Abstract
CD1d-restricted natural killer T (NKT) cells represent a heterogeneous population of innate memory immune cells expressing both NK and T-cell markers distributed into two major subsets, i.e., invariant NKT (iNKT) cells, which express exclusively an invariant T-cell receptor (TCR) α chain (Vα14Jα18 in mice), and non-iNKT cells, which express more diverse TCRs. NKT cells quickly produce Th1- and/or Th2-type cytokines following stimulation with glycolipid antigen (Ag) and, through this property, play potent immunoregulatory roles in autoimmune diseases, cancer, and infection. No study has addressed the role of NKT cells in metazoan parasite infections so far. We show that during murine schistosomiasis, the apparent frequency of both iNKT cells and non-iNKT cells decreased in the spleen as early as 3 weeks postinfection (p.i.) and that both populations expressed a greater amount of the activation marker CD69 at 6 weeks p.i., suggesting an activated phenotype. Two different NKT-cell-deficient mouse models, namely, TCR Jα18−/− (exclusively deficient in iNKT cells) and CD1d−/− (deficient in both iNKT and non-iNKT cells) mice, were used to explore the implication of these subsets in infection. We show that whereas both iNKT and non-iNKT cells do not have a major impact on the immune response during the early phase (1 and 4 weeks) of infection, they exert important, although opposite, effects on the immune response during the acute phase of the disease (7 and 12 weeks), after schistosome egg production. Indeed, iNKT cells contribute to Th1 cell differentiation whereas non-iNKT cells might be mostly implicated in Th2 cell differentiation in response to parasite Ag. Our findings suggest, for the first time, that helminths activate both iNKT and non-iNKT cells in vivo, enabling them to differentially influence the Th1/Th2 balance of the immune response.
CD1d-restricted T cells represent a heterogeneous population of lipid-reactive T cells that play a critical role in directing both adaptive and innate immunity (25, 34). Among them, natural killer (NK) T (herein termed NKT) cells express NK cell markers (such as NK1.1) and contain different categories of unconventional T cells. In mice, the most abundant population expresses a semiconserved, canonical T-cell receptor (TCR) consisting of an invariant Vα14-Jα18 TCRα chain combined with diverse TCRβ chains by using a limited number of Vβ regions (35). In mice, invariant NKT (iNKT) cells are found at the highest frequency in the liver (10 to 40% of liver lymphocytes), but they are present at lower frequencies in the thymus, bone marrow, spleen, lymph nodes (LNs), lungs, and blood (24, 34). In addition to iNKT cells, other NKT-cell subpopulations with more diverse TCRs (termed non-iNKT cells or variant NKT cells) have been described in mice but these cells have been less well studied, mainly because of the absence of known specific markers (5, 9, 39, 51).
NKT cells recognize self and exogenous (microbe-derived) glycolipids presented by antigen (Ag)-presenting cells (APCs), including dendritic cells (DCs), in the context of CD1d (2, 20, 31-33, 37, 54, 56). Upon primary stimulation with CD1d-restricted glycolipid Ag (in particular, in response to the iNKT-cell superagonist α-galactosylceramide [α-GC]), NKT cells produce large amounts of Th1-type and/or Th2-type cytokines (especially gamma interferon [IFN-γ] and interleukin-4 [IL-4]) that lead to downstream activation of DCs, NK cells, B cells, and conventional T cells (8, 16, 18, 22, 23). Through this property, NKT cells have been shown to promote the polarization of conventional CD4 T cells into Th1 and Th2 cells and have been found to regulate different types of immune responses. Indeed, several in vivo models demonstrate that, upon intentional or natural activation, NKT cells are extremely flexible in nature and can either suppress or enhance immune-mediated diseases, including inflammation, cancer, and autoimmune diseases (25, 34). Experimental evidence also suggests that during some infections, NKT cells can become activated and produce immunoregulatory cytokines, in particular, IFN-γ (27, 48). Through this mechanism, NKT cells generally contribute to the polarization of the immune response in a Th1 direction, which can be either beneficial (i.e., pathogen clearance) or detrimental (i.e., excessive inflammation) for the host (3, 4, 14, 15, 26, 44). Although extensively studied during viral, bacterial, protozoan parasite, and fungal infections (27, 48), the role of NKT cells during helminthic infections remains unknown.
Schistosomiasis is a chronic parasitic disease caused by the extracellular parasite Schistosoma. In the case of Schistosoma mansoni, infection is initiated by transcutaneous penetration by cercariae. Following infection, parasites transform into schistosomules that reside in the skin for 3 to 7 days; afterwards, they migrate through the bloodstream to the lungs (1 to 2 weeks) and then to the hepatic portal system, where sexually differentiated males and females mate. A key feature of the immune response in S. mansoni-infected mice is the occurrence of a strong Th2 response triggered by parasite eggs that are gradually deposited in host tissues, as early as 5 weeks postinfection (p.i.) (40). The mechanisms leading to the promotion of this Th2-dominated response are still obscure. Recently, we reported that CD1d-deficient (−/−) mice (BALB/c background) developed a markedly reduced Th2 response during schistosomiasis (19). Moreover, Zaccone et al. showed that infection with S. mansoni inhibits the development of type 1 diabetes in nonobese diabetic mice and that this protective effect could be due to NKT-cell activation (55). In agreement with this hypothesis, we have recently demonstrated that hepatic iNKT cells become activated and expand during infection and that, upon schistosome egg encounter in the liver, they produce both IFN-γ and IL-4 (36). Collectively, these data suggested that NKT cells could be important in acquired immune responses during schistosomiasis. Because new studies show that subsets of NKT cells can be functionally distinct under some circumstances (10, 14, 52), we used two different NKT-cell-deficient mouse strains to identify the effects that are dependent on iNKT cells and non-iNKT cells (CD1d−/− mice) or exclusively on iNKT cells (TCR Jα18−/− mice). Our data show that early (1 and 4 weeks) after infection, NKT cells do not influence the acquired immune response to the larval and adult parasite stages. In marked contrast, during the acute phase of infection (7 and 12 weeks), both NKT-cell subsets seem to have effects, although distinct, on the immune response. Indeed, at these time points and compared to wild-type (WT) mice, TCR Jα18−/− mice mount a decreased Th1 response whereas CD1d−/− mice develop a reduced Th2 response. Thus, iNKT cells might provide help for Th1 cell differentiation whereas non-iNKT cells might promote that of Th2 cells in response to parasite Ag. Together, these data suggest, for the first time, that helminths activate both iNKT and non-iNKT cells in vivo and that these NKT-cell subsets differentially influence the nature of the acquired immune response.
MATERIALS AND METHODS
Animals.
