Abstract
We examined the interactions of live and lysed spirochetes with innate immune cells. THP-1 monocytoid cells were activated to comparable extents by live Borrelia burgdorferi and by B. burgdorferi and Treponema pallidum lysates but were poorly activated by live T. pallidum. Because THP-1 cells poorly internalized live spirochetes, we turned to an ex vivo peripheral blood mononuclear cell system that would more closely reflect spirochete-mononuclear phagocyte interactions that occur during actual infection. In this system, B. burgdorferi induced significantly greater monocyte activation and inflammatory cytokine production than did borrelial lysates or T. pallidum, and only B. burgdorferi elicited gamma interferon (IFN-γ) from NK cells. B. burgdorferi was phagocytosed avidly by monocytes, while T. pallidum was not, suggesting that the enhanced response to live B. burgdorferi was due to phagocytosis of the organism. When cytochalasin D was used to block phagocytosis of live B. burgdorferi, cytokine production decreased to levels comparable to those induced by B. burgdorferi lysates, while the IFN-γ response was abrogated altogether. In the presence of human syphilitic serum, T. pallidum was efficiently internalized and initiated responses resembling those observed with live B. burgdorferi, including the production of IFN-γ by NK cells. Depletion of monocytes revealed that they were the primary source of inflammatory cytokines, while dendritic cells (DCs) directed IFN-γ production from innate lymphocytes. Thus, phagocytosis of live spirochetes initiates cell activation programs in monocytes and DCs that differ qualitatively and quantitatively from those induced at the cell surface by lipoprotein-enriched lysates. The greater stimulatory capacity of B. burgdorferi versus T. pallidum appears to be explained by the successful recognition and phagocytosis of B. burgdorferi by host cells and the ability of T. pallidum to avoid detection and uptake by virtue of its denuded outer membrane rather than by differences in surface lipoprotein expression.
Borrelia burgdorferi and Treponema pallidum are the etiologic agents of Lyme disease and venereal syphilis, respectively. These multisystem, spirochetal diseases share many clinical and immunopathogenic features but also have some important differences. Although transmitted by different means (tick bite versus sexual contact), B. burgdorferi and T. pallidum each cause the development of a characteristic lesion at the site of inoculation, erythema migrans in Lyme disease and the chancre in syphilis, and both pathogens disseminate from these sites to invade diverse organ systems (33, 63). In the case of Lyme disease, constitutional symptoms often accompany erythema migrans despite what appear to be extremely low spirochetal burdens (33, 68). In contrast, systemic symptoms are uncommon in syphilis prior to the secondary stage of the disease, during which spirochetal burdens in blood and tissues are believed to be at their highest levels (44, 63). Both B. burgdorferi and T. pallidum can cause insidious ongoing inflammation and long-term sequelae in affected individuals, although the molecular mechanisms which enable these pathogens to persist are poorly understood and are the subjects of considerable debate (33, 63).
Genomic analysis of B. burgdorferi and T. pallidum has revealed that these pathogens lack orthologs of known exotoxins as well as the specialized secretory machinery required for the delivery of noxious molecules into host cells (14, 25, 26). Therefore, it is now generally accepted that the morbidity and mortality associated with syphilis and Lyme disease are a direct consequence of the inflammatory responses elicited by their etiologic agents (63, 81). B. burgdorferi and T. pallidum lack the classical gram-negative constituent lipopolysaccharide (LPS), which typically stimulates immune cells by Toll-like receptor 4 (TLR4) (2). Instead, they express numerous bacterial lipoproteins (BLPs) (14, 25, 26), which activate innate immune cells via TLR2/1 heterodimers in a CD14-dependent manner (2-4, 11, 35, 70, 80). Intradermal injection of synthetic lipoprotein analogs (lipopeptides) in both animals and humans has confirmed that these molecules can exert proinflammatory activities in vivo and that they have the capacity to recruit diverse leukocyte subsets into tissues (67, 71). In addition to initiating the innate immune response, TLR-derived signals also are essential in directing adaptive immune responses. Engagement of TLR2 by lipoproteins induces dendritic cell (DC) maturation whereby antigen processing is enhanced and major histocompatibility complex and costimulatory molecules are upregulated (10). Activation of DCs via TLR2 also has been shown to be critical for Th1 polarization during the priming of naïve T cells (72).
TLR-ligand interactions originally were studied as cell surface phenomena (18); it is now well documented, however, that some pathogen-associated molecular patterns (PAMPs), such as prokaryotic DNA, engage their cognate TLRs only from within intracellular compartments (1, 41). In addition, receptors such as TLR1, TLR2, and TLR6 that act at the cell surface also can be recruited to phagolysosomes, where they sample vacuolar contents (61, 77). Several years ago, we reported that live T. pallidum is markedly less stimulatory for THP-1 monocytic cells than live B. burgdorferi (69). We attributed this difference to the fact that B. burgdorferi surface lipoproteins are directly accessible to TLRs on monocytes, while cellular activation by T. pallidum, which has no demonstrated surface-exposed lipoproteins (13, 64), likely requires the liberation of sequestered PAMPs following degradation of the organisms within phagolysosomes (69). In this study, we sought to clarify the relative importance of cell surface versus intracellular activation by spirochetal pathogens. Contrary to results obtained using THP-1 cells, we found by using an ex vivo peripheral blood mononuclear cell (PBMC) model that live B. burgdorferi induced a response that differed quantitatively and qualitatively from that of B. burgdorferi lysates and that this augmented response was dependent on phagocytosis of the spirochetes. While B. burgdorferi demonstrated greater stimulatory capacity than unopsonized T. pallidum, this difference was essentially negated when opsonic antibody in human syphilitic serum was employed to drive uptake of treponemes into phagosomes. We also found that phagocytosis of spirochetes not only generated distinctive monocyte responses but also induced DCs to stimulate gamma interferon (IFN-γ) production by innate lymphocytes, potentially setting the stage for the intense Th1 polarization seen in both syphilis and Lyme disease (68, 78). Thus, the greater stimulatory capacity of B. burgdorferi versus T. pallidum appears to be explained by the successful recognition and phagocytosis of B. burgdorferi by host cells and the ability of T. pallidum to avoid detection and uptake by virtue of its denuded outer membrane rather than by differences in surface lipoprotein expression.
MATERIALS AND METHODS
Human subjects.
The University of Connecticut Health Center (UCHC) Institutional Review Board approved all protocols used here. Healthy volunteers with no history of syphilis or Lyme disease were recruited by the General Clinical Research Center at UCHC. Written informed consent was obtained, and blood was collected by venipuncture. Volunteers were confirmed to be seronegative for syphilis and/or Lyme disease by serological tests conducted in the UCHC clinical laboratory.
Bacterial strains.
Low-passage B. burgdorferi 297 was propagated in Barbour-Stoenner-Kelly medium containing 6% rabbit serum (Sigma). Spirochetes grown at 23°C were temperature shifted to 37°C; organisms were harvested at mid-log to late log phase (4 to 8 × 107/ml) by centrifugation at 8,000 × g, washed twice in CMRL (Gibco), and resuspended in RPMI medium (Gibco). For the microscopy studies, virulent B. burgdorferi 297 engineered to express green fluorescent protein (GFP) under the direction of the constitutive flaB promoter (20, 21) was prepared as described above. T. pallidum subsp. pallidum (Nichols strain) was propagated by intratesticular inoculation of adult New Zealand White rabbits as previously described (32) in strict accordance with a protocol approved by the UCHC Animal Care Committee. Treponemes were extracted from rabbit testes in CMRL, pelleted once by centrifugation at 14,000 × g, resuspended in RPMI, and enumerated by dark-field microscopy on a Petroff-Hausser counting chamber (Hausser Scientific).
Reagents.
