Abstract
The NRAMP1 gene encodes a divalent cation transporter, located in the phagolysosomal membrane of macrophages, that has been associated with resistance to intracellular pathogens. In cattle, natural resistance against brucellosis has been associated with polymorphisms at the 3′ untranslated region (3′UTR) of the NRAMP1 gene, which are detectable by single-strand conformational analysis (SSCA). This study aimed to evaluate the association between NRAMP1 3′UTR polymorphisms and resistance against bovine brucellosis in experimental and natural infections. In experimentally infected pregnant cows, abortion occurred in 42.1% of cows with a resistant genotype (SSCAr; n = 19) and in 43.1% of those with a susceptible genotype (SSCAs; n = 23). Furthermore, no association between intensity of pathological changes and genotype was detected. In a farm with a very high prevalence of bovine brucellosis, the percentages of strains of the SSCAr genotype were 86 and 84% in serologically positive (n = 64) and negative (n = 36) cows, respectively. Therefore, no association was found between the NRAMP1-resistant allele and the resistant phenotype in either experimental or naturally occurring brucellosis. To further support these results, bacterial intracellular survival was assessed in bovine monocyte-derived macrophages from cattle with either the resistant or susceptible genotype. In agreement with our previous results, no difference was observed in the rates of intracellular survival of B. abortus within macrophages from cattle with susceptible or resistant genotypes. Taken together, these results indicate that these polymorphisms at the NRAMP1 3′UTR do not affect resistance against B. abortus in cattle and that they are therefore not suitable markers of natural resistance against bovine brucellosis.
Bovine brucellosis is caused by Brucella abortus and is clinically characterized by occurrence of abortion during the last trimester of pregnancy, which results in impaired fertility and decreased milk production (14, 19, 32). In addition to its economic significance, the zoonotic potential of this disease poses a risk to public health.
Although brucellosis has been controlled successfully in a few countries of the Northern hemisphere (18, 40), it is still an important zoonotic disease worldwide. Control of bovine brucellosis is largely based on vaccination and slaughter of infected animals (33). However, complete eradication is not easily achieved, and, therefore, alternative approaches such as genetic selection of naturally resistant cattle may become a useful tool for eradication of the disease. Indeed, genetic markers for natural resistance against brucellosis have been investigated (2, 3, 45).
The Nramp1 gene (coding for natural resistance-associated macrophage protein 1), previously known as Lsh/Ity/Bcg and recently renamed as Slc11a1 (solute carrier family 11 member1), was first recognized in mice. Nramp1 is associated with natural resistance against intracellular pathogens in the mouse, including Mycobacterium sp., Salmonella sp., and Leishmania sp (9, 21, 29, 43, 44). In mice, Nramp1 plays an important role in innate immunity, preventing bacterial growth in macrophages during the initial stages of infection (43). In addition, Nramp1 influences the adaptive immunity through its pleiotropic effects and stabilization of certain cytokine mRNAs (46). The Nramp1 gene encodes a divalent cation transporter that is located in the phagolysosomal membrane of macrophages (22). Nramp1 functions as a pH-dependent transporter of divalent cations such as Fe2+ and Mn2+ through the phagolysosome membrane. Experimental studies suggest that the direction of transport is from the lumen of the phagolysosome towards the cytosol, which prevents acquisition of these cations by intracellular pathogens (17, 27). However, there are also some data indicating that movement of cations occurs in the opposite direction, resulting in an increased concentration of iron in the phagolysosome, which may favor bacterial killing by generation of oxygen intermediates through the Fenton reaction (20, 47).
In cattle, natural resistance against Brucella abortus has been linked to polymorphisms within the 3′ untranslated region (3′UTR) (GT)n microsatellite of the NRAMP1 gene, which are detectable by single-strand conformational analysis (SSCA) (3, 16). These polymorphisms correspond to a variation in the number of GT repeats in a polymorphic (GT)n microsatellite located at the 3′UTR, in which 13 to 16 GT repeats have been identified (3, 26). Experimental evidence indicates that the (GT)13 allele is associated with natural resistance to brucellosis in vivo (3) and control of B. abortus replication within macrophage cell lines (6). A link between 3′UTR microsatellite polymorphisms and resistance to brucellosis has recently been described in water buffalo (8). In contrast, no association between NRAMP1 3′UTR polymorphisms and resistance to Brucella infection was observed in humans (10). Furthermore, these polymorphisms at the 3′UTR of the bovine NRAMP1 gene do not correlate with resistance against Mycobacterium bovis (7).
Considering the potential application of a suitable genetic marker for resistance against bovine brucellosis and the scarcity of studies supporting the use of the polymorphisms at the 3′UTR (GT)n microsatellite as resistance markers, the aim of this study was to investigate the influence of these polymorphisms on resistance to B. abortus infection in both experimentally and naturally challenged cattle as well as cultured bovine macrophages.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
Brucella abortus strain 2308 was used in all experimental inoculations in this study. For in vivo infections, strain 2308 was grown on tryptose agar (Difco, Becton Dickinson, Le Pont de Claux, France) for 48 h at 37°C in an atmosphere of 5% CO2. Prior to inoculation, the plates were scraped and a bacterial suspension was prepared and adjusted to a final concentration of 3.0 × 108 CFU/ml by spectrophotometry. For inoculation of cultured macrophages, strain 2308 was grown for 24 h in Brucella broth (Difco) under agitation.
Experimental infection.