Eight-week-old WT C57BL/6 mice were purchased from Janvier (Le Genest-St-Isle, France). The generation of CD1d−/− and TCR Jα18−/− mice (backcrossed at least 10 times in C57BL/6) has already been described (12, 38). Mice that lack CD1d are devoid of both iNKT and non-iNKT cells. Mice that lack the TCR Jα18 segment are devoid of iNKT cells, but the other lymphoid cell lineages are intact. The T-cell repertoire of these mice includes CD1d-restricted NKT cells that utilize diverse αβ and γδ TCR genes (non-iNKT cells) (12). Both CD1d−/− and TCR Jα18−/− mice were bred in our own facility under pathogen-free conditions.
Abs and reagents.
Monoclonal Abs (MAbs) against mouse CD3ɛ (fluorescein isothiocyanate or allophycocyanin conjugated), CD4 (fluorescein isothiocyanate conjugated), NK1.1 (phycoerythrin conjugated), and CD5 (allophycocyanin conjugated) and purified anti-CD3 MAbs were purchased from BD Pharmingen (BD Pharmingen, San Diego, CA). Allophycocyanin-conjugated CD1d/α-GC tetramer (tetramer) was from ProImmune (Oxford, United Kingdom). α-GC was purchased from Axxora Life Sciences (Coger S.A., Paris, France).
Parasites.
S. mansoni schistosomules were obtained by the skin penetration procedure (42) from cercariae (Puerto Rican strain) shed from infected Biomphalaria glabrata snails. Adult worms and eggs were obtained from infected S. mansoni-infected golden hamsters and purified by classical procedures. Eggs were obtained from the livers of infected golden hamsters after portal-vein perfusion. The absence of contaminating hamster tissue fragments in the egg preparation was checked by microscopic analysis. The absence of endotoxin in the parasite preparations (105 parasites/ml) was checked by a Limulus test (Sigma-Aldrich, St Quentin-Fallavier, France).
Analysis of NKT activation during infection.
Mice were percutaneously infected with 50 S. mansoni cercariae. Spleens were harvested at different time point p.i. and homogenized with a 90-μm-pore-size filter. Red blood cells were removed by lysis in 155 mM NH4Cl (pH 7.4) containing 10 mM NaHCO3 and 0.1 mM EDTA (lysis buffer). Cell suspensions were stained either with anti-CD3ɛ and anti-NK1.1 MAbs or with anti-CD3ɛ, anti-NK1.1, and anti-CD69 MAbs and a tetramer. Briefly, cells were incubated for 30 min with the appropriate dilutions of conjugated MAbs in phosphate-buffered saline containing 2% fetal calf serum and 0.01% NaN3. Cells were acquired and analyzed on a FACScalibur (Becton Dickinson, Rungis, France) cytometer with the CellQuest software.
Preparation of hepatic MNC and purification of splenic non-iNKT cells.
To prepare liver mononuclear cells (MNC), perfused livers from naive WT or TCR Jα18−/− mice were harvested and homogenized with a 90-μm-pore-size filter. After extensive washes, liver homogenates were resuspended in a 33% Percoll gradient, and after centrifugation, the cells in the pellet were recovered. Red blood cells were removed with lysis buffer. For sorting of non-iNKT cells, spleen MNC from TCR Jα18−/− mice, which contain approximately 0.3 to 0.6% CD5+ NK1.1+ cells, were labeled with allophycocyanin-conjugated anti-CD5 and phycoerythrin-conjugated anti-NK1.1 MAbs. After cell surface labeling, cells were electronically sorted with a FACSAria (Becton Dickinson). Sorted CD5+ NK1.1+ populations were always at least 85% pure.
DC-hepatic MNC and DC-splenic non-iNKT-cell coculture systems.
DCs were generated from the bone marrow of mice as described previously (1). DCs were sensitized with live schistosomules (1:200 cells), adult worms (1:106 cells), eggs (1:200 cells), or α-GC (100 ng/ml), extensively washed, and cultured in the presence of liver MNC at a ratio of 1:5 (105 DCs plus 5 × 105 MNC/well) in round-bottom 96-well plates in RPMI supplemented with 5% fetal calf serum. In some cases, parasite-sensitized DCs were cultured with sorted non-iNKT cells (105 DCs plus 105 CD5+ NK1.1+ cells/well). As a positive control, CD5+ NK1.1+ cells were stimulated with plate-bound anti-CD3. After 48 h, IFN-γ and IL-4 production was measured in the culture supernatants by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Infection of mice and analysis of parasitological and immunological parameters.
Mice were percutaneously infected with 150 (sacrifice at 1 week) or 50 (sacrifice at 4 or 7 weeks) cercariae. At 7 weeks p.i., worm burdens were measured by liver perfusion. At the time of perfusion, the small intestines and the livers were also collected for measurement of egg numbers deposited in these organs. Tissues were digested in 4% KOH as previously described (13). Records of the organ weights allowed the calculation of total tissue eggs per organ. Skin LNs that drain the site of infection were harvested at 1 week p.i., and spleens were harvested at 4 or 7 weeks p.i. Cells were stimulated with anti-CD3 Ab (5 μg/ml) or with soluble parasite-derived Ag (20 μg/ml) at 37°C for 2 or 3 days, respectively. During the last 18 h, 0.5 μCi of [3H]thymidine/well was added. In some cases, proliferation was assessed by adding 20 μl Alamar blue to each well and optical density was measured 24 h later in accordance with the manufacturer's (Serotec, Cergy Saint-Christophe, France) protocol. To study the humoral response, mice were bled 7 and 12 weeks p.i. and the anti-soluble egg Ag (SEA) immunoglobulin G1 (IgG1), IgG2b, and IgE titrations were determined for each mouse by ELISA as previously described (19). The serum titer was defined as the dilution which gives an optical density reading at least twofold higher than the mean background of noninfected mouse serum, and results are expressed as the IgG1/IgG2b serum titer ratio and the IgE serum titer.
Analysis of egg-associated liver pathology in infected mice.
Egg-associated pathology was analyzed in the livers of mice at 7 weeks p.i. in a double-blind fashion. Briefly, a small piece of liver from each mouse was fixed in Immunohistofix (Gentaur Molecular Products, Brussels, Belgium) for 2 to 3 days and embedded in wax. Five-micrometer liver sections were then prepared for Rojkind coloration. The staining mixture was made of a saturated solution of picric acid in distilled water containing 0.1% Fast Green FCF (Sigma-Aldrich) and 0.1% Sirius Red. After staining, slides were washed in physiological water and mounted in Acrytol (Surgipath, Labonord, France) after dehydration. Collagen deposition in granulomas (10 to 18/mouse) was quantified on an arbitrary score scale of 0 (no collagen deposition) to 3 (strong collagen deposition all over the granuloma). The diameters of granulomas surrounding single, mature, and viable eggs were measured with the Metaview computing software (Roper Scientific, Evry, France), and the volume of each granuloma was calculated by assuming a spherical shape.
