Abstract
In the Finnish Otitis Media Vaccine Trial, the now-licensed pneumococcal conjugate vaccine containing polysaccharides conjugated to protein CRM197 (PncCRM) and the experimental pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine (PncOMPC), showed similar efficacy profiles against acute otitis media despite different antibody concentrations in sera. We now report the opsonophagocytic activities (OPA) in these sera. OPA, antibody concentration, and avidity for serotypes 6B, 19F, and 23F were determined in sera of infants who received either pneumococcal conjugate (PCV) or control vaccine at 2, 4, and 6 months of age and either the homologous or pneumococcal polysaccharide vaccine at 12 months of age. OPA varied by vaccine and serotype. The majority of PCV recipients had positive OPA after the fourth dose, while OPA was undetectable in the control group. Coinciding with the efficacy data, the concentration of antibodies required for 50% killing was low for 6B and high for 19F for both PCVs. Contradictory to the efficacy data, PncOMPC induced lower functional capacity to 23F than PncCRM. OPA correlated with antibody concentration, while avidity and functional capacity of antibodies showed no correlation. The OPA data provide valuable additional information for serotype-specific differences in protection and when evaluating serotype-specific immunogenicity and should thus be considered when defining serological correlates of protection.
Various pneumococcal conjugate vaccines (PCVs) have been tested in phase II (1, 3, 13, 21, 25, 28, 29, 33, 39, 44) and III (7, 11, 12, 15, 22, 23, 30) clinical trials. The efficacy of seven- or nine-valent pneumococcal conjugate vaccine containing polysaccharides conjugated to protein CRM197 (PncCRM) has been proven in prevention of invasive pneumococcal disease (7, 12, 23, 30), acute otitis media (AOM) (7, 15), and pneumonia (8, 12, 23) in infants. The seven-valent PncCRM (Prevnar, Prevenar) has now been licensed in many countries for prevention of invasive disease and AOM due to Streptococcus pneumoniae (Pnc) in children. PncCRM and another seven-valent PCV, pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine (PncOMPC), were tested in parallel in the Finnish Otitis Media (FinOM) Vaccine Trial. Despite the similar efficacy against vaccine type AOM, 57% (95% confidence interval [CI], 44 to 67%) for PncCRM and 56% (95% CI, 44 to 66%) for PncOMPC (15, 22), the antibody concentrations and the kinetics of antibody concentrations induced by the two PCVs differed (14).
After the licensure of PncCRM, new placebo-controlled efficacy trials with PCVs are, on ethical grounds, unlikely to be conducted. When licensing new PCVs or PCV formulations, the antibody concentration 1 month after the primary series of the new PCV, as measured by enzyme immunoassay (EIA), has been suggested as the primary endpoint in noninferiority studies (18). The antibody concentration alone may, however, be insufficient as a serological correlate of vaccine efficacy. Additional data to demonstrate the functional capacity of the antibody and induction of immunological memory are required for registration (18).
Host protection against Pnc is mainly mediated by opsonin-dependent phagocytosis (10, 40). Therefore, the in vitro opsonophagocytic activity (OPA) of serum is believed to indicate the functional activity of antibodies and serve as a better correlate of protection in vivo than antibody concentration. In animal protection models, both OPA and the concentration of anti-pneumococcal polysaccharide antibodies have been shown to correlate with protection (19, 26, 35-37). Based on meta-analysis of three efficacy trials (7, 23, 30), an antibody concentration of ≥0.35 μg/ml has been recommended as a population-based correlate of protection against invasive disease for all serotypes (42). However, limited data exist on correlates of protection against other clinical outcomes and on the qualitative and functional characteristics of antibodies that contribute to protection in humans. Moreover, the data on functional antibody responses to different PCVs in humans remains limited.
The aim of the current study was to investigate the OPA in sera elicited by two PCVs, PncCRM and PncOMPC, used in parallel in the FinOM Vaccine Trial (15, 22). OPA was measured against serotypes 6B, 19F, and 23F, the most frequent pneumococcal causes of AOM in the FinOM Vaccine Trial (15, 22). The concentration and avidity of antibodies have been reported previously (14). We now report the OPA in sera of children vaccinated with the two PCVs and in sera of unvaccinated children. The specific aims were to determine serotype-specific differences in OPA, the functional capacity of antibodies, i.e., the concentration of antibodies required for 50% killing of Pnc, the correlation between functional capacity and avidity of antibodies, the correlation between OPA and functional capacity of antibodies after a primary series of PncOMPC followed by a pneumococcal polysaccharide (PS) vaccine (PncPS), and the correlation between OPA and concentration of antibodies. Finally, the antibody data were related to clinical protection.
MATERIALS AND METHODS
Study vaccines.
The PncCRM vaccine (Wyeth Vaccines, Pearl River, NY) consisted of 2 μg of capsular PSs 4, 9V, 14, 19F, and 23F, 4 μg of PS 6B, and 2 μg of serotype 18C oligosaccharide, each individually conjugated to the CRM197 protein. The PncOMPC vaccine (Merck & Co., Inc., West Point, PA) contained 1 μg of pneumococcal capsular PSs 4 and 14, 1.5 μg of PS 9V, 2 μg of PSs 18C and 19F, 3 μg of PS 23F, and 5 μg of PS 6B, each individually conjugated to the outer membrane protein complex of Neisseria meningitidis serogroup B. The commercial 23-valent PncPS vaccine (Pneumovax23; Merck & Co., Inc.) contained 25 μg of each capsular PS. The hepatitis B vaccine (Recombivax HB; Merck & Co., Inc.), used as a control vaccine for both study arms, contained 5 μg of recombinant hepatitis B surface protein. All vaccines were administered intramuscularly.