Forty-two crossbred heifers ranging from 20 to 30 months of age were divided into three groups according to their previous vaccination records. The first group was composed of heifers previously vaccinated with the S19 vaccine strain (n = 17); another group was composed of heifers vaccinated with the RB51 vaccine strain prior to becoming pregnant (n = 12), and the third group was composed of nonvaccinated heifers (n = 13). Throughout the experiment, the heifers received a balanced diet containing corn silage, cottonseed, citrus pulp, and a mineral mixture. All heifers were serologically negative for brucellosis before challenge, as assessed by the rose bengal plate agglutination test (RBPAT). The heifers underwent estrous synchronization followed by artificial insemination. Pregnancy was confirmed by ultrasonography at 35 days after insemination. Between 6 and 7 months of pregnancy, the heifers were challenged by conjunctival administration of the virulent B. abortus strain 2308. Each heifer was inoculated at 50 μl in each eye (total of 100 μl per heifer) with a bacterial suspension containing 3.0 × 108 CFU/ml. They were then observed permanently until abortion or calving. This experimental protocol was approved by the local Committee for Ethical Use of Experimental Animals (CETEA—UFMG, protocol 028/05).
Aborted fetuses, newborn calves, and cows were necropsied within 48 h after abortion or parturition. Cows and calves were euthanized by electrocution after intravenous administration of 15 to 20 mg/kg of body weight of xylazine (Coopazine, Coopers, Brazil). Fragments of lymph nodes (prescapular, mammary, internal iliac, and bronchial), mammary gland, lung, spleen, liver, placentome, and endometrium were collected from cows, whereas fragments of bronchial lymph nodes, lung, spleen, and liver were collected from aborted fetuses and calves. Samples from all of these organs were stored at −20°C for bacterial culture and DNA extraction. Tissue fragments were fixed for 24 h in 10% neutral-buffered formalin and further processed for paraffin embedding, sectioning, and staining with hematoxylin and eosin.
Natural infection.
In order to verify any association between the 3′UTR NRAMP1 (GT)n microsatellite polymorphisms and resistance against brucellosis under natural conditions, a crossbred herd with high rate of abortion and large number of cows serologically positive for B. abortus was used in this study. No records of vaccination against brucellosis were available for this herd. One hundred lactating cows were selected for collection of blood and serum. Vaginal swabs for isolation and typing of Brucella were collected from nine cows with a recent history of parturition or abortion.
Serum samples were analyzed for detection of anti-B. abortus antibodies by rose bengal plate agglutination test, standard tube agglutination test (STAT), and 2-mercaptoethanol (2-ME) test. Results were expressed as positive or negative for the RBPAT, and complete agglutination at a dilution of 1:25 or higher was considered positive by 2-ME test. Samples of vaginal swabs were plated on tryptose agar supplemented with antibiotics (Farrell's medium—Brucella selective supplement SR83; Oxoid, United Kingdom), and the isolated biovars were identified by routine methods as previously described (4).
Monocyte-derived macrophage isolation, culture, and infection.
Peripheral blood monocyte-derived macrophages were isolated from 11 male Zebu calves (Bos taurus indicus) at approximately 6 months of age. These calves were genotyped by SSCA and divided into two groups with either resistant or susceptible genotypes. Male calves were used for this study because they are not vaccinated against B. abortus. The option of a Zebu breed was based on our previous findings that Zebu cattle have similar frequencies of resistant and susceptible genotypes, whereas Holstein cattle have a very low frequency of the susceptible genotype (34).
The protocol used for monocyte isolation has been described previously (11, 39). Blood was collected from the jugular vein into a 60-ml syringe containing 8 ml anticoagulant (acid-citrate-dextrose). The blood was diluted in an equal volume of phosphate-buffered saline (pH 7.4) containing 13 mM sodium citrate (PBS-citrate), and layered over a Percoll (Amersham Bioscienses, Uppsala, Sweden) solution with a specific density of 1.077 (mixture of the following solution: 10:1 Percoll, 1.5 M NaCl in 12% NaH2PO4, 130 mM trisodium citrate, 5% bovine serum albumin, and PBS [adjusted for a final refractive index of 1.3460]). After centrifugation at 1,000 × g for 20 min, interface mononuclear cells were transferred to a clean polypropylene tube. The cells were washed three times in PBS-citrate and resuspended in 8 ml of RPMI medium (Gibco, Invitrogen, CA) supplemented with 4 mM l-glutamine (Gibco), 1 mM nonessential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), 2.9 mM 7.5% sodium bicarbonate (Gibco), and 4% autologous serum. The cell suspension was transferred to 50-ml Teflon Erlenmeyer flasks (Nalgene Company, NY) and incubated at 37°C with 5% CO2 for 24 h. Nonadherent cells were removed with medium, and 8 ml of supplemented RPMI medium with 12.5% autologous serum were added to each flask. The flasks were incubated at 37°C in 5% CO2 for 11 days, changing the medium every 3 days.