Statistical analysis.
Results are expressed as the mean ± standard deviation (SD). All P values were determined by using Student's t test.
RESULTS
Splenic NKT cells become activated during S. mansoni infection.
We have previously shown that during the course of murine schistosomiasis, NKT cells in the liver (which predominantly has iNKT cells and few non-iNKT cells) become activated (36). Here, we investigated whether infection also results in the activation of NKT cells in the spleen, which contains approximately the same proportion of iNKT cells and non-iNKT cells (25). For this, we took advantage of the fact that in vivo activation of NKT cells leads to an apparent decrease in their frequency, a phenomenon due to reduced expression of cell surface markers, including NK1.1 (C57/BL6 background) (11, 53). Because a large fraction of mature splenic NKT cells from C57BL/6 mice coexpress CD3 (or CD5) and NK1.1 (17, 25, 41), we therefore determined, in a kinetic manner, the apparent frequency of CD3+ NK1.1+ cells in the spleens of S. mansoni-infected mice. As depicted in Fig. 1A, the percentage of detectable splenic CD3+ NK1.1+ cells dramatically diminished from 3 to 11 weeks p.i., a time at which the experiment was stopped. A representative dot plot is shown in Fig. 1B (at 7 weeks p.i.). On the other hand, the proportion of conventional B (CD3− CD19+) cells and T (CD3+ CD19−) cells did not vary during the course of infection (data not shown). These results suggest that during murine schistosomiasis, the NKT-cell population in the spleen appears to become activated as early as 3 weeks p.i. We then analyzed the relative proportions of both iNKT-cell and non-iNKT-cell populations by using the CD1d/α-GalCer tetramer. We found that among the CD3+ NK1.1+ cells, the proportions of tetramer+ (iNKT) and tetramer− (herein termed non-iNKT) cells remained unchanged whatever the time point analyzed (Fig. 1B, a representative dot plot at 7 weeks p.i.). This indicates that both NKT-cell populations become activated after 3 weeks p.i. To confirm this, we monitored the expression of CD69, an early activation marker, on NKT cells. We found that at 6 weeks p.i., the level of CD69 expression on splenic iNKT cells (CD3+ NK1.1+ tetramer+) was enhanced, compared to the basal level detected in noninfected mice (Fig. 1C). Moreover, although the basal level of CD69 expression on CD3+ NK1.1+ tetramer− cells (mostly non-iNKT) was low, approximately 40% of these cells expressed a higher level of CD69 at 6 weeks p.i. (Fig. 1C). These data support the notion that both iNKT cells and non-iNKT cells become activated during infection, at least at 6 weeks p.i. On the other hand, at other time points, no major variations in the frequency of CD69-expressing cells or in the intensity of CD69 labeling were observed, whatever the NKT-cell population studied.
FIG. 1.
Analysis of splenic NKT-cell activation during S. mansoni infection. Spleens were harvested at different time points after infection, and the frequency (A) and absolute numbers (D) of detectable CD3+ NK1.1+ cells were assessed by fluorescence-activated cell sorter staining. In panel A, week (Wk) 0 refers to mice not infected. In panel D, the total number of MNC in the spleen during infection is shown in the upper left corner. Data represent the mean percentage ± SD (A) or the total number ± SD (D) of CD3+ NK1.1+ cells (four mice per group per time point). Significant differences are designated by asterisks (P < 0.01). One representative experiment out of three is shown. (B) Representative dot plots of total CD3+ NK1.1+ cells (0 and 7 weeks p.i., left side) and tetramer+ and tetramer− cells among CD3+ NK1.1+ cells (right side). (C) Expression of CD69 (solid line) on CD3+ NK1.1+ tetramer+ (left side) and CD3+ NK1.1+ tetramer− (right side) cells at 0 (top) and 6 (bottom) weeks p.i. The isotype control is also shown (dotted line).
Despite the apparent decreased frequency of NKT cells, the absolute number of detectable CD3+ NK1.1+ cells gradually increased in the spleen during infection, starting at 3 weeks p.i. and culminating at 6 weeks p.i. (Fig. 1D). Similarly, the proportion of conventional B cells and T cells also increased during infection (approximately threefold at 7 weeks p.i.) (data not shown). This suggests that splenic NKT cells expand during infection, although increased NKT-cell recruitment in the spleen and/or reduced apoptosis might explain the latter observation.
S. mansoni schistosomules and adult worms do not activate NKT cells via DCs in vitro.
Activation of NKT cells during infection precedes the occurrence of schistosome eggs (∼5 weeks p.i.), suggesting that schistosomules and/or adult worms could be involved in their primary activation. We thus investigated the possibility that these parasite stages could promote NKT-cell activation in vitro via conventional DCs, which are known to be extremely potent in activating NKT cells (22, 30). We have recently shown that DCs sensitized with schistosome eggs can activate iNKT cells to produce IFN-γ and IL-4, but the other parasite stages were not tested in this system (36). To investigate this, DCs were sensitized with the different developmental stages of the parasite, extensively washed, and then cocultured with liver MNC, a rich source of iNKT cells. As a positive control, the iNKT-cell superagonist α-GC was used. As shown in Fig. 2, α-GC- and schistosome egg-sensitized DCs activated liver cells from WT mice, but not those from TCR Jα18−/− mice (which lack iNKT cells) (reference 36 and data not shown), to produce IFN-γ and IL-4. On the other hand, the other parasite stages were inactive. We next addressed the question of whether schistosomes can activate non-iNKT cells. To this end, because the spleens of WT mice contain both iNKT and non-iNKT cells, CD5+ NK1.1+ cells were sorted from the spleens of TCR Jα18−/− mice (to avoid iNKT-cell contamination) and cocultured with parasite-sensitized DCs. As a positive control, CD5+ NK1.1+ cells were stimulated with anti-CD3. As shown in Fig. 2B, whatever the parasite stage analyzed, sensitized DCs failed to activate non-iNKT cells to produce cytokines. By contrast, CD5+ NK1.1+ cells secreted IFN-γ and IL-4 upon CD3 stimulation, indicating that they responded normally to TCR stimulation. Altogether, these data suggest that by interacting with DCs, the egg stage of Schistosoma parasites is capable of activating iNKT cells but not non-iNKT cells. Our data also show that the schistosomule and the adult worm stages do not activate NKT cells, at least after in vitro contact with conventional DCs.
FIG. 2.