Vaccinees, vaccinations, and sampling.
Written informed consent was obtained from the parents or guardians of the study children prior to enrollment. The study protocol was approved by the Ethics Committee of the National Public Health Institute, Helsinki, Finland, by the National Agency for Medicines, and by the relevant local health authorities. The subjects of this study formed a subcohort of infants participating in the FinOM Vaccine Trial (15, 22). In this subcohort, serum samples were obtained at 2, 4, 6, 7, 12, 13, and 24 months of age from 166 infants, while samples from the rest of the vaccinees in the FinOM study were taken at either 7 or 13 months only (22). In the subcohort, 56 infants were vaccinated with PncCRM and 46 with PncOMPC at 2, 4, 6, and 12 months of age. Serum samples taken at the ages of 7, 12, 13, and 24 months were used for this study. In the PncOMPC arm of the trial, 187 of the total of 805 infants received three doses of PncOMPC, followed by a dose of PncPS at 12 months of age (22). In the subcohort, only six infants had received a PncPS booster. To also assess this vaccination scheme, instead of using the samples obtained from these six infants, we randomly selected 50 serum samples taken at 13 months from the recipients of three doses of PncOMPC and PncPS as a booster. We report here the antibody concentrations and OPA results of these samples for serotypes 6B and 19F. The control group of 58 infants received hepatitis B vaccine at the same ages as the PCVs were administered. The detailed data on concentration, kinetics, and avidity of immunoglobulin G (IgG) antibodies in the two PCV groups and the control group have been reported previously (14, 15, 22).
Laboratory methods.
All serological determinations were performed blinded at the Finnish National Public Health Institute. The laboratory personnel were not aware of the vaccination status or the age of the child at the time of sampling.
The concentrations of IgG antibodies to pneumococcal capsular PSs 6B, 19F, and 23F in sera taken at 7, 12, 13, and 24 months of age were measured by a standardized enzyme immunoassay (EIA) (14, 21) without heterologous polysaccharide (22F) absorption.
The relative avidities of IgG antibodies to capsular PSs 6B, 19F, and 23F were determined by EIA as described by Anttila et al. (4) for sera taken at 7, 12, 13, and 24 months of age in the PCV groups and for the 24-month sample in the control group (14). The assay is based on dissociation of antibody-antigen complexes by sodium thiocyanate. Serotype-specific concentrations of sodium thiocyanate (0.5 M for antibodies to 6B and 23F and 0.65 M for antibodies to 19F) were used.
OPA in sera against Pnc was determined by a standard method measuring the killing of pneumococci by differentiated HL-60 cells (ATCC, Manassas, VA) in the presence of baby rabbit complement (Pel-Freez Clinical Systems, Brown Deer, WI) (34). The results are given as titers: the reciprocal of the serum dilution with 50% killing compared with the bacterial growth in the control wells without serum. For sera with OPA titers of ≤8, a value of 4 was assigned. Repeatability was followed by including a control serum on each plate. The coefficient of variance was ≤30% for all serotypes. OPA titers were determined for serotypes 6B, 19F, and 23F for sera taken at 7, 12, 13, and 24 months of age, except for serotype 23F in the control group, for which an OPA titer was determined only for the 24-month sample. For the 13-month sample of children who received PncPS boosting, OPA titers were determined for serotypes 6B and 19F.
Statistical methods.
Antibody concentrations are given as geometric mean concentrations (GMC) and serum opsonophagocytic activities as geometric means of OPA titers (GMOPA) with 95% CI. The avidity results are expressed as avidity indices (AI) and assigned as percentages of antibodies that remained bound to the antigens after thiocyanate treatment. The relative avidities are given as mean AIs. GMCs were here calculated only for the sera for which OPA analyses were performed. Therefore, GMCs differ slightly from the previously published data (14). The functional capacity of antibodies (antibody concentration required for 50% killing of Pnc) was calculated by dividing the IgG anti-pneumococcal PS antibody concentration of a sample by the OPA titer. This was done only for the samples with a detectable OPA titer (≥8). The results are given as geometric means of antibody concentration (ng/ml needed for 50% killing) with 95% CIs. The concentration of IgG antibodies remaining after elution with thiocyanate was calculated by multiplying the IgG antibody concentration by the AI. The percentage of reduction in IgG antibody concentration after elution with thiocyanate was calculated by (1 − AI) × 100%. A linear regression model and Spearman's correlation coefficient test were used to analyze the correlation between IgG antibody concentrations and respective OPA titers. Spearman's correlation coefficient test was used to analyze the correlation between the functional capacity and avidity of IgG antibodies, between OPA titers and the concentration of IgG antibodies remaining after elution with thiocyanate, and between OPA titers and percentages of reduction in IgG antibody concentration after elution with thiocyanate. All the comparisons were made within a time point and for each vaccine group and serotype separately. In the statistical analyses, log-transformed data of concentrations and OPA titers were used.
RESULTS
IgG antibody concentrations and avidity by EIA.
The antibody concentrations and antibody avidity were determined for a subcohort of children participating in the FinOM Vaccine Trial. The antibody concentration results were in agreement with those of the whole study population (15, 22). In the control group, the GMCs of antibodies remained low for all antigens, varying between 0.09 and 0.22 μg/ml at 7 months of age, and started to increase slightly during the second year of life (Table 1).
