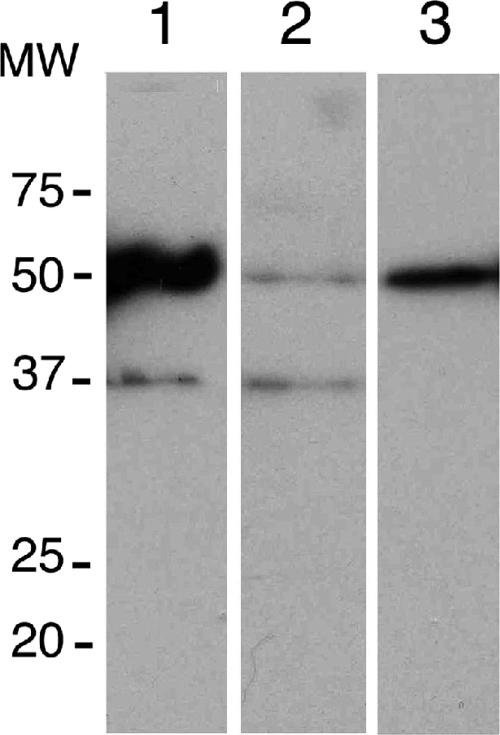
To characterize the correlation between CPB expression and P. falciparum development, we tested the effect of anti-CPB antibodies on the inhibition of CPB activity and parasite development. A rabbit serum raised against a recombinant CPBAg1 protein (17) which recognizes recombinant CPBAg1 and CPBAg2 proteins was used (Fig. 3A, lane 1 and 3), as well as two midgut proteins having the expected size of the zymogen and mature forms of CPBAg1 and CPBAg2 (Fig. 3A, lane 2). These proteins were not recognized by a pool of naive rabbit serum that was used as a control, and no protein from P. falciparum gametocytes was specifically recognized by the anti-CPBAg1 serum, further confirming the specificity of the antibodies (data not shown).
FIG. 3.
Specificity of anti-CPBAg1 serum. Proteins were separated on a 12% SDS-polyacrylamide gel, and a transfer blot was probed with either anti-CPBAg1 rabbit serum or naive rabbit serum (data not shown) at a dilution of 1:500. Lane 1, CPBAg1 recombinant protein (0.1 ng); lane 2, midgut protein extract from blood-fed mosquito (24 h PBM, 1 μg); lane 3, CPBAg2 recombinant protein (20 ng). The anti-CPBAg1 serum, unlike the nonimmune rabbit serum, recognizes two midgut proteins corresponding to the zymogen (48.2-kDa) and mature (37-kDa) forms of CPBAg1 and to the zymogen form of CPBAg2. MW, molecular weight (in thousands).
Firstly, we tested the ability of anti-CPBAg1 serum to inhibit CPB activity in vivo. The addition of anti-CPBAg1 serum to a noninfected blood meal decreased midgut CPB activity in comparison to midgut CPB activity in mosquitoes fed on a pool of naive rabbit serum. CPB enzymatic activity was inhibited by 54%, 36%, and 33% at 14 h, 24 h, and 48 h PBM, respectively, using the mean value from each series of three experiments. Secondly, we assessed the effect of adding anti-CPBAg1 serum to an infected blood meal on P. falciparum development. As shown in Fig. 4A, the addition of anti-CPBAg1 serum to a P. falciparum gametocyte-containing blood meal blocked parasite development, leading to a reduction of more than 92% in the number of infected mosquitoes on day 7 postinfection compared to the control experiments using a naive rabbit serum. These results were consistently obtained in four independent infection experiments using either undiluted or diluted anti-CPBAg1 rabbit serum from two rabbits. In addition, the small number of infected mosquitoes fed on anti-CPBAg1 antibodies harbored few oocysts compared to the control groups fed on the same infected blood but supplemented with naive rabbit serum (Fig. 4B). In summary, serum directed against CPBAg1 reduces CPB activity and exhibits a blocking effect against P. falciparum development in A. gambiae midgut, suggesting that CPB activity influences P. falciparum development and that CPB could be used as target component for a TBV. To address this question further, a series of experiments using immunized mice and the rodent parasite P. berghei were performed, described below.