Analysis of NKT-cell activation in response to schistosomule-, adult-worm-, and egg-sensitized DCs. Schistosomules, adult worms, or eggs were incubated with DCs or without (not stimulated [NS]), and after extensive washing, sensitized DCs were cocultured for 2 days with liver MNC (containing iNKT cells) isolated from WT mice (A) or with splenic CD5+ NK1.1+ cells (mostly non-iNKT cells) sorted from TCR Jα18−/− mice (B). As positive controls, liver MNC were cultured with α-GC-sensitized DCs (A) or sorted CD5+ NK1.1+ cells were incubated with plate-bound anti-CD3 (B). Cytokine production was measured by ELISA. Data represent the mean ± SD of three independent experiments. *, P < 0.001 (compared to cytokine production by unstimulated cells).
NKT cells are not essential in the development of the immune response early after infection.
It is now established that during some infections, NKT cells can provide help to naive T cells during their priming and can therefore modulate the nature and/or the intensity of the adaptive immune response that occurs at later time points (34). Therefore, we aimed to determine the in vivo contribution of NKT cells in the promotion and/or the polarization of conventional T cells during the early stage of infection. For this purpose, WT mice, CD1d−/− mice (which lack both iNKT and non-iNKT cells), and TCR Jα18−/− mice (which lack only iNKT cells) were infected with S. mansoni and 1 week later, the immune response that develops in the skin was studied by restimulating skin-draining LN cells with anti-CD3. Whatever the mouse strain analyzed, CD3 restimulation resulted in identical levels of T-cell priming as determined by LN cell proliferation in culture (Fig. 3A). Relative to WT mice, no significant difference in the production of both Th1-type (IFN-γ) and Th2-type (IL-4, IL-5, and IL-10) (Fig. 3A and not shown) cytokines by skin-draining LN cells was observed in CD1d−/− mice or in TCR Jα18−/− mice. Of note, similar data were obtained after restimulation with schistosomule-derived Ag (not shown). This experiment shows that, early after infection, the absence of NKT cells does not have a quantitative or qualitative impact on the nature of the immune response. Next we investigated the consequences of NKT-cell deficiency on the immune response at 4 weeks p.i., just before the occurrence of schistosome eggs. As shown in Fig. 3B, upon CD3 or adult worm Ag (not shown) restimulation, spleen cells from CD1d−/− or TCR Jα18−/− mice proliferated equally and produced equivalent amounts of both IFN-γ and IL-4 (as well as IL-5 and IL-10 [not shown]) than those from WT mice. Thus, iNKT cells, as well as non-iNKT cells, are dispensable in the Th1/Th2 balance of the immune response early after infection.
FIG. 3.
Analysis of the cellular immune responses of S. mansoni-infected WT, CD1d−/−, and TCR Jα18−/− mice at 1 and 4 weeks p.i. Skin-draining LN cells (A) or spleen cells (B) from infected mice were cultured for 2 days with or without anti-CD3; afterwards, IFN-γ and IL-4 in the supernatants were assayed by ELISA. Proliferation was measured after 2 days of culture. Results represent the mean of triplicate cultures ± SD (six mice per group). P < 0.05 was considered significant. One representative experiment out of two is shown.
iNKT cells and non-iNKT cells exert opposite effects on the cellular immune response during the acute phase of the disease.
The impact of NKT-cell deficiency was next assessed during the acute phase of the disease (7 weeks p.i.). First, we analyzed the parasitological parameters in WT, CD1d−/−, and TCR Jα18−/− mice. In CD1d−/− and TCR Jα18−/− mice, the worm burden was slightly, but not significantly, decreased compared to that in WT mice (Table 1). In addition, tissue (liver and intestine) egg numbers were comparable in the three animal groups. We next analyzed the impact of iNKT-cell and both iNKT- and non-iNKT-cell deficiencies on the acquired immune response (Fig. 4). To this end, spleen cells were restimulated with anti-CD3 or SEA (20 μg/ml). Whatever the mouse strain analyzed, restimulation with anti-CD3 (not shown) or with SEA resulted in identical levels of T-cell priming as assessed by spleen cell proliferation. However, as represented in Fig. 4, major differences in cytokine production were observed among WT, CD1d−/−, and TCR Jα18−/− mice. In agreement with our previous observations (19) and compared to WT mice, CD1d−/− mice developed a markedly reduced Th2 response. Indeed, spleen cells from CD1d−/− mice produced dramatically less IL-4, IL-5, and IL-10, but an identical level of IFN-γ, upon SEA (or CD3, not shown) restimulation compared to those from their WT counterparts. Interestingly, the inverse phenomenon was observed in TCR Jα18−/− mice (Fig. 4). In these mice, compared to their WT counterparts, the production of IFN-γ was dramatically reduced whereas that of Th2-type cytokines was unchanged. Thus, during Schistosoma infection, both iNKT cells and non-iNKT cells appear to exert a regulatory effect on the immune response, albeit their effects on the Th1/Th2 balance are diametrically different.
TABLE 1.
Analysis of worm burdens and total tissue eggs in S. mansoni-infected WT, TCR Jα18−/−, and CD1d−/− mice at 49 days postinfectiona
| Mouse strain | Worm burden | No. of eggs/worm pair/g of:
|
|
|---|---|---|---|
| Liver | Intestine | ||
| WT | 23.14 ± 4.14 | 860 ± 295 | 1,094 ± 265 |
| Jα18−/− | 16.75 ± 4.65 | 700 ± 99 | 926 ± 310 |
| CD1d−/− | 16.83 ± 3.60 | 852 ± 286 | 1,289 ± 394 |
Results represent the mean number ± SD (five to eight mice per group). One representative experiment out of three is shown.
FIG. 4.
Analysis of the cellular immune responses of S. mansoni-infected WT, CD1d−/−, and TCR Jα18−/− mice at 7 weeks p.i. Spleen cells from infected mice were cultured with increasing doses of SEA (shown is 20 μg/ml) or left unstimulated. Cytokine production and proliferation were measured after 3 days of culture. Results represent the mean of triplicate cultures ± SD (five mice per group). Significant differences are designated by asterisks (P < 0.05). One representative experiment out of three is shown.
iNKT cells and non-iNKT cells regulate the humoral immune response differently.