A synthetic bacterial lipohexapeptide (BLP) corresponding to the N terminus of Escherichia coli murine lipoprotein (Bachem Bioscience) was used at a concentration of 10 μg/ml. LPS Re595 (Sigma) was suspended at 5 mg/ml in 0.2% triethylamine, repurified as previously described (36), and used at a final concentration of 100 ng/ml. B. burgdorferi and T. pallidum lysates were prepared from live organisms by sonication. The equivalence of live and lysed spirochete preparations was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining.
Cell preparation and stimulation.
THP-1 cells were propagated in RPMI supplemented with 10% fetal bovine serum (FBS) to a density of 1 × 106/ml and then matured with 160 ng/ml vitamin D3 (Biomol) for 72 to 96 h. The matured cells were washed in Hanks balanced salt solution (HBSS), plated at 1 × 106/ml in 24-well tissue culture plates, and stimulated with live or lysed spirochetes at multiplicities of infection (MOIs) of 1, 10, and 100 or with LPS (100 ng/ml) or BLP (10 μg/ml). Cells were incubated for 6 h prior to collection of supernatants for cytokine analysis. For phagocytosis studies, 1-μm Fluoresbrite carboxylated polystyrene microspheres (Polysciences, Inc.) were used as controls. Bead dilutions were prepared to mimic the spirochete MOIs.
Human PBMCs were isolated by Ficoll density gradient centrifugation according to the instructions of the manufacturer (Amersham Biosciences). Cells were washed three times with HBSS, counted on a hemocytometer, and resuspended at 1 × 106/ml in RPMI-10% FBS. Cells were plated and stimulated with live or lysed spirochetes or control agonists as described above and incubated for 8 h at 37°C with 5% CO2 prior to analysis. Selected samples were incubated with 10 μM cytochalasin D (CytoD) (Sigma) or LysoTracker Red endosomal dye (Molecular Probes). Some experiments with live T. pallidum included samples containing 10% heat-inactivated (56°C for 30 min) pooled human syphilitic serum (HSS) or normal human serum (NHS). When intracellular staining was performed, brefeldin A (Golgiplug; BD) was added at 4 h into the incubation at a dilution of 1 μl/ml. As a control for the effects of opsonic antibody, beads were incubated with 400 μg/ml of purified human immunoglobulin G (IgG) (Sigma) for 1 h at 37°C and then washed twice to remove unbound antibody. Antibody coating was confirmed by counterstaining with anti-human IgG-allophycocyanin (APC) antibody (BD). Microscopy experiments were performed on PBMCs incubated with spirochetes or beads for 4 h. LPS levels in culture media and reagents were confirmed by Limulus assay (Cambrex) to be <10 pg/ml.
Cell Staining and flow cytometry.
All antibody conjugates were purchased from either BD Pharmingen or eBioscience. The antibody panels used in the four-parameter staining of PBMCs are listed in Table 1. Unstimulated or stimulated PBMCs were harvested from tissue culture plates and washed once in fluorescence-activated cell sorting (FACS) buffer (phosphate-buffered saline, 0.1% bovine serum albumin, 0.055% Na azide) in preparation for flow cytometry. Cells were incubated for 10 min at 4°C with 10 μg of purified human IgG (Sigma) for Fc receptor (FcR) blocking, followed by a 20-min incubation with fluorochrome-conjugated antibodies. After a final wash in FACS buffer, cells were fixed in FACS buffer plus 6.25% paraformaldehyde. Individual cell populations were selectively gated for analysis based on the following expression phenotypes: monocytes, CD14+; monocytoid DCs (mDCs), lineage− HLA-DR+ CD11c+; plasmacytoid DCs (pDCs), lineage− HLA-DR+ CD11c−; NK cells, = CD56 or CD57+ CD3−; NKT cells, CD56 or CD57+ CD3+; γδ T cells, γδ T-cell receptor+ CD3+.
TABLE 1.
Antibody staining panels
| Panel no. | Fluorochrome label
|
Immunophenotypes detected | |||
|---|---|---|---|---|---|
| FITC | Phycoerythrin | PerCPa | APC | ||
| 1 | Lineage cocktail | CD83 | HLA-DR | CD11c | DCs and activated monocytes |
| 2 | GFP beads | CD14 | HLA-DR | CD11c | Monocytes and DCs with internalized beads |
| 3 | CD14 | CD40 | CD19 | Monocytes and activated B cells | |
| 4 | CD56 or CD57 | CD69 | CD3 | CD4 | Activated T, NK, and NKT cells |
| 5 | CD56 or CD57 | CD69 | CD3 | IFN-γ | IFN-γ-producing T, NK, and NKT cells |
| 6 | γδ T-cell receptor | CD8 | CD3 or CD21 | IFN-γ | IFN-γ-producing T-cell subsets |
PerCP, peridinin chlorophyll protein.
For intracellular cytokine staining (ICS), the cells were surface stained as described above and then permeabilized in 250 μl of Cytofix/Cytoperm solution (BD) for 20 min at 4°C, followed by one wash in PermWash solution (FACS buffer plus 0.5% saponin; Sigma). Cells were then resuspended in 50 μl of PermWash plus 3 μl of anti-IFN-γ-APC antibody and incubated for 30 min at 4°C. After two washes in PermWash, the cells were resuspended in FACS buffer and analyzed immediately. Flow cytometry was performed using a FACSCalibur dual-laser flow cytometer (BD); a minimum of 100,000 events and a maximum of 500,000 events were collected, depending on the cell type of interest. Multiparameter files were analyzed using WinMDI v2.8 software (Joseph Trotter, Scripps Clinic).
Cytokine analysis.
Simultaneous measurements of IFN-γ, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) were performed using the human Th1/Th2 Cytokine Bead Array (BD) per the manufacturer's protocol. Alternatively, the human inflammatory Cytokine Bead Array kit was used to measure TNF-α, IL-6, IL-1β, and IL-12p70. IL-12p70 also was measured by ultrasensitive enzyme-linked immunosorbent assay (Biosource).
Microscopy.
Following a 4-h incubation with GFP-expressing B. burgdorferi (B. burgdorferi-GFP), cells were harvested, mounted onto a microscope slide, and viewed immediately on an Olympus Bx60 mercury arc lamp fluorescence microscope at ×400 or ×1,000 under oil immersion. Incorporation of GFP fluorescence was visualized using a 470- to 490-nm excitation filter cube (Olympus). T. pallidum-cell associations were visualized by immunofluorescence assay (IFA) as described by Lukehart and Miller (45). HSS pooled from five secondary syphilis patients was diluted 1:100 in CMRL-10% goat serum and used as the primary antibody. NHS was used as an assay control. Secondary goat anti-human IgG antibody conjugated to fluorescein isothiocyanate (FITC) (Zymed) was used at a dilution of 1:250. Cells were visualized as described above for B. burgdorferi-GFP.
Depletion of monocytes from PBMCs by magnetic cell sorting.
PBMCs were suspended in ice-cold sorting buffer (phosphate-buffered saline, 2 mM EDTA, 0.5% bovine serum albumin, pH 7.2) and incubated with metallic bead-conjugated antibodies specific for CD14 or CD56 for the collection of monocytes or NK cells, respectively. Cells were then passed twice through separate ferromagnetic columns held in a magnetic field. The flowthrough was collected as the “depleted” cell fraction; the retained cells were harvested by flushing the column outside of the magnetic field. Sorted cells were washed twice with HBSS and resuspended in RPMI-10% FBS. The sorting magnet, separation columns, and all antibody conjugates were purchased from Miltenyi Biotech.
Statistical analysis.
Statistical analysis was performed using Microsoft Excel software. For comparison of each parameter, paired or unpaired Student t tests were performed, depending on whether the values were generated from within the same experiments or from parallel studies. All tests were two tailed and specified to a 95% confidence interval. For each value, both the standard deviation and the standard error about the mean were calculated. P values of ≤0.05 were considered significant.