After 11 days in culture, monocyte-derived macrophages were resuspended by chilling the Teflon flasks on ice for 20 to 30 min followed by agitation. Viable cells were counted in a hemocytometer chamber with trypan blue exclusion and resuspended to a concentration of 5 × 105 cells/ml in supplemented RPMI with 15% complement-inactivated bovine fetal serum (Gibco), and 5 × 104 cells per well were seeded in quadruplicates into 96-well plates (Sarstedt, Nümbrecht, Germany) and incubated overnight (37°C in 5% CO2). Bacteria were quantified by spectrophotometer and suspended to a final concentration of 2.5 × 107 CFU/ml. For inoculation, medium from each well was replaced with 100 μl of this suspension (multiplicity of infection, 50:1). The plates were then incubated for 2 h at 37°C in 5% CO2. The cells were washed three times with medium, 100 μl of a 50-μg/ml solution of gentamicin (Gibco) in supplemented RPMI was added to each well, and the wells were incubated for 1 h (37°C in 5% CO2) to kill extracellular bacteria. The macrophages were washed three times with sterile PBS and lysed with 100 μl of 0.01% Triton X-100 or incubated for a further 24 h with supplemented RPMI containing 25 μg/ml of gentamicin. The lysates were serially diluted in sterile PBS and plated in duplicates on tryptose agar (Difco) for CFU counting. CFU numbers obtained after 1 h of incubation with gentamicin and 24 h after inoculation were compared to calculate the percentage of intracellular bacterial survival. The concentration of inoculum was confirmed by plating. The inoculum was also incubated with medium containing gentamicin for 1 h to confirm the activity of the antibiotic.
Genotyping by SSCA.
Blood samples collected into tubes containing EDTA or fragments of liver were used for DNA extraction and genotyping. DNA extraction was performed using the guanidinium thiocyanate protocol as previously described (35). Genotyping was performed by SSCA for detection of polymorphisms at the 3′UTR of the bovine NRAMP1 gene. Specific primers (5′-AAGGCAGCAAGACAGACAGG-3′ and 5′-ATGGAACTCACGTTGGCTG-3′) targeting the 3′UTR of the bovine NRAMP1 gene were used as previously described (7). Briefly, PCR was performed with 50 ng of genomic DNA in a total reaction volume of 15 μl containing 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphates (dNTPs), 5 μM of the primers described above, and 0.3 U of Taq DNA polymerase (Phoneutria, Belo Horizonte, Brazil). PCR was carried out with an initial denaturation step at 94°C for 5 min followed by 40 cycles of denaturation (94°C for 1 min), annealing (60°C for 40s), and extension (72°C for 1 min), with a final extension step at 72°C for 7 min. The PCR products were denatured at 94°C for 3 min, run in a 6% acrylamide gel at 250 V for 90 min, and silver stained on the gel. A DNA sample from genotypically and phenotypically resistant cattle previously genotyped in a reference laboratory (Garry Adams, Texas A&M University, College Station) was processed to serve as a control for the specificity of the reaction.
Statistical analysis.
The association between resistant phenotype and resistant allele of the 3′UTR NRAMP1 gene was verified by Fisher's exact, Student's t, Kruskal-Wallis, or Mann-Whitney test, depending on the parameter analyzed, using Graphpad Instat software, version 3.05 (Graphpad Software, Inc., CA).
RESULTS
Genotyping.
In this study, four different PCR products were amplified by SSCA: one single band with 175 bp, one single band with 177 bp, and double bands with 175 and 177 bp or 175 and 179 bp. According to previous reports (6, 7), DNA from cattle considered genotypically resistant (SSCAr) to brucellosis results in amplification of a single band with 175 bp, corresponding to a homozygous (GT)13 allele, whereas amplification of DNA from genotypically susceptible (SSCAs) cattle may result in a single band of 177 bp, corresponding to the homozygous (GT)14 allele or double bands of 175, 177, or 179 bp, corresponding to the heterozygous (GT)13/(GT)14 or (GT)13/(GT)15 allele (Fig. 1). Previous studies have demonstrated that the allele (GT)13 is associated with natural resistance, whereas the other alleles, namely (GT)14, (GT)15, and (GT)16, are associated with susceptibility to brucellosis in cattle (3, 6, 7). In addition to processing control DNA samples previously genotyped by the same method in a reference laboratory, the specificity of this method was confirmed by genotyping all fetuses and calves delivered by the experimentally infected cows. The semen used for insemination was obtained from a single sire which was homozygous (GT)14, and indeed all the fetuses or calves were either homozygous (GT)14 or heterozygous (GT)13/(GT)14, depending on the genotype of the cow.
FIG. 1.
Representative results of SSCA for identification of resistant and susceptible genotypes of the bovine NRAMP1 3′UTR (GT)n microsatellite. Arrows indicate product sizes of 175 and 177 bp, corresponding to the (GT)13 and (GT)14 alleles, respectively.
Polymorphisms of the NRAMP1 3′UTR do not influence the occurrence of abortion and necrotic placentitis in cows experimentally infected with B. abortus.
It has been previously reported that the (GT)13 allele is apparently associated with natural resistance of cattle experimentally challenged with B. abortus (3). However, it was not clear whether that could be extrapolated to different breeds of cattle since our previous study has demonstrated a marked variation in allelic frequencies when European breeds (Bos taurus taurus) are compared with Zebu cattle (Bos taurus indicus) (34). This prompted us to assess the influence of NRAMP1 3′UTR polymorphisms on the outcome of experimental infection in crossbred cattle. The inclusion of vaccinated cattle in this study was due to the fact that a significant percentage of the female bovine population are vaccinated, and therefore a marker for natural resistance should be suitable for both vaccinated and nonvaccinated cattle.
Twelve (28.6%) of the 42 experimentally infected cows had abortion, 6 (14.3%) had weak calves, and 24 (57.1%) had normal calving. B. abortus was isolated from 27 (64.3%) cows, including all cows that aborted or had weak calves. For the purpose of this study, susceptibility to brucellosis was characterized by the occurrence of abortion and by the intensity of inflammatory lesions in several organs. Although vaccinated cattle had a tendency to a lower frequency of abortions and premature weak calves (61.5%, 35.3%, and 33.4% in nonvaccinated cattle and those vaccinated with S19 or RB51, respectively), these differences were not statistically significant. Furthermore, no significant differences in the score of placentitis were observed between vaccinated and nonvaccinated cows (data not shown). Therefore, all cows were regrouped according to their genotypes into two groups (genetically resistant or genetically susceptible). No significant differences in the rates of abortion were observed between cows with resistant or susceptible genotypes, as summarized in Table 1.
