Abstract
Neonates are considered highly susceptible to gastrointestinal infections. This susceptibility has been attributed partially to immaturity in immune cell function. To study this phenomenon, we have developed a model system with murine neonates, using the natural orogastric route of transmission for the enteropathogen Yersinia enterocolitica. The susceptibilities of 7-day-old and adult mice to orogastric Y. enterocolitica infection were assessed in 50% lethal dose experiments. Remarkably, neonatal mice of either the BALB/c or C57BL/6 mouse strain showed markedly enhanced survival after infection compared to adult mice. The resistance of neonates was not due to failure of the bacteria to colonize neonatal tissues; Y. enterocolitica was readily detectable in the intestine and mesenteric lymph nodes (MLN) for at least 1 week after infection. In adult mice, Y. enterocolitica rapidly disseminated to the spleen and liver. In striking contrast, bacterial invasion of the spleen and liver in neonates was limited. Using flow cytometry and histology, we found substantial increases in the percentages of neutrophils and macrophages in the neonatal MLN, while influx of these cells into the adult MLN was limited. Similar results were obtained using two different high-virulence Y. enterocolitica strains. Importantly, depletion of neutrophils with a specific antibody led to increased translocation of the bacteria to the spleens and livers of neonates. Together, these experiments support the hypothesis that the neonatal intestinal immune system can rapidly mobilize innate phagocytes and thereby confine the bacterial infection to the gut, resulting in a high level of resistance.
One of the most critical stages in immune system development occurs in neonatal life. During the first days and months following birth, newborns are exposed to countless new antigens to which they need to mount appropriate immune responses. For both humans and mice, it has been recognized that immune responses are frequently diminished during neonatal life. It is generally thought that immature responses are due in part to qualitative and quantitative deficiencies in immune cell components (3, 20, 47). However, whether and how innate immune cell populations contribute to immune system immaturity are controversial (reviewed in reference 39). For example, human studies indicate deficiencies in a variety of neutrophil properties, including chemotaxis, adhesion to and extravasation into inflamed tissues, oxidative burst, and cytokine production (reviewed in reference 32). However, other studies have clearly demonstrated that innate immune cell functions can be comparable to those of adults under the proper conditions (19, 21, 35, 48).
Adaptive immune responses are thought to be compromised in both human and murine neonates (reviewed in references 3 and 20). Notably, murine neonates have been shown to generate Th2 memory responses to a variety of antigens in vivo and are typically deficient in the development of protective Th1 memory (2, 20). In addition, B-cell responses are often qualitatively and quantitatively diminished in neonates (3, 47). Nonetheless, as for neonatal innate responses, mature adaptive immunity can be modulated to generate adult-like responses by using potent microbial products (7, 8, 13, 29, 34, 38).
Although both the adaptive and innate arms of immunity are fully mature under some circumstances, their responses are often largely ineffective against infectious agents. Thus, neonates are often susceptible to infectious agents that cause little to no pathology in adults (3, 20, 27). For example, Yersinia enterocolitica is a gram-negative enteric pathogen that causes gastroenteritis, inflammation of the mesenteric lymph nodes (MLN), and in some rare cases, septicemia (10, 43). Study of this pathogen is particularly relevant since it is considered a prevalent and emerging cause of childhood gastrointestinal disease in the United States (1, 33, 40). Indeed, it has been reported that two-thirds of Y. enterocolitica infections occur among infants and children (9). However, at present, there are few animal systems to model infection of human neonates with enteropathogens. To approach this issue, we have developed a model system to study Y. enterocolitica infection of murine neonates through the natural orogastric (o.g.) route of transmission. To best mimic pediatric disease, we selected 7-day-old mice because they are considered to be most reflective of human newborns (47). Using this system, we unexpectedly found that neonatal mice are more resistant than adults to primary o.g. infection, as assessed by median lethal dose (LD50) survival experiments. Bacterial colonization experiments revealed that the majority of the bacterial load was contained in the intestines of the neonates, with translocation as far as the MLN but with limited colonization of peripheral organs. However, in adult mice we observed colonization of deeper tissues (liver and spleen), which correlated with their susceptibility to lethality. This led to the hypothesis that neonatal mice are competent to mount a strong antibacterial response via enhanced intestinal innate immune responses. Flow cytometric and histological analyses demonstrated that, indeed, neonatal mice exhibited a marked influx of neutrophils and macrophages into the MLN compared to infected adult mice. In addition, depletion of neutrophils by antibody treatment revealed an increase in the translocation of Y. enterocolitica to the spleens and livers of infected neonates. The combined results presented here suggest that neonatal mice may be well equipped to promote a robust intestinal inflammatory response that is highly protective toward at least some types of bacterial enteropathogens.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Wild-type high-virulence Y. enterocolitica A127/90 serotype 0:8/biotype IB (provided by G. R. Cornelis, Universitat Basel, Basel, Switzerland) and Y. enterocolitica WA serotype 0:8/biotype IB (provided by G. Plano, University of Miami, Miami, FL) were used in this study. Prior to any experiments, the Y. enterocolitica WA strain was passaged in vivo to maintain virulence by infecting C57BL/6 mice o.g. (6). The spleen of an infected mouse was homogenized in Ca2+-Mg2+ Hanks' balanced salt solution (HBSS), and diluted aliquots were plated on yersinia selective agar (YSA) plates (Difco, Sparks, MD). For both strains, bacterial stocks were prepared by overnight culture of fresh bacterial colonies in Luria-Bertani (LB) broth (EMD, San Diego, CA) at 27°C. Cultures were diluted 1:20 in LB broth the next day and grown to early stationary phase. Each strain was stored in 1-ml aliquots in 30% glycerol-LB at −80°C. The titer of the frozen stock was determined by plating a diluted aliquot on LB plates and then counting colonies after incubation for 48 h at 27°C.
Mice.