RESULTS
Differential surface activation of human monocytic cells by B. burgdorferi and T. pallidum.
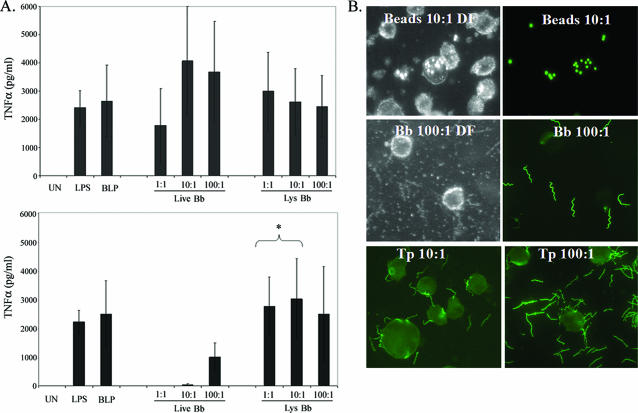
Initially, we used vitamin D3-matured THP-1 cells as a surrogate for human monocytes and compared their responses to individual PAMPs (LPS and BLP), live B. burgdorferi or T. pallidum, and sonicated preparations of spirochetes. As shown in Fig. 1A, live B. burgdorferi induced a strong TNF-α response, which was evident at the lowest MOI examined (1:1) and which was similar to the responses elicited by corresponding lysates. In contrast, live T. pallidum proved to be a much poorer activator of these cells in comparison to the equivalent doses of treponemal lysates; an appreciable response to live T. pallidum was evident only at an MOI of 100:1. To determine whether live spirochetes were taken up by the THP-1 cells, we used B. burgdorferi-GFP and IFA for T. pallidum. Although occasional binding of spirochetes to the cells was apparent, neither B. burgdorferi nor T. pallidum appeared to be internalized by this cell line (Fig. 1B). Thus, activation of these cells by spirochetes appears to occur predominantly or exclusively at the cell surface, while the difference in potency between the two spirochetal pathogens presumably is due to the presence of abundant lipoproteins on the surface of B. burgdorferi and the paucity of these and other PAMPs on the surface of T. pallidum (13, 64). As a test of general phagocytic capacity, we incubated cells with fluorescent polystyrene beads and found that our cell line was indeed capable of phagocytosis (Fig. 1B).
FIG. 1.
THP-1 cells are strongly activated by live B. burgdorferi and spirochetal lysates but not by live T. pallidum. A. Supernatants from vitamin D3-matured THP-1 cells incubated for 6 h with graded doses of live spirochetes or spirochetal lysates (Lys) were assayed for the presence of TNF-α. Shown are the average values ± standard errors from seven and four independent experiments conducted, respectively, with B. burgdorferi (Bb) and T. pallidum (Tp). Asterisks indicate a statistically significant difference between cytokine levels induced by live and lysed T. pallidum at the equivalent MOI. UN, unstimulated. B. Fluorescence microscopy of THP-1 cells incubated for 4 h with fluorescent beads, B. burgdorferi-GFP, or T. pallidum at the indicated MOIs. Cells incubated with B. burgdorferi-GFP were washed once prior to their application to microscope slides. Fluorescent beads and B. burgdorferi-GFP were visualized directly; T. pallidum was visualized by IFA. DF, dark-field image.
Live B. burgdorferi is a far more potent immune cell activator than lysates and live T. pallidum.
The observation that THP-1 cells did not internalize spirochetes prompted us to utilize a system that would more accurately reflect spirochete-monocyte interactions that occur during infection. We reasoned that an ex vivo model employing freshly isolated PBMCs would enable us to examine not only the interactions between spirochetes and native monocytes but also the responses of other mononuclear cell types that spirochetes are likely to encounter as they disseminate through blood and into tissues. We first used this system to compare the responses elicited by live B. burgdorferi and borrelial lysates. Surprisingly in light of our findings with THP-1 cells, fresh monocytes were more strongly activated by live organisms as determined by surface expression of CD40 and CD83 (Fig. 2A). Moreover, compared to lysates, live B. burgdorferi induced much greater production of TNF-α and IL-1β (Fig. 2B), as well as of IL-6 (data not shown). The results for IFN-γ were particularly striking; although the relative amounts of this cytokine in culture supernatants varied widely between different cell donors, it was consistently and reproducibly detected only in response to live B. burgdorferi (Fig. 3A). ICS revealed that NK cells were the primary producers of IFN-γ in response to B. burgdorferi (Fig. 3B); also evident is the high degree of sensitivity of ICS for detecting production of IFN-γ in minor lymphocyte populations. In contrast to live B. burgdorferi, which elicited a strong inflammatory response even at a spirochete-to-cell ratio of 1:1, monocyte activation and cytokine production in response to live T. pallidum were significantly above background only at MOIs of 10:1 or greater. Furthermore, compared to B. burgdorferi, live T. pallidum induced significantly less surface expression of CD40 and CD83 by monocytes (Fig. 4A), decreased production of TNF-α and IL-1β (Fig. 4B) and IL-6 (data not shown), and no IFN-γ (see Fig. 6D, left panels). Duplicate head-to-head comparisons of B. burgdorferi and T. pallidum confirmed that the difference in potency between the live organisms was not due to experiment-to-experiment variation (not shown).
FIG. 2.
Enhanced activation of peripheral blood monocytes by live versus lysed B. burgdorferi. PBMCs were incubated for 8 h with live or lysed (Lys) B. burgdorferi (Bb), and monocytes were selectively gated on as described in Materials and Methods. A. Surface expression of CD40 and CD83. B. TNF-α and IL-1β levels in culture supernatants. Bars depict the means ± standard errors from a minimum of four independent experiments. Asterisks indicate a statistically significant difference between values induced by live and lysed B. burgdorferi at the equivalent MOI. UN, unstimulated.
FIG. 3.
Live, but not lysed (Lys), B. burgdorferi (Bb) induces the production of IFN-γ by NK cells. A. IFN-γ levels in supernatants following incubation of PBMCs with spirochetes for 8 h. Shown are the averages ± standard errors from six experiments. B. Intracellular staining for IFN-γ. Lymphocyte subpopulations within whole PBMCs were delineated via marker expression as described in Materials and Methods. The cytograms shown are representative of three independent experiments. Numbers in the dot plots represent the percentage of cells positive for IFN-γ. UN, unstimulated; TCR, T-cell receptor.
FIG. 4.
Live B. burgdorferi is a much more potent activator of human monocytes than live T. pallidum. A. Monocyte expression of CD40 and CD83 following an 8-h incubation of PBMCs with live B. burgdorferi (Bb) or live T. pallidum (Tp). Monocytes were gated as the CD14+ population. B. TNF-α and IL-1β levels in culture supernatants. Data for B. burgdorferi represent a minimum of five experiments, including the four shown in Fig. 2; data for T. pallidum represent at least three experiments. Statistical comparisons between B. burgdorferi and T. pallidum data sets were performed using an unpaired t test. Asterisks indicate a statistically significant difference between values induced by B. burgdorferi and T. pallidum at equivalent MOIs. UN, unstimulated.
FIG. 6.
Enhanced inflammatory cytokine production following opsonophagocytosis of T. pallidum. A. Effect of HSS on the uptake of T. pallidum. PBMCs were incubated with live T. pallidum at a density of 10:1 for 4 h in the presence of 10% HSS and LysoTracker Red before IFA. The merged image (yellow) demonstrates colocalization of T. pallidum material (green) with lysosomal vacuoles (red). B and C. Internalization of T. pallidum (Tp) leads to enhanced macrophage activation (i.e., surface expression of CD40) (B) and greater production of TNF-α and IFN-γ (C). Monocytes were gated as the CD14+ population. Bar graphs represent the averages ± standard deviations from at least three experiments. Asterisks indicate a statistically significant difference between unopsonized and opsonized T. pallidum at equivalent MOIs. D. Intracellular cytokine staining for IFN-γ. Lymphocyte subpopulations within whole PBMCs were delineated via marker expression as described in Materials and Methods. Numbers in the dot plots represent the percentage of cells positive for IFN-γ; cytograms shown are representative of three experiments. TCR, T-cell receptor; PerCP, peridinin chlorophyll protein; UN, unstimulated.
