Abstract
Successful colonization of the upper respiratory tract by Streptococcus pneumoniae is an essential first step in the pathogenesis of pneumococcal disease. However, the bacterial and host factors that provoke the progression from asymptomatic colonization to invasive disease are yet to be fully defined. In this study, we investigated the effects of single and combined mutations in genes encoding pneumolysin (Ply), pneumococcal surface protein A (PspA), and pneumococcal surface protein C (PspC, also known as choline-binding protein A) on the pathogenicity of Streptococcus pneumoniae serotype 2 (D39) in mice. Following intranasal challenge with D39, stable colonization of the nasopharynx was maintained over a 7-day period at a level of approximately 105 bacteria per mouse. The abilities of the mutant deficient in PspA to colonize the nasopharynx and to cause lung infection and bacteremia were significantly reduced. Likewise, the PspC mutant and, to a lesser extent, the Ply mutant also had reduced abilities to colonize the nasopharynx. As expected, the double mutants colonized less well than the parent to various degrees and had difficulty translocating to the lungs and blood. A significant additive attenuation was observed for the double and triple mutants in pneumonia and systemic disease models. Surprisingly, the colonization profile of the derivative lacking all three proteins was similar to that of the wild type, indicating virulence gene compensation. These findings further demonstrate that the mechanism of pneumococcal pathogenesis is highly complex and multifactorial but ascribes a role for each of these virulence proteins, alone or in combination, in the process.
Streptococcus pneumoniae (the pneumococcus) continues to be responsible for significant mortality and morbidity worldwide, causing a broad spectrum of diseases, including pneumonia, meningitis, bacteremia, and otitis media (28, 40). The burden of pneumococcal infection is particularly large among children and the elderly and is exacerbated by the rising numbers of isolates resistant to antibiotics (36). In developing countries, at least a million deaths per annum among children under 5 years of age are attributed to pneumococcal pneumonia (63a). Thus, there is an urgent need for an effective and affordable vaccine that can be used worldwide.
Asymptomatic colonization of the upper respiratory tract by S. pneumoniae almost invariably precedes disease, which occurs when colonizing organisms in the nasopharynx translocate to the middle ear, alveolar space, or bloodstream (23, 40). Occasionally, the bacteria traverse the blood-brain barrier, causing meningitis (51). The factors and/or events that trigger the progression from colonization to invasive disease are as yet unclear. However, invasive pneumococcal disease often occurs shortly after the acquisition of a new serotype and is rarely associated with prolonged carriage (11, 23). Therefore, the development of vaccines capable of interfering with asymptomatic carriage could protect the individual as well as confer herd immunity.
The pneumococcal polysaccharide capsule is strongly antiphagocytic and enhances virulence (27, 42), and hitherto, it has been the focus of vaccine development against pneumococcal disease. However, at least 90 distinct serotypes exist (21, 29) and antibodies against one serotype do not protect against another, complicating the design of polysaccharide-based vaccines. A protein-based vaccine overcomes this limitation and also may offer protection against nasopharyngeal carriage if delivered by the mucosal route (16). Furthermore, the development of an effective protein-based vaccine requires a thorough understanding of the roles and relative contributions to virulence of the various putative virulence proteins. To reduce cost, the number of different antigens that might be included in the formulation would be restricted to the most important virulence determinants. Promising vaccine candidates include the choline-binding proteins pneumococcal surface protein A (PspA) (16, 17) and pneumococcal surface protein C (PspC) (also known as choline-binding protein A [CbpA]) (18, 52), pneumolysin (Ply) (2, 47), and combinations thereof (43, 44). PspA is believed to play a pivotal role in preventing complement-mediated opsonization (1, 61, 66) and is also capable of binding to, and preventing killing by, lactoferrin (25, 54). PspC, on the other hand, has putative roles in adherence to the nasopharyngeal and lung epithelia and the brain microvascular endothelium (19, 51, 52). There is also evidence that PspC may mediate the invasion of host cells at these locations (45, 67). PspC also specifically binds the secretory component of human secretory immunoglobulin A and human factor H (20, 26), as well as complement component C3 (31, 55). Ply, a thiol-activated or cholesterol-dependent cytotoxin, is implicated in multiple steps of pneumococcal pathogenesis, including the activation of complement, inhibition of ciliary beating in the human respiratory epithelium, and disruption of tight junctions between epithelial cells (12, 46, 48, 57).
Wu et al. (65) established a pneumococcal carriage model with adult BALB/c and CBA/CaHN-XID/J (CBA/N; xid) inbred mice using several pneumococcal serotypes. In this study, we evaluated the relative contributions of PspA, PspC, and Ply to nasopharyngeal colonization, lung infection, and bacteremia in mice by using defined mutants lacking these proteins and combinations thereof. To our knowledge, this is the first study in which S. pneumoniae D39 and its isogenic derivative strains lacking one or more of the three proteins were compared in parallel in the three disease models.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pneumoniae serotype 2 strains used in this study are listed in Table 1. Strains were routinely grown in Todd-Hewitt broth supplemented with 1% yeast extract (THY) (9) or on blood agar at 37°C in 95% air-5% CO2. Where appropriate, gentamicin, erythromycin, and/or spectinomycin was added at 5 μg/ml, 0.2 μg/ml, and 100 μg/ml, respectively. Pneumococcal phase morphology was determined on THY-catalase plates as described by Weiser et al. (62). Strains were confirmed as S. pneumoniae by optochin sensitivity and production of a serotype 2 capsule was confirmed by Quellung reaction using diagnostic antiserum obtained from Statens Seruminstitut, Copenhagen, Denmark, as described previously (9).
TABLE 1.
S. pneumoniae strains used in this study
| Strain | Description | Reference |
|---|---|---|
| D39 | Capsular serotype 2 (NCTC7466) | 4 |
| PspC− (CbpA−) | pspC insertion-duplication mutant of D39 (Eryr) | 9 |
| PspA− | pspA insertion-duplication mutant of D39 (Eryr) | 41 |
| PLN-A | ply insertion-duplication mutant of D39 (Eryr) | 10 |
| ΔPly | ply deletion mutant of D39 | 8 |
| ΔPly PspC− | D39 derivative with ply deletion and pspC insertion-duplication mutations (Eryr) | 9 |
| ΔPly PspA− | D39 derivative with ply deletion and pspA insertion-duplication mutations (Eryr) | 9 |
| PspA− PspC− | D39 derivative with pspA and pspC insertion-duplication mutations (Eryr/Sptr) | This study |
| ΔPly PspA− PspC− | D39 derivative with ply deletion and pspA and pspC insertion-duplication mutations (Eryr/Sptr) | This study |
Construction of PspA− PspC− and ΔPly PspA− PspC− mutants of D39.