BALB/c (Charles River, Wilmington, MA) and C57BL/6 (The Jackson Laboratory, Bar Harbor, ME) mice were bred and housed under barrier conditions in the Division of Veterinary Resources of the University of Miami Miller School of Medicine. Mice were confirmed to be free of common infectious agents through periodic colony screenings. Adult female mice (7 to 9 weeks of age) and neonatal mice (7 days of age) were used in all experiments. Adult mice were given sterile food and water ad libitum prior to infection. Neonatal mice from different litters were separated from the dams and randomly mixed prior to infection. Immediately after infection, the neonates were returned to the dams. Neonatal mice were nursing throughout all the experiments. All animal experiments were approved by the University of Miami Miller School of Medicine Animal Care and Use Committee.
o.g. and i.p. mouse infections and LD50 experiments.
Bacterial frozen stocks were washed twice with HBSS and diluted in consecutive 10-fold dilutions to the desired concentrations in sterile filtered 0.1% blue food coloring (McCormick, Baltimore, MD) in HBSS. Blue food coloring was used to facilitate visualization of delivery of the inoculum to the neonatal stomach. For LD50 analyses, groups of four or five adult mice per dose were inoculated o.g. with the bacterial suspensions via a 1-in.-long, 22-gauge, round-tipped feeding needle (Fine Science Tools, Foster City, CA) attached to a 1-ml syringe. Five to seven neonates were intubated o.g. with PE-10 tubing (polyethylene tube with an outside diameter of 0.61 mm; Clay Adams, Sparks, MD) attached to a 30-gauge needle and a Hamilton syringe (42). Adults received 100 μl and neonates received 25 μl of 10-fold dilutions of bacterial suspensions ranging from 2 × 103 to 2 × 108 CFU of Y. enterocolitica A127/90, depending on the experiment, or from 5 × 105 to 5 × 107 CFU of Y. enterocolitica WA. Mice were excluded from all experiments when signs of inoculum aspiration were visible, including sneezing and excess fluid in the mouth and nostrils. For infections by the intraperitoneal (i.p.) route, neonatal and adult mice were infected i.p. in parallel, using 30- and 25-gauge needles, respectively. Doses ranged from 10 to 200 CFU Y. enterocolitica A127/90 in a 50-μl volume. For all experiments, the actual administered dose was determined by plating serial dilutions of the suspensions on LB plates and incubating them at 27°C for 48 h. For each experiment, there were four or five adult mice and six or seven neonatal mice per dose. The LD50 means were calculated from three independent experiments. All mice were monitored for signs of distress twice daily for 21 days; mice that had become unable to move were euthanized, and their deaths were included in the analysis.
Bacterial enumeration from organs of infected mice.
To measure Yersinia titers in whole gut tissue after o.g. infection, the intestines were excised, from 0.5 cm below the pylorus to about 0.5 cm above the rectum, and placed intact into cold HBSS. The intestines were weighed and homogenized in 5 to 10 ml of cold HBSS, using a Seward Biomaster 80 stomacher (Brinkmann, Westbury, NY) for 2 to 3 min at high speed. The MLN, spleens, and livers of infected mice were excised sterilely at 2 to 9 days postinfection (p.i.) and were homogenized for 2 to 3 min at medium speed in 3 to 6 ml or 3 to 10 ml of cold HBSS, for neonatal and adult organs, respectively. Viable duplicate plate counts were done by spreading serial dilutions of the suspensions on YSA plates and incubating them at 27°C for 48 h. Control experiments with age-matched uninfected mice demonstrated that intestinal commensal bacteria were undetectable using this selective medium (26; data not shown); therefore, we were confident that all bacterial colonies were indeed Y. enterocolitica. The average titer was calculated and expressed as log CFU per gram of tissue. The lower limit of detection for the assay was estimated using similar calculations, assuming the presence of one bacterial colony in the lowest dilution. The limit of detection for all organs of infected neonates and adults ranged from 1.78 to 2.94 log CFU/g tissue.
Cell staining, antibodies, and flow cytometry analysis.
Neonates and adults were infected o.g. in parallel with Y. enterocolitica A127/90 or Y. enterocolitica WA. Individual Peyer's patches (PP) from adults and MLN and spleens from both groups were harvested at 3 and 5 days p.i. and placed in cold HBSS containing 1% calf serum (Gibco), 10 mM HEPES (Gibco), and 4 mM sodium azide. Age-matched uninfected mice served as controls. For neutrophil and macrophage analysis, 5 × 105 to 1 × 106 cells were incubated in mouse Fc Block (CD16/CD32; BD Pharmingen, San Diego, CA) for 5 min on ice, followed by a 30-min incubation with phycoerythrin-conjugated anti-Gr-1 (Ly6C/Ly6G) (BD Pharmingen). To detect intracellular CD68 expression, the cells were fixed, permeabilized, and stained with Alexa Fluor 647-conjugated anti-CD68 antibody (FA-11) per the manufacturer's instructions (Serotec, Raleigh, NC). Samples were analyzed on a Becton Dickinson LSR I flow cytometer. Neutrophils were characterized as Gr-1hi CD68int cells, and macrophages were characterized as Gr-1lo-int CD68hi cells.
Wright-Giemsa staining.
In parallel to the flow cytometric studies, MLN suspensions from infected neonatal and adult mice were used for histological identification of neutrophils. Age-matched uninfected mice served as controls. Cells (5 × 104) were spun onto positively charged slides (VWR, West Chester, PA) at 800 rpm for 8 min, using a cytocentrifuge. Slides were allowed to air dry for 1 h, followed by fixation in 100% methanol. Dry slides were submerged in Wright stain (Sigma, St. Louis, MO) for 6 min, followed by soaking in Sorensen buffer (0.15 M Na2HPO4, 0.15 M KH2PO4, pH 6.5) for 3 min. Finally, slides were stained with Giemsa stain (Sigma) for 4 min and washed with distilled water. All cells were viewed under a Leitz Laborlux S microscope at a magnification of ×630.