Phagocytosis enhances monocyte and cytokine responses to viable spirochetes.
It has been reported that monocytes avidly phagocytose B. burgdorferi (8, 54), while the uptake of T. pallidum is inefficient in the absence of opsonins (45). We therefore hypothesized that the augmented response to live B. burgdorferi, compared to lysates and live T. pallidum, resulted from uptake of B. burgdorferi by monocytes and liberation of borrelial PAMPs following degradation within phagolysosomes. When PBMCs were incubated with B. burgdorferi-GFP, we observed numerous vacuoles containing degraded organisms, but no intact spirochetes, inside a substantial fraction of the cells (Fig. 5A); colocalization of GFP fluorescence with LysoTracker dye revealed that the digested borreliae were inside phagolysosomal vacuoles (Fig. 5A). In contrast, we were unable to detect uptake of T. pallidum by IFA (Fig. 5B). On average, 10% of the PBMCs contained ingested spirochetes, a percentage highly similar to the average monocyte composition of the mixture determined by FACS analysis (12%). Flow cytometry subsequently confirmed that monocytes avidly internalize B. burgdorferi-GFP (see Fig. 9, top row). The cytochalasins have been used extensively to inhibit phagocytosis of many different types of bacteria, and they also have been shown to have no effect on the production of cytokines in response to agonists acting at the cell surface (15, 28, 56, 66). When CytoD was used to block phagocytosis of live B. burgdorferi, production of proinflammatory cytokines decreased to levels comparable to those induced by B. burgdorferi lysates, while the IFN-γ response was abrogated altogether (Fig. 5C). Flow cytometry confirmed that CytoD at the dosage used (10 μg/ml) virtually eliminated the uptake of B. burgdorferi-GFP by monocytes at an MOI of 10:1 and markedly inhibited uptake of spirochetes at the highest MOI (100:1) employed. (Fig. 5D).
FIG. 5.
Internalization is a critical event for stimulation of cytokine production by live spirochetes. A. B. burgdorferi-GFP was incubated with PBMCs in the presence of LysoTracker Red for 4 h at an MOI of 10:1 prior to fluorescence microscopy. The merged image (yellow) shows colocalization of GFP (green) with endosomal compartments (red). B. PBMCs were incubated with T. pallidum at an MOI of 10:1 in the presence of LysoTracker Red for 4 h prior to IFA and fluorescence microscopy. C. Effect of CytoD on the TNF-α response of PBMCs to live and lysed B. burgdorferi (Bb). Shown are results from one of two experiments; error bars represent the standard deviations for duplicate samples. D. CytoD inhibits uptake of live B. burgdorferi by monocytes. PBMCs were preincubated with cytochalasin D (10 μg/ml) for 1 h prior to the addition of B. burgdorferi-GFP at various MOIs for 8 hours. The cells then were stained with CD14 and analyzed by flow cytometry to determine the percentage of monocytes that had internalized spirochetes (GFP-positive cells) and the number of spirochetes taken up per monocyte (represented by the mean fluorescence intensity [MFI] of GFP-positive cells). Shown are the means ± standard deviations from four independent experiments. Asterisks indicate P values of ≤0.05 for CytoD-treated and untreated samples. UN, unstimulated.
FIG. 9.
Monocytes and DCs internalize B. burgdorferi. Whole PBMCs were incubated with B. burgdorferi-GFP for 4 h, stained with antibodies for surface antigens, and then analyzed by flow cytometry to determine the percentage of GFP-positive cells in monocytes (Monos), mDCs, and pDCs. Cell populations were gated as described in Materials and Methods. Cytograms shown are representative of three experiments. Numbers in the dot plots represent the percentage of cells positive for GFP fluorescence.
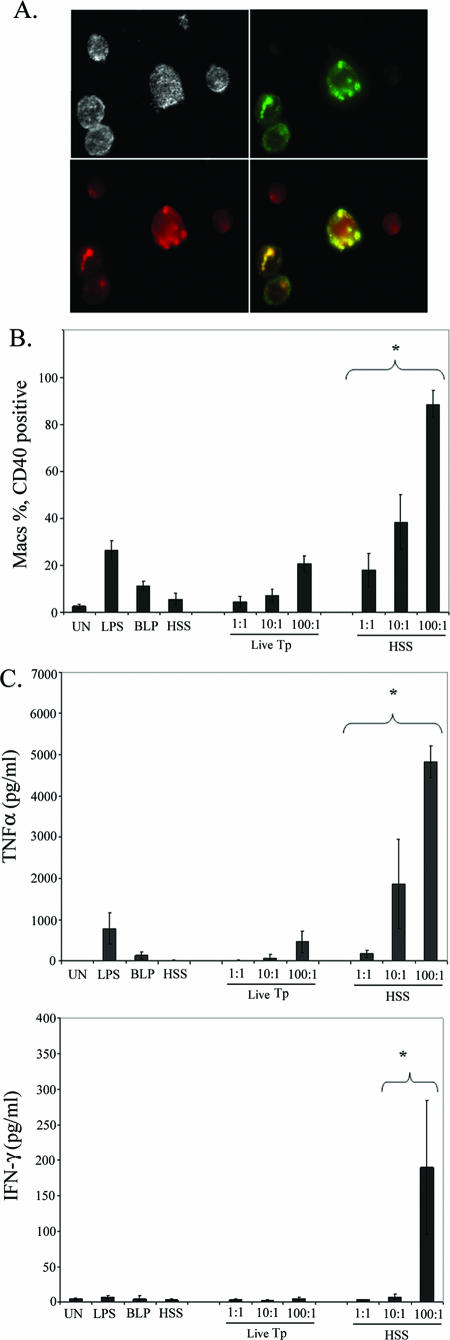
In the rabbit model of experimental syphilis, ingestion and clearance of T. pallidum by macrophages are markedly facilitated by the addition of rabbit immune serum but can be blocked by cytochalasin B (6, 43, 45); the ability of antibodies in HSS to opsonize T. pallidum, however, has not been reported. Based on our results with B. burgdorferi, we hypothesized that incubation of treponemes with HSS would promote their internalization by PBMCs and that increased uptake would result in enhanced immune cell activation. Indeed, as shown in Fig. 6A, degraded treponemes within phagolysosomes were readily visualized when T. pallidum was incubated with PBMCs in the presence of 10% heat-inactivated HSS but not heat-inactivated NHS (not shown). Enumeration of residual spirochetes was consistent with the microscopy data; less than one-half of the input treponemes were recovered from cell suspensions incubated at an MOI of 10 or 100 with HSS, while spirochete counts in cells incubated with NHS were essentially unchanged from input values (data not shown). Opsonophagocytosis of T. pallidum markedly increased macrophage activation as assessed by the surface expression of CD40 (Fig. 6B) and CD83 (not shown) and the secretion of TNF-α (Fig. 6C), IL-6 (not shown), and IL-1β (not shown). The de novo induction of IFN-γ by opsonized T. pallidum (Fig. 6C and D) further differentiated this response from that induced by unopsonized treponemes. The IFN-γ response to low MOIs of T. pallidum resembled that induced by B. burgdorferi in that it was mediated primarily by NK cells (compare Fig. 6D with 3D). High concentrations of opsonized T. pallidum, however, induced the production of this cytokine from a broader spectrum of innate lymphocytes, namely, NK cells, NKT cells, and γδ T cells (Fig. 6D). A small number of CD3+ CD8+ cells, likely representing CD8+ γδ and/or NKT cells (or iNKT cells) (37, 40), also produced IFN-γ in response to high levels of opsonized T. pallidum (Fig. 6D, bottom row). To confirm that the responses observed were due to uptake and degradation of T. pallidum and not FcR signaling, we incubated PBMCs with carboxylate beads coated with human IgG; opsonized beads were highly internalized but did not induce cytokines (Fig. 7).