A PspA− PspC− mutant of D39 was constructed by insertion-duplication mutagenesis of pspC in a previously constructed PspA− strain (41), as follows. A 405-bp internal fragment of pspC was PCR amplified from D39 genomic DNA by using primers AD28 (5′-GAA AAC GAA GGA AGT ACC CAA GCA-3′) and AD29 (5′-GCG TTT TGT AAG TAT TGG TTG GGT-3′) and cloned into the suicide vector pR350, generating pR350-pspC, essentially as described previously (22). A ΔPly PspA− PspC− mutant (a derivative of D39 with an in-frame deletion mutation in ply encoding a derivative of Ply lacking amino acids 55 to 437) was constructed using pR350-pspC to transform a competent ΔPly PspA− mutant (Table 1) (9). Mutants were confirmed by Southern hybridization analysis (not shown), and their phenotypes were confirmed by Western blotting.
Mice.
Male 5- to 6-week-old CD1 and BALB/c mice were used for colonization and intraperitoneal (i.p.) challenge experiments, respectively, at the University of Adelaide. Six-week-old female CBA/N mice were used for pneumonia/sepsis experiments at the University of Alabama at Birmingham. CBA/N mice have an X-linked genetic defect and thus do not make protective antipolysaccharide antibodies even as adults (3, 14, 63). All animal experiments were approved by the respective ethics committees of the University of Adelaide or University of Alabama at Birmingham.
Colonization studies.
Bacteria for intranasal (i.n.) challenge of CD1 mice were grown in THY broth containing the appropriate antibiotic(s) at 37°C to an A600 of 0.25, corresponding to approximately 108 CFU/ml. Inocula were then centrifuged and resuspended in sterile phosphate-buffered saline (pH 7.2) such that 10 μl of bacterial suspension contained approximately 2 × 107 to 3 × 107 CFU. In these studies, two essentially identical experiments were performed. In each experiment, mice (16 per group) were challenged and analyzed for colonizing CFU and CFU in the blood and lung at days 1, 2, 4, and 7 essentially as described by Wu et al. (65). However, the nasopharynx was washed with 1 ml trypsin buffer (0.5% trypsin and 0.02% EDTA in sterile phosphate-buffered saline, pH 7.2) and the entire nasopharynx was also excised, homogenized, and plated.
Bacterial counts obtained from the nasopharynx, lungs, and blood were compared using Mann-Whitney two-tailed tests. Most analyses were performed using Graphpad Prism version 4.03. The Fisher exact test was used to analyze the differences in numbers of mice in each group exhibiting colonization, lung infection, or bacteremia.
Virulence studies.
For i.p. virulence studies, groups of 10 6-week-old male BALB/c mice were challenged with approximately 1 × 106 CFU of each strain grown in serum broth as described previously (9). For i.n. virulence studies, pneumococci were prepared as described previously (6, 13). For i.n. challenge, CBA/N mice were anesthetized with methoxyflurane (Metofane; Shering-Plough) in order to facilitate aspiration (32), and approximately 2 × 107 bacteria were given in a 40-μl volume into a single nostril. Mice were observed for 21 days for survival. Differences in median survival time between groups were analyzed by the Mann-Whitney U test (two tailed). Differences in the overall survival rates between groups were analyzed by the Fisher exact test.
SDS-PAGE and Western blotting.
Whole-cell lysates of bacteria were prepared as described previously (44). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the method described by Laemmli (35) and electroblotted onto nitrocellulose (Pall Life Sciences, MI) as described by Towbin et al. (60). The membrane was then probed with specific polyclonal mouse antisera. Bound antibody was detected using goat anti-mouse-alkaline phosphatase conjugate.
RESULTS
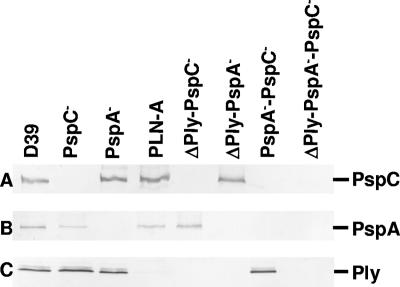
Previous work has shown outbred CD1 mice to be susceptible to colonization by several different pneumococcal serotypes (7, 53). In this study, each mouse was infected with approximately 2 × 107 to 3 × 107 CFU of D39 or its isogenic mutant derivatives, as previous work by Wu et al. (65) suggests that stable colonization of the nasopharynx can be achieved at this dose. Prior to challenge, whole-cell lysates of all strains were analyzed by Western blotting using polyclonal sera raised against purified PspA, PspC, and Ply to confirm the phenotypes of various mutants (Fig. 1). Distinct immunoreactive bands were observed for D39 at ∼100 kDa, ∼75 kDa, and ∼50 kDa, corresponding to the expected mobilities of PspC, PspA, and Ply, respectively. Although PspC has a deduced molecular mass of 75 kDa, it is known to migrate at a larger apparent size on SDS-PAGE (44, 52). Such anomalous migration is also characteristic of PspA (59).
FIG. 1.
Western immunoblot analysis of lysates of D39 and its isogenic derivatives deficient in PspA, PspC, and/or Ply after they were electroblotted onto nitrocellulose. The samples were reacted with polyclonal sera specific for the indicated antigens. Immunoreactive bands correspond to PspC (∼100 kDa) (row A), PspA (∼75 kDa) (row B), and Ply (∼50 kDa) (row C).
Colonization studies.
A previous study (37) observed that 200 μl of Ringer's solution appeared sufficient to wash the majority of pneumococci (serotypes 2 and 3) from the nasopharynx of inbred CBA/N mice. However, despite a 1-ml wash with trypsin buffer in this study, there was significant retention of colonizing bacteria in the nasopharyngeal tissue after the wash for all the strains, especially for those challenged with the D39 parent. Consequently, the nasopharyngeal tissues from all mice in all sets of experiments were excised and plated, and the numbers of bacteria recovered from the tissue and the nasal wash were added to give the total count recovered from the nasopharynx. Furthermore, a trend towards predominantly opaque or transparent phenotype was apparent on THY-catalase plates for samples from the nasal tissue or the nasal wash, respectively, for all the strains (data not shown).