Neutrophil depletion.
RBC-8C5 antibody (anti-Gr-1) was collected from ascites fluids grown in nu/nu mice (University of Miami, Miami, FL) and purified over a protein G Sepharose column (GE Healthcare, Piscataway, NJ). Seven-day-old BALB/c mice were injected i.p. with either control rat IgG antibody (Jackson ImmunoResearch Inc., West Grove, PA) or RBC-8C5 antibody 1 day before and 1 day after o.g. infection with 5 × 107 CFU Y. enterocolitica A127/90. All mice received a total of 200 μg of the appropriate antibody in 50-μl injections. The whole intestine, MLN, spleen, and liver were collected at 8 days p.i. and processed as previously described for bacterial counts. To ensure that neutrophils had been depleted, flow cytometric analysis of individual MLN and spleen cells was performed after antibody treatment and o.g. infection. Neutrophils were characterized as Gr-1hi CD68int cells and found to be reduced to ≤0.02% of the cells in each tissue (data not shown).
Statistical analysis.
LD50 values were estimated using the method of Reed and Muench (44). Survival curves were generated by the Kaplan-Meier method, and survival kinetics between neonatal and adult groups were analyzed by the Mantel-Haenszel log rank test (GraphPad Prism 4). Survival curves were considered significantly different when the P value was ≤0.05. For colonization experiments, the data from two or three independent experiments were pooled before analysis. Flow cytometric and histological analyses were performed twice for each Y. enterocolitica strain. The means between groups were analyzed by the Mann-Whitney test, with significance for P values of ≤0.05. Neutrophil depletion was performed twice, and the mean bacterial titers were analyzed by an unpaired t test, with significance for P values of ≤0.05, after confirming that each group followed a normal distribution.
RESULTS
Neonatal mice are highly resistant to infection with Y. enterocolitica via the natural (o.g.) route of exposure.
Since the susceptibility of neonatal mice to Y. enterocolitica has not been reported, we wished to compare infections in neonates and adults. For this purpose, we infected 7-day-old and adult BALB/c and C57BL/6 mice, using the natural (o.g.) route of transmission. LD50 survival experiments with the high-virulence Y. enterocolitica strain A127/90 (biotype IB/serotype 0:8) were performed. Surprisingly, we found that neonatal mice were more resistant than adult mice to lethal o.g. Y. enterocolitica infection (Table 1). The differences in the average LD50 values between neonates and adults of the BALB/c and C57BL/6 strains were approximately 50- and 46-fold, respectively. Comparison of the geometric means between groups showed similar differences (38- and 61-fold for BALB/c and C57BL/6 mice, respectively) (Table 1). These results indicated that the patterns of resistance for both mouse strains were similar in that 7-day-old mice were highly resistant to o.g. infection compared to adult mice. This is clearly demonstrated in Fig. 1A and B, which show significant differences in the survival rates of neonates and adults of both mouse strains (BALB/c [P < 0.0001] and C57BL/6 [P = 0.0315]) infected with this isolate of Y. enterocolitica.
TABLE 1.
Average LD50 values after o.g. infection of BALB/c and C57BL/6 neonatal and adult mice with Y. enterocolitica A127/90a
| Mouse strain | Group | LD50 (CFU)
|
Geometric mean LD50c | Avg LD50 | ||
|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||||
| BALB/c | Neonates | 2.7 × 105 | 1.9 × 107b | 2.7 × 105 | 1.1 × 106 | 6.5 × 106 |
| Adults | 8.2 × 103 | 3.8 × 105b | 7.8 × 103 | 2.9 × 104 | 1.3 × 105 | |
| C57BL/6 | Neonates | 2.4 × 106 | 1.7 × 106 | 1.5 × 108b | 8.5 × 106 | 5.1 × 107 |
| Adults | 2.3 × 105 | 3.4 × 104 | 3.3 × 106b | 1.4 × 105 | 1.2 × 106 | |
Mice were infected o.g. with 10-fold dilutions of bacterial suspensions ranging from 2×103 to 2×108 CFU.
LD50 values were derived from infections performed in parallel for each mouse strain.
The geometric mean was derived by calculating the mean of the logarithms of the values and then calculating the antilog of the mean.
FIG. 1.
Survival curves for neonatal and adult BALB/c and C57BL/6 mice after o.g. infection with Y. enterocolitica A127/90 and Y. enterocolitica WA. (A) Seven-day-old (•) and 7- to 9-week-old (○) BALB/c mice were infected o.g. with 2 × 105 CFU Y. enterocolitica A127/90 as described in Materials and Methods. For each survival curve, data from 9 adult mice and 10 neonatal mice from two independent experiments are depicted. (B) Five C57BL/6 adults and seven neonates were infected o.g. in parallel with 2 × 106 CFU Y. enterocolitica A127/90. (C) Six neonates and five BALB/c adults were infected o.g. in parallel with 5 × 106 CFU Y. enterocolitica WA. Survival curves were generated by Kaplan-Meier survival analysis. The rates of death were compared using the Mantel-Haenszel log rank test. *, P = 0.0315; ***, P < 0.0001.
The Y. enterocolitica strain A127/90 is a clinical isolate which has been used by other researchers for multiple in vitro characterizations (17). However, this is the first time that the LD50 for the A127/90 strain has been reported in the literature. The LD50 value we observed for the A127/90 strain in adult mice was nearly a log lower than the values reported for other strains of Y. enterocolitica (25). Therefore, to ensure the universality of our findings, we performed an o.g. LD50 experiment using another high-virulence strain, Y. enterocolitica WA (biotype IB/serotype 0:8). The LD50 value we obtained for adult BALB/c mice with Y. enterocolitica WA (3 × 106 CFU) was similar to that previously reported in the literature (37). Furthermore, as found for the A127/90 strain, neonatal BALB/c mice were also more resistant to o.g. infection with the WA strain; the LD50 was still at least a log higher for neonates (5 × 107 CFU) than for adults (3 × 106 CFU). The proportions of neonates and adults surviving infection with the same dose of Y. enterocolitica WA were also significantly different (P = 0.0315) (Fig. 1C). Although the difference (17-fold) in o.g. LD50 values between BALB/c neonates and adults after Y. enterocolitica WA infection was not as high as that for Y. enterocolitica A127/90 (50-fold), this result extends and supports the conclusion that neonatal mice are highly resistant to o.g. Y. enterocolitica exposure.