FIG. 7.
Antibody-coated polystyrene beads are avidly internalized by monocytes but do not induce cellular activation. A. Fluorescent polystyrene beads were incubated with purified human IgG and counterstained with an anti-human IgG-APC conjugate prior to flow cytometric analysis. B. Antibody-coated beads were incubated with PBMCs for 4 h and uptake by monocytes (CD14+ events) was assessed by flow cytometry; numbers indicate the percentage of cells containing fluorescent beads. PE, phycoerythrin. y axis, beads fluorescing in channel 1 (FITC). C. Opsonized beads do not elicit an inflammatory cytokine response. TNF-α and IL-1β in PBMC culture supernatants were measured following 8 h of incubation with the indicated stimuli. UN, unstimulated. The results shown in all panels are representative of three independent experiments.
The enhanced response to internalized spirochetes is mediated by both monocytes and DCs.
Our PBMC system contains several types of mononuclear phagocytes (monocytes, mDCs [CD11c+], and pDCs [CD11c−]), with the capacity to activate innate lymphocytes by contact-dependent and/or -independent mechanisms (29, 48, 73). To determine if monocytes stimulate IFN-γ production in response to live spirochetes, we depleted the CD14+ cells from the mixture via magnetic cell sorting, typically achieving an absorption efficiency of ≥99%. Monocyte-depleted PBMCs stimulated with live B. burgdorferi (Fig. 8A) or opsonized T. pallidum (Fig. 8B) produced dramatically less TNF-α, IL-1β, and IL-6 (not shown) than whole PBMCs, demonstrating conclusively that monocytes were the primary source of these proinflammatory cytokines. In contrast, the IFN-γ response elicited by B. burgdorferi and T. pallidum (Fig. 8A and B) was unchanged by the depletion of monocytes from PBMCs, indicating that DCs stimulated production of this cytokine from innate lymphocytes. This conclusion was buttressed by the observation that purified NK cells, incubated alone or in coculture with purified monocytes, did not produce IFN-γ in response to live B. burgdorferi (data not shown). Interestingly, using ultrasensitive cytokine assays, we were unable to detect IL-12p70, a cytokine known to stimulate NK and γδ T cells (9, 51), even in response to high MOIs of B. burgdorferi or opsonized T. pallidum (data not shown).
FIG. 8.
Depletion of monocytes from PBMCs markedly diminishes production of TNF-α and IL-6, but not IFN-γ, following incubation for 8 h with live B. burgdorferi (Bb) (A) or opsonized T. pallidum (Tp) (B). Data for TNF-α and IL-1β depict the averages ± standard deviations from three (B. burgdorferi) or two (T. pallidum) individual experiments. Dot plots for IFN-γ are representative of at least two experiments; numbers in the dot plots denote the percentage of cells positive for IFN-γ. Lymphocyte subpopulations within whole PBMCs were delineated via marker expression as described in Materials and Methods. Only NK cells are shown for B. burgdorferi experiments in panel A. CD4 T cells did not produce IFN-γ and are not shown (B). UN, unstimulated; TCR, T-cell receptor; PerCP, peridinin chlorophyll protein.
To shed further light on how DCs interact with B. burgdorferi and T. pallidum, we characterized their capacity to internalize spirochetes and their subsequent activation patterns. When we used B. burgdorferi-GFP to track the uptake of spirochetes by DCs, we were able to detect fluorescence in both mDCs and pDCs (Fig. 9). Notably, the percentage of mDCs containing spirochetes was approximately threefold greater than the proportion of GFP-positive pDCs. As with monocytes, pDCs were activated to a greater degree by live B. burgdorferi than by borrelial lysates (Fig. 10A, left panel). Furthermore, pDC activation by opsonized T. pallidum exceeded that induced by unopsonized treponemes (Fig. 10B, left panel). Interestingly, in our system, we routinely noticed significant pDC activation in response to spirochete preparations as well as to LPS and BLP (Fig. 10), yet these cells are not believed to express TLR4 or TLR2 (2, 38). When monocytes were depleted from the PBMCs, however, pDC activation by all stimuli was notably diminished (data not shown). Assessment of mDC activation by surface CD83 expression was complicated by the relatively high background levels of activation caused by even minor manipulations, as noted by others (23). Nonetheless, we still detected significantly greater activation of mDCs in response to opsonized versus unopsonized T. pallidum at MOIs of 1:1 and 10:1 (Fig. 10B, right panel). Live B. burgdorferi also activated mDCs to a greater degree than lysed B. burgdorferi, although these differences were significant only at an MOI of 100:1 (Fig. 10A, right panel). In contrast to the situation with pDCs, mDC activation by all stimuli was unchanged in monocyte-depleted PBMCs (not shown).
FIG. 10.
Activation of DCs by live B. burgdorferi and T. pallidum. Whole PBMCs were incubated with the indicated stimuli, and CD83 expression on gated pDCs or mDCs in response to live versus lysed (Lys) B. burgdorferi (Bb) (A) or to unopsonized versus opsonized T. pallidum (Tp) (B) was determined. Shown are the averages ± standard errors for four experiments. Asterisks indicate a statistically significant difference when comparing live and lysed B. burgdorferi or opsonized and unopsonized T. pallidum at equivalent MOIs. UN, unstimulated.
DISCUSSION
Investigations of cellular responses to bacterial pathogens have often utilized monocytic cell lines along with bacterial lysates and/or isolated PAMPs to model pathogen-monocyte/macrophage interactions. The limited phagocytic capacity of THP-1 cells, as determined here, suggested that such in vitro models might not fully recapitulate spirochete-mononuclear phagocyte interactions as they occur during infection. Indeed, using freshly isolated human mononuclear cells, we found that phagocytosis of live organisms initiated activation programs in both monocytes and DCs that differed quantitatively and qualitatively from those elicited by lipoprotein-enriched lysates or individual PAMPs, which act at the cell surface. Microscopy studies demonstrated that internalized B. burgdorferi and T. pallidum were completely degraded within phagolysosomes, suggesting that the augmented responses of phagocytic cells are triggered by the release of spirochetal products within maturing phagosomes.
Amplification of the proinflammatory cytokine response to spirochetes from within the phagosome may occur by a number of nonexclusive mechanisms. TLR responses initiated from within the phagosome may be augmented by a high concentration of PAMPs within a relatively small space. In addition, TLR2 and its cooperative pairing partners TLR1 and TLR6 have been shown to be actively recruited to the phagosome (61, 77), and this may lead to a greater receptor density in this compartment than at the cell surface. Phagolysosomes are dynamic organelles that mature, transmit signals to the cytosol, and direct their own interactions with other cellular compartments via a complex set of membranous proteins on the organelle surface (76). Thus, the presence of specific “docking” molecules may provide a particularly effective scaffold for TLR machinery. Yet another possibility is that TLR signaling pathways distinct from those at the cell surface are activated within the phagosome upon the liberation of sequestered PAMPs by bacterial disruption. The fact that some TLRs, such as TLR9, signal only from within phagolysosomes supports this conjecture (1, 41). Several spirochetal constituents, such as flagellin, DNA, and lipoproteins in the case of T. pallidum, would be available to cognate pattern recognition receptors only following spirochetal degradation. The activation of multiple TLRs may then lead to synergistic amplification of the signaling cascade, resulting in greater cytokine secretion. The phenomenon of TLR cooperation to generate immune responses is becoming increasingly recognized (5, 59).
