Abstract
Interleukin-22 (IL-22) is a recently discovered proinflammatory cytokine, structurally related to IL-10. Since IL-22 is induced by lipopolysaccharide in vivo, we studied the role of IL-22 in a model of polymicrobial peritonitis. Quantitative real-time reverse transcription-PCR analysis showed marked induction of IL-22 and IL-22 receptor in spleen and kidney during the course of sepsis. The biological activity of IL-22 is modulated by IL-22-binding protein (IL-22BP), which is considered a natural antagonist of IL-22. To further analyze the role of IL-22 during septic peritonitis, mice were treated with recombinant IL-22BP generated as Fcγ2a fusion protein. IL-22BP-Fc completely blocked IL-22-induced STAT3 activation in hepatocytes in vitro. Treatment of mice with IL-22BP-Fc 4 h before sepsis induction led to enhanced accumulation of neutrophils and mononuclear phagocytes and a reduced bacterial load at the site of infection. In addition, IL-22 blockade led to an enhanced bacterial clearance in liver and kidney and reduced kidney injury. These results imply an important proinflammatory role of IL-22 during septic peritonitis, contributing to bacterial spread and organ failure. IL-22 therefore appears to play an important role in the regulation of inflammatory processes in vivo.
Sepsis is still a major cause of postoperative morbidity and mortality after abdominal surgery (11). Although activation of the innate immune system by microbial pathogens and their products was reported to contribute to hyperinflammation and organ injury, many aspects of sepsis immunopathology need further elucidation. Specifically, engagement of Toll-like receptors may lead to the production of numerous immune mediators and to the induction of systemic immune responses (4, 16). Although cytokine production is essential for the generation of protective host response mechanisms during septic peritonitis, overwhelming or uncontrolled mediator production may activate several cellular systems and damage multiple organs, thereby leading to irreversible organ failure and death (4, 16, 28, 29). Thus, interference with the amplification of cytokine expression during sepsis may influence sepsis outcome.
Interleukin-22 (IL-22) is a recently discovered T-cell-derived cytokine belonging to the IL-10 family (9, 37). IL-22 was found to be induced by IL-9 in murine T cells in vitro (9) and is expressed by activated T cells, mast cells, and NK cells (10, 34, 35). IL-22 mediates signal transduction through a receptor complex consisting of the specific IL-22R1 and the common IL-10R2 subunits (18, 37), both members of the type II cytokine receptor family. Notably, IL-22R1 is not expressed on immune cells but is detected on nonimmune cells such as epithelial cells, keratinocytes, or hepatic cell lines, which, however, do not respond to IL-10. This indicates an exclusive role for IL-22 for nonimmune cell function, while IL-10 is known to act only on immune cells (18, 35, 37). IL-22 signaling activates STAT1, STAT3, and STAT5 and leads to the induction of mitogen-activated protein kinases in a rat hepatoma cell line (7, 10, 18, 22, 37). Furthermore, IL-22 function is regulated by IL-22-binding protein (IL-22BP). IL-22BP was cloned as a soluble member of the class II cytokine receptor family and was proposed to function as a natural antagonist of IL-22 (8, 19).
IL-22 expression could be demonstrated during rheumatoid arthritis (17), Crohn's disease (3), and psoriasis (2, 36), where IL-22 levels correlate with disease severity. IL-22 was shown to act in a proinflammatory manner in the lung (33). It induces the expression of acute-phase proteins in vitro and in vivo (1, 10) and triggers production of proinflammatory cytokines and defensins in keratinocytes (2, 35, 36) and intestinal epithelial cells (3). Both IL-22 and its antagonist, IL-22BP, are induced by lipopolysaccharide (LPS) in vivo (32). In addition, IL-22R1 was demonstrated to be upregulated by proinflammatory stimuli in vitro and during Crohn's disease (3), thus indicating a role in proinflammatory processes.
In this study, the roles of IL-22 and its receptor in severe bacterial infections were examined. IL-22 and IL-22R1 were induced during the course of sepsis, pointing to a role for IL-22 during bacterial infections. To interfere with IL-22 function during infections, we produced recombinant IL-22BP as a fusion protein with a noncytolytic Fcγ2a fragment. rIL-22BP-Fc was shown to specifically inhibit IL-22, but not IL-10, function in vitro. Furthermore, pretreatment of mice with rIL-22BP-Fc was found to modulate cytokine levels during septic peritonitis and to enhance accumulation of effector cells and it reduced bacterial load at the site of infection and in peripheral organs. Accordingly, septic kidney failure was attenuated by IL-22BP-Fc treatment.
MATERIALS AND METHODS
Mouse model of septic peritonitis.
C57BL/6 mice were purchased from Harlan Winkelmann (Borchem, Germany). Mice were used at 8 to 12 weeks of age for all experiments. The colon ascendens stent peritonitis (CASP) procedure used for induction of polymicrobial septic peritonitis was described in detail previously (38). Briefly, the colon ascendens was exteriorized and a 7/0 ethilon thread (Ethicon, Norderstedt, Germany) was stitched through the antimesenteric portion of the colon ascendens approximately 10 mm distal of the ileocecal valve. A 16-gauge venous catheter was inserted by puncture antimesenterically through the colonic wall into the intestinal lumen, directly proximal of the pretied knot, and fixed. To ensure proper intraluminal position of the stent, stool was milked from the cecum into the colon ascendens until a small drop appeared. Fluid resuscitation of the animals was performed by flushing 0.5 ml sterile saline into the peritoneal cavity prior to closure of the abdominal wall. Mice were pretreated intraperitoneally with either 10 μg rIL-22BP-Fc or rFc (10 μg/mouse) 4 h before CASP surgery.
Gene expression analysis by real-time quantitative reverse transcription-PCR.