FIG. 4.
Effect of anti-CPBAg1 serum on P. falciparum development in A. gambiae. A. gambiae mosquitoes were fed on P. falciparum-infected red blood cells supplemented with either undiluted or diluted (1:1) anti-CPBAg1 serum. The proportion of infected mosquitoes (A) and intensity of infection (B) were determined on day 7 postfeeding. The proportion of infected mosquitoes was determined by using the mean value from each series of four infections using anti-CPBAg1 serum from two different rabbits. Each infection experiment was performed with gametocytes from a single carrier. The frequency distribution of oocysts pooled from four independent assays is displayed, with bars indicating the percentage of mosquitoes with an oocyst number in the range indicated on the x axis. Asterisks indicate statistically significant differences at the 95% confidence level, based on the P value from the chi-square test (A) and ANOVA (B). Numbers above bars (A) indicate the sample size.
CPBAg1 triggers the production of high-titer antibodies in mice.
To evaluate the immunogenicity of A. gambiae CPBAg1 as a vaccine candidate, mice were immunized with a recombinant CPBAg1 protein produced in the baculovirus/insect cell expression system (17). Two immunization regimens were used, a single injection (group 1) or two injections at a 3-week interval (group 2) (Fig. 5A). As shown in Fig. 5B, all mice receiving a single injection developed high titers of specific anti-CPBAg1 antibodies 24 days after immunization (endpoint titer ∼12,150), whereas the control mice injected with adjuvant and PBS alone showed undetectable CPBAg1-binding antibody levels, indicating that CPBAg1 is immunogenic even after a single injection. In group 2, a second injection increased the antibody titers (endpoint titer ∼36,450).
The specificity of the serum from each immunized mouse was assessed by Western blot analysis on mosquito midgut extracts; representative data are presented in Fig. 5C. All sera specifically recognized recombinant CPBAg1 and two bands in mosquito midgut extracts having the expected size of the zymogen and mature forms of CPBAg1 and CPBAg2. However, recombinant CPBAg2 was not detected at the dilution used (data not shown). As expected, the pooled serum from control mice did not detect any specific antigen in mosquito extracts and did not react with any of the recombinant CBP proteins (data not shown).
CPBAg1-immunized mice exhibit Plasmodium TBI.
To determine if the anti-CPBAg1 antibodies generated by mouse immunization were effective at blocking the transmission of Plasmodium, immunized and control mice were infected with P. berghei and used to feed mosquitoes. On day 3 after parasite injection, parasitemias and gametocytemias were in the same range in control mice and immunized mice within each experimental group. As shown in Fig. 6A, the rate of infection was significantly reduced in mosquitoes fed on CPBAg1-immunized mice from both groups compared to their respective controls, which consisted of mosquitoes fed on mice injected with adjuvant and PBS alone. In mosquitoes that developed oocysts, the mean number of oocysts was similar between control and immunized mice in all instances but three (Fig. 6B and data not shown). Interestingly, the transmission-blocking immunity (TBI) was effective as early as 3 weeks after the first immunization (group 1). In group 1, immunization with CPBAg1 led to a mean reduction of 63% in the number of infected mosquitoes. In group 2 (booster group), the mean reduction in the rate of infection reached only 51%, despite the fact that the antibody titer in this group was higher than in the group receiving a single injection. The lower TBI observed in group 2 might be due to the smaller size of this experimental group.
FIG. 6.
P. berghei transmission-blocking assays. The development of P. berghei was monitored in A. gambiae mosquitoes fed on control mice and mice immunized with CPBAg1. Data are expressed as the rate of infected mosquitoes determined by counting oocysts on mosquito midguts on day 9 postfeeding (A) and rate of infection (B). Group 1 mice received a single injection and group 2 mice a booster injection as shown in Fig. 5A. The percent reduction in rate of infection was calculated as 100 × [1 − (% infected mosquitoes fed on immunized mice/% infected mosquitoes fed on control mice)]. All data were statistically significant (P < 0.05) compared to the mean value from the control group, determined by the chi-square test.
Anti-CPBAg1 serum reduces mosquito reproductive capacity.
