Abstract
Group B streptococcus (GBS) expresses a hemolysin/cytolysin that plays an important role in pathogenesis. Using the Himar1 transposon mutagenesis system, a hypohemolytic mutant carrying an interrupted cylJ gene was characterized. cylJ, encoding a putative glycosyltransferase, and cylK, whose product is unknown, are both required for the full hemolytic/cytolytic activity, pigment formation, and virulence of GBS.
Streptococcus agalactiae (also known as group B streptococcus [GBS]) is a commensal organism found in the gastrointestinal and genitourinary tracts of healthy individuals. However, in certain circumstances, mostly in neonates, GBS can become a life-threatening pathogen, causing invasive infections such as pneumonia, sepsis, and meningitis. The hemolysin/cytolysin expressed by GBS is an important virulence factor (8). Using ISS1 transposon mutagenesis, Spellerberg et al. identified a cluster of genes that are required for the hemolytic activity of S. agalactiae (16). This cluster comprises at least 12 open reading frames that appear to belong to a single operon called cyl (15). It comprises genes encoding an ABC transporter (cylA, cylB), an acyl carrier protein homologue (acpC), additional proteins involved in fatty acid biosynthesis (cylD, cylG, cylZ, and cylI), a putative aminomethyltransferase (cylF), and a putative glycosyltransferase (cylJ); it also comprises three GBS-specific genes (cylX, cylE, and cylK) of unknown function. The ABC transporter encoded by this operon (cylA and cylB) was proposed to be required for hemolysin export at the bacterial surface (16). cylAB was recently shown to encode an ABC-type multidrug-resistant (MDR) transporter (5). Until now, 8 out of 12 genes composing the cyl operon have been insertionally inactivated (acpC, cylZ, cylA, cylB, cylE, cylF, cylI, and cylK), and the corresponding mutants all displayed a hypohemolytic phenotype, except for the cylE mutant, which clearly was nonhemolytic (5, 13, 17). Indeed, the gene cylE, which confers a hemolytic activity to the recombinant Escherichia coli strain, was identified as the structural gene for the GBS hemolysin/cytolysin (13). Using the Himar1 transposon mutagenesis system developed for Streptococcus equi (7), a bank of about 2,000 mutants was generated in GBS strain NEM316. Efficient and random transposition of the minitransposon onto the chromosome of NEM316 was demonstrated by Southern blot analysis and insertion site sequencing of 24 randomly chosen mutants (data not shown). Screening of this bank on Todd-Hewitt agar containing 5% defibrinated horse blood revealed one hypohemolytic mutant in which the transposon had inserted in the 3′ end of cylJ, the penultimate gene of the cyl operon (Fig. 1). Quantitative reverse transcription-PCR analysis revealed that transcription of the downstream gene cylK was abolished in the cylJ::Himar1 mutant (data not shown). This result indicates that cylJ and cylK are cotranscribed. In this work, we have analyzed the contributions of cylJ and of the downstream cylK gene to the hemolytic/cytolytic activity, its associated phenotype (i.e., pigment production), and GBS virulence.
FIG. 1.
Structure of the cyl operon in the serotype III GBS strain NEM316 (3). The 12 genes belonging to this cluster are shown, and similarities of the deduced proteins to gene products with known functions in the databases are indicated by shading. The CovR binding site in the promoter region is depicted.
Variations of hemolysin production in clinical isolates are difficult to quantitate precisely due to low levels of hemolysin activity in culture supernatants. To circumvent this problem, we deleted the CovR binding site in the cyl promoter region (bp 661363 to 661423 in the NEM316 genome, as defined at http://genolist.pasteur.fr/SagaList/), since CovR acts as a repressor of the cyl operon (6, 7). As expected, a 50-fold increase in β-galactosidase activity was observed in NEM316 when the mutated promoter was cloned into pTCV-lac, in comparison with the β-galactosidase activity of the wild-type cyl promoter (data not shown). The resulting mutant strain, strain CCH206, thereafter called Pcyl+, displayed a hyperhemolytic and hyperpigmented phenotype similar to that of the CovSR− mutant (data not shown). Therefore, to evaluate the respective roles of cylJ and cylK in the hemolytic/cytolytic activity of S. agalactiae, in-frame deletions of these genes were constructed in a CCH206 genetic background (ΔcylJ [NEM2457] and ΔcylK [NEM2458], respectively). An in-frame deletion of cylE was also carried out in CCH206 (ΔcylE [NEM2456]) to further characterize this mutation in the same hyperhemolytic genetic background. The ΔcylJ, ΔcylK, and ΔcylE deletions were also constructed using the wild-type NEM316 strain, and similar results were obtained (data not shown).