The expression of IL-22, IL-10, gamma interferon (IFN-γ), IL-22R1, IL-10R2, IL-10R2, and IL-22BP was analyzed using quantitative real-time PCR (ABI 7300 Real Time PCR System; Applied Biosystems, Foster City, CA) in liver, kidney, and spleen RNA samples from mice 0 h, 3 h, 6 h, and 12 h after sepsis induction. Total RNA was prepared using the QIAGEN RNeasy kit according to the recommendations of the manufacturer (QIAGEN, Hilden, Germany). Two micrograms of total RNA treated with RNase inhibitor (Fermentas, St. Leon-Rot, Germany) was reverse transcribed using RevertAid H Minus Moloney murine leukemia virus reverse transcriptase (Fermentas, St. Leon-Rot, Germany). iQSYBR Green Supermix (Eurogentec, Seraing, Belgium) was used to detect accumulation of PCR products of IL-22, IL-10, IL-22R1, and IL-10; TaqMan gene expression arrays (Applied Biosystems, Foster City, CA) were used to detect IFN-γ, IL-10R1, and IL-22BP during cycling on the SDS7300 cycler (Applied Biosystems). Expression levels of cytokines and cytokine receptors of samples of septic animals were normalized to β-actin and displayed as change (n-fold) relative to samples of control mice used as calibrator (set to 1). Primers used in this study are as follows: IL-22 sense, 5′-ATA CAT CGT CAA CCG CAC CTT T-3′; IL-22 antisense, 5′-AGC CGG ACA TCT GTG TTG TTA T-3′; IL-22R1 sense, 5′-CTA CGT GTG CCG AGT GAA GA-3′; IL-22R1 antisense, 5′-AAG CGT AGG GGT TGA AAG GT-3′; IL-10 sense, 5′-CCC AAG TAA CCC TTA AAG TCC TGC-3′; IL-10 antisense, 5′-ATA ACT GCA CCC ACT TCC CAG TC-3′; IL-10R2 sense, 5′-ACA TTC GGA GTG GGT CAA TGT C-3′; IL-10R2 antisense, 5′-TCT GCA TCT CAG GAG GTC CAA T-3′; β-actin sense, 5′-ACC CAC ACT GTG CCC ATC TAC-3′; β-actin antisense, 5′-AGC CAA GTC CAG ACG CAG G-3′.
Isolation of spleen cell populations.
Spleens were collected before and 6 h after CASP induction. T and B cells were isolated by magnetically assisted cell sorting (MACS) using anti-Thy-1- and anti-B220-conjugated beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocols. Purification of the isolated cell populations was analyzed by fluorescence-activated cell sorting (FACS) using Thy-1.2 (53-2.1), CD3 (145-2C11), CD19 (1D3), and B220 (RA3-6B2) antibodies and was over 90%. Non-T/non-B cells (Thy1.2− B220− cells) were isolated from the flowthrough of the MACS purification using a Moflo-Sorter (Cytomation, Fort Collins, CO). RNA was prepared immediately after sorting using the RNeasy minikit (QIAGEN, Hilden, Germany). Real-time PCR analysis was performed as described above.
Differentiation of BMDC.
Bone marrow-derived dendritic cells (BMDC) were differentiated as described previously (31). Briefly, femurs of mice were flushed with phosphate-buffered saline (PBS) and unfractionated cell populations were plated at a density of 5 × 105 cells/ml in suspension culture petri dishes (Greiner, Frickenhausen, Germany) in RPMI medium supplemented with 10 ng/ml mouse recombinant granulocyte-macrophage colony-stimulating factor (tebu-bio, Offenbach, Germany). Cell cultures were used at day 10. The purity of the dendritic cell population was assessed by FACS analysis (FACSCalibur flow cytometer and CellQuest software; BD Biosciences, San Diego, CA) using CD11c (HL30) and CD11b (M1/70) antibodies (all from BD Pharmingen, San Diego, CA) and was 80 to 85% in all experiments.
Cloning and purification of rIL-22BP-Fc.
IL-22BP cDNA was amplified from murine spleen cDNA and genetically linked to a mutated Fcγ2a (39) to obtain the IL-22BP/Fcγ2a fusion protein (recombinant IL-22BP-Fc [rIL-22BP-Fc]). After transient transfection into HEK 293T cells, the fusion protein was purified from the supernatants by protein A affinity chromatography, followed by dialysis against PBS. The protein was stored at −20°C before use. Fcγ2a protein was purified similarly and served as a control in each experiment.
The purity of the recombinant proteins was checked by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, and LPS content was analyzed by Limulus assay and was below 0.01 endotoxin units/ml for all protein charges used in this study.
Analysis of STAT3 activation.
Total cell lysates were prepared in 50 mM Tris (pH 8.0), 150 mM NaCl, 1% NP-40, 0.5% deoxycholic acid, 0.1% SDS, and 1 mM EDTA. Samples were sonicated for 30 s and boiled at 95°C for 5 min. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and probed with antibodies to STAT3 and p-STAT3 (Cell Signaling, Danvers, MA). Specific binding was visualized with the ECL Western Blotting Detection System (GE Healthcare Biosciences AB, Uppsala, Sweden) according to the manufacturer's instructions.
In vitro characterization of rIL-22BP-Fc function.
Hepa1-6 cells (1 × 106 cells; mouse liver hepatoma cells; ATCC 1830) were cultured for 2 h in serum-deprived medium (0.5% fetal calf serum) to reduce the endogenous level of phosphorylated STAT3. IL-22BP-Fc or rFc was mixed with 50 ng/ml murine rIL-22 or murine rIL-10 (both from R&D Systems, Minneapolis, MN) and was incubated on ice for 30 min. Hepa1-6 cells were treated for 10 min with the mixtures and subsequently analyzed for activation of STAT3 by Western blotting as described above.
Systemic cytokine and chemokine production and serum creatinine determination.