TABLE 1.
Frequencies of abortion and delivery of clinically healthy calves in genotypically SSCAr and SSCAs cows experimentally infected with Brucella abortus
| Group (n) | No. (%) of cowsa
|
|
|---|---|---|
| SSCAr (n = 19) | SSCAs (n = 23) | |
| Abortion (18)b | 08 (42.1) | 10 (43.5) |
| Healthy calves (24) | 11 (57.9) | 13 (56.5) |
No significant differences were detected by Fisher's exact test (P > 0.05).
The number of abortions includes premature weak calves.
Histologically, the most important lesion observed in cows was a necrotizing suppurative and acute placentitis, which was present in 40 cows (95%). In the fetuses and calves, inflammatory changes were observed in the lung, pleura, bronchial lymph nodes, liver, and spleen. Histopathological changes were scored according to the intensity of inflammation as follows: absent (score = 0), mild (score = 1), moderate (score = 2), and severe (score = 3). The scores of inflammation in organs of the cows or fetuses were then compared between genetically resistant or susceptible cows. As demonstrated in Table 2, no significant association between genotype and intensity of lesions was observed. In addition, a qualitative analysis was performed comparing the frequency of lesions in cows genetically resistant or susceptible, and again no significant differences were observed (data not shown).
TABLE 2.
Mean scores of inflammatory lesions in organs of cows and fetuses or calves according to the genotype of cows experimentally infected with Brucella abortus
| Genotype (n) | Mean score of inflammatory lesions ina:
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cows
|
Fetuses/calves
|
||||||||||||
| Plac | End | MG | Liver | Spleen | MLN | PLN | BLN | IILN | Lung | Liver | Spleen | BLN | |
| SSCAr (19) | 1.95 | 1.68 | 0.53 | 0.63 | 1.10 | 0.79 | 0.42 | 0.39 | 0.42 | 0.94 | 0.47 | 1.31 | 0.87 |
| SSCAs (23) | 1.91 | 1.81 | 0.78 | 0.43 | 0.74 | 0.48 | 0.61 | 0.43 | 0.52 | 0.73 | 0.78 | 1.23 | 0.95 |
Scores: 0, absent; 1, mild; 2, moderate; and 3, severe. Abbreviations: Plac, placentome; End, endometrium; MG, mammary gland; MLN, mammary lymph node; PLN, prescapular lymph node; BLN, bronchial lymph node; IILN, internal iliac lymph node. No significant difference was detected by the nonparametric Mann-Whitney test (P > 0.05).
Finally, for a thorough analysis, phenotypically resistant and susceptible cattle were reclassified stringently considering susceptible cows those that met all of the following criteria: (i) positive isolation of B. abortus, (ii) abortion, and (iii) severe placentitis. Resistant cows were (i) bacteriologically negative, (ii) had normal calving, and (ii) were had no or only mild placentitis. When these parameters of phenotypic resistance were applied, 5 out of 8 resistant cows (62.5%) had the resistant genotype, whereas 7 out of 14 susceptible cows (50%) had the resistant genotype, which indicates no significant difference between these frequencies (P > 0.05).
Natural resistance against brucellosis in cattle does not correlate with NRAMP1 3′UTR polymorphisms.
Considering that the results above were based on experimental infections, in which a relatively high infectious dose was administered at the optimal time for infection (late gestation), we generated further data to assess the influence of the NRAMP1 3′UTR polymorphisms in resistance against brucellosis in natural infections under field conditions.
Serologically, 64 (64%) of the 100 cows selected for this study were positive by RBPAT, standard tube agglutination test, and 2-ME test, while the remaining 36 (36%) were negative by RBPAT. The 64 positive cows were considered infected with Brucella sp., since the lowest titer observed by 2-ME test was 1:50. In addition, 55% of the serologically positive cows were reactive at the highest dilution tested (1:200) by 2-ME test. Brucella sp. was isolated from vaginal swabs collected from two cows with recent history of abortion or parturition. The isolates were identified as B. abortus based on standard biochemical tests (4, 30). Isolation of B. abortus from vaginal swabs associated with high serologic prevalence of Brucella and history of high frequency of abortion at the last trimester of gestation strongly suggests that B. abortus was an important, if not the only, cause of abortion in this herd. Importantly, all cows selected for this study had been kept in direct contact in the same premises for a prolonged period of time preceding the collection of samples for this study, which suggests that all cows had a very high risk of previous exposure to B. abortus. Therefore, for the purpose of this analysis, serologically negative cows were considered resistant, assuming their prolonged exposure to B. abortus and absence of serologic evidence of infection.
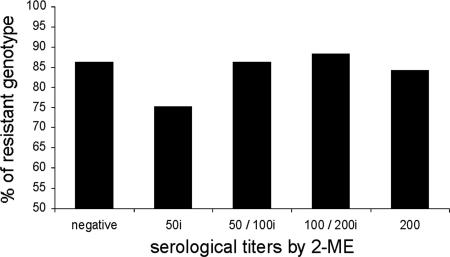
Genotyping resulted in the identification of 85 cows (85%) as genetically resistant and 15 cows (15%) as genetically susceptible. The percentages of serologically positive cows were statistically similar among genotypically resistant or susceptible cows (Table 3). Unfortunately, reliable reproductive records were not available, and therefore a retrospective comparison of abortion rates was not possible. Interestingly, the two cows from which B. abortus was isolated were SSCAr, which further supported the notion of a lack of correlation between polymorphisms of the NRAMP1 3′UTR (GT)n microsatellite and actual resistance against brucellosis. Furthermore, no correlation was observed between genotype and serologic titers (Fig. 2).