Effect of mutations in pspA, pspC, and ply on colonization.
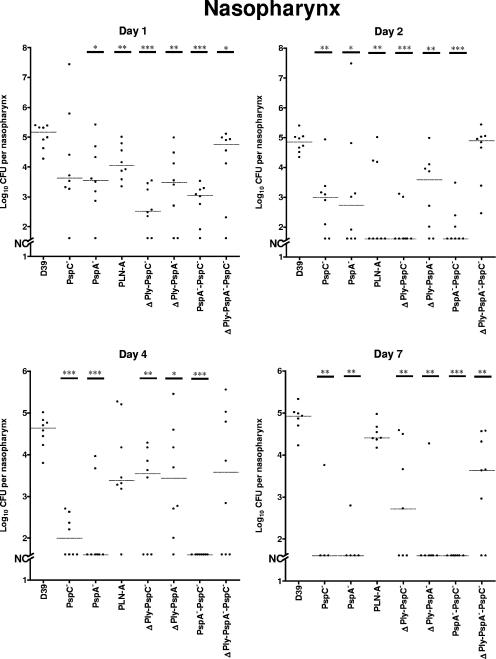
Two essentially identical experiments were carried out to determine the impact of mutation in the genes encoding PspA, PspC, and Ply on colonization. The colonization profile of the D39 parent was highly reproducible in both experiments, yielding approximately 105 CFU per nasopharynx at all time points. Similarly, the colonization profiles of the single mutants were also comparable in both experiments. Pooled results for the two experiments are shown in Fig. 2.
FIG. 2.
Bacterial recovery from the nasopharynx of CD1 mice after i.n. challenge with 2 × 107 to 3 × 107 CFU of D39 or its isogenic derivatives over a 7-day period. Pooled data from two independent experiments are shown. The horizontal broken lines indicate the median levels of colonization of each strain at each time point. Asterisks indicate points of statistical significance in comparison to the values for the D39 parent (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [Mann-Whitney two-tailed tests]). The broken segment on the y axis (NC) indicates that no mouse was colonized at the indicated time points (limit of detection, 40 CFU).
The PspA− and PspC− mutants colonized the nasopharynx at comparable levels but to levels lower than that of the D39 parent, throughout the course of these experiments. The differences were statistically significant at all of the time points except for day 1 for the PspC− strain (Fig. 2). D39 colonized mice at significantly higher levels than the Ply insertion-duplication mutant PLN-A at days 1 and 2 (Fig. 2), but the number of mice colonized by D39 was not significantly different from the number of mice colonized by the PLN-A strain, apart from those sacrificed on day 2 (Table 2).
TABLE 2.
Statistical analysis of numbers of mice colonized by mutants compared to numbers of mice colonized by D39
| Day |
P value for indicated strain compared to D39a
|
||||||
|---|---|---|---|---|---|---|---|
| PspC− | PspA− | PLN-A | ΔPly PspC− | ΔPly PspA− | PspA− PspC− | ΔPly PspA− PspC− | |
| 1 | NS | NS | NS | NS | NS | NS | NS |
| 2 | NS | <0.05 | <0.025 | <0.01 | <0.025 | <0.025 | NS |
| 4 | NS | <0.025 | NS | NS | NS | <0.005 | NS |
| 7 | <0.025 | <0.025 | NS | NS | <0.005 | <0.005 | NS |
P values were obtained by the Fisher exact probability test; a P value of <0.05 was considered significant. NS, not significant.
Effects of multiple virulence gene mutations on colonization.
To determine whether there are any additive or conflicting effects of multiple mutations in virulence genes on colonization of the nasopharynx, lung infection, or bacteremia, groups of mice were challenged with D39 derivative ΔPly PspC−, ΔPly PspA−, PspA− PspC−, and ΔPly PspA− PspC− strains (Table 1) in two independent experiments. The parental D39 strain was again used as a control, and pooled results are shown in Fig. 2.
The numbers of ΔPly PspC−, ΔPly PspA−, and PspA− PspC− bacteria recovered from the nasopharynx were significantly lower than those for the D39 parent at all time points (Fig. 2). Surprisingly, the triple mutant (ΔPly PspA− PspC−) colonized the nasopharynx better than all the other double mutants, and the difference between the levels of colonization of this mutant and the D39 parent was not statistically significant, except on days 1 and 7.
The levels of nasopharyngeal colonization by the single-mutant strains were compared to those of the double and triple mutants. We observed less colonization with the PspA and PspC double mutant than with the single mutants on each day assayed. The situation with mutants lacking Ply was slightly different. PLN-A did not have much of an effect as a single mutant, but when its deletion was combined with a PspA or a PspC deletion in a mutant, there actually appeared to be an increased level of colonization of mice on some days compared to the level for mice with PspA or PspC single mutants. For the ΔPly PspC− mutant, the colonization increased on days 4 and 7 over what had been observed on days 1 and 2, exactly the opposite of what was observed for the PspC mutant by itself. With the PspA single mutant, colonization had decreased 30-fold from day 1 to day 4, whereas with the ΔPly PspA− mutant, the day 4 value was indistinguishable from the day 1 and day 2 values. In the case of the triple mutant, colonization rates were quite high. Compared to the addition of the PspA− PspC− mutant, the addition of the Ply mutant caused the strain to carry bacterial levels 10 to 100 times higher than the PspA− PspC− mutant. Thus, overall, we saw that the Ply mutant had little effect itself on colonization, but when a Ply deletion was combined with a PspA, a PspC, or a PspA-PspC deletion in a mutant, the general result was, surprisingly, an increase rather than a decrease in the level of colonization.
Lung infection.