Peripheral blood samples were collected 0 h and 12 h after CASP surgery. Immune mediator concentrations in serum were measured by enzyme-linked immunosorbent assay specific for tumor necrosis factor alpha (TNF-α), IL-6, IL-10, and CXCL1 (all from R&D Systems, Minneapolis, MN). Serum creatinine levels were measured by standardized protocols at the Institute of Clinical Chemistry, Technische Universität München, Munich, Germany.
Determination of bacterial load of peripheral organs and the peritoneal cavity.
Peritoneal lavage fluid, liver, and kidney samples were collected 12 h after CASP surgery. Serial dilutions of organ homogenates and peritoneal lavage in PBS were plated onto blood agar plates (BD Biosciences, Heidelberg, Germany). CFU were counted after incubation at 37°C for 24 h and calculated as CFU per whole organ or peritoneal cavity.
Flow cytometry analysis.
Peritoneal lavage cells were counted and differentiated by staining with antibodies to Mac-1 (M1/70) and Ly-6G/Gr-1 (RB6-8C5) using appropriate isotype-matched controls (all from BD Pharmingen, San Diego, CA) and analyzed by FACS (FACSCalibur flow cytometer and CellQuest software; BD Biosciences, San Diego, CA).
Statistical analysis.
Statistical analysis of the data was performed by the chi-square test, the Fisher exact test, or the Mann-Whitney U test where appropriate. The level of significance was P < 0.5.
RESULTS
Induction of IL-22 and IL-22R during acute polymicrobial peritonitis.
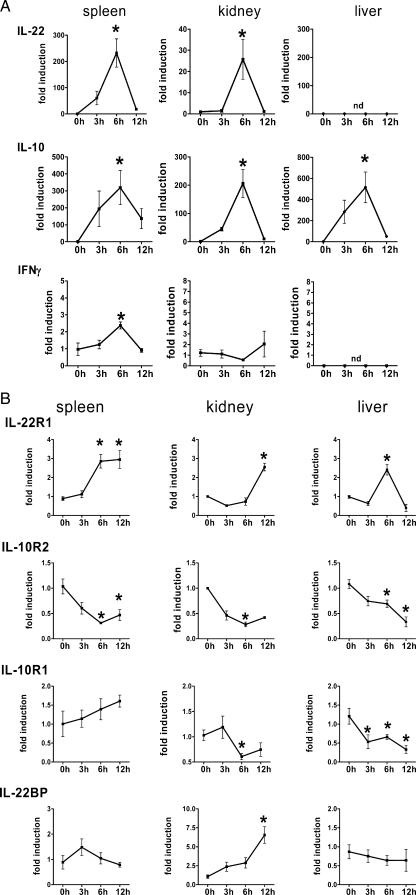
IL-22 is a recently discovered proinflammatory member of the family of IL-10-related cytokines. Since it was shown to be induced after LPS administration in vivo and is related to proinflammatory processes (2, 3, 35), we analyzed the expression of IL-22 and its specific receptor subunit IL-22R1, as well as IL-10 and IL-10R2, in peripheral organs in a model of polymicrobial peritonitis in mice by quantitative real-time PCR (Fig. 1A). Induction of IL-22 RNA could be observed in spleen and kidney, peaking 6 h after sepsis induction while returning to baseline levels at 12 h. The highest expression levels for IL-22 could be observed in the spleen. In liver, however, IL-22 was not induced during peritonitis. In parallel, the expression of the IL-22 family member IL-10 was monitored during the course of sepsis. IL-10 was induced in either spleen, kidney, or liver 3 h after sepsis induction. Peak levels were observed 6 h after CASP, returning to low levels at 12 h after sepsis induction in all organs analyzed. Expression levels of IL-10 were comparable in spleen, kidney, and liver, and the expression kinetics of IL-10 correlated with the expression kinetics of IL-22 in spleen and kidney. Furthermore, moderate induction of IFN-γ expression was observed in spleen but not in kidney and liver.
FIG. 1.
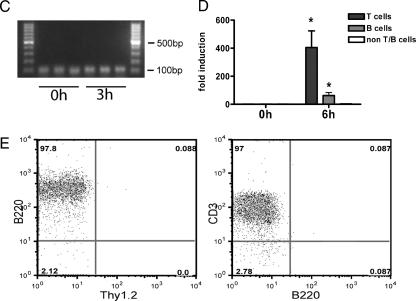
Expression of IL-22 and IL-22R during septic peritonitis. (A and B) Organs were removed from wild-type C57BL/6 mice before (0 h) and 3 h, 6 h, and 12 h after CASP. Organs were homogenized, and RNA was prepared. IL-22, IL-22R1, IL-10, IFN-γ, IL-10R2, IL-10R2, and IL-22BP levels were determined by quantitative PCR. Expression levels are given relative to those before CASP as calibrator. Results are derived from four mice per group and time point. (C) Constitutive expression of IL-22BP mRNA was detected by reverse transcription-PCR in spleen 0 h and 3 h, respectively, after sepsis induction in three individual mice. (D) Spleens were removed from wild-type C57BL/6 mice before (0 h) and 6 h after CASP. T, B, and non-T/B cells were isolated by MACS and flow cytometry cell sorting, and RNA was prepared. IL-22 levels were determined by quantitative PCR. Expression levels are given relative to those before CASP as calibrator. Results are derived from four individual experiments. (E) Purity of B220- (left panel) or Thy1.2-positive (right panel) cell populations used for IL-22 quantitative PCR determinations after magnetic sorting. *, P < 0.05 (compared to 0 h); nd, not detectable.
In additional experiments, the expression levels of IL-22R were analyzed during the course of sepsis (Fig. 1B). Expression of the IL-22R1 subunit was induced in spleen, liver, and kidney. In spleen, expression levels were upregulated after 6 h and stayed unaltered until 12 h after sepsis induction. In kidney, the induction of IL-22R1 was detectable 12 h after sepsis induction. In liver, however, expression levels peaked at 6 h after sepsis induction and, in contrast to spleen, returned to baseline levels at the 12-h observation point. Expression levels of the IL-10R2 subunit, however, were downregulated during the course of sepsis with the same kinetics in spleen, kidney, and liver (Fig. 1B). Expression of the IL-10R1 subunit was downregulated in kidney and liver (Fig. 1B).