TABLE 3.
Frequencies of SSCAr and SSCAs genotypes in serologically positive or negative cows under high risk of natural infection with Brucella abortus
| Serology (n) | No. (%) of cows with genotypea:
|
|
|---|---|---|
| SSCAr | SSCAs | |
| Negative (36) | 31 (86) | 05 (14) |
| Positive (64) | 54 (84) | 10 (16) |
No significant differences were detected by Fisher's exact test observed (P > 0.05).
FIG. 2.
Frequency of the SSCAr genotype according to the 2-ME test serological titers. No significant differences in the percentages of the resistant genotype were observed (P > 0.05). i (x axis), incomplete.
Polymorphisms of the NRAMP1 3′UTR do not influence the ability of bovine monocyte-derived macrophages to control intracellular replication of B. abortus.
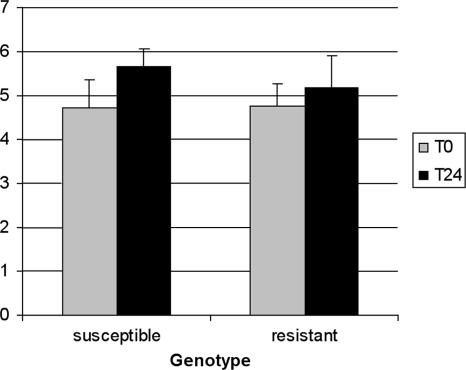
It has been demonstrated that macrophages from cattle phenotypically resistant to brucellosis have an enhanced ability to prevent intracellular growth of B. abortus (11, 24, 38, 39), although these findings have not been correlated with polymorphisms of the NRAMP1 3′UTR. Thus, we aimed to assess the ability of macrophages from animals with resistant or susceptible genotypes to control intracellular growth of B. abortus. Peripheral blood monocyte-derived macrophages from six genotypically resistant and five susceptible calves were inoculated with B. abortus in two independent experiments. No significant differences were observed in invasion when comparing macrophages from calves with different genotypes, and no significant difference was observed in the number of intracellular bacteria at 24 h after inoculation (Fig. 3), resulting in a similar rate of intracellular survival and no measurable influence of the NRAMP1 3′UTR polymorphisms on intracellular survival of B. abortus in macrophages.
FIG. 3.
Invasion and intracellular survival of Brucella abortus in blood monocyte-derived macrophages from cattle with SSCAr or SSCAs genotypes. Macrophages were challenged with B. abortus at a multiplicity of infection of 1:50 in quadruplicates. Columns and error bars indicate the average and standard deviation of two independent experiments (totaling six genotypically resistant and five susceptible calves) performed in quadruplicates. CFU numbers were counted at 0 and 24 h after inoculation, as described in Materials and Methods. No statistically significant differences were observed at 0 or 24 h after inoculation between macrophages from genotypically resistant or susceptible calves (P > 0.05).
DISCUSSION
Our long-term goal was to generate supporting data for the use of a previously described SSCA-based genotyping method (7) for selection of cattle that are naturally resistant against B. abortus infection. This type of approach may prove valuable under conditions of high prevalence of bovine brucellosis, as is the case in certain areas in Brazil (36). Surprisingly, our data clearly indicated that (i) polymorphisms of the NRAMP1 3′UTR did not influence the incidence of abortion and placentitis in pregnant cows experimentally infected with B. abortus, (ii) these polymorphisms did not correlate with resistance against B. abortus in naturally infected cows under field conditions, and (iii) intracellular survival of B. abortus in bovine macrophages was not influenced by polymorphisms at the NRAMP1 3′UTR (GT)n microsatellite. These data contrast sharply with previous reports, which were based on experimental infection of a reduced number of cattle (3) and in vitro inoculation of murine macrophages transfected with alleles of bovine NRAMP1 (6). Transfection of the murine macrophage cell line RAW264.7 with the resistant allele (GT)13 of the bovine NRAMP1 cDNA driven by the bovine NRAMP1 promoter results in a decrease of intracellular survival of B. abortus and increase in expression of NRAMP1 compared to cells transfected with the susceptible allele (GT)16 (6). However, it is not possible to compare these data with in vivo studies or even with infections of primary macrophages from cattle with resistant or susceptible genotypes since levels of expression may not be comparable. Furthermore, the susceptible construct used in this transfection study was a (GT)16 allele, which is an allele that is not often found under natural conditions (34). Finally, murine macrophage cell lines transfected with the susceptible construct had lower levels of phagocytosis of B. abortus (6), which was not observed in primary bovine macrophages with the susceptible genotype (GT)14, which is the most commonly found putative susceptible allele in cattle (34).
Although previous studies have shown that natural resistance to brucellosis in cattle correlates with the ability of cultured macrophages to control intracellular replication of B. abortus (11, 24, 38, 39), these studies did not evaluate the genotype of the cells with regard to the 3′UTR of the NRAMP1 gene; therefore, it is unclear whether these phenotypic features correlate with NRAMP1 polymorphisms or level of NRAMP1 expression. To our knowledge, this is the first study in which macrophages from cattle genotyped as resistant and susceptible were infected in vitro. The mechanism of resistance described in these early studies is not clear, but it has been demonstrated that resistant macrophages have a more intense oxidative burst, which correlates with a stronger bactericidal activity (24). Furthermore, other differences in immune response have been identified when comparing phenotypically resistant and susceptible cattle such as immunoglobulin allotypes and oligoclonal T-cell responses to B. abortus (42).