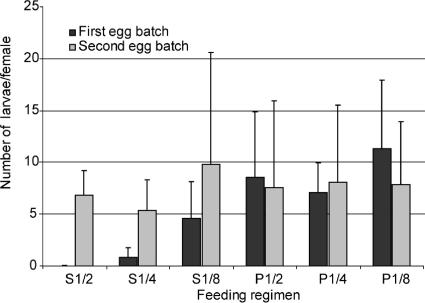
CPB are digestive exopeptidases that contribute with digestive endopeptidases (trypsin and chymotrypsin) to the degradation of the blood meal ingested by a female mosquito, providing amino acids that are necessary for the maturation of its eggs (1). The addition of anti-CPBAg1 antibodies, which reduce midgut CPB activity, to a blood meal would likely affect the maturation and quality of eggs and thus limit the reproductive capacity of the mosquitoes. Such an effect will likely affect disease transmission, as it is tightly linked to the vector population size. To assess whether ingestion of anti-CPBAg1 antibodies would affect mosquito reproductive capacity, female mosquitoes were fed on human red blood cells complemented with serial dilutions of anti-CPBAg1 serum using a membrane feeding system. Reproductive capacity was quantified as the number of larvae hatching from the first egg batch per female mosquito. As shown in Fig. 7, females which ingested anti- CPBAg1 serum had a reduced number of progeny, with progeny being almost absent at the highest concentrations of ingested antibodies. The effect was highly significant [F(1,22) = 16.9; P < 0.001]. To rule out a mosquito population effect on the observed data, we determined the number of hatching larvae from the second egg batch triggered by a normal blood meal. The numbers of progeny from the second egg batches were similar whether females received a first blood meal supplemented with anti-CPBAg1 serum or one supplemented with normal rabbit serum [F(1,22) = 0.03; P = 0.857], indicating that there was no bias among the different groups. This effect of anti-CPBAg1 serum on mosquito reproductive capacity will likely contribute to further reduction of the basic reproductive rate (R0) for malaria by reducing the number of mosquitoes per human host.
FIG. 7.
Ingestion of anti-CBAg1 antibodies reduces A. gambiae reproductive capacity. Groups of 50 mosquitoes were given a first blood meal composed of human red blood cells mixed with human AB serum and serial dilutions of anti-CPBAg1 serum starting at a 1/2 dilution (S1/2, S1/4, S1/8) or serial dilutions of a pool of naive rabbit serum (P1/2, P1/4, P1/8). After egg laying, mosquitoes received a second blood meal composed of human red blood cells mixed with human AB serum. Fecundity was estimated as the number of larvae hatching from the first and second egg batches corresponding to the first and second blood meals, respectively. Bars indicate standard deviations from four independent biological replicates.
DISCUSSION
We demonstrate here that serum recognizing A. gambiae CPB, CPBAg1, reduces the infectivity of malaria parasites to the mosquito, thereby validating it as a candidate for a malaria TBV subunit. CPBAg1 is the first molecule from Anopheles mosquitoes to be characterized and proposed as a TBV component.
The concept of a malaria TBV strategy arose in the 1950s from work with avian malaria (12), and it was confirmed for human malaria when it was demonstrated that antibodies targeting protein expressed in sexual stages of Plasmodium could block its development in the mosquito midgut (6, 20, 31). Since then, the impact of a TBV strategy on malaria control has been discussed extensively using diverse models (3, 4, 8, 9), most of which emphasize the idea that the impact of a TBV will be most significant in areas of malaria endemicity where the R0 is low. Nevertheless, in areas of endemicity with moderate or even high R0, the association of a TBV with a liver or blood stage vaccine, or in combination with pharmacological interventions, would be beneficial for malaria control by limiting the spread of drug-resistant parasites or parasites escaping a liver/blood stage vaccine (5, 13).