In addition, expression of the IL-22 antagonist IL-22BP was analyzed during septic peritonitis. We found constitutive expression of IL-22BP in spleen, kidney, and liver. Figure 1C shows IL-22BP expression 0 h and 3 h after sepsis induction, confirming constitutive expression of IL-22BP in spleen. IL-22BP was upregulated 12 h after sepsis induction in kidney (Fig. 1B).
To further analyze the expression of IL-22 at the cellular level, splenic T and B cells were isolated 0 h and 6 h after sepsis induction using anti-Thy-1.2- and anti-B220-conjugated magnetic beads (Fig. 1D). Non-T/B cells (Thy-1.2−/B220− cells) were further purified by flow cytometry-based cell sorting. Figure 1E shows the purity of sorted cell populations. Notably, B220-sorted populations did not contain Thy1.2-positive cells and the Thy1.2-sorted populations almost uniformly expressed CD3 and did not contain B220-positive cells. Additional flow cytometry analyses confirmed coexpression of Thy1.2 and CD3, indicating Thy1.2-positive cells to be T cells, as well as coexpression of B220 and CD19, indicating that double-positive cells represent B cells (data not shown). The 6-h time point was chosen because the expression of IL-22 peaked 6 h after sepsis induction (Fig. 1A). During septic peritonitis, IL-22 mRNA was induced mainly in T cells and was found to a lesser amount in B-cell preparations, whereas non-T/B cells did not produce IL-22.
These data show that the expression of IL-22 is induced in either spleen or kidney during septic peritonitis, with the cellular source of IL-22 being primarily T lymphocytes. Induction of IL-22 and its receptor during septic peritonitis suggests a role for this cytokine during polymicrobial infection.
Construction and functional in vitro characterization of rIL-22BP-Fc.
IL-22BP is known to bind and to antagonize IL-22 function in vitro (8, 19). To further investigate the role of IL-22 during polymicrobial peritonitis, we aimed at counteracting sepsis-induced IL-22 with recombinant IL-22BP in vivo. Recombinant murine IL-22BP was produced as fusion protein with a noncytolytic Fcγ2a fragment. Fusion of IL-10 with the Fcγ2a fragment was previously shown to result in prolonged half-life in vivo (39). The recombinant Fcγ2a fragment (rFc) was used as control for all experiments.
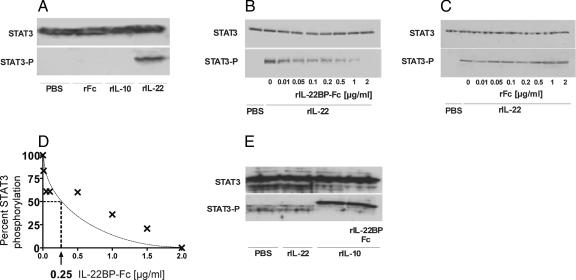
To functionally characterize rIL-22BP-Fcγ2a (rIL-22BP-Fc), its blocking activity of IL-22-induced STAT3 activation in the murine hepatoma line Hepa1-6 was tested. This cell line was chosen as an in vitro model because of its abundant IL-22R expression (data not shown). Whereas rIL-22 induced STAT3 phosphorylation in Hepa1-6 cells, rIL-10 as well as the control rFc protein did not (Fig. 2A). To analyze the inhibitory capacity of rIL-22BP-Fc in vitro, different amounts of rIL-22BP-Fc and rFc were incubated with 50 ng/ml mrIL-22, to allow the binding of rIL-22BP-Fc to IL-22. Subsequently, Hepa1-6 cells were treated with the cytokine mixture for 10 min and STAT3 phosphorylation was analyzed. Figures 2B and C show that rIL-22BP-Fc was able to inhibit IL-22-induced STAT3 phosphorylation in a dose-dependent manner, whereas rFc did not show any inhibitory effects, indicating a specific blockade of IL-22 by IL-22BP fusion protein. Furthermore, the inhibitory capacity of rIL-22BP-Fc was determined after densitometric analysis of the Western blots (Fig. 2D). A concentration of 0.25 μg/ml of rIL-22BP-Fc was calculated to result in half-maximal inhibition of IL-22-induced STAT3 phosphorylation.
FIG. 2.
Functional characterization of IL-22BP-Fc. (A) Treatment of Hepa1-6 cells with rIL-22, but not rIL-10 or rFc, leads to STAT3 activation. Hepa1-6 cells were treated with 50 ng/ml rIL-22 or rIL-10 for 30 min. Lysates of Hepa1-6 cells were analyzed by Western blotting against STAT3 and phosphorylated STAT3. (B and C) rIL-22BP-Fc, but not rFc, inhibits rIL-22-induced STAT3 phosphorylation in Hepa1-6 cells. Fifty nanograms of rIL-22 per milliliter was incubated with indicated concentrations of rIL-22BP-Fc or rFc (0 to 2 μg/ml) for 10 min. Then, the mixtures were incubated for 30 min with Hepa1-6 cells. Cell lysates were analyzed by Western blotting with antibodies to STAT3 and phosphorylated STAT3. Western blots representative of three independent experiments are shown. (D) After densitometry, the concentration of IL-22BP-Fc yielding 50% inhibition of IL-22-induced STAT3 phosphorylation was calculated to be 0.25 μg/ml. (E) BMDC were treated with 50 ng/ml rIL-22 or rIL-10 for 30 min in the presence of rIL-22BP-Fc (10 μg/ml). Lysates were analyzed by Western blotting against STAT3 and phosphorylated STAT3.