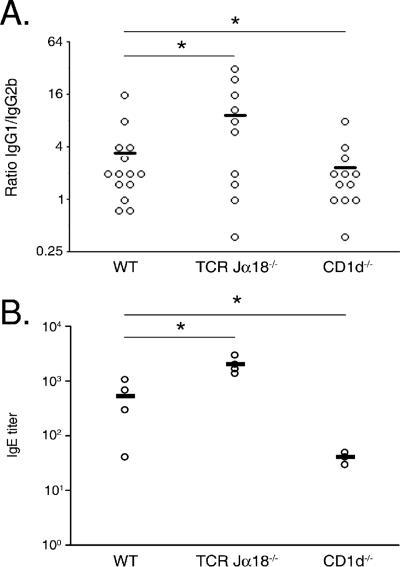
Previous studies have demonstrated that NKT cells can influence the Ab responses against microbial Ag during infection (15, 29, 46). Thus, we determined the titers of SEA-specific IgG1 (a marker of a Th2 response) and IgG2b (a marker of a Th1 response) in infected mice at 7 weeks p.i. Although the overall Ig levels were identical in the two groups (not shown), the average IgG1/IgG2b ratio was reduced (by 35%) in CD1d−/− mice compared to that in WT mice, whereas the inverse phenomenon was observed in TCR Jα18−/− mice (threefold increase compared to WT mice) (Fig. 5A). Interestingly, this modified Th1/Th2 balance of the humoral response observed in CD1d−/− and TCR Jα18−/− mice, compared to WT controls, was supported by determination of the IgE serum titer at 12 weeks p.i. (Fig. 5B).
FIG. 5.
SEA-specific Ab isotype responses of S. mansoni-infected WT, CD1d−/−, and TCR Jα18−/− mice (7 and 12 weeks p.i.). Results are expressed as the IgG1/IgG2b titer ratio (A) and IgE titer (B) of each mouse (4 to 14 mice per group). The mean number ± SD is presented. Significant differences are designated by asterisks (P < 0.05). One representative experiment out of three is shown.
Collectively, these data suggest that iNKT cells are important in the Th1 bias of SEA-reactive T cells and in the subsequent generation of SEA-specific Th1-associated Ab isotypes during infection, whereas non-iNKT cells have the opposite effect.
NKT-cell deficiency influences the formation of liver granulomas in S. mansoni-infected mice.
We then proceeded to evaluate the influence of NKT-cell deficiency on the egg-induced liver granuloma. As shown in Fig. 6A, the volume of the granulomas surrounding eggs was more important in TCR Jα18−/− mice, compared to WT and CD1d−/− mice. Since fibrosis is a sequel to egg granuloma formation during infection, we then visualized collagen deposition within the liver. Rojkind staining demonstrated differences in the deposition of collagenous material (red stain) in the granulomas of CD1d−/− and TCR Jα18−/− mice, compared to control mice (Fig. 6 B and C). In agreement with reference 19 and compared to WT animals, granulomas from CD1d−/− mice had less intense, but more diffuse, collagen deposition, whereas the level of collagen deposition was more pronounced in TCR Jα18−/− mice.
FIG. 6.
Analysis of egg-induced pathology in the livers of WT, CD1d−/−, and TCR Jα18−/− mice. In panel A, the volumes of granulomas are indicated for each animal group. Panels B and C show Rojkind staining of liver sections from WT, CD1d−/−, and TCR Jα18−/− mice. In panel B, the collagen deposition score of each animal group is indicated. In panel C, note the decreased or increased intensity of collagen deposition (red staining) in CD1d−/− or TCR Jα18−/− mice compared to that in WT mice (×100 magnification). Significant differences are designated by asterisks (P < 0.01).
DISCUSSION
NKT cells are important players in the development and regulation of both innate and acquired immune responses during infection (27, 48). Their natural role during helminthiasis has, however, not been explored. Here, we used two different NKT-cell-deficient mouse strains (CD1d −/− and TCR Jα18−/−) to investigate the functions of iNKT and non-iNKT cells during murine schistosomiasis. We report that during the acute, but not the early, phase of the disease, CD1d−/− and TCR Jα18−/− mice not only develop a different type of immune response than WT mice but also display striking differences compared to each other.
We have recently shown that during murine schistosomiasis, iNKT cells become activated and expand in the liver (the main site of egg deposition) as early as 2 to 3 weeks p.i. (36). Since the spleen contains diverse NKT-cell subsets (17, 25) and is critically involved in the immune response against the parasite, we investigated NKT-cell activation in this organ during infection. Relative to uninfected animals, we observed a sustained decreased proportion of splenic CD3+ NK1.1+ cells within MNC in infected mice, starting at 3 weeks p.i. Despite this apparently reduced frequency, their absolute number increased during infection, a phenomenon likely to be due to increased expansion and/or recruitment of NKT cells in this organ, although reduced apoptosis cannot be ruled out. Interestingly, the apparent frequency of both iNKT cells (tetramer+) and non-iNKT (tetramer−) cells was reduced during infection. Thus, although splenic CD3+ NK1.1+ cells in normal mice comprise a minor proportion of non-NKT cells (10 to 20%) (17) and during infection, some activated T cells might have acquired the NK1.1 marker (49), our data suggest that iNKT and non-iNKT cells become activated as early as 3 weeks p.i., before the occurrence of parasite eggs. This was confirmed by analyzing CD69 expression on NKT cells. Indeed, the remaining detectable iNKT cells and a proportion of the remaining detectable non-iNKT cells up-regulated CD69 at 6 weeks p.i. The fact that no variation in CD69 expression was observed at other time points might be explained by the apparent disappearance of strongly activated cells. The activation of NKT cells as early as 3 weeks p.i. supposes that schistosomules and/or adult worms could be involved in their primary activation in vivo. However, our coculture system using parasite-sensitized DCs and liver MNC (a rich source of iNKT cells) or sorted non-iNKT cells indicates that these parasite stages do not promote NKT-cell stimulation via DCs in vitro (Fig. 2). This implies that during infection, schistosomules and/or adult worms stimulate NKT cells via other APCs and/or that in vitro DC-parasite interactions do not mimic in vivo interplays. On the other hand, our coculture system indicates that schistosome eggs activate iNKT cells, but not non-iNKT cells, via DCs. Although our previous finding suggests that self (DC-derived), but not parasite, CD1d-restriced Ags are required (probably in concert with costimulatory signals) to activate iNKT cells (36), the exact mechanism of this activation is still unclear.