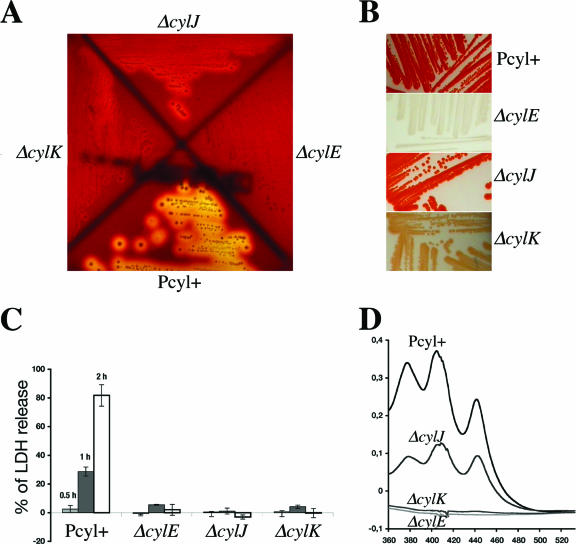
The primers used for the construction of deletion alleles are listed in Table 1. In-frame deletions of cylE (using primers O1-O2 and O3-O4), cylJ (O5-O6 and O7-O8), and cylK (O9-O10 and O11-O12) were constructed by using splicing-by-overlap-extension PCR. A similar strategy was employed to delete the CovR binding site in the cylX promoter region (using primers O13-O14 and O15-O16). Chromosomal gene exchanges were carried out as described previously (1, 2). In-frame deletions of all genes were confirmed by PCR and sequence analysis. Macroscopic analyses of the mutants, colony morphology, and growth curves in complex Todd-Hewitt medium and in RPMI medium (used as a chemically defined minimal medium) did not reveal any differences from the parental strain. As shown in Fig. 2A, the ΔcylE strain is nonhemolytic, whereas the ΔcylK and ΔcylJ strains are significantly less hemolytic than the parental strain on a horse blood agar plate. GBS hemolysin was reported to act as a cytolysin on lung epithelial cells (9). We thus measured the cytotoxic activity of the different mutants on A549 human pulmonary epithelial cells as described previously (11). Release of the cytoplasmic lactate dehydrogenase (LDH) enzyme was used as an indicator of cell lysis. The ΔcylE, ΔcylJ, and ΔcylK mutants were found to be noncytotoxic (Fig. 2C), whereas the parental strain shows a time-dependent cytotoxic effect. Taken together, these results demonstrate that CylJ and CylK are required for full expression of the hemolytic/cytolytic activity of S. agalactiae, whereas only CylE appears essential to promote hemolysis.