Because IL-22 shows homology to IL-10, we analyzed whether rIL-22BP-Fc would interfere with IL-10-induced STAT3 signaling. These experiments were performed with BMDC, since Hepa1-6 cells do not express the IL-10R (data not shown). As shown in Fig. 2E, rIL-22BP-Fc was not able to inhibit IL-10-induced STAT3 activation in these cells, indicating that IL-22BP does not interfere with IL-10 function. rIL-22 was not able to induce STAT3 phosphorylation of BMDC, because they do not express IL-22R1 (data not shown). These data show that rIL-22BP-Fc is a potent antagonist of IL-22, but not IL-10, action in vitro.
Modulation of cytokine production after in vivo administration of rIL-22BP-Fc during septic peritonitis.
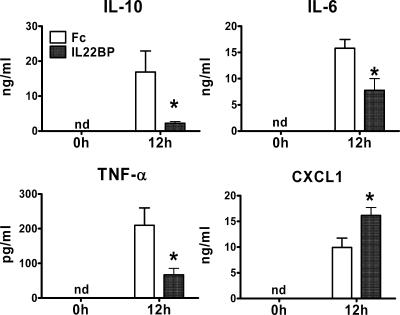
To further analyze the contribution of IL-22 to sepsis pathology, we blocked IL-22 function in vivo by the administration of rIL-22BP-Fc. Mice were pretreated intraperitoneally 4 h before CASP with 10 or 40 μg of rIL-22BP-Fc or rFc. The systemic inflammatory reaction was examined 12 h after sepsis induction (Fig. 3). Systemic levels of IL-10, TNF-α, and IL-6 were significantly attenuated in mice receiving rIL-22BP-Fc compared with mice pretreated with the control protein. In contrast, levels of the chemokine CXCL1 were increased in rIL-22BP-Fc-treated mice. Comparable reduction of IL-10 production could be detected when mice were administered either 10 or 40 μg of rIL-22BP-Fc prior to CASP (data not shown). Thus, blockade of IL-22 substantially alters the systemic inflammatory response during septic peritonitis.
FIG. 3.
Treatment of mice with rIL-22BP-Fc modulates systemic cytokine response in sepsis. Peripheral blood was collected from rIL-22BP-Fc-treated (filled bars) and control rFc-treated (open bars) mice before (0 h) and 12 h after CASP. Cytokine concentrations were determined by enzyme-linked immunosorbent assay. Results are derived from nine mice per group and time point. *, P < 0.05 (rIL-22BP-Fc versus rFc treatment); nd, not detectable.
Improved host defense and attenuation of organ failure after pretreatment of mice with rIL-22BP-Fc.
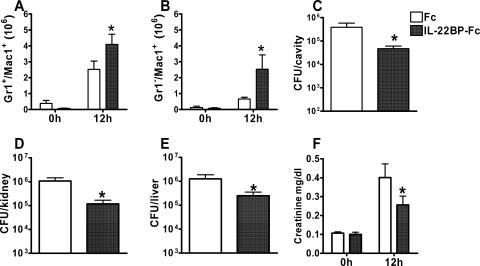
To further analyze the impact of pretreatment of mice with the IL-22 antagonist, the mechanisms of innate host defense against invading pathogens were analyzed at the infectious focus. Infiltration of effector neutrophils is crucial for the antibacterial host defense during septic peritonitis (5, 13, 14, 30). Neutrophils invading the peritoneal cavity express high levels of Gr-1 (Ly-6B/Ly-6C) and CD11b (Mac-1) (20). Mice pretreated with rIL-22BP-Fc showed enhanced accumulation of neutrophils (Gr-1hi Mac-1hi) and macrophage/monocyte-like cells (Mac-1+ Gr-1−) in the peritoneal cavity (Fig. 4A and B). Notably, enhanced infiltration of effector cells was accompanied by a reduced bacterial load (Fig. 4C). Reduced bacterial burden could be demonstrated also for kidney and liver (Fig. 4D and E).
FIG. 4.
Enhanced antibacterial defense and reduced septic renal failure after rIL-22BP-Fc treatment. (A and B) Peritoneal cells were harvested from rIL-22BP-Fc-treated and control rFc-treated mice before (0 h) and 12 h after CASP. Neutrophils were identified by high expression of Gr-1 and Mac-1 (A) and macrophages by expression of Mac-1 but not Gr-1 (B). Cell numbers were determined from five to six independent mice per group and time point. (C to E) Peritoneal lavage fluid (C) as well as kidneys (D) and livers (E) was obtained 12 h after CASP from mice treated with rIL-22BP-Fc or rFc. Total bacterial counts were determined after plating of serial dilutions of lavage fluid or organ extracts on blood agar. Results are derived from nine independent mice per group. (F) Serum samples of mice treated with rIL-22BP-Fc or rFc were obtained before (0 h) or 12 h after CASP and analyzed for creatinine levels as an indicator of renal failure. Data are derived from nine independent mice per group. *, P < 0.05 (rIL-22BP-Fc treatment versus rFc treatment).
Acute organ failure is a hallmark of sepsis (16, 28) and can be correlated with the bacterial burden of peripheral organs. We therefore investigated whether reduced bacterial load in organs of mice treated with rIL-22BP-Fc may be associated with attenuated organ failure. To analyze acute renal failure, serum creatinine levels were determined 12 h after sepsis induction (Fig. 4F). Sepsis resulted in increased serum creatinine levels in control and IL-22BP-treated mice, but the elevation in rIL-22BP-Fc-treated mice was significantly attenuated compared with control mice, indicating an attenuated renal failure after IL-22 blockade.
Thus, blockade of IL-22 function by the antagonist rIL-22BP-Fc leads to decreased bacterial load and attenuated organ failure during peritonitis and indicates a possible role for IL-22 in the pathogenesis of severe systemic inflammation.