TABLE 1.
Geometric mean concentrations of IgG antibodies measured by EIA to pneumococcal serotype 6B, 19F, and 23F polysaccharides at indicated ages in infants immunized with a 7-valent pneumococcal conjugate vaccine, PncCRM or PncOMPC, or control vaccine at 2, 4, and 6 months of age and with a homologous vaccine or 23-valent polysaccharide vaccine (PncPS) at 12 months of age
| Serotype | Vaccine(s) | GMC (95% CI) (μg/ml) at age (mo):
|
||
|---|---|---|---|---|
| 7 (n = 53-55) | 13 (n = 44-55) | 24 (n = 43-54) | ||
| 6B | PncCRM | 1.99 (1.35-2.95) | 9.02 (6.48-12.54) | 1.48 (1.11-1.99) |
| PncOMPC | 0.40 (0.29-0.56) | 1.89 (1.24-2.88) | 0.95 (0.65-1.39) | |
| PncOMPC + PncPSa | NDb | 3.73 (2.65-5.26) | ND | |
| Control | 0.09 (0.07-0.12) | 0.15 (0.12-0.20) | 0.25 (0.19-0.32) | |
| 19F | PncCRM | 3.28 (2.56-4.20) | 4.96 (3.86-6.36) | 2.14 (1.47-3.12) |
| PncOMPC | 3.52 (2.69-4.59) | 8.88 (6.24-12.65) | 4.88 (2.96-8.03) | |
| PncOMPC + PncPS | ND | 63.04 (43.66-91.02) | ND | |
| Control | 0.22 (0.17-0.29) | 0.40 (0.31-0.53) | 0.62 (0.47-0.82) | |
| 23F | PncCRM | 2.56 (1.87-3.50) | 6.20 (4.51-8.52) | 1.28 (0.98-1.68) |
| PncOMPC | 0.61 (0.45-0.82) | 2.14 (1.51-3.03) | 1.05 (0.76-1.46) | |
| Control | 0.10 (0.07-0.12) | 0.15 (0.12-0.20) | 0.23 (0.17-0.31) | |
A group of infants (n = 50) who received a primary series of PncOMPC at 2, 4, and 6 months of age followed by a booster dose of 23-valent PncPS vaccine at 12 months of age.
ND, not done.
At 7 months of age, the GMCs of antibodies were highest in both PCV groups for serotype 19F (Table 1). The lowest GMCs were detected for anti-6B and -23F antibodies in the PncOMPC group. During the 5 months after the primary series of PCV (data not shown), the GMCs of antibodies had decreased (PncCRM group) or stayed unchanged (PncOMPC group), as reported previously (14).
At 13 months of age, the GMCs were higher than at 7 months of age (Table 1). After the PCV boosting, the GMCs of anti-6B and -23F, but not of anti-19F, antibodies were higher in the PncCRM than in the PncOMPC group. After the PncPS booster, the GMCs of antibodies to serotype 6B and especially to 19F were higher than after PncOMPC boosting (Table 1). At 24 months of age, 12 months after the PCV booster dose, the GMCs of antibodies had declined for all antigens, three- to sixfold in the PncCRM group and two- to threefold in the PncOMPC group. The GMCs of antibodies were, however, higher in both PCV groups than in the control group (Table 1).
The mean AI of antibodies increased between the ages of 7 and 12 months in both PCV groups for all three serotypes studied. The increase was most notable for serotypes 6B and 23F. By the age of 24 months, the mean AIs of antibodies to serotypes 6B and 23F, but not to 19F, had increased further in both PCV groups. Between 7 and 24 months of age, the mean AIs to serotypes 6B and 23F increased notably, and that to 19F stayed unchanged in both PCV groups. In general, the kinetics of avidity development were similar in the two PCV groups, but the mean AIs of antibodies tended to be higher in the PncCRM group than in the PncOMPC group. The antibody avidity results have been described in detail elsewhere (14).
OPA.
In the control group, the number of infants with an OPA titer of ≥8 in their sera remained low during the whole follow-up period (Table 2).
TABLE 2.