Neonatal mice are very susceptible to ectopic (i.p.) infection with Y. enterocolitica.
The finding that neonatal mice showed increased survival after o.g. infection was surprising, since neonates are generally thought to be more susceptible to bacterial infections. However, in contrast to our studies, where we have used the natural route of transmission, many previous studies introduced bacteria ectopically (22, 23, 46). To determine if the resistance of neonates was restricted to the o.g. route of administration, survival experiments were carried out using Y. enterocolitica A127/90 injected i.p. into BALB/c mice. The results, shown in Table 2, revealed that neonates were at least one-half a log more susceptible to Y. enterocolitica by i.p. infection than were adult mice; the neonatal survival rate after i.p. injection was also significantly (P < 0.001) decreased compared to that for adults (Fig. 2). These findings indicated that in contrast to the case for o.g. infection, neonates could not efficiently control infection when bacteria were administered ectopically. Note that the difference in LD50 values between o.g. and i.p. infections was quantitatively greater for neonates (>200,000 times) than it was for adults (>700 times). These results suggest that Y. enterocolitica administered through the natural route triggers a local response in neonates that is highly protective, in contrast to what occurs after i.p. infection. Together, the results from these survival experiments demonstrate that 7-day-old mice are capable of efficiently controlling infection with this pathogen only if it is encountered through the natural, o.g. route of exposure.
TABLE 2.
Average LD50 values after i.p. infection of neonatal and adult BALB/c mice with Y. enterocolitica A127/90a
| Group | LD50 (CFU)
|
Avg LD50 | ||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| Neonates | 19 | 21 | 40 | 30 |
| Adults | 138 | 189 | 178 | 168 |
Mice were infected i.p. in parallel with 50 μl of Y. enterocolitica A127/90 suspensions ranging from 10 to 200 CFU.
FIG. 2.
Survival curves after i.p. infection of neonatal and adult BALB/c mice with Y. enterocolitica A127/90. Seven-day-old (•) and 7- to 9-week-old (○) BALB/c mice were infected i.p. with 50 CFU Y. enterocolitica. The curves depict the survival of 12 infected neonates and 9 infected adults. Each curve shows the pooled data from two independent experiments. Survival curves were compared by the log rank test. **, P < 0.001.
Following o.g. infection, Y. enterocolitica is largely confined to the intestinal tissues of neonates.
The enhanced resistance of neonates to Y. enterocolitica could potentially be due to developmental or physiological differences between neonates and adults. For example, an immature intestinal environment might preclude efficient colonization of the neonatal gut, leading to rapid clearance of the bacterium from the body. To investigate this possibility, kinetic studies of bacterial colonization of the intestines were performed. Initially, we examined colonization of the whole intestine in neonates and adults infected with the same dose (5 × 106 CFU) of Y. enterocolitica. This dose corresponds to approximately 0.8× LD50 for neonates and 40× LD50 for adults. The adult intestine harbored low but detectable levels of bacteria at 1 day p.i. (Fig. 3A). In contrast, high levels of Y. enterocolitica were detected in all of the neonatal mice 1 day after infection and persisted for at least the first 3 days p.i. By this time point, the mean titers for both groups were not significantly different, and at 5 days p.i., high titers were detected in 100% of adult mice, in contrast to 57% of neonatal mice. These data indicate that there is high-level colonization of the neonatal intestine immediately after inoculation and that the enhanced resistance of neonates is not caused by prompt elimination of the infectious inoculum.
FIG. 3.
Colonization kinetics following o.g. infection of neonates and adults with similar doses of Y. enterocolitica A127/90. (A) Seven-day-old (•) and adult (○) BALB/c mice were infected o.g. with 5 × 106 CFU Y. enterocolitica A127/90. On the days indicated, the whole intestine was removed, and bacterial titers were determined as described in Materials and Methods. The graph represents the pooled data from three experiments for a total of 5 to 10 neonatal or adult mice. (B to D) At 2, 6, and 7 days p.i., the MLN (B), spleens (C), and livers (D) from mice infected o.g. with 5 × 106 CFU were collected and processed as described in the text. Each graph depicts the pooled data from three experiments, with a total of 9 to 14 neonatal or adult mice per time point. For all graphs, each symbol is the average titer from duplicate plate counts for an individual mouse. The bold lines represent the mean for each group, and the dashed lines are at the limit of detection for each organ. The Mann-Whitney test was used for analyses comparing neonates and adults on the indicated days. *, P = 0.045; **, P = 0.0025; ***, P ≤ 0.0004.
Next, we wished to address whether the enhanced resistance of neonates after o.g. infection was due to failure of the bacteria to invade past the intestinal lumen to establish systemic infection. For this purpose, we compared the colonization kinetics of the MLN, spleen, and liver in neonates and adults during the first week after infection. Y. enterocolitica was detected as early as 2 days p.i. in the MLN of 36% of infected neonates, in contrast to 7% of infected adults (Fig. 3B). These relative titers were maintained in at least 38% of the neonatal mice analyzed 6 days after infection. By 7 days p.i., the pattern was reversed, with a greater proportion of adult mice (50%) having detectable Y. enterocolitica in the MLN, in contrast to 11% of neonates. In contrast, the relative colonization of the spleen and liver followed a completely different pattern. Viable bacteria were readily detected at 2 days p.i. in the spleens (Fig. 3C) and livers (Fig. 3D) of 43% and 28% of infected adult mice, respectively. At this time point, the bacterial titers were already high in the spleen. By 6 days p.i., at least 54% of the adult mice showed high titers in the spleen and liver. These titers increased significantly in both organs by 7 days p.i. (P ≤ 0.0048) (Fig. 3C and D). In striking contrast, Y. enterocolitica was detectable only in the spleen (Fig. 3C) and liver (Fig. 3D) of 1 of 36 infected neonates over the entire 7-day period of analysis. Note that the high bacterial levels in the adult spleen and liver correlate with their susceptibility to the lethal effects of the infection. Together, these findings show that Y. enterocolitica is able to efficiently colonize the intestinal tissues of adults and neonates but that further dissemination of bacteria beyond the gut is limited in neonates.