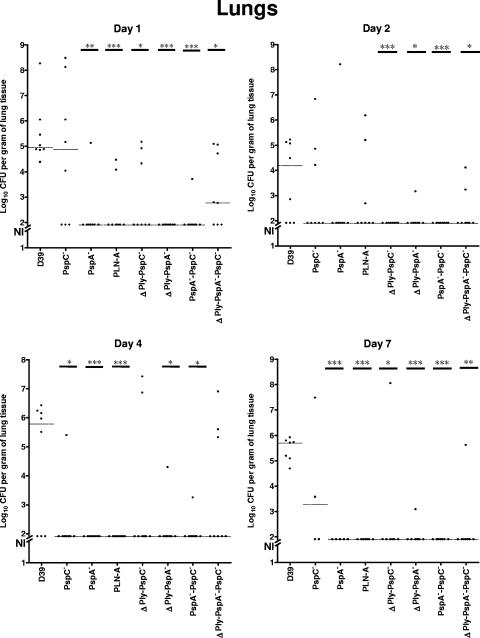
The pathogenesis of pneumococcal pneumonia involves bacterial translocation from the nasopharyngeal niche to the lungs and, subsequently, in some cases, to the bloodstream (40). In the colonization studies, the mice were inoculated i.n. without anesthesia. Thus, the transfer of bacteria from nasal tissue to the lung is spontaneous rather than induced. The abilities of strains carrying mutations in PspA, PspC, or Ply (or combinations thereof) to survive in the lungs were examined by removing the lungs from sacrificed mice and enumerating pneumococci in the homogenates. Data were from the same mice for whom the bacterial colonization is described in Table 2. As shown in Fig. 3, approximately 105 wild-type D39 organisms had infected the lungs by day 1; this number declined 100-fold by day 2 but then increased again to approximately 105 organisms at the end of the experiment (day 7). In general, most of the mice challenged with the D39 strain exhibited lung infection throughout the course of the experiments, whereas the vast majority of the mutant strains (the exceptions being the PspC− and triple mutants) seemed to have difficulty in stably existing in the lungs, especially from day 2 onwards. On days 1, 4, and 7 the numbers of CFU and the fractions of mice with pneumococci in their lungs were significantly less for mice infected with PspA and Ply mutants than for mice infected with the D39 parent. The effect of the PspC mutant on numbers of pneumococci in the lung was less apparent, but a statistically significant difference was observed on day 4. The PspC and Ply double mutant gave a result somewhat similar to that of PLN-A alone. The mice infected with the Ply and PspA double mutant and the PspA and PspC double mutant showed less CFU in the lungs on days 1 and 2 than mice infected with either of the two mutants in each pair. On days 4 and 7, numbers of CFU were also extremely low, as was the case for either the Ply or PspA single mutant. The mice infected with the triple mutant generally showed more CFU than those infected the double mutants lacking PspA, but at days 1 and 7, its CFU levels were significantly lower than those of mice infected with the D39 parent. It was of interest that the effects of PspC and Ply on colonization and lung infection appeared to be reversed. Ply had a larger effect on lung infection than it did on colonization, and PspC had a bigger effect on colonization than it did on lung infection.
FIG. 3.
Bacterial recovery from the lungs of CD1 mice after i.n. challenge with 2 × 107 to 3 × 107 CFU of D39 or its isogenic derivatives over a 7-day period. Pooled data from two independent experiments are shown. The horizontal broken lines indicate the median levels of lung invasion of each strain at each time point. Asterisks indicate points of statistical significance in comparison to the values for the D39 parent (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [Mann-Whitney two-tailed tests]). The broken segment on the y axis (NI) indicates no lung infection at the indicated time points (limit of detection, 80 CFU). The lung CFU data are from the same mice for which data are shown in Fig. 2.
Bacteremia.
Most commonly, pneumococcal bacteremia results from either leakage or transcytosis of pneumococci from the lung epithelia into the bloodstream (40). Considering the progression of disease and the high virulence of strain D39, some mice were expected to exhibit bacteremia at the later time points (days 4 and 7 postchallenge). It was expected that mutations in PspA, PspC, and Ply would attenuate pneumococcal virulence, resulting in decreased numbers of bacteria in the bloodstream compared to the numbers of D39 bacteria. Not surprisingly, the majority of the mutant strains did not exhibit bacteremia, corroborating the lung infection data. Again, only the mutants that exhibited lung infection (the PspC− and triple mutants) showed detectable bacteremia (approximately 103 to 106 CFU/ml of blood) in a limited number of mice over the course of the experiments (data not shown). Notably, a greater number of deaths were observed for mice challenged with the PspC− strain than for mice challenged with the D39 parent in both experiments. These mice most likely died of pneumococcal bacteremia, as blood samples taken from some mice soon after death were found to contain approximately 109 pneumococci per ml of blood.
Virulence studies.
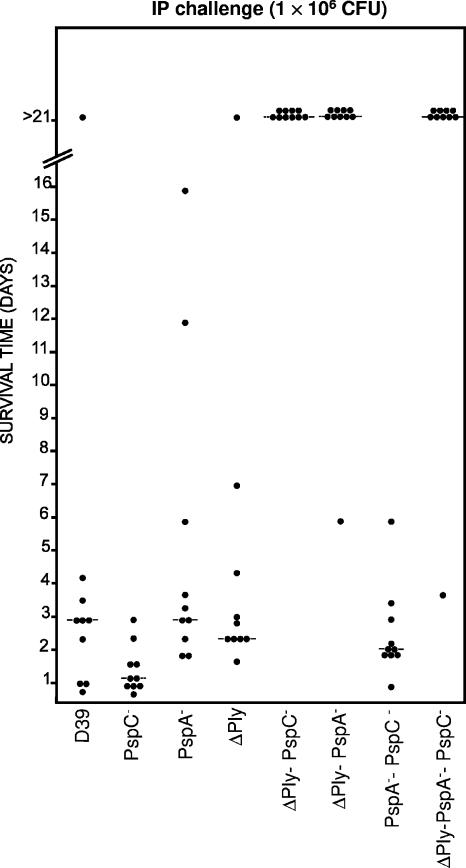
The effect of combined mutations in PspA, PspC, and Ply on the virulence of S. pneumoniae was investigated by challenging mice via the i.p. and i.n. routes. In the i.p. challenge experiment, high challenge doses (approximately 1 × 106 CFU of each strain) were used in an effort to enhance our ability to detect any additive attenuation of virulence due to the combined mutations. As expected, none of the derivatives carrying single mutations were significantly attenuated relative to the D39 parent (Fig. 4). However, the ΔPly PspC−, ΔPly PspA−, and ΔPly PspA− PspC− mutants were all severely attenuated, with almost all the mice in these groups remaining alive and well for the duration of the experiment (>21 days). For these three groups, both the median survival times and overall survival rates of mice were significantly greater than those for mice challenged with the D39 parent (P values of <0.01 and <0.001, respectively). The attenuation of the ΔPly PspC− and ΔPly PspA− mutants is comparable to that obtained in earlier work (9). The only double mutant that did not show significant attenuation was PspA− PspC−, a result that underscores the extreme importance of Ply in the invasive pathogenesis of D39 in mice. This basic scenario remained unchanged when mice were challenged with an even higher dose of each strain (1.5 × 107 CFU) (result not shown).