Irrespective of the mechanisms of activation, we next investigated whether NKT cells could modulate the intensity or the nature of the adaptive immune response triggered by schistosomules and adult worms. The use of infected CD1d−/− and TCR Jα18−/− C57BL/6 mice indicates that iNKT cells, as well as non-iNKT cells, are not required for the control of the immune response early (1 and 4 weeks) after infection. We next focused on the acute stage of the disease by comparing parasitological and immunological parameters in infected WT, CD1d−/−, and TCR Jα18−/− mice. In the three animal groups, no differences in worm and egg burdens were noticed. Thus, although host defense responses to many pathogens are generally impaired in NKT-deficient mice (27, 48), the lack of NKT cells does not influence parasite survival and fecundity during murine schistosomiasis. Among the immunological parameters analyzed, we found no significant differences in the relative percentage or in the total number of splenic B cells (CD3− CD19+) and T cells (CD3+ CD19−) (both CD4+ and CD8+) among the three infected animal groups (not shown). Similarly, upon CD3 (not shown) or SEA (Fig. 4) restimulation, identical levels of spleen cell proliferation were observed, implying that NKT cells do not exacerbate or curb the priming of conventional T cells during infection. In contrast, major differences in the cytokine profile of restimulated spleen cells were observed (7 weeks p.i.). Compared to WT mice, CD1d−/− mice developed a markedly reduced Th2 response but maintained their ability to promote a Th1 response. This result is important since it not only confirms our previous observations of BALB/c mice (19) but also suggests that, unlike in other infection models (28, 50), differences in the genetic background of the host (C57BL/6 versus BALB/c) do not appear to influence the regulatory impact of NKT-cell functions on the global immune response in our model. On the other hand, C57BL/6 TCR Jα18−/− mice mounted a dramatically decreased Th1 cytokine response, relative to WT mice. In addition, although no changes in the overall Ig levels were noticed, the anti-SEA antibody isotypic responses of both CD1d−/− (decreased IgG1/IgG2b ratio and decreased IgE titer) and TCR Jα18−/− (increased IgG1/IgG2b ratio and enhanced IgE titer) mice deviated from those of WT mice, albeit in opposite directions. These striking differences in the Th1/Th2 balance of the cellular immune response between these mouse strains were also observed during the chronic stage of infection (12 weeks p.i. [not shown]). Importantly, these data argue that schistosome infection stimulates not only iNKT cells but also non-iNKT cells. Secondly, these results support new findings showing that, in some cases, NKT-cell subsets can behave differently in terms of their capacities to regulate immune responses. For instance, a recent report shows that iNKT cells and non-iNKT cells exert different protective effects in some, but not all, mouse tumor models (52), whereas another paper demonstrates differential functions of CD4+ and CD4− iNKT cells in some natural antitumor responses (10). Duthie and coworkers recently demonstrated that NKT-cell subsets display distinct regulatory functions during Trypanosoma cruzi infection, with non-iNKT cells being involved in the proinflammatory response and iNKT cells being involved in the antiinflammatory response to the parasite (14). However, to the best of our knowledge, our study suggests, for the first time, that during the same infection, iNKT-cell and non-iNKT-cell subsets contribute differentially to the Th1 and Th2 arms of the immune response. Of note, this Th1 or Th2 cytokine bias in CD1d−/− mice and TCR Jα18−/− mice, respectively, was accompanied by some differences in egg-induced pathology, although these differences did not fully mirror the dysregulated Th1/Th2 balance observed in these mice. As observed in BALB/c mice (19), the intensity of collagen deposition in liver granulomas in S. mansoni-infected CD1d−/− mice was less intense compared to that in WT control mice whereas that in TCR Jα18−/− mice was more pronounced. These differences may be explained by variation of the Th1/Th2 balance of the immune response in these animal groups and/or by a more direct role of NKT cells in the control of liver pathology (14).
During murine schistosomiasis, iNKT cells and non-iNKT cells might have distinct functions in the immune response triggered by schistosome eggs. Non-iNKT cells, which are absent in CD1d−/− mice, appear to help in Th2 cell differentiation, whereas iNKT cells, which are absent in TCR Jα18−/− mice, might be more efficient at promoting the development of Th1 cells. Why animals lacking both NKT subsets (CD1d−/− mice) did not also present an impaired Th1 response, in concert with a decreased Th2 response, is unknown. It is possible that the absence of iNKT cells in these mice is compensated for by other (CD1d-independent) cellular populations. Although during infection, iNKT cells generally contribute to the development of Th1 responses (2, 26, 43, 44), the role of this NKT subset in the promotion of the Th1 response during Schistosoma infection is surprising. Indeed, we have recently reported that intravenous immunization of naive mice with DCs previously sensitized with schistosome eggs polarizes the immune response in a Th2 direction in WT, but not TCR Jα18−/−, mice, suggesting that iNKT cells provide help for Th2 responses in this model (36). These apparently conflicting results can be explained by differences in the organs analyzed (skin-draining LNs versus the spleen). Indeed, it is known that the functions of iNKT cells (i.e., their role in the Th1/Th2 balance) can greatly vary according to the organ (i.e., the spleen versus the liver) (10). Moreover, the functions of iNKT cells dramatically depend on the frequency of their TCR stimulation (7, 21, 47). Therefore, in our immunization model, primary and single stimulation of iNKT cells by egg-sensitized DCs may be efficient at promoting Th2 immunity (36) whereas during infection, chronic activation of iNKT cells may rather polarize the immune response in a Th1 direction. Moreover, recent observations suggest that the type of CD1d-expressing APCs (as well as the environment where APCs and iNKT cells encounter each other) are crucial in dictating iNKT-cell functions (6, 21, 45). In the immunization model (36), conventional DCs were used to promote the Th2 adjuvant bystander effects of iNKT cells. On the other hand, during infection, DCs and other APCs (including B cells, macrophages, nonconventional DCs) might stimulate peripheral iNKT cells to have an impact on the Th1 response. As discussed above, our data also suggest that during Schistosoma infection, unlike iNKT cells, non-iNKT cells rather favor the production of Th2 cytokines by T cells. Attempts are now under way to identify their mechanisms of activation (which appear to be DC independent; Fig. 2) and to more firmly demonstrate their role on the Th2 response, for instance, by transferring non-iNKT cells into CD1d−/− mice reconstituted with WT APCs.
In summary, our data provide the first evidence that NKT cells are important players in the acquired immune response during helminthiasis and suggest that iNKT- and non-iNKT-cell subsets have opposite, and perhaps complementary, effects on the Th1/Th2 balance during murine schistosomiasis. In the future, it will be interesting to better define the regulatory functions of NKT-cell subsets during murine schistosomiasis but also during other helminth infections that affect human populations.
Acknowledgments
We gratefully acknowledge T. Nakayama and M. Taniguchi (Chiba University, Japan) and L. Van Kaer (Vanderbilt University, Nashville, TN) for the respective gifts of TCR Jα18−/− and CD1d−/− C57BL/6 mice. N. Messiaen (Institut Pasteur de Lille) is gratefully acknowledged for efforts in breeding the mice used in this study.
This study was supported by the Institut National de la Santé et de la Recherche Médicale, l'Institut Pasteur de Lille, and l'Université de Lille 2. We also thank l'Agence Nationale de la Recherche (Program Microbiologie, Infections et Immunités, grant APV05103ESA) for supporting the NKTschisto project. T.M. and L.B. were recipients of a doctoral fellowship from the Ministère de l'Education Nationale de la Recherche et Technique and from the Fondation pour la Recherche Médicale (for T.M.). A.C.-K. was the recipient of a postdoctoral fellowship from CNPq, Brazil. F.T. and M.L.M. are supported by the Centre National de la Recherche Scientifique, and C.F. is supported by the Inserm.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 12 March 2007.