The observation that Y. enterocolitica is largely retained in the guts of neonates offers a potential explanation for their high-level resistance, since containment of the bacteria in the intestine or MLN could potentially spare vital organs from the harmful effects of an inflammatory response. However, this result also raised the possibility that the lack of detectable bacteria in the spleen was due to developmental immaturity in the neonate that prevented systemic colonization. Therefore, we wished to determine whether it was possible to colonize the neonatal spleen and liver with Y. enterocolitica under any conditions of infection. Initially, we addressed this question by increasing the infectious dose given o.g. to neonates. As shown in Fig. 4A, once the titer of bacteria was increased to 10× LD50 for neonates (5 × 107 CFU), colonization of the MLN was evident at 4 days p.i., when 80% of infected neonates had substantial bacterial titers in the MLN. The bacterial levels in the neonatal MLN increased significantly (P = 0.0046), by 2 log, 3 days later and persisted at high numbers as late as 9 days p.i. in 100% of analyzed mice. In contrast, viable Y. enterocolitica was not detectable in the neonatal spleen at 4 days p.i., even in the presence of high bacterial titers in the MLN (Fig. 4B). Only one neonate had detectable bacteria in the liver at this time point. By 7 days p.i., it was possible to recover Y. enterocolitica from the spleens and livers of 28.6% and 57% of infected neonates, respectively. However, the average levels of bacteria in these tissues were reduced over 4 log compared with the titers found in the MLN at the same time point. By 9 days p.i., greater percentages of mice had higher but comparable bacterial levels in the spleen (75%) and liver (100%). Interestingly, the mean Y. enterocolitica level found in the liver at 9 days p.i. was at least 1 log lower than that detected in the adult liver 7 days after infection with a comparable dose (Fig. 3D). Strikingly, the mean bacterial level detected in the neonatal spleen (4.6 log) at 9 days p.i. was at least 3 log lower than that found in the adult spleen (8.0 log) after 7 days of infection (Fig. 3C). This may indicate that despite translocation of the bacteria to peripheral tissues, neonates carry lower titers than infected adult mice.
FIG. 4.
Pattern of Y. enterocolitica colonization beyond the neonatal gut after o.g. infection with a high dose or when introduced ectopically. Seven-day-old BALB/c mice were infected o.g. with 10× LD50 for neonates (5 × 107 CFU Y. enterocolitica A127/90). MLN (A), spleens (B) (▵), and livers (B) (▴) were collected from infected neonatal mice at 4, 7, and 9 days p.i. and processed as described previously. (C) Neonatal BALB/c mice were infected i.p. with 150 CFU Y. enterocolitica A127/90. The bacterial levels in the spleen (▵) and liver (▴) were measured at 7 days p.i. in two independent experiments. For all graphs, each symbol represents the titer from an individual mouse. The solid lines show the means, and the dashed lines are at the limit of detection for each organ. *, P = 0.0289 for spleens on day 9 compared to spleens on day 7; **, P = 0.0046 for MLN on day 7 compared to MLN on day 4.
To demonstrate independently the ability of Y. enterocolitica to spread to deeper tissues following infection, colonization experiments were done using another route of infection. When neonatal mice were infected i.p. with 5× LD50 (150 CFU), we observed a substantial colonization of the spleen and liver at 7 days p.i. in 86% of mice analyzed (Fig. 4C). Collectively, these data indicate that Y. enterocolitica is efficiently confined to the intestinal tissues upon o.g. exposure and when the infective doses are sublethal. However, some systemic spread can be observed when neonates are exposed to lethal bacterial doses o.g. or when the bacteria are introduced ectopically.
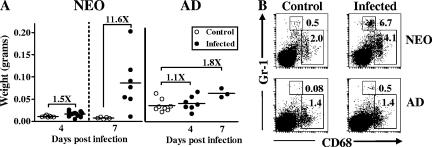
Following o.g. infection in neonates, there is a marked influx of innate phagocytes into the neonatal MLN.