A particularly intriguing finding was the marked amplification of IL-1β production in response to internalized spirochetes. Unlike the case for other proinflammatory cytokines, which are linearly induced by TLR signaling, the production of biologically active IL-1β requires the integration of at least two signaling events: NF-κB-mediated induction of pro-IL-1β synthesis and conversion of the procytokine into its active form by caspase-1 (16, 19). Interestingly, while TLRs are potent inducers of pro-IL-1β, they are inefficient at initiating caspase-1 activity, which requires the assembly and activation of the cytosolic multimolecular complex known as the inflammasome (50). Therefore, pure PAMPs acting at the cell surface, such as LPS and BLPs, elicit only low levels of active IL-1β, as demonstrated here (Fig. 2 and 4) and elsewhere (62, 82). We envision two potential mechanisms by which internalized spirochetes activate cytosolic signaling cascades resulting in robust IL-1β production. First, signals may be transmitted into the cytosol by receptors that traverse the phagosomal membrane. Such intercompartmental signaling may be mediated by TLRs or by as-yet-unidentified phagosomal receptors that may be recruited to the phagosome and activated by spirochetal PAMPs to direct inflammasome assembly. Second, as recently proposed (76), it is possible that the phagosome leaks pathogen components into the cytosol for delivery to cytosolic receptors in a manner analogous to the transfer of antigen for cross-presentation. It is interesting to note that in other models of monocyte-pathogen interactions, activation of caspase-1 is achieved by viable microorganisms due to their ability to escape endosomal compartments and enter the cytoplasm (28, 34) or to secrete material directly into the cytosol via dedicated secretory systems (24, 53, 79). Live spirochetes possess neither of these capabilities.
One of our most striking observations was that IFN-γ was produced by innate lymphocytes in an accessory cell-dependent manner exclusively in response to internalized spirochetes. Moreover, in contrast to production of the proinflammatory cytokines, which virtually disappeared when monocytes were removed from the system, IFN-γ production was refractory to monocyte depletion. This observation led us to conclude that the activation of innate lymphocytes was mediated by DCs. Both DC subsets internalized spirochetes and were activated to a greater degree by live organisms; several observations, however, suggest that mDCs direct this response. For one, mDCs internalized B. burgdorferi-GFP with greater efficiency than pDCs, consistent with the current paradigm that these cells are more phagocytic and function as the primary antigen-processing and -presenting cells for the initiation of adaptive responses (7, 17). Second, mDCs were highly activated in a direct manner by live spirochetes. In contrast, the strong pDC response to live spirochetes was diminished by the depletion of monocytes, while the IFN-γ response remained unchanged, demonstrating a discordance between pDC activation and IFN-γ induction in our ex vivo system. Finally, mDCs are well known to activate NK, NKT, and γδ T cells by a wide variety of mechanisms (27, 29, 40, 57, 58). Nonetheless, pDCs have been shown to activate NK cells (65) and therefore have not been ruled out as a stimulator of IFN-γ production in our ex vivo system. Furthermore, pDC activation in response to B. burgdorferi and T. pallidum is reported to occur in vivo (68, 68a).
The clinical responses observed in both syphilis and Lyme disease are intensely Th1 polarized (68, 78). IFN-γ produced by DC-activated NK (and other) cells has been shown to provide a reciprocal signal to DCs for the induction of IL-12, ultimately generating a cytokine milieu that promotes Th1 development in T cells as they are primed in the lymph nodes (46, 49, 74). Thus, the production of IFN-γ by innate lymphocytes, as observed in our study, could provide the Th1 priming signal during spirochetal infection. As IFN-γ was elicited only by live organisms, and not by lysates or individual PAMPs, the induction of this cytokine in innate lymphocytes could act as a gauge at the innate-adaptive interface for the immune system to differentiate between the presence of proinflammatory agonists and viable pathogens. There is considerable debate about the benefit of a Th1 response in the case of Lyme disease. Th1 responses are classically believed to target intracellular pathogens by providing a signal for enhanced bactericidal killing by macrophages in the form of IFN-γ. As B. burgdorferi is an extracellular pathogen that is easily killed by monocytes, as shown here, and by macrophages in the absence of IFN-γ (55), the advantage of a Th1 response in the case of B. burgdorferi infection is unclear. Studies with the murine model have demonstrated no requirement for IFN-γ in bacterial clearance or disease susceptibility (12, 30) but have suggested that the Th1 response is actually deleterious in the course of Lyme disease (52). On the other hand, a study that carefully examined the kinetics of Th cell polarization in Lyme arthritis-susceptible and -resistant mice demonstrated that the Th1 response in fact correlated with better control of infection early in disease and was detrimental only when prolonged (39). IFN-γ has been shown to enhance monocyte effector functions in response to spirochetal constituents (47) and so may function to expedite the recruitment of key immune cells to the site of infection during the initial phases of the disease. In contrast to the case for Lyme disease, the requirement for opsonic antibody helps to explain the benefit of a Th1 response in syphilis. The IgG isotypes generated in a Th1 context, such as IgG2a (75), are bound with a high affinity by activating Fc receptors (FcγRI and FcγRIIIa) but poorly by the inhibitory FcγRIIb (22, 60). Furthermore, IFN-γ promotes the process of opsonophagocytosis via the upregulation of activating FcγRs and the downregulation of the inhibitory FcγRs on monocytes (42).
The work presented here has helped to clarify the mechanisms by which B. burgdorferi and T. pallidum initiate innate and adaptive immune responses. Taken collectively, our data demonstrate that the difference in immunostimulatory capacity between B. burgdorferi and T. pallidum is a result of dramatically different levels of phagocytosis of these two organisms, not the relative abundance of surface-expressed lipoproteins per se as we previously proposed (69). Presumably, live B. burgdorferi displays at least one moiety on its surface, which T. pallidum lacks, that promotes recognition and phagocytosis by host cells. When the host counters this immune evasion strategy via the production of opsonic antibody, the efficiency of T. pallidum internalization is dramatically enhanced, leading to cellular and cytokine responses similar to those generated by B. burgdorferi. FcRs are known to initiate numerous signaling pathways and are reported to downregulate immune responses (31). Our data indicate, however, that the primary purpose of FcRs is to deliver T. pallidum to phagolysosomes for PAMP-mediated recognition, rather than to modulate the pathogen-specific immune response. The delayed onset of constitutional symptoms in syphilis, compared to Lyme disease, may reflect the fact that phagocytosis of T. pallidum and the subsequent generation of inflammatory responses do not occur efficiently until opsonic antibody is generated as part of the adaptive immune response. The intrinsic ability of T. pallidum to evade phagocytosis highlights how exquisitely adapted it is as a human pathogen.
Acknowledgments
We thank Morgan LaVake, Cynthia Gonzales, and Ken Bourell for superb technical support; Melissa Caimano for assistance with passaging of B. burgdorferi; Timothy Sellati for insightful comments regarding the manuscript; and Sheila Lukehart for thoughtful comments and discussions.
This work was supported by Public Health Service grants AI-26756, AI-29735, and AI-38894 to J.D.R. and by General Clinical Research Center grant M01RR06192 from the National Institutes of Health.