REFERENCES
- 1.Aksoy, E., C. S. Zouain, F. Vanhoutte, J. Fontaine, N. Pavelka, N. Thieblemont, F. Willems, P. Ricciardi-Castagnoli, M. Goldman, M. Capron, B. Ryffel, and F. Trottein. 2005. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J. Biol. Chem. 280:277-283. [DOI] [PubMed] [Google Scholar]
- 2.Amprey, J. L., J. S. Im, S. J. Turco, H. W. Murray, P. A. Illarionov, G. S. Besra, S. A. Porcelli, and G. F. Spath. 2004. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J. Exp. Med. 200:895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arrunategui-Correa, V., and H. S. Kim. 2004. The role of CD1d in the immune response against Listeria infection. Cell Immunol. 227:109-120. [DOI] [PubMed] [Google Scholar]
- 4.Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16:583-594. [DOI] [PubMed] [Google Scholar]
- 5.Behar, S. M., and S. Cardell. 2000. Diverse CD1d-restricted T cells: diverse phenotypes, and diverse functions. Semin. Immunol. 12:551-560. [DOI] [PubMed] [Google Scholar]
- 6.Bezbradica, J. S., A. K. Stanic, N. Matsuki, H. Bour-Jordan, J. A. Bluestone, J. W. Thomas, D. Unutmaz, L. Van Kaer, and S. Joyce. 2005. Distinct roles of dendritic cells and B cells in Va14Ja18 natural T cell activation in vivo. J. Immunol. 174:4696-4705. [DOI] [PubMed] [Google Scholar]
- 7.Burdin, N., L. Brossay, and M. Kronenberg. 1999. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur. J. Immunol. 29:2014-2025. [DOI] [PubMed] [Google Scholar]
- 8.Carnaud, C., D. Lee, O. Donnars, S.-H. Park, A. Beavis, Y. Koezuka, and A. Bendelac. 1999. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 163:4647-4650. [PubMed] [Google Scholar]
- 9.Chiu, Y. H., J. Jayawardena, A. Weiss, D. Lee, S. H. Park, A. Dautry-Varsat, and A. Bendelac. 1999. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J. Exp. Med. 189:103-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowe, N. Y., J. M. Coquet, S. P. Berzins, K. Kyparissoudis, R. Keating, D. G. Pellicci, Y. Hayakawa, D. I. Godfrey, and M. J. Smyth. 2005. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 202:1279-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowe, N. Y., A. P. Uldrich, K. Kyparissoudis, K. J. Hammond, Y. Hayakawa, S. Sidobre, R. Keating, M. Kronenberg, M. J. Smyth, and D. I. Godfrey. 2003. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J. Immunol. 171:4020-4027. [DOI] [PubMed] [Google Scholar]
- 12.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623-1626. [DOI] [PubMed] [Google Scholar]
- 13.Doenhoff, M., R. Musallam, J. Bain, and A. McGregor. 1978. Studies on the host-parasite relationship in Schistosoma mansoni-infected mice: the immunological dependence of parasite egg excretion. Immunology 35:771-778. [PMC free article] [PubMed] [Google Scholar]
- 14.Duthie, M. S., M. Kahn, M. White, R. P. Kapur, and S. J. Kahn. 2005. Critical proinflammatory and antiinflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect. Immun. 73:181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duthie, M. S., M. Wleklinski-Lee, S. Smith, T. Nakayama, M. Taniguchi, and S. J. Kahn. 2002. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect. Immun. 70:36-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberl, G., P. Brawand, and H. R. MacDonald. 2000. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J. Immunol. 165:4305-4311. [DOI] [PubMed] [Google Scholar]
- 17.Eberl, G., R. Lees, S. T. Smiley, M. Taniguchi, M. J. Grusby, and H. R. MacDonald. 1999. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J. Immunol. 162:6410-6419. [PubMed] [Google Scholar]
- 18.Eberl, G., and H. R. MacDonald. 2000. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur. J. Immunol. 30:985-992. [DOI] [PubMed] [Google Scholar]
- 19.Faveeuw, C., V. Angeli, J. Fontaine, C. Maliszewski, A. Capron, L. Van Kaer, M. Moser, M. Capron, and F. Trottein. 2002. Antigen presentation by CD1d contributes to the amplification of Th2 responses to Schistosoma mansoni glycoconjugates in mice. J. Immunol. 169:906-912. [DOI] [PubMed] [Google Scholar]
- 20.Fischer, K., E. Scotet, M. Niemeyer, H. Koebernick, J. Zerrahn, S. Maillet, R. Hurwitz, M. Kursar, M. Bonneville, S. H. Kaufmann, and U. E. Schaible. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA 101:10685-10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii, S., K. Shimizu, M. Kronenberg, and R. M. Steinman. 2002. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat. Immunol. 3:867-874. [DOI] [PubMed] [Google Scholar]
- 22.Fujii, S., K. Shimizu, C. Smith, L. Bonifaz, and R. M. Steinman. 2003. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J. Exp. Med. 198:267-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galli, G., S. Nuti, S. Tavarini, L. Galli-Stampino, C. De Lalla, G. Casorati, P. Dellabona, and S. Abrignani. 2003. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J. Exp. Med. 197:1051-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godfrey, D. I., K. J. Hammond, L. D. Poulton, M. J. Smyth, and A. G. Baxter. 2000. NKT cells: facts, functions and fallacies. Immunol. Today 21:573-583. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey, D. I., H. R. MacDonald, M. Kronenberg, M. J. Smyth, and L. Van Kaer. 2004. NKT cells: what's in a name? Nat. Rev. Immunol. 4:231-237. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Aseguinolaza, G., L. Van Kaer, C. C. Bergmann, J. M. Wilson, J. Schmieg, M. Kronenberg, T. Nakayama, M. Taniguchi, Y. Koezuka, and M. Tsuji. 2002. Natural killer T cell ligand α-galactosylceramide enhances protective immunity induced by malaria vaccines. J. Exp. Med. 195:617-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen, D. S., and L. Schofield. 2004. Regulation of immunity and pathogenesis in infectious diseases by CD1d-restricted NKT cells. Int. J. Parasitol. 34:15-25. [DOI] [PubMed] [Google Scholar]
- 28.Hansen, D. S., M. A. Siomos, L. Buckingham, A. A. Scalzo, and L. Schofield. 2003. Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18:391-402. [DOI] [PubMed] [Google Scholar]