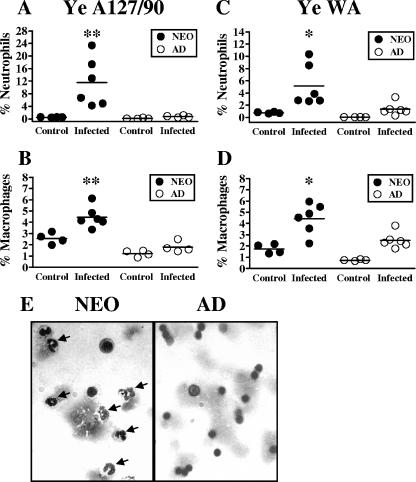
The observation that the majority of Y. enterocolitica organisms are contained in the intestine and MLN and do not reach peripheral tissues is consistent with the idea that the infection may be controlled by regional innate immune responses in the neonatal gut. A substantial difference between neonates and adults infected with a lethal dose is in the extent of gross tissue changes that occur in the MLN. Indeed, we observed that the neonatal MLN increase dramatically in size and weight (>2- to 11-fold increase in weight compared with those of age-matched uninfected controls) by 7 days after o.g. infection with 5 × 107 CFU Y. enterocolitica (Fig. 5A). Adults infected with the same dose showed little to no change in MLN weight (≥1.8-fold increase) (Fig. 5A). Based on the pattern of bacterial colonization and the changes in organ size, we hypothesized that resistance in neonates may be attributed to enhanced recruitment of innate phagocytes that function to limit the spread of the bacteria beyond the neonatal gut. To test this idea, we analyzed the influx of neutrophils and macrophages, two innate phagocytes which have been shown to be important in controlling Y. enterocolitica infection in adult mice (5, 15). Flow cytometric analysis of individual MLN of neonates and adult mice infected o.g. with 5 × 107 CFU Y. enterocolitica A127/90 was conducted. As previously described by others (31, 41), neutrophils were identified as Gr-1hi CD68int cells, and macrophages were identified as Gr-1lo-int CD68hi cells (Fig. 5B). There was an increase in the percentages of both neutrophils (Fig. 6A) and macrophages (Fig. 6B) in the MLN of most infected neonates analyzed as early as 3 days p.i. compared with age-matched uninfected controls. However, there were no significant changes in the percentages of neutrophils (Fig. 6A) and macrophages (Fig. 6B) in the adult MLN analyzed at 3 (Fig. 6) and 5 days p.i. (data not shown). We observed significant differences (P = 0.0095) in the percentages of neutrophils (16-fold) and macrophages (2.5-fold) in the MLN of infected neonates compared to those in the MLN of infected adults. The limited phagocyte infiltration in the adult MLN could not be accounted for solely by the absence of Y. enterocolitica in this organ because there were similar mean titers (mean ± standard deviation) at 4 days p.i. in the MLN of neonates (6.1 ± 2.1) and adults (7.7 ± 0.3) infected with this dose. In addition, the low percentages of neutrophils and macrophages in the adult MLN could not be explained as a failure to detect these cell populations because staining of PP and spleen cells from the same mice revealed increases in both populations as early as 3 days p.i. (data not shown), which is consistent with previous reports (5, 25). These results indicated that even at very high doses of bacteria (Fig. 4A), inflammatory infiltration into the adult MLN was decreased compared to that for neonates. To determine that these results were not limited to the A127/90 strain, we then measured neutrophils and macrophages infiltrating the neonatal MLN after infection with 5 × 108 CFU of Y. enterocolitica strain WA. Similar to what was observed with a high dose of Y. enterocolitica A127/90, we detected significantly greater percentages of neutrophils (3.8-fold; P = 0.0152) (Fig. 6C) and macrophages (1.8-fold; P = 0.0411) (Fig. 6D) in infected neonates than in infected adult mice. To confirm that the large influx observed in neonates by flow cytometry was indeed composed mostly of neutrophils, Wright-Giemsa staining of MLN cells from the same mice was performed (Fig. 6E). Using this method, percentages of neutrophils comparable to those observed by flow cytometry were found for the MLN of uninfected and infected neonatal and adult mice (Table 3). The differences in percentages of neutrophils in the neonatal MLN compared to adult mice infected with Y. enterocolitica A127/90 and WA were 17- and 10-fold, respectively. Altogether, these results indicate that neonatal mice have a proportionally greater neutrophil infiltration than do adult mice infected with two different high-virulence Y. enterocolitica strains.
FIG. 5.
Distinct anatomical and cellular changes in the MLN of neonates infected with high titers of Y. enterocolitica A127/90. Seven-day-old and adult BALB/c mice were infected o.g. with 5 × 107 CFU Y. enterocolitica A127/90. (A) MLN from neonates and adults were harvested on different days p.i. and weighed. Controls are age-matched uninfected mice. Data were pooled from three or four independent infections. (B) Individual MLN from neonates and adults were harvested at 3 days p.i., and cell suspensions were stained with anti-Gr-1 and anti-CD68 antibodies. Age-matched uninfected mice served as controls. Fluorescent staining profiles for representative mice are shown. The top box is the neutrophil gate (Gr-1hi CD68int) and the bottom box is the macrophage gate (Gr-1lo-int CD68hi), with their respective percentages indicated.
FIG. 6.
Flow cytometric and histological analyses of innate immune cell infiltration into the MLN of neonates infected with different Y. enterocolitica strains. Seven-day-old and adult BALB/c mice were infected o.g. in parallel with 5 × 107 CFU Y. enterocolitica A127/90 (A, B, and E) or 5 × 108 CFU Y. enterocolitica WA (C and D). Individual MLN from neonates and adults were harvested 3 days p.i., and cell suspensions were stained with anti-Gr-1 and anti-CD68 antibodies and analyzed as described in the legend to Fig. 5. The percentages of Gr-1hi CD68int cells (neutrophils) are shown in panels A and C, and the percentages of Gr-1lo-int CD68hi cells (macrophages) are shown in panels B and D. The graphs depict the pooled data from two independent experiments for a total of four control and four to six infected mice. Age-matched uninfected mice served as controls. Each symbol represents an individual mouse, with the horizontal lines showing the mean for each group. (E) Individual MLN suspensions from neonates (left) and adults (right) were analyzed by Wright-Giemsa staining. Arrows point to neutrophils in a representative field. Total magnification, ×600. *, P ≤ 0.041; **, P = 0.0095 between infected neonates and infected adults.
TABLE 3.
Histological analysis of neutrophils in the MLN of infected mice
| Group | Neutrophils in MLN infected with Y. enterocolitica strain
|
|||
|---|---|---|---|---|
| A127/90
|
WA
|
|||
| Mean (%)a | Range (%) | Mean (%)a | Range (%) | |
| Uninfected neonates | 0.97 | 0.22-2.02 | 0.92 | 0.76-1.14 |
| Infected neonates | 14.74b,c | 0.97-36.53 | 10.02b,c | 1.04-19.36 |
| Uninfected adults | 0.08 | 0.00-0.24 | 0.02 | 0.00-0.09 |
| Infected adults | 0.87d | 0.15-1.42 | 1.00d | 0.64-2.27 |
Percentage of neutrophils analyzed by Wright-Giemsa staining 3 days after infection with 5×107 CFU Y. enterocolitica A127/90 or 5×108 CFU Y. enterocolitica WA. Three or four uninfected and four to six infected mice were analyzed per group.