GMOPA titers, percentage of infants with detectable (≥8) OPA titer, and GMC required to kill 50% of Pnc antibodies to pneumococcal serotype 6B, 19F, and 23F polysaccharides were measured in infants at 7, 13, and 24 months of age. The children were immunized with a seven-valent pneumococcal conjugate vaccine (PncCRM, PncOMPC) or control vaccine at 2, 4, and 6 months of age and either with the homologous vaccine or 23-valent polysaccharide vaccine (PncPS) at 12 months of age
| Serotype | Vaccine(s) | GMOPA titer (95% CI) at age (mo):
|
Infants (%) with detectable OPA titer at age (mo):
|
GMC (ng/ml) needed for 50% killing (95% CI) at age (mo):
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7 (n = 53-55) | 13 (n = 44-55) | 24 (n = 43-54) | 7 | 13 | 24 | 7 | 13 | 24 | ||
| 6B | PncCRM | 143 (82-249) | 606 (394-932) | 42 (23-78) | 83 | 95 | 67 | 10 (7-15) | 13 (11-17) | 17 (10-27) |
| PncOMPC | 15 (9-26) | 91 (49-170) | 15 (8-27) | 40 | 77 | 41 | 9 (5-17) | 13 (8-22) | 27 (15-47) | |
| PncOMPC + PncPSa | NDb | 46 (28-78) | ND | ND | 74 | ND | ND | 56 (41-75) | ND | |
| Control | <8 | <8 (4-4.4) | <8 (4-5) | 0 | 2 | 2 | ND | ND | ND | |
| 19F | PncCRM | 26 (18-37) | 78 (55-110) | 11 (7-19) | 78 | 95 | 33 | 97 (81-117) | 59 (45-76) | 81 (45-145) |
| PncOMPC | 51 (35-75) | 84 (50-142) | 27 (14-55) | 83 | 84 | 56 | 50 (40-63) | 78 (63-96) | 101 (73-139) | |
| PncOMPC + PncPS | ND | 478 (332-690) | ND | ND | 98 | ND | ND | 128 (112-147) | ND | |
| Control | <8 | <8 | <8 (4-6) | 0 | 0 | 6 | ND | ND | ND | |
| 23F | PncCRM | 39 (26-58) | 173 (109-273) | 14 (9-20) | 83 | 93 | 52 | 59 (46-75) | 31 (25-37) | 58 (43-79) |
| PncOMPC | <8 (5-7) | 17 (11-25) | <8 (5-10) | 23 | 64 | 19 | 92 (45-189) | 96 (78-117) | 65 (34-123) | |
| Control | ND | ND | <8 (4-5) | ND | ND | 4 | ND | ND | ND | |
A group of infants (n = 50) who received PncOMPC at 2, 4, and 6 months of age followed by a 23-valent PS vaccine at 12 months of age.
ND, not done.
At 7 months of age, 78 to 83% (depending on the serotype) of infants in the PncCRM group and 23 to 83% in the PncOMPC group had a positive (≥8) OPA titer (Table 2). In the PncCRM group, the GMOPA was substantially higher for serotype 6B than for 19F or 23F, despite similar GMCs of IgG antibodies (Tables 1 and 2). In the PncOMPC group, by contrast, the GMOPA was highest for serotype 19F (Table 2), agreeing with the GMC data. However, while the GMCs of IgG antibodies differed up to 10-fold (Table 1), the difference between GMOPAs was less.
At 12 months of age, the proportion of infants with a detectable OPA titer was low in both PCV groups (7 to 46%), with the exception of serotype 6B in the PncCRM group (61%). Due to the low percentages of positive samples, GMOPAs were not calculated.
The majority (64 to 95%) of sera obtained at 13 months of age, 1 month after the booster dose, were positive for OPA in both PCV groups (Table 2). In the sera of the PncCRM recipients, the GMOPAs were highest for serotype 6B and lowest for 19F (Table 2). In the PncOMPC group, the GMOPAs for serotypes 19F and 6B were similar, even though the GMC for anti-19F antibodies was five times as high as for anti-6B antibodies (Table 1). Further, the GMOPA for 6B was five times as high as for 23F, despite similar GMCs of IgG antibodies (Tables 1 and 2).
The majority of sera obtained from infants boosted with PncPS had a positive OPA titer for serotypes 6B and 19F (Table 2). The GMOPA for serotype 6B was lower after PncPS than PncOMPC boosting despite the greater GMC of anti-6B antibodies after PncPS boosting (Tables 1 and 2). For serotype 19F, the remarkably higher GMC of IgG antibodies induced by PncPS than PncOMPC (Table 1) resulted also in a substantially higher GMOPA. Furthermore, for serotype 19F, the GMOPA was clearly higher after PncPS boosting than after PncCRM boosting (Table 2).
At 24 months of age, 19 to 67% of sera, depending on the serotype and PCV used, had a positive OPA titer (Table 2). The GMOPAs for the two PCV groups were similar, despite the clear difference in GMOPAs for serotypes 6B and 23F at 13 months. The GMOPAs were higher in both PCV groups than in the control group, except for serotype 23F in the PncOMPC group (Table 2).
Functional capacity of antibodies.
In both PCV groups and at both 7 and 13 months of age, 1/3 to 1/10 concentrations of anti-6B IgG antibodies were required for 50% killing of Pnc compared to anti-19F or anti-23F antibody concentrations required for the same effect (Table 2). The functional capacity of anti-6B antibodies was similar in the two PCV groups. After the third PCV dose, a nearly twice as high anti-19F concentration was required for 50% killing in the PncCRM group than in the PncOMPC group, and after the fourth dose, more anti-23F antibodies were required for killing in the PncOMPC group than in the PncCRM group (Table 2). After boosting with PncPS, the IgG concentrations required for 50% killing of 6B and 19F Pnc were two to five times as high as after boosting with the PCVs, suggesting lower functional capacity of antibodies induced by the PncPS (Table 2).
In sera taken at the age of 24 months, there were no differences in the functional capacity of antibodies between the two PCV groups (Table 2).
Correlation between functional capacity and avidity.
No significant correlation was found between the antibody concentration required for 50% killing of Pnc and the relative avidity of antibodies within each serotype, time point, or PCV group (data not shown).
Correlation between OPA and antibody concentration.