FIG. 4.
Survival times for mice after i.p. challenge. Groups of 10 BALB/c mice were challenged i.p. with approximately 1 × 106 CFU of the indicated strains. Each datum point represents one mouse. The horizontal broken lines denote the median survival times for each group.
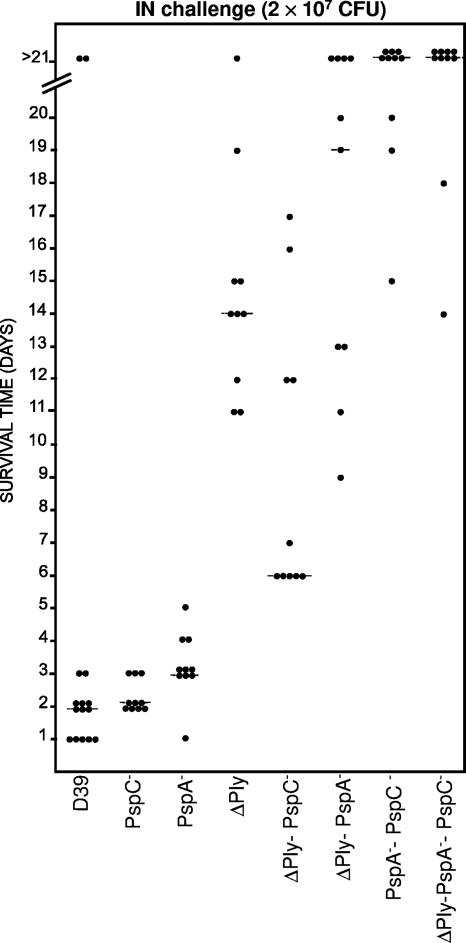
We also challenged CBA/N mice intranasally with approximately 2 × 107 CFU of each strain. Because this mouse strain is much more susceptible than BALB/c (3, 14, 63) and CD1 mice (unpublished observations), the i.n. dose of D39 used was able to cause fatal sepsis in 100% of the challenged mice. The median survival times of mice challenged with all the mutants except for mice challenged with the PspC− mutant were significantly longer than that of mice challenged with the D39 parent (Fig. 5). P values were <0.002 for mice challenged with the PspA− mutant and <0.0001 for mice challenged with the remainder of the mutants. Of the mice challenged with the single mutants, those challenged with the Ply mutant showed the longest median survival time. The mice challenged with ΔPly PspA−, PspA− PspC−, and ΔPly PspA− PspC− mutants all showed longer survival times than mice challenged with either parent, and the PspA and PspC single mutants and the ΔPly PspA− PspC− mutant were almost completely avirulent in this assay. In the case of PspA− PspC−, and ΔPly PspA− PspC− mutants, the differences between the survival times of mice challenged with double and triple mutants and each parent were statistically significant. Interestingly, the ΔPly PspC− mutant gave a value intermediate between each parent and statistically different from each parent.
FIG. 5.
Survival times for mice after i.n. challenge. Groups of 10 to 15 CBA/N (xid) mice were challenged i.n. with approximately 2 × 107 CFU of the indicated strains. Each datum point represents one mouse. The horizontal broken lines denote the median survival times for each group.
DISCUSSION
In this study, the relative contributions of Ply, PspA, and PspC (or CbpA) to the pathogenicity of S. pneumoniae were investigated. Each of these proteins has been ascribed putative roles in adherence to the nasopharyngeal and lung epithelia and invasion into other host cells (12, 19, 25, 46, 48, 52, 57, 67).
After i.n. infection of mice with wild-type D39, stable and reproducible colonization of the nasopharynx at a level of approximately 105 bacteria was maintained over 7 days. Accurate enumeration of colonizing bacteria was achieved by plating nasopharyngeal wash and nasopharyngeal tissue homogenates. While a strong correlation between recovered and retained wild-type bacteria following a nasopharyngeal wash with Ringer's solution was previously identified (65), this study suggests that derivatives of the same strain may adhere to the nasopharynx with different efficiencies. Taken together, our findings are consistent with recent observations that asymptomatic nasopharyngeal colonization involves two populations of pneumococci: those with a transparent phenotype loosely associated with the nasal surface and those with an opaque phenotype that is intimately associated with the nasal mucosa (15).
The D39 nasopharyngeal colonization model was used to compare the colonization capacities of D39 and its otherwise isogenic PspA−, PspC−, PLN-A, ΔPly PspA−, ΔPly PspC−, PspA− PspC−, and ΔPly PspA− PspC− derivative strains. In these experiments, D39 colonized significantly better than the PspC− mutant, except on day 1. This finding corroborates a previous report of a study using an infant rat model (52) and other studies showing that PspC is required for prolonged bacterial numbers in the nasopharynx (5, 45). However, a deficiency in PspC does not appear to attenuate the level of invasion of the lung epithelia or bacteremia, despite this protein being implicated in the process (19, 67). This is supported by the observation that the median survival time of mice challenged i.p. and i.n. with the PspC− strain was very similar to that of mice challenged with the D39 parent, corroborating previous findings (9, 45). In contrast, attenuation of virulence of a type 2 PspC− strain was demonstrated by others (5, 30). The discrepancy between our findings and the other studies may be primarily due to a difference in challenge doses and challenge strains used. Interestingly, in a recent study (34), the deletion of pspC in serotypes 2, 3, and 19F strains did not significantly attenuate virulence in a pneumonia model, in agreement with our findings. The study also demonstrated that the contribution of PspC to virulence in pneumonia varies in pneumococcal strains through its interaction with complement and through other complement-independent mechanisms.