Candidate subunits of a TBV should possess the following qualities: (i) the capacity to induce the production of antibodies that impede the development of Plasmodium in its mosquito vector; (ii) limited antigenic diversity, such as that which might arise from selection by the human immune pressure; and (iii) the ability to induce the production of high antibody titers after a single injection of the vaccinated individual, as minimal or no natural boosting will occur. Molecules possessing these properties could be parasite-derived molecules as well as mosquito-derived molecules. To date, research has focused predominantly on parasite-derived molecules, such as the Pfs25 protein, which is expressed exclusively when the parasite develops in the mosquito midgut and therefore is not under selective pressure in the human host. A few reports documented the feasibility of a TBV based upon mosquito-derived molecules, using whole mosquito midgut extracts to generate transmission-blocking serum, or monoclonal antibodies in various mosquito-Plasmodium systems (7, 15, 16, 19). In addition, it was demonstrated that antibodies that inhibit midgut trypsin activity in Aedes aegypti are capable of blocking the development of the avian parasite Plasmodium gallinaceum, suggesting that mosquito trypsin could be a target for a TBV (22).
Our data show that CPBAg1 possesses properties defining candidacy as a component for a TBV: (i) antibodies directed against CPBAg1 impede P. falciparum development in the major African vector, A. gambiae; (ii) CPBAg1 is expressed exclusively in the mosquito midgut and is not exposed to selective pressure in humans; and (iii) mouse immunization experiments show that a single injection can induce high antibody titers and transmission-blocking activity. Moreover, CPBAg1 antiserum reduces mosquito reproductive capacity, an effect that may increase the impact of vaccination by reducing the local vector population, a major component of malaria transmission (23). It has been argued that a major drawback for TBV based on mosquito digestive proteases would be the resulting selection of resistant mosquitoes due to decrease reproductive fitness of the vector (13). However, as A. gambiae possesses two midgut CPB, targeting both will likely limit the selection of resistant mosquitoes.
Our current data suggest that the development of a TBV that is based upon both CPBAg1 and CPBAg2 would, in addition to limiting the selection of resistant mosquitoes, have an additive effect in limiting P. falciparum development. Indeed, the expression pattern of cpbAg2 suggests that CPBAg2 contributes substantially to the overall midgut CPB in both noninfected and infected mosquitoes. Despite the fact that anti-CPBAg1 antibodies only partially inhibit midgut CPB activity and only weakly recognize CPBAg2, they nevertheless have a significant effect on the development of P. falciparum. Thus, targeting both CPBAg1 and CPBAg2 should presumably result in a combinatorial increase in the efficiency of a TB-based vaccine.
The transmission-blocking effect of anti-CPBAg1 antibodies was greater when P. falciparum was used than when P. berghei was used. This might reflect either methodological differences between membrane feeding and direct feeding on immunized animals that led to variations in the number and amount of ingested gametocytes and immune serum or a lower efficiency of the mouse sera at blocking A. gambiae CPB activity. It might also reflect true differences between the two systems, as previously shown (26). Experiments are in progress to decipher the functional role of A. gambiae midgut carboxypeptidases in the development of the early sporogonic stages of Plasmodium. This will likely provide additional clues to explain the differences in the transmission-blocking effect on P. falciparum and P. berghei.
In conclusion, the work described in this report demonstrates that A. gambiae CPB are candidates for a TBV subunit that is capable of eliciting antibodies which limit P. falciparum development in the mosquito vector. These results further validate the concept that mosquito molecules can be targets for a P. falciparum TBV. Furthermore, the presumably high conservation of midgut carboxypeptidases in Anopheles mosquitoes, and the ability of these mosquitoes to transmit multiple species of human malaria parasites, suggests that the development of a TBV based on A. gambiae CPB might be an effective control measure in most countries where malaria is endemic.
Acknowledgments
We thank R. Ménard for critical reading of the manuscript and helpful suggestions. We are grateful to P. Baldacci, G. Milon, P. Druihle, S. Mecheri, R. Paul, and T. Templeton for discussion and comments. We are grateful to C. Thouvenot and J.-C. Jacques, members of CEPIA (Centre for Production and Infection of Anopheles, Pasteur Institute), for mosquito rearing and Alain Cosson for assistance with ELISA. We thank members of Paludologie Afrotropicale (IRD, Dakar, Senegal) for mosquito rearing and mass screening to detect parasite carriers in Senegalese villages. We thank A. Waters for supplying P. berghei ANKA strain 2.34.