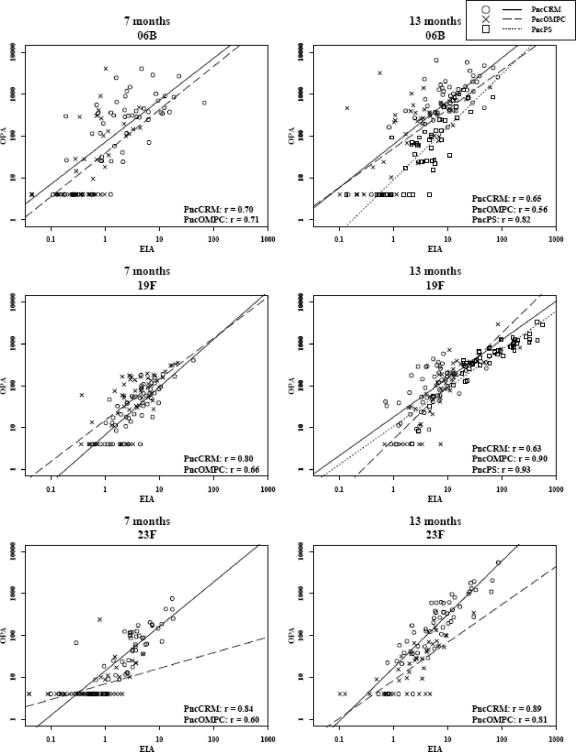
The detection threshold for the OPA method was approximately 1 μg/ml for the three serotypes. Individual antibody concentrations correlated positively with OPA titers for all serotypes, as follows: at 7 months, r = 0.70 to 0.84 (P < 0.001) for PncCRM and r = 0.60 to 0.71 (P < 0.001) for PncOMPC; at 13 months, r = 0.63 to 0.89 (P < 0.001) for PncCRM, r = 0.56 to 0.90 (P < 0.001) for PncOMPC, and r = 0.82 to 0.93 (P < 0.0001) for PncPS (Fig. 1).
FIG. 1.
The correlation (Spearman's correlation coefficient; P < 0.001 for all serotypes and time points) and a linear regression model for the IgG anti-6B, -19F, and -23F antibody concentrations (EIA, μg/ml) and OPA titers after three (7 months) and four (13 months) doses of vaccine. The children were immunized with 7-valent pneumococcal conjugate vaccine (PncCRM or PncOMPC) at 2, 4, and 6 months of age and either with the homologous vaccine or 23-valent polysaccharide vaccine (PncPS) at 12 months of age. All children boosted with PncPS had previously received three doses of PncOMPC.
By 24 months of age, the proportion of sera with detectable OPA had decreased, yet the antibody concentrations correlated well with the OPAs: r = 0.64 to 0.78 (P < 0.001) in the PncCRM group and r = 0.50 to 0.88 (P < 0.001) in the PncOMPC group.
The correlation between OPA titers and the concentration of high-avidity IgG antibodies, i.e., the concentration of IgG antibodies remaining after elution with thiocyanate was, for all serotypes and vaccine groups, similar to the correlation between OPA and total IgG antibody concentration. No significant correlation was found between OPA titers and the percentage of reduction in antibody concentration after elution with thiocyanate (data not shown).
DISCUSSION
The decision on the acceptance of a new pneumococcal vaccine, vaccine formulation, or vaccination schedule should be based on the efficacy of the vaccination. However, after the licensure of a PCV, new efficacy trials are not feasible on ethical grounds. In this situation, serologic correlates of protection would be useful. The protective concentrations of serotype-specific pneumococcal antibodies have not been clearly defined, although recent meta-analysis has provided guidelines (42). In addition, it has been suggested that a functional antibody assay rather than binding of antibody as measured by EIA is a more useful surrogate assay. In this study, we measured the functional antibody activity elicited by PncCRM and PncOMPC, used in parallel in the FinOM Vaccine Trial.
There were serotype-dependent differences in the OPA results. Although GMCs of antibodies were generally highest for serotype 19F and lowest for 6B, the antibody concentrations required for 50% killing were substantially lower for 6B than for 19F for both PCVs. This suggests better functional capacity of anti-6B than anti-19F antibodies. The results are in agreement with the previous studies, demonstrating low functional activity of anti-19F antibodies (6, 32, 34, 43), and with the data of Jokinen et al. (20), showing that a higher mean antibody concentration is needed for protection against serotype 19F than for 6B AOM after PCV vaccination. Serotypes 6B and 23F elicited similar antibody concentrations, yet the concentration required for 50% killing of serotype 23F was 2 to 10 times as high as for 6B, indicating again the superiority of anti-6B antibodies. The finding was unexpected, since previously published data for different PCVs (6, 32, 34, 43) indicate equal functional activity of anti-6B and -23F antibodies. However, similar results have been presented by Nurkka et al. for an 11-valent PnPD vaccine (29a).
The low functional activity of anti-19F antibodies could be due to the lack of avidity maturation. There was no increase in the avidity of anti-19F antibodies between 7 and 24 months of age (14). By contrast, a clear increase was found in the avidity of anti-6B and -23F antibodies (14). In previous studies, avidity has been shown to have an effect on the functional activity of antibodies (2, 24, 38, 41). The low functional activity of anti-19F antibodies could also be due to bacterial factors: serotypic differences have been documented in the deposition and degradation of the opsonic complement protein C3b on the surface of the pneumococcus, resulting in differences in resistance to phagocytosis (16). However, our preliminary studies have not indicated differences in C3b deposition between pneumococcal serotypes 6B and 19F (25a).
The functional capacity of anti-23F antibodies differed between the PCV groups. After the fourth dose of vaccine, the GMC of antibodies required for 50% killing was substantially lower for PncCRM than for PncOMPC. The same trend was seen after the third dose. Interestingly, the mean relative avidity of anti-23F IgG antibodies was substantially higher in the PncCRM group than in the PncOMPC group (14). This suggests that the lower functional capacity of anti-23F antibodies induced by PncOMPC is associated with lower avidity. In agreement with these findings, previous studies have demonstrated higher functional activity and avidity of antibodies to the PS of Haemophilus influenzae type b evoked by CRM than by OMPC conjugate vaccine (24, 38).