TABLE 1.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3 ′) | Gene target |
|---|---|---|
| O1 | GGGATCGAATTCTTAGGGACTGTTTTATCTGCGGCG | cylE |
| O2 | GCGGCAGCACCCGGGCGTGCCAGTTAAAGGCCTACCGTCTGG | cylE |
| O3 | GGCACGCCCGGGTGCTGCCGCATCCGCCAAGGGGAACATGTC | cylE |
| O4 | ATAAGGATCCAAAGCTTTATGACTAGCCAACC | cylE |
| O5 | TTCAGCAGAATTCCTCTCTCGTCAAGCATTGGA | cylJ |
| O6 | GCGGCAGCACCCGGGCGTGCCTCCAAGGATATGAATATCATGTCC | cylJ |
| O7 | GGCACGCCCGGGTGCTGCCGCGCGGAGGCAGAAATTGAATCCTTT | cylJ |
| O8 | CTATAGGGATCCCACATTTATAGGAAATTTC | cylJ |
| O9 | GCTTTGGGAATTCATTTAAACGAGATTGGGTGGA | cylK |
| O10 | GCGGCAGCACCCGGGCGTGCCCTCCAATGTAGACTGCCTATT | cylK |
| O11 | GGCACGCCCGGGTGCTGCCGCGATGGCTATATTTATGGTTATGCT | cylK |
| O12 | AGTAAGGATCCCGTCTCTTTAATGCGG | cylK |
| O13 | GTTTAGAATTCTTAGGCTTACTAACTTAGC | Pcyl |
| O14 | CATTATTATGTTAAAATAGTAAC | Pcyl |
| O15 | GTTACTATTTTAACATAATAATGTGTTTGAAGTAGATGTTTAGA | Pcyl |
| O16 | TGTTAGGATCCACGACACTGCCATCAGCAC | Pcyl |
| O17 | GTAAAGGGATCCGCTGTCTTGAAAGAGGCTATG | cylK |
| O18 | AAGTATCTGCAGACTTAGCACTATTCGCATCA | cylK |
FIG. 2.
Hemolytic/cytolytic activities and pigment formation in GBS strains. The in-frame deletion mutants NEM2456 (ΔcylE), NEM2457 (ΔcylJ), and NEM2458 (ΔcylK) derived from the hyperhemolytic and hyperpigmented strain CCH206 (Pcyl+). (A) Hemolytic phenotype of the mutants on horse blood agar plates (BioMérieux). (B) Pigment formation on Granada medium plates. (C) The cytolytic activity of GBS on A549 human alveolar epithelial cells (multiplicity of infection, 100 bacteria per cell) was assessed using the cytotoxicity detection kit (LDH) (Roche) at different time points (30 min, 1 h, and 2 h). The values were standardized such that the positive control phosphate-buffered saline-Triton 0.1% gave 100% of LDH release. (D) Absorbance profile of pigment extracts from the parental strain Pcyl+ and its ΔcylE, ΔcylJ, and ΔcylK isogenic mutants. The results shown are representative of three independent experiments.
Until now, in GBS, hemolysis and pigment production have never been dissociated (6, 9, 12, 16, 17). The GBS pigment has been recently identified as a glycopolyene (14). The detection of orange-red pigmented colonies in Granada medium is an easy way to screen and identify GBS in clinical laboratories (13). We therefore analyzed pigment production of the different mutants on Granada medium plates incubated under anaerobiosis at 37°C (Fig. 2B). The ΔcylE mutant was clearly not pigmented, whereas the ΔcylK and ΔcylJ mutants produced lesser amounts of pigment than did the parental strain. The degree of pigmentation correlates with the hemolytic activity of the mutants, with the ΔcylE mutant < the ΔcylK mutant < the ΔcylJ mutant < Pcyl+. Spectral analysis of pigment extract from GBS cultivated in Todd-Hewitt broth supplemented with 0.1% starch and 1% glucose at 37°C (6, 17) shows a characteristic triple peak in the parental strain that was reduced in the ΔcylJ mutant and absent from the ΔcylE and ΔcylK mutant extracts (Fig. 2D). Thus, the amplitude of spectral absorbance correlates with the level of hemolysin activity of the strains. To be certain of the essential role of the GBS-specific cylK in hemolytic activity and pigment biosynthesis, single-gene complementation was performed. The cylK gene was amplified in its entirety with primers O17-O18 (Table 1) and cloned into the low-copy-number shuttle vector pTCV-erm to be transcribed from the gram-positive kanamycin resistance gene promoter PaphA-3 (11). The vectors pTCV-erm and pTCV-erm (PaphA-3-cylK) were introduced by electrotransformation in NEM2458 (ΔcylK). Transcomplementation with a plasmid-borne cylK restored pigment production and hemolytic activity in the ΔcylK deletion mutant (see Fig. S1 in the supplemental material). These results point out the role of CylJ and CylK in GBS hemolysin and pigment biosynthesis and suggest a complex enzymatic pathway involving CylE as an essential component and CylJ and CylK as cofactors required for synthesis of a fully potent hemolysin. Polyene and fatty acid biosynthesis share common pathways (4). Of note, about half of the genes of the cyl operon code for enzymes involved in the biosynthesis of fatty acids (5), which can explain the close relationship found between hemolysin and pigment production in GBS.