P ≤ 0.024 compared to uninfected neonates by Mann-Whitney test.
P ≤ 0.004 compared to infected adults by Mann-Whitney test.
P ≤ 0.017 compared to uninfected adults by Mann-Whitney test.
Neutrophil depletion leads to a greater proportion of neonates with high bacterial titers in the spleen and liver.
Since neutrophils are highly phagocytic, the increased neutrophil infiltration in the neonatal MLN suggested that this cell population in particular may contribute to the resistance of neonates after o.g. infection. In this case, neutrophils would be important in controlling bacterial replication in neonatal intestinal tissues. To address this possibility, we analyzed the impact of neutrophil depletion prior to infection with a high bacterial dose. We used the monoclonal antibody RBC-8C5 (anti-Gr-1), which selectively targets neutrophils and has previously been used successfully in neonatal mice (21). To verify that neutrophils were depleted by this antibody under our infection conditions, flow cytometric analysis of cells from infected mice determined that Gr-1hi CD68int cells were reduced to <0.02% of cells in the neonatal MLN and spleen after injection with the anti-Gr-1 antibody (data not shown). Therefore, we were confident that the antibody treatment effectively reduced the neutrophil population. Neonatal mice were treated with anti-Gr-1 antibody 1 day prior to and 1 day following infection with 10× LD50, and their tissues were analyzed 8 days p.i. for bacterial colonization. The bacterial titers found in the intestines and MLN did not differ between groups, indicating that all mice were productively infected (data not shown). However, the anti-Gr-1 antibody treatment increased the proportion of neonatal mice with detectable Y. enterocolitica to 100% for the spleen and liver, in contrast to 43% and 71% for the control group (Fig. 7). The mean bacterial titers in the neutrophil-depleted neonates for both organs were at least 2 log higher than those found in the control mice, although statistical significance (P = 0.038) was only evident for the spleen. These results suggest that in the absence of neutrophils, the bacteria are able to replicate and disseminate into peripheral tissues at greater rates, resembling the colonization pattern observed in adults. These observations indicate that neonatal neutrophils may contribute substantially to the increased resistance observed in neonates. The combined results from these experiments support our main hypothesis that the innate immune system of neonates rapidly mobilizes phagocytes to the gut and that these phagocytes efficiently protect against Y. enterocolitica introduced through the natural route of infection.
FIG. 7.
Bacterial colonization of the neonatal spleen and liver following neutrophil depletion. Neonatal BALB/c mice were injected i.p. with either control IgG (▵) or RBC-8C5 (anti-Gr-1) antibody (▴) on days −1 and +1 in reference to o.g. infection with 5 × 107 CFU Y. enterocolitica A127/90. The spleens and livers from all neonates were harvested at 8 days p.i., and bacterial counts were measured as described previously. Each symbol represents an individual mouse, with the lines showing the means from two independent experiments (n = 7). The dashed line is at the limit of detection. The number above each group is the percentage of mice with colonized organs. *, P = 0.038 between control IgG- and anti-Gr-1-treated neonates.
DISCUSSION
The studies presented here were initiated in an effort to identify the pattern of immune responsiveness in the developing gut to the food-borne enteropathogen Y. enterocolitica. Remarkably, we found that 7-day-old mice infected o.g. with the highly pathogenic Y. enterocolitica A127/90 strain (17, 28) show enhanced survival compared to that of infected adult mice. This was a very surprising finding because for a number of other murine models of infection, including Salmonella enterica serovar Typhimurium and Listeria monocytogenes infection, neonates have been reported to be more sensitive to the pathogenic effects of bacterial agents administered both through the natural route of exposure and parenterally (12, 16, 21, 22, 45). It is remarkable that neonates, despite their smaller size and lower immune cell numbers (3, 18), were able to control o.g. Y. enterocolitica infection better than adult mice. In fact, normalization of the LD50 values suggests that, on the basis of body size, neonatal mice may be at least 200 times more resistant to infection than adults. To our knowledge, this is the first report in the literature of an experimental model of infection where neonatal mice have improved survival over adult mice in the absence of exogenous treatment with immunomodulatory agents. These striking results raise the possibility that Y. enterocolitica may elicit distinct responses in neonates that lead to highly efficient mucosal immune antimicrobial function. We proposed that the increased survival of neonates and containment of Y. enterocolitica in the gut tissues would be due to a quantitative difference in the recruitment of innate phagocytes. This idea was supported by flow cytometric and histological analyses. Neonatal mice infected with doses above the LD50 showed significantly increased levels of neutrophil and macrophage infiltration in the MLN early after infection. However, increased infiltration was only apparent in the PP and spleens of infected adults and was minimal in their MLN. Importantly, depletion of neutrophils increased the rates of colonization of peripheral tissues in infected neonates. Thus, the improved survival of o.g. infected neonates may be partially attributable to innate phagocytes actively containing the infection to the neonatal intestinal tissues.