A deficiency in PspA was seen to significantly reduce nasopharyngeal colonization by pneumococci over the 7-day period, contradicting earlier observations using a similar D39 PspA− mutant (5). However, in that study, the numbers of colonizing bacteria were derived entirely from bacterial counts in nasal wash. The findings in this study may therefore more accurately reflect murine colonization by a PspA− mutant, implying a more significant role for PspA in carriage. This correlates with other reports that preexisting antibodies (immunoglobulin G and immunoglobulin A) to PspA, in particular, to the N-terminal region, are protective against carriage in humans (39) and that i.n. immunization of mice with PspA elicited protection against carriage (64). Furthermore, the PspA− mutant exhibited attenuated virulence compared to D39 in the intranasal virulence study. Significant attenuation of virulence of a PspA− strain following intravenous infection of mice has been demonstrated (41, 61), consistent with the role for PspA in preventing complement-mediated opsonization and its capacity to bind lactoferrin (24, 25, 50), thereby preventing its iron-free from, apolactoferrin, from killing pneumococci (50). In the i.p. virulence studies, given the high challenge dose used, no significant difference in virulence between D39 and the PspA− strain was detected, similar to previous observations (9).
Previous studies investigating nasopharyngeal colonization by a Ply mutant yielded conflicting results (32, 33, 49, 53). In our study, appreciable numbers of PLN-A bacteria were recovered from the nasopharynx up to 7 days postinfection, and the number of mice colonized by D39 was not significantly different from those colonized by the PLN-A strain at most time points examined. While these discrepancies may be due to mouse strain differences, it seems that Ply has a relatively minor role in colonization compared to those of PspA and PspC (and other as yet unidentified components) in nasopharyngeal colonization by pneumococci, as previously suggested (53). However, the observation that the PLN-A strain was severely attenuated in the lungs in this study is in agreement with other findings (33, 45) and would account for the increased median survival time of mice challenged with this strain compared to that of mice challenged with the D39 parent. Thus, although PLN-A could colonize, it was not successful at surviving in the lungs. However, due to the high challenge dose used in the i.p. challenge experiment, the Ply mutant was not significantly attenuated relative to the D39 parent.
Considering the fact that mutations in PspA and PspC individually had a significant impact on pneumococcal colonization, a deficiency in both proteins was expected to result in further reduction in colonization. This was indeed the case, corroborating previous findings (5). However, the differences between the PspC mutant and the double mutant reached statistical significance only on days 2 and 4, and the difference between the PspA mutant and the double mutant did not reach statistical significance at any time point. This was surprising, as the two proteins have been proposed to have complementary roles (18). Choline-binding proteins as a whole have also been reported to contribute to adherence of pneumococci through effects on surface charge (58), and so the absence of both PspA and PspC could affect this property. However, the present study suggests that such combined effects are minimal in the nasopharyngeal niche. Nevertheless, the mutant deficient in both PspA and PspC was significantly attenuated in the i.n. virulence study; this was illustrated by the massive increase in the median survival time of mice challenged with the double-mutant strain over that of mice challenged with the PspA− and PspC− single mutants, an observation that supports an earlier report that the PspA− PspC− double mutant was cleared from the blood more rapidly than either the PspA or PspC single mutant or the D39 parent (5). In the i.p. virulence study, the PspA− PspC− double mutant did not show significantly more attenuation than the PspA or PspC single mutants. This is not surprising, considering lethality of the model and the high challenge dose used. Furthermore, the PspC mutant was so virulent in the model that it masked any possible additive attenuation due to the PspA mutation. This result also emphasizes the significance of Ply in the systemic virulence of D39 in mice, as it could be seen that multiple mutants carrying the Ply mutation were significantly attenuated.
Perhaps the most unexpected result from this study was the apparent capability of the triple mutant to colonize the nasopharynx and invade the blood of mice to almost the same extent as D39. A likely explanation could be derived from studies demonstrating that innate resistance to pneumococcal infection in mice was due to the recognition of Ply by Toll-like receptor 4 via an enhancement of Ply-induced apoptosis (38, 56). This suggests that a mutant deficient in Ply could at least colonize the nasopharynx as well as the wild type and, in the case of the triple mutant, compensate for the absence of PspA and PspC. Alternatively, it is conceivable that carriage involves a careful balance between having sufficient virulence to establish stable colonization and yet being not so virulent as to provoke significant local inflammation. Ply may elicit sufficient host inflammatory responses to keep colonization under control, whereas triple-mutant pneumococci may be unable to cause enough tissue inflammation to trigger innate defenses, resulting in little host resistance to carriage. Another noticeable observation was that no mice infected with the triple-mutant strain died in the CD1 colonization experiments, despite one sacrificed mouse having approximately 106 bacteria per ml of blood. This suggested that the triple mutant exhibited reduced systemic virulence. This is supported by data showing that mice infected i.p. and i.n. with the triple mutant had a significantly increased median survival time compared to the D39 parent or single mutants.
This study further strengthens accumulating evidence for the roles of characterized pneumococcal virulence proteins in colonization and systemic disease caused by S. pneumoniae and points to the fact that the dynamics of these events may be complex. Colonized individuals are the principal reservoir for pneumococcal infection, and understanding the role of these pneumococcal virulence proteins will facilitate development of more-effective vaccines capable of reducing transmission of the pathogen in the community.
Acknowledgments
We thank Yvette Hale, Judy Morona, David Miller, and Katie Spackman for assistance with animal experiments.
This work was supported by Program Grant 284214 from the National Health and Medical Research Council of Australia.
A.D.O. and K.S.L. contributed equally to this work.