The differences in the OPA titers and antibody concentrations for serotype 23F were not, however, reflected in the protection. PncCRM and PncOMPC elicited similar protection against type 23F AOM (59% [95% CI, 35 to 75%] for PncCRM and 52% [95% CI, 28 to 68%] for PncOMPC) (15, 22). Furthermore, even though the anti-6B antibody concentrations and OPA titers were clearly lower in the PncOMPC group, no substantial differences between the two PCVs were found in the anti-6B antibody concentrations required for 50% killing of Pnc or in the efficacy against AOM due to serotype 6B (84% [95% CI, 62 to 93%] for PncCRM and 79% [95% CI, 58 to 89%] for PncOMPC) (15, 22). This suggests that both vaccines had induced a satisfactory immune response to serotypes 6B and 23F, even if there were quantitative and qualitative differences. Further, a different PCV, protein D conjugate (GlaxoSmithKline Biologicals), induced a very similar efficacy profile to PncCRM and PncOMPC for serotypes 6B (88% [95% CI, 58 to 96%]) and 23F (72% [95% CI, 25 to 90%]) (31), suggesting that this range of protection is the maximum that can be evoked by the present vaccines. The lack of good correlation between differences in OPA titers and protection could also suggest that protection against AOM, a mucosal infection, may be mediated by other factors in addition to opsonophagocytosis. The functional capacity of anti-19F antibodies was, after three doses of vaccine, higher in the PncOMPC than in the PncCRM group. After the fourth dose, no such difference was found anymore. The low clinical efficacy estimates (25% [95% CI, −14 to 51%] for PncCRM and 37% [95% CI, 1 to 59%] for PncOMPC) are concordant with the low OPA titers but not with the high antibody concentrations.
A number of studies have shown a relationship between avidity and in vitro functional activity of antibodies (2, 27, 38, 41). We could, however, not find any correlation between the concentration required for 50% killing and the relative avidity of antibodies when the comparisons were made within a time point for each PCV group and serotype. Accordingly, in the study by Wuorimaa et al. (43), no such correlation was found. However, relatively small numbers of samples obtained at the same age may have resulted in the relatively narrow variation of AIs, which, in turn, may have obstructed us from observing a truly existing correlation. The lack of correlation may also be due to the fact that the OPA assay includes all antibody isotypes and specificities, while EIA measures only IgG antibodies to pneumococcal polysaccharides.
A subcohort of the FinOM Vaccine Trial received a primary series of PncOMPC followed by a PncPS booster (22). A PncPS booster would be cheaper and benefit countries for which the cost of the vaccine is a major hurdle to its introduction to the national immunization program. In accordance with previous studies (9, 17, 44), boosting with the PncPS evoked higher antibody concentrations than boosting with PCV. The concentration of antibodies required for 50% killing was, however, higher after PncPS than after PCV booster. Accordingly, antibody avidity has been shown to be lower after PncPS than PCV boosting (5). The vaccine efficacy against AOM after PncPS or PCV booster was not different (22), suggesting that the differences shown in antibody concentrations and functional capacities have low clinical relevance for short-term protection in the case of AOM. The clinical relevance against other pneumococcal outcomes and long-term effects on the persistence of protection remain to be seen.
This study shows that though antibody concentrations and OPA titers correlate well within each serotype, the data of OPA and functional capacity provide valuable additional information for serotype-specific differences in protection. Therefore, the OPA and functional capacity of antibodies should, in addition to the antibody concentrations, be taken into consideration when defining correlates of protection.
Acknowledgments
We thank Maijastiina Karpala, Hannele Lehtonen, Raili Haikala, Piia Pihlajamaa, Leena Saarinen, and Nina Nikkanen for excellent technical assistance, Jaason Haapakoski and Esa Ruokokoski for experienced data management, Mika Lahdenkari for statistical advice, P. Helena Mäkelä for critical reading of the manuscript, the personnel of the study health centers, and all the children and their parents who volunteered to participate in the FinOM study.
This work was supported by Merck & Co. Inc., sanofi pasteur, and Wyeth Vaccines.
T.K. and H.K. have served as consultants to Wyeth Vaccines and GSK Bio, and H.K. has served as a consultant to sanofi pasteur.