After thoroughly analyzing several parameters of either experimental or natural in vivo infections, our results do not support the notion that in vivo resistance against brucellosis is largely due to the (GT)13 allele of NRAMP1 as previously postulated (3). A recent report (28) attempted to correlate the (GT)13 allele with resistance to brucellosis in crossbred Holstein cattle, but the study was incomplete since all the genotyped cattle were homozygous (GT)13, which is in good agreement with our previous study in which 100% of the genotyped Holstein cattle had the (GT)13 allele (34). Interestingly, in spite of the notorious differences in allelic frequencies between Hostein and Zebu (34), no specific breed predisposition to brucellosis has been reported (36), which corroborates the lack of association between these alleles and resistance to B. abortus infection.
In the mouse, macrophage killing of Mycobacterium bovis is dependent on the NRAMP1 gene (43). However, similarly to the results reported here, no association between the NRAMP1 3′UTR microsatellite polymorphisms and resistance to M. bovis in cattle can be identified (7). In addition, NRAMP1 expression in macrophages is increased in M. bovis-infected cattle, but this upregulation of the gene is associated with progression of clinical disease (15). Importantly, the susceptible phenotype in the mouse is due to a nucleotide substitution (G is replaced by A at position 783) that results in the nonconservative replacement of Gly105 with Asp105 (31) rather than to polymorphisms in the 3′UTR sequences. Although it is not completely clear how polymorphisms in the 3′UTR microsatellite influence Nramp1 function, it has been demonstrated that they may be due to altered levels of Nramp1 expression in both naïve and infected macrophages, which is associated with macrophage bactericidal activity (12).
In contrast with our results, it has been recently demonstrated that polymorphisms at the 3′UTR of the NRAMP1 gene of water buffalos are linked to an increased ability of macrophages to prevent intracellular growth of B. abortus and higher levels of expression of NRAMP1 (8). Further studies confirmed that the so-called BB genotype in water buffalo is associated with higher levels of Nramp1 expression, enhanced bactericidal activity of macrophages, and stronger induction of oxygen and nitrogen intermediates (12). However, it is important to emphasize that the structure of the (GT)n 3′UTR microsatellite in water buffalo differs from that present in cattle.
In mice, resistance against B. abortus is genetically determined and dependent on macrophage function (25). However, resistance in this case is not mediated by the Nramp1 genotype (41). For instance, mouse strains that are either resistant (C57BL/10) or susceptible (BALBc) to B. abortus carry the same susceptible genotype of Nramp1. Resistance against B. abortus in mice is associated with expression of cytokines responsible for macrophage activation such as gamma interferon (5). Furthermore, polymorphisms and expression of murine Nramp1 are not linked with control of B. melitensis infection by macrophage lines (23).
It is noteworthy that in spite of our data indicating that (GT)n 3′UTR microsatellite polymorphisms do not play a significant role in resistance against bovine brucellosis, these findings obviously do not imply that NRAMP1 does not play a role in controlling intracellular growth of B. abortus. Other polymorphisms at the 3′UTR of NRAMP1 have been identified in cattle and water buffalo (1, 13), but the suitability of these polymorphisms as markers for natural resistance to Brucella and other intracellular pathogens has not yet been investigated.
Although our data indicate a lack of statistical significance in the rate of abortion between vaccinated and nonvaccinated cattle, that result is likely due to the relative low number of cows per group, since this study has not been designed to assess vaccine protection. Indeed, in a recent study under the same experimental conditions, RB51 proved efficient in a larger group of vaccinated cows (37).
In conclusion, taken together, our data support the notion that the NRAMP1 3′UTR (GT)n microsatellite polymorphisms do not affect the resistance against B. abortus in cattle, and therefore 3′UTR (GT)n is not a suitable genetic maker of resistance to bovine brucellosis.
Acknowledgments
This study was supported by FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais, Brazil) grants EDT-2207/03 and CAG-1091/05 to R.L.S. T.A.P., A.V.C.N., A.P.L., and R.L.S. are partially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil).
We thank L. Garry Adams for providing control samples, Joaquim Marcos P. Carneiro for technical assistance, and Reneé M. Tsolis and Christelle Roux for reviewing the manuscript.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Ables, G. P., M. Nishibori, M. Kanemaki, and T. Watanabe. 2002. Sequence analysis of the NRAMP1 genes from different bovine and buffalo breeds. J. Vet. Med. Sci. 64:1081-1083. [DOI] [PubMed] [Google Scholar]
- 2.Adams, L. G., R. Barthel, J. Feng, T. Qureshi, J. Piedrahita, and J. W. Templeton. 1996. Genes associated with innate killing of Brucella abortus and Mycobacterium bovis by macrophages from genetically resistant cattle. Vet. Immunol. Immunopathol. 54:135. [DOI] [PubMed] [Google Scholar]
- 3.Adams, L. G., and J. W. Templeton. 1998. Genetic resistance to bacterial diseases of animals. Rev. Sci. Tech. Off. Int. Epizoot. 17:200-219. [DOI] [PubMed] [Google Scholar]
- 4.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. INRA, Paris, France.