This project was supported by fellowships to C. Lavazec (F. Lacoste, CANAM, Fondation des Treilles) and research funds from the Pasteur Institute and the French Ministry of Research (PAL+ Special Program). We are especially grateful to F. Lacoste for constant support of C. Lavazec.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Attardo, G. M., I. A. Hansen, and A. S. Raikhel. 2005. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem. Mol. Biol. 35:661-675. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet, S., G. Prevot, J. C. Jacques, C. Boudin, and C. Bourgouin. 2001. Transcripts of the malaria vector Anopheles gambiae that are differentially regulated in the midgut upon exposure to invasive stages of Plasmodium falciparum. Cell. Microbiol. 3:449-458. [DOI] [PubMed] [Google Scholar]
- 3.Carter, R. 1999. Epidemiological considerations for malaria reduction by transmission blocking vaccination. Parassitologia 41:415-420. [PubMed] [Google Scholar]
- 4.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 19:2309-2314. [DOI] [PubMed] [Google Scholar]
- 5.Carter, R., K. N. Mendis, L. H. Miller, L. Molineaux, and A. Saul. 2000. Malaria transmission-blocking vaccines: how can their development be supported? Nat. Med. 6:241-244. [DOI] [PubMed] [Google Scholar]
- 6.Carter, R., L. H. Miller, J. Rener, D. C. Kaushal, N. Kumar, M. Graves, C. A. Grotendorst, R. W. Gwadz, C. French, and D. Wirth. 1984. Target antigens in malaria transmission blocking immunity. Philos. Trans. R. Soc. Lond. Ser. B 307:201-213. [DOI] [PubMed] [Google Scholar]
- 7.Dinglasan, R. R., I. Fields, M. Shahabuddin, A. F. Azad, and J. B. Sacci, Jr. 2003. Monoclonal antibody MG96 completely blocks Plasmodium yoelii development in Anopheles stephensi. Infect. Immun. 71:6995-7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta, S., and R. M. Anderson. 1996. Predicting the effects of malaria vaccines on the population dynamics of infection and disease, p. 249-276. In S. L. Hoffman (ed.), Malaria vaccine development: a multi-immune response approach. ASM Press, Washington, DC.
- 9.Halloran, M. E., and C. J. Struchiner. 1992. Modeling transmission dynamics of stage-specific malaria vaccines. Parasitol. Today 8:77-85. [DOI] [PubMed] [Google Scholar]
- 10.Hay, S. I., C. A. Guerra, A. J. Tatem, P. M. Atkinson, and R. W. Snow. 2005. Urbanization, malaria transmission and disease burden in Africa. Nat. Rev. Microbiol. 3:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hisaeda, H., and K. Yasutomo. 2002. Development of malaria vaccines that block transmission of parasites by mosquito vectors. J. Med. Investig. 49:118-123. [PubMed] [Google Scholar]
- 12.Huff, C. G., D. F. Marchbank, and T. Shiroishi. 1958. Changes in infectiousness of malarial gametocytes. II. Analysis of the possible causative factors. Exp. Parasitol. 7:399-417. [DOI] [PubMed] [Google Scholar]
- 13.Kaslow, D. C. 1996. Transmission blocking vaccines, p. 181-227. In S. L. Hoffman (ed.), Malaria vaccine development: a multi-immune response approach. ASM Press, Washington, DC.