In addition to T.K., H.K., M.V., and J.V., the members of the FinOM Study Group are Juhani Eskola, Mervi Eerola, Tapani Hovi, Pekka Karma, Maija Leinonen, P. Helena Mäkelä, Arto Palmu, Esa Ruokokoski, Aino K. Takala, Kari S. Lankinen, Petri Mattila, Pirjo-Riitta Saranpää, Anna-Stina Leinonen, Terhi Hulkko, Wilhelm Bredenberg, Kaisu Hattela, Tuija-Leena Huupponen, Marja-Leena Hyypiä, Elina Hyödynmaa, Päivi Leinonen, Päivi Limnell, Merja Mölsä, Hanna Rautio, Auli Räsänen, Päivi Savikurki-Heikkilä, Heljä Savolainen, Anneli Siro, Ritva Syrjänen, Sirpa Vesa, Sari Vikström, Hannele Holli, Marja-Leena Hotti, Helena Jokinen, Marjo-Riitta Kauppinen, Eija Lahtinen, Johanna Laitinen, Ella Lehto, Taina Nissinen, Sirkka Oikarinen, Sirkka-Liisa Piirto, Minna Ranta, Päivi Sirén, Terttu Suikkanen, Päivi Tervonen, Eija Kujanne, Hannamari Salonen, Marjo Virkki, Arja Katila, Maire Selin, Elja Herva, Aili Hökkä, Tarja Kaijalainen, Eeva-Liisa Korhonen, Hilkka Ohukainen, Maijastiina Karpala, Minna Koivuniemi, Hannele Lehtonen, Piia Pihlajamaa, Satu Rapola, Leena Saarinen, Heidi Åhman, Soile Blomqvist, Marjaana Kleemola, Marko Grönholm, Jaason Haapakoski, Eeva Koskenniemi, Satu Nahkuri, Matti Sarjakoski, Jukka Jokinen, Mika Lahdenkari, Ulla Johansson, Paula Solukko, Olli Ruuskanen, Paul Fine, Jussi Mertsola, Richard Moxon, Patrick Olin, and Karin Prellner.
Editor: D. L. Burns
Footnotes
Published ahead of print on 29 January 2007.
REFERENCES
- 1.Åhman, H., H. Käyhty, P. Tamminen, A. Vuorela, F. Malinoski, and J. Eskola. 1996. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr. Infect. Dis. J. 15:134-139. [DOI] [PubMed] [Google Scholar]
- 2.Amir, J., X. Liang, and D. M. Granoff. 1990. Variability in the functional activity of vaccine-induced antibody to Haemophilus influenzae type b. Pediatr. Res. 27:358-364. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, E. L., D. J. Kennedy, K. M. Geldmacher, J. Donnelly, and P. M. Mendelman. 1996. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J. Pediatr. 128:649-653. [DOI] [PubMed] [Google Scholar]
- 4.Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1998. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J. Infect. Dis. 177:1614-1621. [DOI] [PubMed] [Google Scholar]
- 5.Anttila, M., J. Eskola, H. Åhman, and H. Käyhty. 1999. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine 17:1970-1977. [DOI] [PubMed] [Google Scholar]
- 6.Anttila, M., M. Voutilainen, V. Jäntti, J. Eskola, and H. Käyhty. 1999. Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae. Clin. Exp. Immunol. 118:402-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, K. Edwards, et al. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 8.Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. [DOI] [PubMed] [Google Scholar]
- 9.Blum, M. D., R. Dagan, P. M. Mendelman, V. Pinsk, M. Giordani, S. Li, N. Bohidar, and T. B. McNeely. 2000. A comparison of multiple regimens of pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine and pneumococcal polysaccharide vaccine in toddlers. Vaccine 18:2359-2367. [DOI] [PubMed] [Google Scholar]
- 10.Brown, E. J., S. W. Hosea, and M. M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 5:S797-805. [DOI] [PubMed] [Google Scholar]
- 11.Capeding, M. Z., T. Puumalainen, C. P. Gepanayao, H. Käyhty, M. G. Lucero, and H. Nohynek. 2003. Safety and immunogenicity of three doses of an eleven-valent diphtheria toxoid and tetanus protein-conjugated pneumococcal vaccine in Filipino infants. BMC Infect. Dis. 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutts, F. T., S. M. Zaman, G. Enwere, S. Jaffar, O. S. Levine, J. B. Okoko, C. Oluwalana, A. Vaughan, S. K. Obaro, A. Leach, K. P. McAdam, E. Biney, M. Saaka, U. Onwuchekwa, F. Yallop, N. F. Pierce, B. M. Greenwood, and R. A. Adegbola. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365:1139-1146. [DOI] [PubMed] [Google Scholar]
- 13.Dagan, R., R. Melamed, O. Zamir, and O. Leroy. 1997. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr. Infect. Dis. J. 16:1053-1059. [DOI] [PubMed] [Google Scholar]
- 14.Ekström, N., H. Åhman, J. Verho, J. Jokinen, M. Väkeväinen, T. Kilpi, and H. Käyhty. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Käyhty, P. Karma, R. Kohberger, G. Siber, and P. H. Mäkelä. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 16.Hostetter, M. K. 1986. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J. Infect. Dis. 153:682-693. [DOI] [PubMed] [Google Scholar]
- 17.Huebner, R. E., N. Mbelle, B. Forrest, D. V. Madore, and K. P. Klugman. 2004. Long-term antibody levels and booster responses in South African children immunized with nonavalent pneumococcal conjugate vaccine. Vaccine 22:2696-2700. [DOI] [PubMed] [Google Scholar]
- 18.Jodar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Käyhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, S. E., L. Rubin, S. Romero-Steiner, J. K. Dykes, L. B. Pais, A. Rizvi, E. Ades, and G. M. Carlone. 1999. Correlation of opsonophagocytosis and passive protection assays using human anticapsular antibodies in an infant mouse model of bacteremia for Streptococcus pneumoniae. J. Infect. Dis. 180:133-140. [DOI] [PubMed] [Google Scholar]
- 20.Jokinen, J. T., H. Åhman, T. M. Kilpi, P. H. Mäkelä, and M. H. Käyhty. 2004. Concentration of antipneumococcal antibodies as a serological correlate of protection: an application to acute otitis media. J. Infect. Dis. 190:545-550. [DOI] [PubMed] [Google Scholar]
- 21.Käyhty, H., H. Åhman, P. R. Rönnberg, R. Tillikainen, and J. Eskola. 1995. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J. Infect. Dis. 172:1273-1278. [DOI] [PubMed] [Google Scholar]
- 22.Kilpi, T., H. Åhman, J. Jokinen, K. S. Lankinen, A. Palmu, H. Savolainen, M. Grönholm, M. Leinonen, T. Hovi, J. Eskola, H. Käyhty, N. Bohidar, J. C. Sadoff, and P. H. Mäkelä. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37:1155-1164. [DOI] [PubMed] [Google Scholar]
- 23.Klugman, K. P., S. A. Madhi, R. E. Huebner, R. Kohberger, N. Mbelle, and N. Pierce. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349:1341-1348. [DOI] [PubMed] [Google Scholar]
- 24.Lucas, A. H., and D. M. Granoff. 1995. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J. Immunol. 154:4195-4202. [PubMed] [Google Scholar]
- 25.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 25a.Melin, M., H. Jarva, S. Meri, and H. Käyhty. 2004. Abstr. 4th Int. Symp. Pneumococci Pneumococcal Dis., abstr. IMM-41.