DISCUSSION
IL-22 is a member of the IL-10 cytokine family showing proinflammatory function. It is induced during chronic infections such as psoriasis and colitis (2, 3, 36) and is also upregulated by LPS (9). In this study, we assessed the role of IL-22 during acute bacterial infection. We show the induction of IL-22 as well as its specific receptor subunit (IL-22R1) in the course of acute polymicrobial sepsis in vivo. Inhibition of IL-22 function during sepsis by the application of recombinant IL-22BP-Fc, which serves as an IL-22 antagonist, indicates that IL-22 might influence cytokine production and antibacterial host defense mechanisms.
Analysis of cytokine expression during sepsis revealed that IL-22 as well as IL-10 was induced during peritonitis with similar kinetics. Thus, pro- and anti-inflammatory mediators of the IL-10 family are induced in parallel during polymicrobial peritonitis. Whereas IL-10 is induced in all organs analyzed, the expression of IL-22 is limited to spleen and kidney, suggesting a more restricted function of IL-22 compared to IL-10 during acute infection. Further analysis of splenic cell populations revealed that IL-22 is produced primarily by T cells, which is in line with observations that T cells, especially when differentiated to the Th1 subtype, express high amounts of IL-22 (35). This finding might also explain the strong expression of IL-22 in septic spleen. T-cell infiltration in the kidney during sepsis (26) may explain the expression of IL-22 in this organ. T-cell activation during sepsis has been reported previously (15, 16) and is consistent with our present and previous findings of IFN-γ expression in spleen (38). In addition, weak expression of IL-22 was detected in B cells isolated ex vivo from septic mice. It appears unlikely that IL-22 was derived from contaminating cells, because B-cell preparations were devoid of T cells and non-T/B cells did not produce IL-22. Expression of IL-22 was not shown for B cells so far, since human B cells activated in vitro by Staphylococcus aureus did not produce IL-22 (35). The discrepancy can possibly be explained by the different stimulus in our in vivo model of mixed bacterial infection. Thus, during sepsis, B cells might be activated directly via Toll-like receptor signals or indirectly through multiple Toll-like receptor-induced cytokines.
In addition to IL-22, IL-22R1 is upregulated during the course of sepsis in spleen, liver, and kidney, thus indicating a role for IL-22 action in the amplification of cytokine burst during polymicrobial sepsis. The different induction kinetics of IL-22R1 in spleen, liver, and kidney might be due to differences in the subsets of immune cells recruited to these organs during sepsis or cell-type-specific differences in the regulation of receptor expression by resident parenchymal cells. IL-22R1 was shown to be upregulated by LPS or the proinflammatory cytokines TNF-α and IL-1β on epithelial cells in vitro (3). The induction of IL-22R1 during sepsis might be, as expected for IL-22, directly caused by Toll-like receptor or caused by sepsis-mediating cytokines. The expression of IL-10R2 and IL-10R1, however, was downregulated during sepsis. In line with our data, in vitro zymosan-induced signaling also leads to downregulation of IL-10R and inhibition of IL-10-induced signaling in macrophages (6). As IL-10R2 is the common subunit of IL-22R and IL-10R and IL-22R1 is upregulated during infection, IL-22R may be expressed at the expense of IL-10R. This may indicate preferential activation of the IL-22 pathway and proinflammatory responses. Consistent with these observations, IL-22R was also found to be upregulated in vivo during inflammation in lung and pancreas as well as in skin and intestine (1, 2, 3, 33, 36). IL-10R2, which is involved not only in the recognition of IL-22 but also in IL-10, IL-26, IL-28, and IL-29 signaling (21), is important for several distinct processes during ongoing infections and might therefore be regulated more tightly.
IL-22BP is a natural antagonist of IL-22, which is expressed by human monocytes and by dendritic cells in spleen and lymph nodes (8, 24, 37). IL-22BP competes with IL-22R for binding of IL-22, thereby dampening IL-22 action. Expression analysis of IL-22BP during sepsis revealed constitutive levels in spleen and a moderate upregulation in kidney. To further analyze the role of the IL-22 pathway during sepsis, we counteracted IL-22 function with a recombinant IL-22BP-Fc fusion protein. Functionality of the fusion protein was clearly demonstrated by blockade of IL-22-induced STAT3 phosphorylation in the murine hepatoma line Hepa1-6. In contrast to STAT3, IL-22 did not induce p38 phosphorylation (data not shown). In the rat hepatoma cell line H4IIE, however, p38 was phosphorylated after IL-22 stimulation (22). Since differences in the activation of STAT1 and STAT5, in addition to STAT3, occurred in this cell line, cell-type- or species-specific differences may lead to activation of different signaling programs (10, 22, 35, 37). Importantly, no interference of IL-22BP-Fc with IL-10-induced STAT3 phosphorylation was detected, demonstrating the specificity for IL-22 and confirming previous results (37).
Blockade of IL-22 action in vivo resulted in the modulation of cytokine production during septic peritonitis. The expression of IL-10, IL-6, and TNF-α was attenuated after IL-22 blockade, whereas the expression of the chemokine CXCL1 was increased. Notably, we also found the recruitment of macrophage-like cells (Mac-1+ Gr-1−) and neutrophils (Gr-1hi Mac-1hi) to the infected peritoneal cavity to be augmented and the bacterial clearance to be improved by rIL-22BP-Fc application. It therefore appears likely that the reduced pathogen load is associated with an attenuated stimulation of immune cells, which, in turn, may result in a reduced cytokine response. IL-10 was among the cytokines with decreased production during sepsis after rIL-22BP-Fc administration. Since it was shown elsewhere that blockade of IL-10 improves bacterial clearance during sepsis (25, 27), it is conceivable that the reduced amount of IL-10 during IL-22BP-Fc treatment contributes to the accumulation of effector cells at the site of infection and overall bacterial clearance.