- 14.Kaslow, D. C. 1997. Transmission-blocking vaccines: uses and current status of development. Int. J. Parasitol. 27:183-189. [DOI] [PubMed] [Google Scholar]
- 15.Lal, A. A., P. S. Patterson, J. B. Sacci, J. A. Vaughan, C. Paul, W. E. Collins, R. A. Wirtz, and A. F. Azad. 2001. Anti-mosquito midgut antibodies block development of Plasmodium falciparum and Plasmodium vivax in multiple species of Anopheles mosquitoes and reduce vector fecundity and survivorship. Proc. Natl. Acad. Sci. USA 98:5228-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal, A. A., M. E. Schriefer, J. B. Sacci, I. F. Goldman, V. Louiswileman, W. E. Collins, and A. F. Azad. 1994. Inhibition of malaria parasite development in mosquitoes by anti-mosquito-midgut antibodies. Infect. Immun. 62:316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavazec, C., S. Bonnet, I. Thiery, B. Boisson, and C. Bourgouin. 2005. cpbAg1 encodes an active carboxypeptidase B expressed in the midgut of Anopheles gambiae. Insect Mol. Biol. 14:163-174. [DOI] [PubMed] [Google Scholar]
- 18.Moore, S., and W. H. Stein. 1948. Photometric ninhydrin method for use in the chromatography of amino acids. J. Biol. Chem. 176:367-388. [PubMed] [Google Scholar]
- 19.Ramasamy, M. S., and R. Ramasamy. 1990. Effect of anti-mosquito antibodies on the infectivity of the rodent malaria parasite Plasmodium berghei to Anopheles farauti. Med. Vet. Entomol. 4:161-166. [DOI] [PubMed] [Google Scholar]
- 20.Rener, J., P. M. Graves, R. Carter, J. L. Williams, and T. R. Burkot. 1983. Target antigens of transmission-blocking immunity on gametes of plasmodium falciparum. J. Exp. Med. 158:976-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert, V., G. le Goff, L. C. Gouagna, M. Sinden, J. Kieboom, R. Kroneman, and J. P. Verhave. 1998. Kinetics and efficiency of Plasmodium falciparum development in the midguts of Anopheles gambiae, An. funestus and An. nili. Ann. Trop. Med. Parasitol. 92:115-118. [DOI] [PubMed] [Google Scholar]
- 22.Shahabuddin, M., F. J. A. Lemos, D. C. Kaslow, and M. Jacobs-Lorena. 1996. Antibody-mediated inhibition of Aedes aegypti midgut trypsins blocks sporogonic development of Plasmodium gallinaceum. Infect. Immun. 64:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, D., and F. Ellis McKenzie. 2004. Statics and dynamics of malaria infection in Anopheles mosquitoes. Malaria J. 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srikrishnaraj, K. A., R. Ramasamy, and M. S. Ramasamy. 1995. Antibodies to Anopheles midgut reduce vector competence for Plasmodium vivax malaria. Med. Vet. Entomol. 9:353-357. [DOI] [PubMed] [Google Scholar]
- 25.Stowers, A., and R. Carter. 2001. Current developments in malaria transmission-blocking vaccines. Expert Opin. Biol. Ther. 1:619-628. [DOI] [PubMed] [Google Scholar]
- 26.Tahar, R., C. Boudin, I. Thiery, and C. Bourgouin. 2002. Immune response of Anopheles gambiae to the early sporogonic stages of the human malaria parasite Plasmodium falciparum. EMBO J. 21:6673-6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchuinkam, T., B. Mulder, K. Dechering, H. Stoffels, J. P. Verhave, M. Cot, P. Carnevale, J. H. E. T. Meuwissen, and V. Robert. 1993. Experimental infections of Anopheles gambiae with Plasmodium falciparum of naturally infected gametocyte carriers in Cameroon—factors influencing the infectivity to mosquitoes. Trop. Med. Parasitol. 44:271-276. [PubMed] [Google Scholar]
- 28.Tsuboi, T., M. Tachibana, O. Kaneko, and M. Torii. 2003. Transmission-blocking vaccine of vivax malaria. Parasitol. Int. 52:1-11. [DOI] [PubMed] [Google Scholar]
- 29.UNICEF and World Health Organization. 2005. World malaria report. http://rbm.who.int/wmr2005/.
- 30.Vaughan, J. A., B. H. Noden, and J. C. Beier. 1992. Population dynamics of Plasmodium falciparum sporogony in laboratory-infected Anopheles gambiae. J. Parasitol. 78:716-724. [PubMed] [Google Scholar]
- 31.Vermeulen, A. N., T. Ponnudurai, P. J. Beckers, J. P. Verhave, M. A. Smits, and J. H. Meuwissen. 1985. Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J. Exp. Med. 162:1460-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson, K. C. 2003. Pfs230: from malaria transmission-blocking vaccine candidate toward function. Parasite Immunol. 25:351-359. [DOI] [PubMed] [Google Scholar]