- 29.Hansen, D. S., M. A. Siomos, T. De Koning-Ward, L. Buckingham, B. S. Crabb, and L. Schofield. 2003. CD1d-restricted NKT cells contribute to malarial splenomegaly and enhance parasite-specific antibody responses. Eur. J. Immunol. 33:2588-2598. [DOI] [PubMed] [Google Scholar]
- 30.Hermans, I. F., J. D. Silk, U. Gileadi, M. Salio, B. Mathew, G. Ritter, R. Schmidt, A. L. Harris, L. Old, and V. Cerundolo. 2003. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J. Immunol. 171:5140-5147. [DOI] [PubMed] [Google Scholar]
- 31.Jahng, A., I. Maricic, C. Aguilera, S. Cardell, R. C. Halder, and V. Kumar. 2004. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199:947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki, and M. Taniguchi. 1997. CD1d-restricted and TCR-mediated activation of vα14 NKT cells by glycosylceramides. Science 278:1626-1629. [DOI] [PubMed] [Google Scholar]
- 33.Kinjo, Y., D. Wu, G. Kim, G. W. Xing, M. A. Poles, D. D. Ho, M. Tsuji, K. Kawahara, C. H. Wong, and M. Kronenberg. 2005. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434:520-525. [DOI] [PubMed] [Google Scholar]
- 34.Kronenberg, M., and L. Gapin. 2002. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2:557-568. [DOI] [PubMed] [Google Scholar]
- 35.Lantz, O., and A. Bendelac. 1994. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180:1097-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mallevaey, T., J. P. Zanetta, C. Faveeuw, J. Fontaine, E. Maes, F. Platt, M. Capron, M. Leite-de-Moraes, and F. Trottein. 2006. Activation of invariant NKT cells by the helminth parasite Schistosoma mansoni. J. Immunol. 176:2476-2485. [DOI] [PubMed] [Google Scholar]
- 37.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525-529. [DOI] [PubMed] [Google Scholar]
- 38.Mendiratta, S. K., W. D. Martin, S. Hong, A. Boesteanu, S. Joyce, and L. Van Kaer. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6:469-477. [DOI] [PubMed] [Google Scholar]
- 39.Park, S. H., A. Weiss, K. Benlagha, T. Kyin, L. Teyton, and A. Bendelac. 2001. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J. Exp. Med. 193:893-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499-511. [DOI] [PubMed] [Google Scholar]
- 41.Pellicci, D. G., K. J. Hammond, J. Coquet, K. Kyparissoudis, A. G. Brooks, K. Kedzierska, R. Keating, S. Turner, S. Berzins, M. J. Smyth, and D. I. Godfrey. 2005. DX5/CD49b-positive T cells are not synonymous with CD1d-dependent NKT cells. J. Immunol. 175:4416-4425. [DOI] [PubMed] [Google Scholar]
- 42.Ramalho-Pinto, F. J., G. Gazzinelli, R. E. Howells, T. A. Mota-Santos, E. A. Figueiredo, and J. Pellegrino. 1974. Schistosoma mansoni: defined system for stepwise transformation of cercaria to schistosomule in vitro. Exp. Parasitol. 36:360-372. [DOI] [PubMed] [Google Scholar]
- 43.Ranson, T., S. Bregenholt, A. Lehuen, O. Gaillot, M. C. Leite-de-Moraes, A. Herbelin, P. Berche, and J. P. Di Santo. 2005. Invariant Vα14+ NKT cells participate in the early response to enteric Listeria monocytogenes infection. J. Immunol. 175:1137-1144. [DOI] [PubMed] [Google Scholar]
- 44.Ronet, C., S. Darche, M. Leite de Moraes, S. Miyake, T. Yamamura, J. A. Louis, L. H. Kasper, and D. Buzoni-Gatel. 2005. NKT cells are critical for the initiation of an inflammatory bowel response against Toxoplasma gondii. J. Immunol. 175:899-908. [DOI] [PubMed] [Google Scholar]
- 45.Schmieg, J., G. Yang, R. W. Franck, N. Van Rooijen, and M. Tsuji. 2005. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc. Natl. Acad. Sci. USA 102:1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schofield, L., M. J. McConville, D. Hansen, A. S. Campbell, B. Fraser-Reid, M. J. Grusby, and S. D. Tachado. 1999. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science 283:225-229. [DOI] [PubMed] [Google Scholar]
- 47.Singh, N., S. Hong, D. C. Scherer, I. Serizawa, N. Burdin, M. Kronenberg, Y. Koezuka, and L. Van Kaer. 1999. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J. Immunol. 163:2373-2377. [PubMed] [Google Scholar]
- 48.Sköld, M., and S. M. Behar. 2003. Role of CD1d-restricted NKT cells in microbial immunity. Infect. Immun. 71:5447-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slifka, M. K., R. R. Pagarigan, and J. L. Whitton. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 164:2009-2015. [DOI] [PubMed] [Google Scholar]
- 50.Smiley, S. T., P. A. Lanthier, K. N. Couper, F. M. Szaba, J. E. Boyson, W. Chen, and L. L. Johnson. 2005. Exacerbated susceptibility to infection-stimulated immunopathology in CD1d-deficient mice. J. Immunol. 174:7904-7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stenstrom, M., M. Skold, A. Ericsson, L. Beaudoin, S. Sidobre, M. Kronenberg, A. Lehuen, and S. Cardell. 2004. Surface receptors identify mouse NK1.1+ T cell subsets distinguished by function and T cell receptor type. Eur. J. Immunol. 34:56-65. [DOI] [PubMed] [Google Scholar]
- 52.Terabe, M., J. Swann, E. Ambrosino, P. Sinha, S. Takaku, Y. Hayakawa, D. I. Godfrey, S. Ostrand-Rosenberg, M. J. Smyth, and J. A. Berzofsky. 2005. A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J. Exp. Med. 202:1627-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson, M. T., C. Johansson, D. Olivares-Villagomez, A. K. Singh, A. K. Stanic, C. R. Wang, S. Joyce, M. J. Wick, and L. Van Kaer. 2003. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc. Natl. Acad. Sci. USA 100:10913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, D. Y., N. H. Segal, S. Sidobre, M. Kronenberg, and P. B. Chapman. 2003. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J. Exp. Med. 198:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaccone, P., Z. Fehervari, F. M. Jones, S. Sidobre, M. Kronenberg, D. W. Dunne, and A. Cooke. 2003. Schistosoma mansoni antigens modulate the activity of the innate immune response and prevent onset of type 1 diabetes. Eur. J. Immunol. 33:1439-1449. [DOI] [PubMed] [Google Scholar]
- 56.Zhou, D., J. Mattner, C. Cantu III, N. Schrantz, N. Yin, Y. Gao, Y. Sagiv, K. Hudspeth, Y. P. Wu, T. Yamashita, S. Teneberg, D. Wang, R. L. Proia, S. B. Levery, P. B. Savage, L. Teyton, and A. Bendelac. 2004. Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786-1789. [DOI] [PubMed] [Google Scholar]