Phagocytes have been shown to be crucial for efficient host defense during sepsis (12, 23). Therefore, enhanced effector cell functions might also influence the development of septic organ failure. Application of rIL-22BP-Fc led to enhanced antibacterial clearance in liver and kidney, as well as to attenuated kidney failure. Acute kidney failure is a hallmark of sepsis pathology and is associated with the infiltration of neutrophils and T lymphocytes (26). Thus, IL-22 production by infiltrating T cells could contribute to acute renal failure and might explain the expression of IL-22 mRNA in septic kidneys. In liver, the lack of IL-22 expression might be due to insufficient T-cell recruitment. In addition, IL-22 production was reported neither for hepatocytes nor for Kupffer cells.
Collectively, these data show that the proinflammatory cytokine IL-22 might play a role not only in chronic inflammatory diseases but also during acute bacterial infections. IL-22 appears to contribute to the amplification of cytokine burst, leading to organ failure and death. The recombinant receptor antagonist IL-22BP, provided as an Fcγ2a fusion protein, may act as a helpful tool to counteract IL-22-mediated inflammatory reactions in vivo and to limit inflammatory damage during acute and chronic inflammatory diseases.
Editor: R. P. Morrison
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Aggarwal, S., M.-H. Xie, M. Maruka, J. Foster, and A. Gurney. 2001. Acinar cells of the pancreas are a target of interleukin-22. J. Interferon Cytokine Res. 21:1047-1053. [DOI] [PubMed] [Google Scholar]
- 2.Boniface, K., F.-X. Bernard, M. Garcia, A. L. Gurney, J.-C. Lecron, and F. Morel. 2005. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol. 174:3695-3702. [DOI] [PubMed] [Google Scholar]
- 3.Brand, S., F. Beigel, T. Olszak, K. Zitzmann, S. T. Eichhorst, J. M. Otte, H. Diepolder, A. Marquardt, W. Jagla, A. Popp, S. Leclair, K. Hermann, J. Seiderer, T. Ochsenkühn, B. Göke, C. J. Auernhammer, and J. Dambacher. 2006. IL-22 is increased in active Crohn's disease and promotes proinflammatory gene expression and intestinal cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 290:G827-G838. [DOI] [PubMed] [Google Scholar]
- 4.Buras, J. A., B. Holzmann, and M. Sitkovsky. 2005. Animal models of sepsis: setting the stage. Nat. Rev. Drug. Discov. 4:854-865. [DOI] [PubMed] [Google Scholar]
- 5.Dalrymple, S. A., R. Slattery, D. M. Aud, M. Krishna, L. A. Lucian, and R. Murray. 1996. Interleukin-6 is required for a protective immune response to systemic Escherichia coli infection. Infect. Immun. 64:3231-3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du, Z., E. Kelly, I. Mecklenbräuker, L. Agle, C. Herrero, P. Paik, and L. B. Ivashkiv. 2006. Selective regulation of IL-10 signaling and function by zymosan. J. Immunol. 176:4785-4792. [DOI] [PubMed] [Google Scholar]
- 7.Dumoutier, L., C. Leemans, D. Lejeune, S. V. Kotenko, and J. C. Renauld. 2001. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J. Immunol. 167:3545-3549. [DOI] [PubMed] [Google Scholar]
- 8.Dumoutier, L., D. Lejeune, D. Colau, and J. C. Renauld. 2001. Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166:7090-7095. [DOI] [PubMed] [Google Scholar]
- 9.Dumoutier, L., J. Louahed, and J. C. Renauld. 2000. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 164:1814-1819. [DOI] [PubMed] [Google Scholar]
- 10.Dumoutier, L., E. Van Roost, D. Colau, and J. C. Renauld. 2000. Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl. Acad. Sci. USA 97:10144-10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emmanuel, K., H. Weighardt, H. Bartels, J. R. Siewert, and B. Holzmann. 2005. Current and future concepts of abdominal sepsis. World J. Surg. 29:3-9. [DOI] [PubMed] [Google Scholar]
- 12.Feterowski, C., M. Mack, H. Weighardt, B. Bartsch, S. Kaiser-Moore, and B. Holzmann. 2004. CC chemokine receptor 2 regulates leukocyte recruitment and 10 production during acute polymicrobial sepsis. Eur. J. Immunol. 34:3664-3673. [DOI] [PubMed] [Google Scholar]
- 13.Feterowski, C., A. Novotny, S. Kaiser-Moore, P. F. Muhlradt, T. Rossmann-Bloeck, M. Rump, B. Holzmann, and H. Weighardt. 2005. Attenuated pathogenesis of polymicrobial peritonitis in mice after TLR2 agonist pre-treatment involves ST2 up-regulation. Int. Immunol. 17:1035-1046. [DOI] [PubMed] [Google Scholar]
- 14.Haziot, A., N. Hijiya, S. C. Gangloff, J. Silver, and S. M. Goyert. 2001. Induction of a novel mechanism of accelerated bacterial clearance by lipopolysaccharide in CD14-deficient and Toll-like receptor 4-deficient mice. J. Immunol. 166:1075-1078. [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss, R. S., K. C. Chang, P. E. Swanson, K. W. Tinsley, J. J. Hui, P. Klender, S. Xanthoudakis, S. Roy, C. Black, E. Grimm, R. Aspiotis, Y. Han, D. W. Nicholson, and I. E. Karl. 2000. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat. Immunol. 1:496-501. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss, R. S., and I. E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138-150. [DOI] [PubMed] [Google Scholar]
- 17.Ikeuchi, H., T. Kuroiwa, N. Hiramatsu, Y. Kaneko, K. Ueki, and Y. Nojima. 2005. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 52:1037-1046. [DOI] [PubMed] [Google Scholar]