- 26.Musher, D. M., B. Johnson, Jr., and D. A. Watson. 1990. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect. Immun. 58:3871-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahm, M. H., K. H. Kim, P. Anderson, S. V. Hetherington, and M. K. Park. 1995. Functional capacities of clonal antibodies to Haemophilus influenzae type b polysaccharide. Infect. Immun. 63:2989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nurkka, A., J. Joensuu, I. Henckaerts, P. Peeters, J. Poolman, T. Kilpi, and H. Käyhty. 2004. Immunogenicity and safety of the eleven valent pneumococcal polysaccharide-protein D conjugate vaccine in infants. Pediatr. Infect. Dis. J. 23:1008-1014. [DOI] [PubMed] [Google Scholar]
- 29.Nurkka, A., H. Åhman, M. Yaich, J. Eskola, and H. Käyhty. 2001. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine 20:194-201. [DOI] [PubMed] [Google Scholar]
- 29a.Nurkka, A., J. Poolman, I. Henckaerts, T. Kilpi, and H. Käyhty. 2004. Abstr. 4th Int. Symp. Pneumococci Pneumococcal Dis., abstr. PSV-41.
- 30.O'Brien, K. L., L. H. Moulton, R. Reid, R. Weatherholtz, J. Oski, L. Brown, G. Kumar, A. Parkinson, D. Hu, J. Hackell, I. Chang, R. Kohberger, G. Siber, and M. Santosham. 2003. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 362:355-361. [DOI] [PubMed] [Google Scholar]
- 31.Prymula, R., P. Peeters, V. Chrobok, P. Kriz, E. Novakova, E. Kaliskova, I. Kohl, P. Lommel, J. Poolman, J. P. Prieels, and L. Schuerman. 2006. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 367:740-748. [DOI] [PubMed] [Google Scholar]
- 32.Puumalainen, T., N. Ekström, R. Zeta-Capeding, J. Ollgren, K. Jousimies, M. Lucero, H. Nohynek, and H. Käyhty. 2003. Functional antibodies elicited by an 11-valent diphtheria-tetanus toxoid-conjugated pneumococcal vaccine. J. Infect. Dis. 187:1704-1708. [DOI] [PubMed] [Google Scholar]
- 33.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604-611. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero-Steiner, S., D. M. Musher, M. S. Cetron, L. B. Pais, J. E. Groover, A. E. Fiore, B. D. Plikaytis, and G. M. Carlone. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 36.Saeland, E., H. Jakobsen, G. Ingolfsdottir, S. T. Sigurdardottir, and I. Jonsdottir. 2001. Serum samples from infants vaccinated with a pneumococcal conjugate vaccine, PncT, protect mice against invasive infection caused by Streptococcus pneumoniae serotypes 6A and 6B. J. Infect. Dis. 183:253-260. [DOI] [PubMed] [Google Scholar]
- 37.Saeland, E., G. Vidarsson, and I. Jonsdottir. 2000. Pneumococcal pneumonia and bacteremia model in mice for the analysis of protective antibodies. Microb. Pathog. 29:81-91. [DOI] [PubMed] [Google Scholar]
- 38.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 39.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 40.Tuomanen, E. I., R. Austrian, and H. R. Masure. 1995. Pathogenesis of pneumococcal infection. N. Engl. J. Med. 332:1280-1284. [DOI] [PubMed] [Google Scholar]
- 41.Usinger, W. R., and A. H. Lucas. 1999. Avidity as a determinant of the protective efficacy of human antibodies to pneumococcal capsular polysaccharides. Infect. Immun. 67:2366-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. 2005. Recommendations for the production and control of pneumococcal conjugate vaccines. Technical Report Series no. 927, annex 2. World Health Organization, Geneva, Switzerland.
- 43.Wuorimaa, T. K., R. Dagan, F. Bailleux, R. Haikala, N. Ekstrom, J. Eskola, M. Yaich, and H. Kayhty. 2005. Functional activity of antibodies after immunization of Finnish and Israeli infants with an 11-valent pneumococcal conjugate vaccine. Vaccine 23:5328-5332. [DOI] [PubMed] [Google Scholar]
- 44.Zangwill, K. M., D. P. Greenberg, C. Y. Chiu, P. Mendelman, V. K. Wong, S. J. Chang, S. Partridge, and J. I. Ward. 2003. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants. Vaccine 21:1894-1900. [DOI] [PubMed] [Google Scholar]