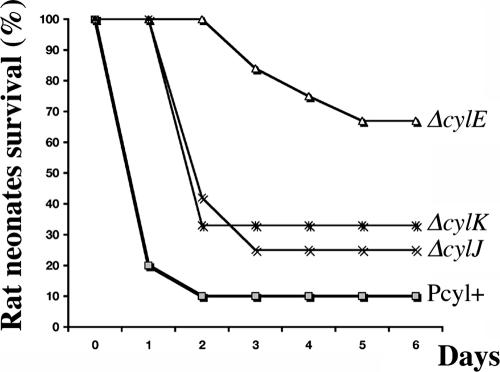
CylE has been shown to be involved in GBS virulence (8), but the roles of CylJ and CylK have not been studied. Bacterial virulence levels were compared using a neonatal rat sepsis model (10), where the parental strain Pcyl+ and the isogenic ΔcylE, ΔcylJ, and ΔcylK mutant derivatives were injected intraperitoneally (i.p.) (Fig. 3). Randomized groups of 12 rat pups were inoculated i.p. with 100 μl of bacterial suspensions containing 5 × 106 GBS cells in 0.9% NaCl. The survival of the pups was monitored for 7 days, and the statistical significance of differences between groups observed was evaluated using the Mann-Whitney U test. A P value of <0.05 was considered statistically significant. The virulence of all mutants was significantly more attenuated than was that of the parental Pcyl+ strain, with the ΔcylE mutant being the less-virulent mutant (Fig. 3). Interestingly, the ΔcylJ and ΔcylK mutants displayed similar levels of virulence, which were intermediate to those of Pcyl+ and the ΔcylE mutant (Fig. 3). At day 7 postinfection, the percentages of mortality of the rat pups injected with Pcyl+ and the ΔcylK, ΔcylJ, and ΔcylE mutants were 90%, 75% (P < 0.0213), 70% (P < 0.0213), and 30% (P < 0.0178), respectively. Thus, these results seem to show a positive correlation between the virulence of these strains and their hemolytic activity.
FIG. 3.
Mortality curves in neonatal rats infected with the GBS parental strain Pcyl+ and its ΔcylE, ΔcylJ, and ΔcylK isogenic mutants. Two-day-old Sprague-Dawley rat pups (12 per strain) were inoculated i.p. with 5 × 106 bacteria.
In summary, we have studied the role of two uncharacterized genes, cylJ and cylK, belonging to the cyl operon. cylJ, the penultimate gene of the cyl operon, encodes a putative glycosyltransferase of 403 amino acids related to UDP-glucuronosyltransferase. cylK, the last gene of the cyl operon, encodes a 191-amino-acid protein and does not exhibit any similarity with other genes in the current databases. We showed that both cylJ and cylK are involved in, but not essential for, hemolytic/cytolytic activity and pigment production, two linked phenotypes in GBS, and that the level of hemolytic activity correlates with the virulence in a neonatal rat sepsis model. Elucidation of the biochemical nature of GBS hemolysin represents the next important challenge, and the CCH206 hyperhemolytic strain may be useful for that purpose.
Supplementary Material
Acknowledgments
We thank Josh Slater for the gift of the pCAM-45 SD1 plasmid and all the students of the Cours de Microbiologie Générale de l'Institut Pasteur 2005-2006 for the screening of the Himar1 transposon bank.
This work was supported by research funds from the Institut Pasteur (GPH no. 9) and the Centre National de la Recherche Scientifique (CNRS).