- 18.Kotenko, S. V., L. S. Izotova, O. V. Mirochnitchenko, E. Esterova, H. Dickensheets, R. P. Donnelly, and S. Pestka. 2001. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rβ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 276:2725-2732. [DOI] [PubMed] [Google Scholar]
- 19.Kotenko, S. V., L. S. Izotova, O. V. Mirochnitchenko, E. Esterova, H. Dickensheets, R. P. Donnelly, and S. Pestka. 2001. Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J. Immunol. 166:7096-7103. [DOI] [PubMed] [Google Scholar]
- 20.Lagasse, E., and I. L. Weissman. 1996. Flow cytometric identification of murine neutrophils and monocytes. J. Immunol. Methods 197:139-150. [DOI] [PubMed] [Google Scholar]
- 21.Langer, J. A., E. C. Cutrone, and S. Kotenko. 2004. The class II cytokine receptor (CRF2) family: overview and patterns of receptor-ligand interactions. Cytokine Growth Factor Rev. 15:33-48. [DOI] [PubMed] [Google Scholar]
- 22.Lejeune, D., L. Dumoutier, S. Constantinescu, W. Kruijer, J. J. Schuringa, and J.-C. Renauld. 2002. Interleukin-22 (IL-22) activates the JAK/STAT, ERK, JNK, and p38 MAP kinase pathways in a rat hepatoma cell line. J. Biol. Chem. 277:33676-33682. [DOI] [PubMed] [Google Scholar]
- 23.Maier, S., K. Emmanuilidis, M. Entleutner, N. Zantl, M. Werner, K. Pfeffer, and C. D. Heidecke. 2000. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock 14:187-192. [DOI] [PubMed] [Google Scholar]
- 24.Nagalakshmi, M. L., A. Rascle, S. Zurawski, S. Menon, and R. W. Malefyt. 2004. Interleukin-22 activates STAT3 and induces IL-10 by colon epithelial cells. Int. Immunopharmacol. 4:679-691. [DOI] [PubMed] [Google Scholar]
- 25.Sewnath, M. E., D. P. Olszyna, R. Birjmohun, F. J. ten Kate, D. J. Gouma, and T. van der Poll. 2001. IL-10 deficient mice demonstrate multiple organ failure and increased mortality during Escherichia coli peritonitis despite an accelerated bacterial clearance. J. Immunol. 166:6323-6331. [DOI] [PubMed] [Google Scholar]
- 26.Singbartl, K., S. G. Bockhorn, A. Zarbock, M. Schmolke, and H. Van Aken. 2005. T cells modulate neutrophil dependent acute renal failure during endotoxemia: critical role for CD28. J. Am. Soc. Nephrol. 16:720-728. [DOI] [PubMed] [Google Scholar]
- 27.Steinhauser, M. L., C. M. Hogaboam, S. L. Kunkel, N. W. Lukacs, R. M. Strieter, and T. J. Standiford. 1999. IL-10 is a major mediator of sepsis-induced impairment in lung antibacterial host defense. J. Immunol. 162:392-399. [PubMed] [Google Scholar]
- 28.van der Poll, T. 2001. Immunotherapy of sepsis. Lancet Infect. Dis. 1:165-174. [DOI] [PubMed] [Google Scholar]
- 29.van der Poll, T., and S. J. Deventer. 1999. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. N. Am. 13:413-426. [DOI] [PubMed] [Google Scholar]
- 30.Weighardt, H., C. Feterowski, M. Veit, M. Rump, H. Wagner, and B. Holzmann. 2000. Increased resistance against acute polymicrobial sepsis in mice challenged with immunostimulatory CpG oligodeoxynucleotides is related to an enhanced innate effector cell response. J. Immunol. 165:4537-4543. [DOI] [PubMed] [Google Scholar]
- 31.Weighardt, H., S. Kaiser-Moore, S. Schlautkötter, T. Rossmann-Bloeck, U. Schleicher, C. Bogdan, and B. Holzmann. 2006. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J. Immunol. 177:5623-5630. [DOI] [PubMed] [Google Scholar]
- 32.Weiss, B., K. Wolk, B. H. Grunberg, H.-D. Volk, W. Strerry, K. Asadullah, and R. Sabat. 2004. Cloning of murine IL-22 receptor alpha2 and comparison with its human counterpart. Genes Immun. 5:330-336. [DOI] [PubMed] [Google Scholar]
- 33.Whittington, H. A., L. Armstrong, K. M. Uppington, and A. B. Millar. 2004. Interleukin-22, a potential immunomodulatory molecule in the lung. Am. J. Respir. Cell Mol. Biol. 31:220-226. [DOI] [PubMed] [Google Scholar]
- 34.Wolk, K., S. Kunz, K. Asadullah, and R. Sabat. 2002. Immune cells as sources and targets of the IL-10 family members? J. Immunol. 168:5397-5402. [DOI] [PubMed] [Google Scholar]
- 35.Wolk, K., S. Kunz, E. Witte, M. Friedrich, K. Asadullah, and R. Sabat. 2004. IL-22 increases the innate immunity of tissues. Immunity 21:241-254. [DOI] [PubMed] [Google Scholar]
- 36.Wolk, K., E. Witte, E. Wallace, W. D. Döcke, S. Kunz, K. Asadullah, H.-D. Volk, W. Sterry, and R. Sabat. 2006. IL-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur. J. Immunol. 36:1309-1323. [DOI] [PubMed] [Google Scholar]
- 37.Xie, M.-H., S. Aggarwal, W.-H. Ho, J. Foster, Z. Zhang, J. Stinson, W. I. Wood, A. D. Goddard, and A. L. Gurney. 2000. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 275:31335-31339. [DOI] [PubMed] [Google Scholar]
- 38.Zantl, N., A. Uebe, B. Neumann, H. Wagner, J. R. Siewert, B. Holzmann, C. D. Heidecke, and K. Pfeffer. 1998. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 66:2300-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, X. X., A. W. Steele, W. W. Hancock, A. C. Stevens, P. W. Nickerson, P. Roy-Chaudhury, Y. Tian, and T. B. Strom. 1997. A noncytolytic IL-10/Fc fusion protein prevents diabetes, blocks autoimmunity, and promotes suppressor phenomena in NOD mice. J. Immunol. 158:4507-4513. [PubMed] [Google Scholar]