- 5.Baldwin, C. L., and M. Parent. 2002. Fundamentals of host immune response against Brucella abortus: what the mouse model has revealed about control of infection. Vet. Microbiol. 90:367-382. [DOI] [PubMed] [Google Scholar]
- 6.Barthel, R., J. Feng, J. A. Piedrahita, D. N. McMurray, J. W. Templeton, and L. G. Adams. 2001. Stable transfection of the bovine Nramp1 gene into murine RAW264.7 cells: effect on Brucella abortus survival. Infect. Immun. 69:3110-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthel, R., J. A. Piedrahita, D. N. McMurray, J. Payeur, D. Baca, F. S. Güemes, V. S. Perumaalla, T. A. Ficht, J. W. Templeton, and L. G. Adams. 2000. Pathologic findings and association of Mycobacterium bovis infection with the bovine NRAMP1 gene in cattle from herds with naturally occurring tuberculosis. Am. J. Vet. Res. 61:1140-1144. [DOI] [PubMed] [Google Scholar]
- 8.Borriello, G., R. Capparelli, M. Bianco, D. Fenizia, F. Alfano, F. Capuano, D. Ercolini, A. Parisi, S. Roperto, and D. Iannelli. 2006. Genetic resistance to Brucella abortus in the water buffalo (Bubalus bubalis). Infect. Immun. 74:2115-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley, D. J., B. A. Taylor, J. Blackwell, E. P. Evans, and J. Freeman. 1979. Regulation of Leishmania populations within the host. III. Mapping of the locus controlling susceptibility to visceral leishmaniasis in the mouse. Clin. Exp. Immunol. 37:7-14. [PMC free article] [PubMed] [Google Scholar]
- 10.Bravo, M. J., J. D. Colmenero, J. Martín, A. Alonso, and A. C. González. 2006. Variation in the NRAMP1 gene does not affect susceptibility or protection in human brucellosis. Microbes Infect. 8:154-156. [DOI] [PubMed] [Google Scholar]
- 11.Campbell, G. A., and L. G. Adams. 1992. The long-term culture of bovine monocyte-derived macrophages and their use in the study of intracellular proliferation of Brucella abortus. Vet. Immunol. Immunopathol. 34:291-305. [DOI] [PubMed] [Google Scholar]
- 12.Capparelli, R., F. Alfano, M. G. Amoroso, G. Borriello, D. Fenizia, A. Bianco, S. Roperto, F. Roperto, and D. Iannelli. 2007. Protective effect of the Nramp1 BB genotype against Brucella abortus in the water buffalo (Bubalus bubalis). Infect. Immun. 75:988-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coussens, P. M., M. J. Coussens, B. C. Tooker, and W. Nobis. 2004. Structure of the bovine natural resistance associated macrophage protein (NRAMP 1) gene and identification of a novel polymorphism. DNA Seq. 15:15-25. [DOI] [PubMed] [Google Scholar]
- 14.Enright, F. M., J. V. Walker, G. Jeffers, and B. L. Deyoe. 1984. Cellular and humoral responses of Brucella abortus-infected bovine fetuses. Am. J. Vet. Res. 45:424-430. [PubMed] [Google Scholar]
- 15.Estrada-Chávez, C., A. L. Pereira-Suárez, M. A. Meraz, C. Arriaga, A. Garcia-Carrancá, C. Sánchez-Rodriguez, and R. Mancilla. 2001. High-level expression of NRAMP1 in peripheral blood cells and tuberculous granulomas from Mycobacterium bovis-infected bovines. Infect. Immun. 69:7165-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng, J., Y. Li, M. Hashad, E. Schurr, P. Gros, L. G. Adams, and J. W. Templeton. 1996. Bovine natural resistance associated macrophage protein 1 (Nramp1) gene. Genome Res. 6:956-964. [DOI] [PubMed] [Google Scholar]
- 17.Forbes, J. R., and P. Gros. 2003. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood 102:1884-1892. [DOI] [PubMed] [Google Scholar]
- 18.Godfroid, J., and A. Kosbohrer. 2002. Brucellosis in the European Union and Norway at the turn of the twenty-first century. Vet. Microbiol. 90:135-145. [DOI] [PubMed] [Google Scholar]
- 19.Gorham, S. L., F. M. Enright, T. G. Snider III, and E. D. Roberts. 1986. Morphologic lesions in Brucella abortus infected ovine fetuses. Vet. Pathol. 23:331-332. [DOI] [PubMed] [Google Scholar]
- 20.Goswami, T., A. Bhattacharjee, P. Babal, S. Searle, E. Moore, M. Li, and J. M. Blackwell. 2001. Natural-resistance-associated macrophage protein 1 is an H+/bivalent cation antiporter. Biochem. J. 354:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gros, P., E. Skamene, and A. Forget. 1981. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J. Immunol. 127:2417-2421. [PubMed] [Google Scholar]
- 22.Gruenheid, S., E. Pinner, M. Desjardins, and P. Gros. 1997. Natural resistance to infection with intracellular pathogens: the Nramp1 protein is recruited to the membrane of the phagosome. J. Exp. Med. 185:717-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guilloteau, L. A., J. Dornand, A. Gross, M. Olivier, F. Cortade, Y. Le Vern, and D. Kerboeuf. 2003. Nramp1 is not a major determinant in the control of Brucella melitensis infection in mice. Infect. Immun. 71:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harmon, B. G., L. G. Adams, J. W. Templeton, and R. Smith III. 1989. Macrophage function in mammary glands of Brucella abortus-infected cows and cows that resisted infection after inoculation of Brucella abortus. Am. J. Vet. Res. 50:459-465. [PubMed] [Google Scholar]
- 25.Ho, M., and C. Cheers. 1982. Resistance and susceptibility of mice to bacterial infection. IV. Genetic and cellular basis of resistance to chronic infection with Brucella abortus. J. Infect. Dis. 146:381-387. [DOI] [PubMed] [Google Scholar]
- 26.Horin, P., I. Rychlík, J. W. Templeton, and L. G. Adams. 1999. A complex pattern of microsatellite polymorphism within the bovine NRAMP1 gene. Eur. J. Immunogenet. 26:311-313. [DOI] [PubMed] [Google Scholar]
- 27.Jabado, B. N., A. Jankowski, S. Dougaparsad, V. Picard, S. Grinstein, and P. Gros. 2000. Natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J. Exp. Med. 192:1237-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar, N., A. Mitra, I. Ganguly, R. Singh, S. M. Deb, S. K. Srivastava, and A. Sharma. 2005. Lack of association of brucellosis resistance with (GT)13 microsatellite allele at 3′UTR of NRAMP1 gene in Indian zebu (Bos indicus) and crossbred (Bos indicus × Bos taurus) cattle. Vet. Microbiol. 111:139-143. [DOI] [PubMed] [Google Scholar]
- 29.Lissner, C. R., R. N. Swanson, and A. D. O'Brien. 1983. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J. Immunol. 131:3006-3013. [PubMed] [Google Scholar]
- 30.MacFaddin, J. F. 1980. Pruebas bioquimicas para la identificación de bacterias de importancia clinica. Panamericana, Buenos Aires, Argentina.