Editor: D. L. Burns
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Ahmad-Nejad, P., H. Hacker, M. Rutz, S. Bauer, R. M. Vabulas, and H. Wagner. 2002. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 32:1958-1968. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. [DOI] [PubMed] [Google Scholar]
- 4.Aliprantis, A. O., R. B. Yang, M. R. Mark, S. Suggett, B. Devaux, J. D. Radolf, G. R. Klimpel, P. Godowski, and A. Zychlinsky. 1999. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science 285:736-739. [DOI] [PubMed] [Google Scholar]
- 5.Bafica, A., C. A. Scanga, C. G. Feng, C. Leifer, A. Cheever, and A. Sher. 2005. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J. Exp. Med. 202:1715-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker-Zander, S. A., J. M. Shaffer, and S. A. Lukehart. 1993. Characterization of the serum requirement for macrophage-mediated killing of Treponema pallidum ssp. pallidum: relationship to the development of opsonizing antibodies. FEMS Immunol. Med. Microbiol. 6:273-279. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 8.Benach, J. L., H. B. Fleit, G. S. Habicht, J. L. Coleman, E. M. Bosler, and B. P. Lane. 1984. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J. Infect. Dis. 150:497-507. [DOI] [PubMed] [Google Scholar]
- 9.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar- Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 10.Blander, J. M., and R. Medzhitov. 2006. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440:808-812. [DOI] [PubMed] [Google Scholar]
- 11.Brightbill, H. D., D. H. Libraty, S. R. Krutzik, R. B. Yang, J. T. Belisle, J. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 12.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron, C. E. 2006. The T. pallidum outer membrane and outer membrane proteins, p. 237-266. In J. D. Radolf and S. A. Lukehart (ed.), Pathogenic treponemes: molecular and cellular biology. Caister Academic Press, Norwich, United Kingdom.
- 14.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. M. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490-516. [DOI] [PubMed] [Google Scholar]
- 15.Chiani, P., C. Bromuro, and A. Torosantucci. 2000. Defective induction of interleukin-12 in human monocytes by germ-tube forms of Candida albicans. Infect. Immun. 68:5628-5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cogswell, J. P., M. M. Godlevski, G. B. Wisely, W. C. Clay, L. M. Leesnitzer, J. P. Ways, and J. G. Gray. 1994. NF-κB regulates IL-1Β transcription through a consensus NF-κB binding site and a nonconsensus CRE-like site. J. Immunol. 153:712-723. [PubMed] [Google Scholar]
- 17.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 18.Creagh, E. M., and L. A. O'Neill. 2006. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27:352-357. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello, C. A. 1998. Interleukin-1Β, interleukin-18, and the interleukin-1Β converting enzyme. Ann. N. Y. Acad. Sci. 856:1-11. [DOI] [PubMed] [Google Scholar]
- 20.Eggers, C. H., M. J. Caimano, M. L. Clawson, W. G. Miller, D. S. Samuels, and J. D. Radolf. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281-295. [DOI] [PubMed] [Google Scholar]
- 21.Eggers, C. H., M. J. Caimano, and J. D. Radolf. 2006. Sigma factor selectivity in Borrelia burgdorferi: RpoS recognition of the ospE/ospF/elp promoters is dependent on the sequence of the −10 region. Mol. Microbiol. 59:1859-1875. [DOI] [PubMed] [Google Scholar]
- 22.Fanger, N. A., D. Voigtlaender, C. Liu, S. Swink, K. Wardwell, J. Fisher, R. F. Graziano, L. C. Pfefferkorn, and P. M. Guyre. 1997. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor Fc gamma RI (CD64) expressed on human blood dendritic cells. J. Immunol. 158:3090-3098. [PubMed] [Google Scholar]
- 23.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1Β in Salmonella-infected macrophages. Nat. Immunol. 7:576-582. [DOI] [PubMed] [Google Scholar]
- 25.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J. F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 26.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, G. G. Sutton, R. Dodson, M. Gwinn, E. K. Hickey, R. Clayton, K. A. Ketchum, E. Sodergren, J. M. Hardham, M. P. McLeod, S. Salzberg, J. Peterson, H. Khalak, D. Richardson, J. K. Howell, M. Chidambaram, T. Utterback, L. McDonald, P. Artiach, C. Bowman, M. D. Cotton, and J. C. Venter. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 27.Fujii, S., K. Shimizu, M. Kronenberg, and R. M. Steinman. 2002. Prolonged IFN-γ-producing NKT response induced with alpha-galactosylceramide-loaded DCs. Nat. Immunol. 3:867-874. [DOI] [PubMed] [Google Scholar]
- 28.Gavrilin, M. A., I. J. Bouakl, N. L. Knatz, M. D. Duncan, M. W. Hall, J. S. Gunn, and M. D. Wewers. 2006. Internalization and phagosome escape required for Francisella to induce human monocyte IL-1β processing and release. Proc. Natl. Acad. Sci. USA 103:141-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grazia, C. M., F. S. Sutterwala, G. Trinchieri, D. M. Mosser, and X. Ma. 2001. Suppression of IL-12 transcription in macrophages following Fcγ receptor ligation. J. Immunol. 166:4498-4506. [DOI] [PubMed] [Google Scholar]
- 32.Hazlett, K. R. O., T. J. Sellati, T. T. Nguyen, D. L. Cox, M. L. Clawson, M. J. Caimano, and J. D. Radolf. 2001. The T. pallidumrK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J. Exp. Med. 193:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hengge, U. R., A. Tannapfel, S. K. Tyring, R. Erbel, G. Arendt, and T. Ruzicka. 2003. Lyme borreliosis. Lancet Infect. Dis. 3:489-500. [DOI] [PubMed] [Google Scholar]
- 34.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 35.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by Toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 36.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 37.Imlach, S., C. Leen, J. E. Bell, and P. Simmonds. 2003. Phenotypic analysis of peripheral blood γδ T lymphocytes and their targeting by human immunodeficiency virus type 1 in vivo. Virology 305:415-427. [DOI] [PubMed] [Google Scholar]
- 38.Kadowaki, N., S. Ho, S. Antonenko, M. R. de Waal, R. A. Kastelein, F. Bazan, and Y. J. Liu. 2001. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang, I., S. W. Barthold, D. H. Persing, and L. K. Bockenstedt. 1997. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect. Immun. 65:3107-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronenberg, M., and L. Gapin. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557-568. [DOI] [PubMed] [Google Scholar]
- 41.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190-198. [DOI] [PubMed] [Google Scholar]
- 42.Liu, Y., E. Masuda, M. C. Blank, K. A. Kirou, X. Gao, M. S. Park, and L. Pricop. 2005. Cytokine-mediated regulation of activating and inhibitory Fcγ receptors in human monocytes. J. Leukoc. Biol. 77:767-776. [DOI] [PubMed] [Google Scholar]
- 43.Lukehart, S. A. 1992. Immunology and pathogenesis of syphilis, p. 141-163. In T. C. Quinn (ed.), Advances in host defense mechanisms, vol. 8. Sexually transmitted diseases. Raven Press, New York, NY. [Google Scholar]
- 44.Lukehart, S. A. 2004. Syphilis, p. 977-985. In E. Brauwald, A. S. Faucci, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrison's principles of internal medicine. McGraw Hill, New York, NY.