Editor: A. Camilli
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility of surface-bound complement and surface antigens of Streptococcus pneumoniae. Infect. Immun. 71:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, J. E., R. A. Lock, C. C. A. M. Peeters, J. T. Poolman, P. W. Andrew, T. J. Mitchell, D. Hansman, and J. C. Paton. 1994. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect. Immun. 62:5683-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amsbaugh, D. F., C. T. Hansen, B. Prescott, P. W. Stashak, D. R. Barthold, and P. J. Baker. 1972. Genetic control of the antibody response to type III pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. J. Exp. Med. 136:931-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandran, P., A. Brooks-Walter, A. Virolainen-Julkunen, S. K. Hollingshead, and D. E. Briles. 2002. Role of pneumococcal surface protein C in nasopharyngeal carriage and pneumonia and its ability to elicit protection against carriage of Streptococcus pneumoniae. Infect. Immun. 70:2526-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran, P., S. K. Hollingshead, J. C. Paton, and D. E. Briles. 2001. The autolytic enzyme LytA of Streptococcus pneumoniae is not responsible for releasing pneumolysin. J. Bacteriol. 183:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergeron, Y., N. Ouellet, A.-M. Deslauriers, M. Simard, M. Olivier, and M. G. Bergeron. 1998. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect. Immun. 66:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berry, A. M., A. D. Ogunniyi, D. C. Miller, and J. C. Paton. 1999. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect. Immun. 67:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry, A. M., and J. C. Paton. 2000. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 68:133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berry, A. M., J. Yother, D. E. Briles, D. Hansman, and J. C. Paton. 1989. Reduced virulence of a defined pneumolysin negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boulnois, G. J. 1992. Pneumococcal proteins and the pathogenesis of disease caused by Streptococcus pneumoniae. J. Gen. Microbiol. 138:249-259. [DOI] [PubMed] [Google Scholar]
- 12.Boulnois, G. J., J. C. Paton, T. J. Mitchell, and P. W. Andrew. 1991. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol. Microbiol. 5:2611-2616. [DOI] [PubMed] [Google Scholar]
- 13.Briles, D. E., S. K. Hollingshead, J. King, A. Swift, P. A. Braun, M. K. Park, L. M. Ferguson, M. H. Nahm, and G. S. Nabors. 2000. Immunization of humans with rPspA elicits antibodies, which passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J. Infect. Dis. 182:1694-1701. [DOI] [PubMed] [Google Scholar]
- 14.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briles, D. E., L. Novak, M. Hotomi, F. W. van Ginkel, and J. King. 2005. Nasal colonization with Streptococcus pneumoniae includes subpopulations of surface and invasive pneumococci. Infect. Immun. 73:6945-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Briles, D. E., R. C. Tart, E. Swiatlo, J. P. Dillard, P. Smith, K. A. Benton, B. A. Ralph, A. Brooks-Walter, M. J. Crain, S. K. Hollingshead, and L. S. McDaniel. 1998. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA). Clin. Microbiol. Rev. 11:645-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briles, D. E., J. Yother, and L. S. McDaniel. 1988. Role of pneumococcal surface protein A in the virulence of Streptococcus pneumoniae. Rev. Infect. Dis. 10:372-374. [DOI] [PubMed] [Google Scholar]
- 18.Brooks-Walter, A., D. E. Briles, and S. K. Hollingshead. 1999. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect. Immun. 67:6533-6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cundell, D. R., and E. I. Tuomanen. 1994. Receptor specificity of adherence of Streptococcus pneumoniae to human type-II pneumocytes and vascular endothelial cells in vitro. Microb. Pathog. 17:361-374. [DOI] [PubMed] [Google Scholar]
- 20.Dave, S., S. Carmicle, S. Hammerschmidt, M. K. Pangburn, and L. S. McDaniel. 2004. Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J. Immunol. 173:471-477. [DOI] [PubMed] [Google Scholar]
- 21.García, E., D. Llull, R. Munoz, M. Mollerach, and R. Lopez. 2000. Current trends in capsular polysaccharide biosynthesis of Streptococcus pneumoniae. Res. Microbiol. 151:429-435. [DOI] [PubMed] [Google Scholar]
- 22.Giammarinaro, P., and J. C. Paton. 2002. Role of RegM, a homologue of the catabolite repressor protein CcpA, in the virulence of Streptococcus pneumoniae. Infect. Immun. 10:5454-5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray, B. M., G. M. Converse III, and H. C. Dillon, Jr. 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142:923-933. [DOI] [PubMed] [Google Scholar]
- 24.Håkansson, A., H. Roche, S. Mirza, L. S. McDaniel, A. Brooks-Walter, and D. E. Briles. 2001. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect. Immun. 69:3372-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammerschmidt, S., G. Bethe, P. H. Remane, and G. S. Chhatwal. 1999. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect. Immun. 67:1683-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammerschmidt, S., M. P. Tillig, S. Wolff, J. P. Vaerman, and G. S. Chhatwal. 2000. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol. Microbiol. 36:726-736. [DOI] [PubMed] [Google Scholar]
- 27.Hardy, G. G., A. D. Magee, C. L. Ventura, M. J. Caimano, and J. Yother. 2001. Essential role for cellular phosphoglucomutase in virulence of type 3 Streptococcus pneumoniae. Infect. Immun. 69:2309-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83-93. [DOI] [PubMed] [Google Scholar]
- 29.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iannelli, F., D. Chiavolini, S. Ricci, M. R. Oggioni, and G. Pozzi. 2004. Pneumococcal surface protein C contributes to sepsis caused by Streptococcus pneumoniae in mice. Infect. Immun. 72:3077-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janulczyk, R., F. Iannelli, A. G. Sjöholm, G. Pozzi, and L. Björck. 2000. Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J. Biol. Chem. 275:37257-37263. [DOI] [PubMed] [Google Scholar]
- 32.Kadioglu, A., N. A. Gingles, K. Grattan, A. Kerr, T. J. Mitchell, and P. W. Andrew. 2000. Host cellular immune response to pneumococcal lung infection in mice. Infect. Immun. 68:492-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadioglu, A., S. Taylor, F. Iannelli, G. Pozzi, T. J. Mitchell, and P. W. Andrew. 2002. Upper and lower respiratory tract infection by Streptococcus pneumoniae is affected by pneumolysin deficiency and differences in capsule type. Infect. Immun. 70:2886-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerr, A. R., G. K. Paterson, J. McCluskey, F. Iannelli, M. R. Oggioni, G. Pozzi, and T. J. Mitchell. 2006. The contribution of PspC to pneumococcal virulence varies between strains and is accomplished by both complement evasion and complement-independent mechanisms. Infect. Immun. 74:5319-5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 36.Lynch, J. P., III, and G. G. Zhanel. 2005. Escalation of antimicrobial resistance among Streptococcus pneumoniae: implications for therapy. Semin. Respir. Crit. Care Med. 26:575-616. [DOI] [PubMed] [Google Scholar]