- 31.Malo, D., K. Vogan, S. Vidal, J. Hu, M. Cellier, E. Schurr, A. Fuks, N. Bumstead, K. Morgan, and P. Gros. 1994. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics 23:51-61. [DOI] [PubMed] [Google Scholar]
- 32.Meador, V. P., B. L. Deyoe, and N. F. Cheville. 1989. Pathogenesis of Brucella abortus infection of the mammary gland and supramammary lymph node of the goat. Vet. Pathol. 26:357-368. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti, P. 1980. The epidemiology of bovine brucellosis. Adv. Vet. Sci. Comp. Med. 24:69-95. [PubMed] [Google Scholar]
- 34.Paixão, T. A., C. Ferreira, A. M. Borges, D. A. Oliveira, A. P. Lage, and R. L. Santos. 2006. Frequency of bovine Nramp1 (Slc11a1) alleles in Holstein and Zebu breeds. Vet. Immunol. Immunopathol. 109:37-42. [DOI] [PubMed] [Google Scholar]
- 35.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 36.Poester, F. P., V. S. P. Gonçalves, and A. P. Lage. 2002. Brucellosis in Brazil. Vet. Microbiol. 90:55-62. [DOI] [PubMed] [Google Scholar]
- 37.Poester, F. P., V. S. Gonçalves, T. A. Paixão, R. L. Santos, S. C. Olsen, G. G. Schurig, and A. P. Lage. 2006. Efficacy of strain RB51 vaccine in heifers against experimental brucellosis. Vaccine 24:5327-5334. [DOI] [PubMed] [Google Scholar]
- 38.Price, R. E., J. W. Templeton, R. Smith III, and L. G. Adams. 1990. Ability of phagocytes from cattle naturally resistant or susceptible to brucellosis to control in vitro intracellular survival of Brucella abortus. Infect. Immun. 58:879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qureshi, T., J. W. Templeton, and L. G. Adams. 1996. Intracellular survival of Brucella abortus, Mycobacterium bovis BCG, Salmonella Dublin and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucella abortus. Vet. Immunol. Immunopathol. 50:55-65. [DOI] [PubMed] [Google Scholar]
- 40.Ragan, V. E. 2002. The Animal and Plant Health Inspection Service (APHIS) brucellosis eradication program in the United States. Vet. Microbiol. 90:11-18. [DOI] [PubMed] [Google Scholar]
- 41.Sathiyaseelan, J., X. Jiang, and C. L. Baldwin. 2000. Growth of Brucella abortus in macrophages from resistant and susceptible mouse strains. Clin. Exp. Immunol. 121:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Templeton, J. W., R. Smith III, and L. G. Adams. 1988. Natural disease resistance in domestic animals. J. Am. Vet. Med. Assoc. 192:1306-1315. [PubMed] [Google Scholar]
- 43.Vidal, S. M., D. Malo, K. Vogan, E. Skamene, and P. Gros. 1993. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell 73:469-485. [DOI] [PubMed] [Google Scholar]
- 44.Vidal, S. M., M. L. Tremblay, G. Govoni, S. Gauthier, G. Sebastiani, D. Malo, E. Skamene, M. Olivier, S. Jothy, and P. Gros. 1995. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J. Exp. Med. 182:655-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Womack, J. E. 1988. Molecular cytogenetics of cattle: a genomic approach to disease resistance and productivity. J. Dairy Sci. 71:1116-1123. [DOI] [PubMed] [Google Scholar]
- 46.Wyllie, S., P. Seu, and J. A. Goss. 2002. The natural resistance-associated macrophage protein 1 Slc11a1 (formerly Nramp1) and iron metabolism in macrophages. Microbes Infect. 4:351-359. [DOI] [PubMed] [Google Scholar]
- 47.Zwilling, B. S., D. E. Kuhn, L. Wikoff, D. Brown, and W. Lafuse. 1999. Role of iron in Nramp1-mediated inhibition of mycobacterial growth, Infect. Immun. 67:1386-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]