- 45.Lukehart, S. A., and J. N. Miller. 1978. Demonstration of the in vitro phagocytosis of Treponema pallidum by rabbit peritoneal macrophages. J. Immunol. 121:2014-2024. [PubMed] [Google Scholar]
- 46.Ma, X., J. M. Chow, G. Gri, G. Carra, F. Gerosa, S. F. Wolf, R. Dzialo, and G. Trinchieri. 1996. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J. Exp. Med. 183:147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma, Y., and J. J. Weis. 1993. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect. Immun. 61:3843-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall, J. D., D. S. Heeke, C. Abbate, P. Yee, and N. G. Van. 2006. Induction of interferon-γ from natural killer cells by immunostimulatory CpG DNA is mediated through plasmacytoid-dendritic-cell-produced interferon-α and tumour necrosis factor-α. Immunology 117:38-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Fontecha, A., L. L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-γ for T(H)1 priming. Nat. Immunol. 5:1260-1265. [DOI] [PubMed] [Google Scholar]
- 50.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1Β. Mol. Cell 10:417-426. [DOI] [PubMed] [Google Scholar]
- 51.Matsuzaki, G., H. Yamada, K. Kishihara, Y. Yoshikai, and K. Nomoto. 2002. Mechanism of murine Vγ1+ γδ T cell-mediated innate immune response against Listeria monocytogenes infection. Eur. J. Immunol. 32:928-935. [DOI] [PubMed] [Google Scholar]
- 52.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Molofsky, A. B., B. G. Byrne, N. N. Whitfield, C. A. Madigan, E. T. Fuse, K. Tateda, and M. S. Swanson. 2006. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203:1093-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montgomery, R. R., D. Lusitani, C. A. de Boisfleury, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 55.Montgomery, R. R., and S. E. Malawista. 1996. Entry of Borrelia burgdorferi into macrophages is end-on and leads to degradation in lysosomes. Infect. Immun. 64:2867-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore, K. J., L. P. Andersson, R. R. Ingalls, B. G. Monks, R. Li, M. A. Arnaout, D. T. Golenbock, and M. W. Freeman. 2000. Divergent response to LPS and bacteria in CD14-deficient murine macrophages. J. Immunol. 165:4272-4280. [DOI] [PubMed] [Google Scholar]
- 57.Morita, C. T., R. A. Mariuzza, and M. B. Brenner. 2000. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin. Immunopathol. 22:191-217. [DOI] [PubMed] [Google Scholar]
- 58.Morita, C. T., Y. Tanaka, B. R. Bloom, and M. B. Brenner. 1996. Direct presentation of non-peptide prenyl pyrophosphate antigens to human gamma delta T cells. Res. Immunol. 147:347-353. [DOI] [PubMed] [Google Scholar]
- 59.Napolitani, G., A. Rinaldi, F. Bertoni, F. Sallusto, and A. Lanzavecchia. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nimmerjahn, F., and J. V. Ravetch. 2006. Fcγ receptors: old friends and new family members. Immunity 24:19-28. [DOI] [PubMed] [Google Scholar]
- 61.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ozoren, N., J. Masumoto, L. Franchi, T. D. Kanneganti, M. Body-Malapel, I. Erturk, R. Jagirdar, L. Zhu, N. Inohara, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Distinct roles of TLR2 and the adaptor ASC in IL-1beta/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176:4337-4342. [DOI] [PubMed] [Google Scholar]
- 63.Radolf, J. D., K. R. O. Hazlett, and S. A. Lukehart. 2006. Pathogenesis of syphilis, p. 197-236. In J. D. Radolf and S. A. Lukehart (ed.), Pathogenic treponemes: cellular and molecular biology. Caister Academic Press, Norfolk, United Kingdom.
- 64.Radolf, J. D. 1995. Treponema pallidum and the quest for outer membrane proteins. Mol. Microbiol. 16:1067-1073. [DOI] [PubMed] [Google Scholar]
- 65.Romagnani, C., C. M. Della, S. Kohler, B. Moewes, A. Radbruch, L. Moretta, A. Moretta, and A. Thiel. 2005. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4(+) T helper cells and CD4(+) CD25(hi) T regulatory cells. Eur. J. Immunol. 35:2452-2458. [DOI] [PubMed] [Google Scholar]
- 66.Rosenshine, I., S. Ruschkowski, and B. B. Finlay. 1994. Inhibitors of cytoskeletal function and signal transduction to study bacterial invasion. Methods Enzymol. 236:467-476. [DOI] [PubMed] [Google Scholar]
- 67.Salazar, J. C., C. D. Pope, M. W. Moore, J. Pope, T. G. Kiely, and J. D. Radolf. 2005. Lipoprotein-dependent and -independent immune responses to spirochetal infection. Clin. Diagn. Lab Immunol. 12:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salazar, J. C., C. D. Pope, T. J. Sellati, H. M. Feder, Jr., T. G. Kiely, K. R. Dardick, R. L. Buckman, M. W. Moore, M. J. Caimano, J. G. Pope, P. J. Krause, and J. D. Radolf. 2003. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171:2660-2670. [DOI] [PubMed] [Google Scholar]
- 68a.Salazar, J. C., A. R. Cruz, C. D. Pope, L. Valderrama, R. Trujillo, N. G. Saravia, and J. D. Radolf. 2007. Treponema pallidum elicits innate and adaptive cellular immune responses in skin and blood during secondary syphilis: a flow-cytometric analysis. J. Infect. Dis. 195:879-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sellati, T. J., D. A. Bouis, M. J. Caimano, J. A. Feulner, C. Ayers, E. Lien, and J. D. Radolf. 1999. Activation of human monocytic cells by Borrelia burgdorferi and Treponema pallidum is facilitated by CD14 and correlates with surface exposure of spirochetal lipoproteins. J. Immunol. 163:2049-2056. [PubMed] [Google Scholar]
- 70.Sellati, T. J., D. A. Bouis, R. L. Kitchens, R. P. Darveau, J. Pugin, R. J. Ulevitch, S. C. Gangloff, S. M. Goyert, M. V. Norgard, and J. D. Radolf. 1998. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J. Immunol. 160:5455-5464. [PubMed] [Google Scholar]
- 71.Sellati, T. J., S. L. Waldrop, J. C. Salazar, P. R. Bergstresser, L. J. Picker, and J. D. Radolf. 2001. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J. Immunol. 166:4131-4140. [DOI] [PubMed] [Google Scholar]
- 72.Sieling, P. A., W. Chung, B. T. Duong, P. J. Godowski, and R. L. Modlin. 2003. Toll-like receptor 2 ligands as adjuvants for human Th1 responses. J. Immunol. 170:194-200. [DOI] [PubMed] [Google Scholar]
- 73.Siren, J., T. Sareneva, J. Pirhonen, M. Strengell, V. Veckman, I. Julkunen, and S. Matikainen. 2004. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J. Gen. Virol. 85:2357-2364. [DOI] [PubMed] [Google Scholar]
- 74.Snijders, A., P. Kalinski, C. M. Hilkens, and M. L. Kapsenberg. 1998. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10:1593-1598. [DOI] [PubMed] [Google Scholar]
- 75. Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 76.Stuart, L. M., and R. A. Ezekowitz. 2005. Phagocytosis: elegant complexity. Immunity 22:539-550. [DOI] [PubMed] [Google Scholar]
- 77.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 78.Van Voorhis, W. C., L. K. Barrett, D. M. Koelle, J. M. Nasio, F. A. Plummer, and S. A. Lukehart. 1996. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J. Infect. Dis. 173:491-495. [DOI] [PubMed] [Google Scholar]
- 79.Viala, J., C. Chaput, I. G. Boneca, A. Cardona, S. E. Girardin, A. P. Moran, R. Athman, S. Memet, M. R. Huerre, A. J. Coyle, P. S. DiStefano, P. J. Sansonetti, A. Labigne, J. Bertin, D. J. Philpott, and R. L. Ferrero. 2004. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 5:1166-1174. [DOI] [PubMed] [Google Scholar]
- 80.Wooten, R. M., T. B. Morrison, J. H. Weis, S. D. Wright, R. Thieringer, and J. J. Weis. 1998. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J. Immunol. 160:5485-5492. [PubMed] [Google Scholar]
- 81.Wooten, R. M., and J. J. Weis. 2001. Host-pathogen interactions promoting inflammatory Lyme arthritis: use of mouse models for dissection of disease processes. Curr. Opin. Microbiol. 4:274-279. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto, M., K. Yaginuma, H. Tsutsui, J. Sagara, X. Guan, E. Seki, K. Yasuda, M. Yamamoto, S. Akira, K. Nakanishi, T. Noda, and S. Taniguchi. 2004. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9:1055-1067. [DOI] [PubMed] [Google Scholar]