- 37.Magee, A. D., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 69:3755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malley, R., P. Henneke, S. C. Morse, M. J. Cieslewicz, M. Lipsitch, C. M. Thompson, E. Kurt-Jones, J. C. Paton, M. R. Wessels, and D. T. Golenbock. 2003. Recognition of pneumolysin by Toll-like receptor 4 confers resistance to pneumococcal infection. Proc. Natl. Acad. Sci. USA 100:1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCool, T. L., T. R. Cate, G. Moy, and J. N. Weiser. 2002. The immune response to pneumococcal proteins during experimental human carriage. J. Exp. Med. 195:359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullers, J. A., and E. I. Tuomanen. 2001. Molecular pathogenesis of pneumococcal pneumonia. Front. Biosci. 6:D877-D889. [DOI] [PubMed] [Google Scholar]
- 41.McDaniel, L. S., J. Yother, M. Vijayakamur, L. McGarry, W. R. Guild, and D. E. Briles. 1987. Use of insertional inactivation to facilitate studies of biological properties of pneumococcal surface protein A (PspA). J. Exp. Med. 165:381-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahm, M. H., M. A. Apicella, and D. E. Briles. 2003. Chapter 41, Immunity to extracellular bacteria, p. 1263-1284. In W. Paul (ed.), Fundamental immunology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 43.Ogunniyi, A. D., R. L. Folland, D. E. Briles, S. K. Hollingshead, and J. C. Paton. 2000. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect. Immun. 68:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogunniyi, A. D., M. C. Woodrow, J. T. Poolman, and J. C. Paton. 2001. Protection against Streptococcus pneumoniae elicited by immunization with pneumolysin and CbpA. Infect. Immun. 69:5997-6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Orihuela, C. J., G. Gao, K. P. Francis, J. Yu, and E. I. Tuomanen. 2004. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 190:1661-1669. [DOI] [PubMed] [Google Scholar]
- 46.Paton, J. C. 1996. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 4:103-106. [DOI] [PubMed] [Google Scholar]
- 47.Paton, J. C., R. A. Lock, and D. J. Hansman. 1983. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect. Immun. 40:548-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paton, J. C., B. Rowan-Kelly, and A. Ferrante. 1984. Activation of human complement by the pneumococcal toxin pneumolysin. Infect. Immun. 43:1085-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rayner, C. F., A. D. Jackson, A. Rutman, A. Dewar, T. J. Mitchell, P. W. Andrew, P. J. Cole, and R. Wilson. 1995. Interaction of pneumolysin-sufficient and -deficient isogenic variants of Streptococcus pneumoniae with human respiratory mucosa. Infect. Immun. 63:442-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren, B., A. J. Szalai, O. Thomas, S. K. Hollingshead, and D. E. Briles. 2003. Both Family 1 and Family 2 PspA proteins can inhibit complement deposition and confer virulence to a capsular serotype 3 strain of Streptococcus pneumoniae. Infect. Immun. 71:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ring, A., J. N. Weiser, and E. I. Tuomanen. 1998. Pneumococcal trafficking across the blood-brain barrier. J. Clin. Investig. 102:347-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenow, C., P. Ryan, J. N. Weiser, S. Johnson, P. Fontan, A. Ortqvist, and H. R. Masure. 1997. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 25:819-829. [DOI] [PubMed] [Google Scholar]
- 53.Rubins, J. B., A. H. Paddock, D. Charboneau, A. M. Berry, J. C. Paton, and E. N. Janoff. 1998. Pneumolysin in pneumococcal adherence and colonization. Microb. Pathog. 25:337-342. [DOI] [PubMed] [Google Scholar]
- 54.Shaper, M., S. K. Hollingshead, W. H. Benjamin, Jr., and D. E. Briles. 2004. PspA protects Streptococcus pneumoniae from killing by apolactoferrin, and antibody to PspA enhances killing of pneumococci by apolactoferrin. Infect. Immun. 72:5031-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, B. L., and M. K. Hostetter. 2000. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 182:497-508. [DOI] [PubMed] [Google Scholar]
- 56.Srivastava, A., P. Henneke, A. Visintin, S. C. Morse, V. Martin, C. Watkins, J. C. Paton, M. R. Wessels, D. T. Golenbock, and R. Malley. 2005. The apoptotic response to pneumolysin is Toll-like receptor 4 dependent and protects against pneumococcal disease. Infect. Immun. 73:6479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steinfort, C., R. Wilson, T. Mitchell, C. Feldman, A. Rutman, H. Todd, D. Sykes, J. Walker, K. Saunders, P. W. Andrew, G. J. Boulnois, and P. J. Cole. 1989. Effects of Streptococcus pneumoniae on human respiratory epithelium in vitro. Infect. Immun. 57:2006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Swiatlo, E., F. R. Champlin, S. C. Holman, W. W. Wilson, and J. M. Watt. 2002. Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae. Infect. Immun. 70:412-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talkington, D. F., D. C. Voellinger, L. S. McDaniel, and D. E. Briles. 1992. Analysis of pneumococcal PspA microheterogeneity in SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb. Pathog. 13:343-355. [DOI] [PubMed] [Google Scholar]
- 60.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tu, A. H., R. L. Fulgham, M. A. McCrory, D. E. Briles, and A. J. Szalai. 1999. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect. Immun. 67:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiser, J. N., R. Austrian, P. K. Sreenivasan, and H. R. Masure. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wicker, L. S., and I. Scher. 1986. X-linked immune deficiency (xid) of CBA/N mice. Curr. Top. Microbiol. Immunol. 124:87-102. [DOI] [PubMed] [Google Scholar]
- 63a.World Health Organization. 2005. Vaccinating African children against pneumococcal disease saves lives. http://www.who.int/mediacentre/news/statements/2005/s03/en/index.htm.
- 64.Wu, H.-Y., M. H. Nahm, Y. Guo, M. W. Russell, and D. E. Briles. 1997. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J. Infect. Dis. 175:839-846. [DOI] [PubMed] [Google Scholar]
- 65.Wu, H.-Y., A. Virolainen, B. Mathews, J. King, M. W. Russell, and D. E. Briles. 1997. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb. Pathog. 23:127-137. [DOI] [PubMed] [Google Scholar]
- 66.Yother, J., and D. E. Briles. 1992. Structural properties and evolutionary relationships of PspA, a surface protein of Streptococcus pneumoniae, as revealed by sequence analysis. J. Bacteriol. 174:601-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, J. R., K. E. Mostov, M. E. Lamm, M. Nanno, S. Shimida, M. Ohwaki, and E. Tuomanen. 2000. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 102:827-837. [DOI] [PubMed] [Google Scholar]