Editor: A. Camilli
Footnotes
Published ahead of print on 5 February 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Biswas, I., A. Gruss, S. D. Ehrlich, and E. Maguin. 1993. High-efficiency gene inactivation and replacement system for gram-positive bacteria. J. Bacteriol. 175:3628-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dramsi, S., E. Caliot, I. Bonne, S. Guadagnini, M. C. Prevost, M. Kojadinovic, L. Lalioui, C. Poyart, and P. Trieu-Cuot. 2006. Assembly and role of pili in group B streptococci. Mol. Microbiol. 60:1401-1413. [DOI] [PubMed] [Google Scholar]
- 3.Glaser, P., C. Rusniok, C. Buchrieser, F. Chevalier, L. Frangeul, T. Msadek, M. Zouine, E. Couve, L. Lalioui, C. Poyart, P. Trieu-Cuot, and F. Kunst. 2002. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol. Microbiol. 45:1499-1513. [DOI] [PubMed] [Google Scholar]
- 4.Goel, A. K., L. Rajagopal, N. Nagesh, and R. V. Sonti. 2002. Genetic locus encoding functions involved in biosynthesis and outer membrane localization of xanthomonadin in Xanthomonas oryzae pv. oryzae. J. Bacteriol. 184:3539-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottschalk, B., G. Bröker, M. Kuhn, S. Aymanns, U. Gleich-Theurer, and B. Spellerberg. 2006. Transport of multidrug resistance substrates by the Streptococcus agalactiae hemolysin transporter. J. Bacteriol. 188:5984-5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu, G. Y., K. S. Doran, T. Lawrence, N. Turkson, M. Puliti, L. Tissi, and V. Nizet. 2004. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc. Natl. Acad. Sci. USA 101:14491-14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May, J. P., C. A. Walker, D. J. Maskell, and J. D. Slater. 2004. Development of an in vivo Himar1 transposon mutagenesis system for use in Streptococcus equi subsp. equi. FEMS Microbiol. Lett. 238:401-409. [DOI] [PubMed] [Google Scholar]
- 8.Nizet, V. 2002. Streptococcal beta-hemolysins: genetics and role in disease pathogenesis. Trends Microbiol. 10:575-580. [DOI] [PubMed] [Google Scholar]
- 9.Nizet, V., R. L. Gibson, E. Y. Chi, P. E. Framson, M. Hulse, and C. E. Rubens. 1996. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 64:3818-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poyart, C., E. Pellegrini, M. Marceau, M. Baptista, F. Jaubert, M. C. Lamy, and P. Trieu-Cuot. 2003. Attenuated virulence of Streptococcus agalactiae deficient in d-alanyl-lipoteichoic acid is due to an increased susceptibility to defensins and phagocytic cells. Mol. Microbiol. 49:1615-1625. [DOI] [PubMed] [Google Scholar]
- 11.Poyart, C., and P. Trieu-Cuot. 1997. A broad-host-range mobilizable shuttle vector for the construction of transcriptional fusions to beta-galactosidase in gram-positive bacteria. FEMS Microbiol. Lett. 156:193-198. [DOI] [PubMed] [Google Scholar]
- 12.Pritzlaff, C. A., J. C. Chang, S. P. Kuo, G. S. Tamura, C. E. Rubens, and V. Nizet. 2001. Genetic basis for the beta-haemolytic/cytolytic activity of group B streptococcus. Mol. Microbiol. 39:236-247. [DOI] [PubMed] [Google Scholar]
- 13.Rosa-Fraile, M., J. Rodriguez-Granger, M. Cueto-Lopez, A. Sampedro, E. B. Gaye, J. M. Haro, and A. Andreu. 1999. Use of Granada medium to detect group B streptococcal colonization in pregnant women. J. Clin. Microbiol. 37:2674-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosa-Fraile, M., J. Rodríguez-Granger, A. Haidour-Benamin, J. M. Cuerva, and A. Sampedro. 2006. Granadaene: proposed structure of the group B streptococcus polyenic pigment. Appl. Environ. Microbiol. 72:6367-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spellerberg, B., S. Martin, C. Brandt, and R. Lutticken. 2000. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol. Lett. 188:125-128. [DOI] [PubMed] [Google Scholar]
- 16.Spellerberg, B., B. Pohl, G. Haase, S. Martin, J. Weber-Heynemann, and R. Lütticken. 1999. Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition. J. Bacteriol. 181:3212-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tapsall, J. W. 1986. Pigment production by Lancefield-group-B streptococci (Streptococcus agalactiae). J. Med. Microbiol. 21:75-81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.