In contrast to our results using the o.g. route, neonatal mice were highly susceptible to infection after exposure by a peripheral route. We propose that the ability to mount a protective inflammatory response in neonates may be dependent on the route of exposure. Since it is recognized that the majority of infectious agents are encountered through the mucosal surfaces of the gastrointestinal and upper respiratory tracts, it is plausible that the neonatal innate immune system may have an important role in preventing the replication or spread of pathogens entering through mucosal surfaces. These responses, however, may be suboptimal when the pathogens bypass the natural defenses elicited by the mucosa. In this regard, Lotz et al. demonstrated that intestinal epithelial cells from 6-day-old mice spontaneously produce the chemokine macrophage inflammatory protein 2 (36), which is thought to recruit neutrophils and macrophages to sites of inflammation (14). In contrast, it has been shown for newborn rats infected i.p. with group B streptococci that there is a delayed recruitment of innate phagocytes to the site of bacterial exposure compared to that in adult mice (46). However, similar to our results for the gut, Garvy and Harmsen (21) showed that there is an early and substantial influx of neutrophils into the lungs after intranasal inoculation of 1-day-old neonatal mice with Streptococcus pneumoniae. This information suggests that neonates may be fully equipped to promote inflammatory responses when infectious agents are encountered through natural routes of infection. With this model, we expand previous reports to include natural o.g. infection of murine neonates with Y. enterocolitica as another example of potential antigenic conditions that may trigger highly protective responses (4, 8, 13, 29, 35, 38, 49). Together, our data suggest that neonatal mice may be better prepared to respond to o.g. Y. enterocolitica infection by means of a very plastic and efficient intestinal innate immune response.
The neonatal infection model we have developed will be valuable for expanding our knowledge of the immunological development of the gut. In addition, we propose that this model will be extremely valuable for understanding pediatric yersiniosis, since it shares several striking characteristics with pediatric disease pathology. First, despite the enhanced survival of neonates from Y. enterocolitica lethality, infection with high doses of Y. enterocolitica led to a pronounced inflammatory response in the neonatal MLN, as measured by high bacterial titers, infiltration of innate phagocytes, and a remarkable increase in the net MLN weight. This last physical characteristic shares similarities with Y. enterocolitica infection in children and young adults, where this pathogen usually causes mesenteric lymphadenitis (10). This suggests that the disease pattern observed in o.g. infected neonatal mice targets the tissues usually affected during human infections and may reflect disease pathology that is not observable in adult mice. Second, similar to infected children, neonatal mice infected with lethal doses of Y. enterocolitica develop diarrhea, as indicated by the absence of formed stools in the large intestine (30), which we did not observe in infected adult mice (unpublished observations). Last, the incidence of bacteremia and systemic infection in young children is very low (40), as we have seen with neonatal mice. The similarity of these clinical signs in mice and humans leads to the suggestion that we have developed a model system that closely resembles Y. enterocolitica infection as it occurs in the human pediatric population.
Our results contrast with those reported for neonatal models of S. enterica serovar Typhimurium infection, where neonates and young mice are more susceptible to o.g. infection (12, 45). One major difference in experimental design is that unlike in our experiments, the neonates used for the S. enterica serovar Typhimurium experiments were treated with antibiotics to eliminate the intestinal commensal flora (45). In these mice, the intestinal immune system may have been relatively underdeveloped compared with that of our neonatal mice. In addition, the modes of delivery of the bacteria are also different, because in our model the bacteria are introduced into the stomach, while in the Salmonella model the bacteria are deposited into the mouth (12). The difference in susceptibility between neonates infected with Y. enterocolitica and those infected with S. enterica serovar Typhimurium may also be related to the environment in which each particular bacterial species prefers to replicate. It is thought that Y. enterocolitica can survive extracellularly in lymphoid tissues (24), while S. enterica serovar Typhimurium is, for the most part, an intracellular pathogen. It is possible that the relative susceptibilities of neonates to these pathogens may be a matter of the type of organism encountered.
Lastly, it is important to consider the potential effects of immune factors transferred from the dams to the infected neonates. The transfer of protective antibodies transplacentally or through the milk is thought to reduce the susceptibility of neonates to gastrointestinal disease (11). However, in our experiments, the nursing dams were not previously exposed to any enteric bacterial pathogens. Therefore, it would be unlikely that Y. enterocolitica-reactive antibodies could be transferred transplacentally or in the milk to the nursing neonates and thereby protect them from infection. In addition, the high bacterial titers found in the intestine (Fig. 3A) and MLN (Fig. 4A) during the course of infection would also suggest that transferred innate immune factors do not have strict bactericidal properties. Therefore, the enhanced survival of neonatal mice may reflect an endogenous immune response rather than the antibacterial function of transferred maternal immune factors.
Acknowledgments
We thank Robert F. Ramig for guidance with the o.g. intubation of neonates, Guy R. Cornelis and Gregory Plano for providing bacterial strains, Bonnie Blomberg for helpful suggestions, Kenneth Fields for an insightful review of the manuscript, and Patricia Guevara for technical assistance and animal care.
This work was supported by NIH grant R01 AI44923-02 from the National Institute of Allergy and Infectious Diseases (B.A.), by Public Health Service grant AI53459 from the NIAID (K.S.), and by the Department of Microbiology and Immunology, University of Miami Miller School of Medicine, Miami, FL.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Abdel-Haq, N. M., B. I. Asmar, W. M. Abuhammour, and W. J. Brown. 2000. Yersinia enterocolitica infection in children. J. Pediatr. Infect. Dis. 19:954-958. [DOI] [PubMed] [Google Scholar]
- 2.Adkins, B., Y. Bu, V. Vincek, and P. Guevara. 2003. The primary responses of murine neonatal lymph node CD4+ cells are Th2-skewed and are sufficient for the development of Th2-biased memory. Clin. Dev. Immunol. 10:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins, B., C. Leclerc, and S. Marshall-Clarke. 2004. Neonatal adaptive immunity comes of age. Nat. Rev. Immunol. 4:553-564. [DOI] [PubMed] [Google Scholar]
- 4.Ascon, M. A., D. M. Hone, N. Walters, and D. W. Pascual. 1998. Oral immunization with a Salmonella typhimurium vaccine vector expressing recombinant enterotoxigenic Escherichia coli K99 fimbriae elicits elevated antibody titers for protective immunity. Infect. Immun. 66:5470-5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autenrieth, I. B., V. Kempf, T. Sprinz, S. Preger, and A. Schnell. 1996. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Autenrieth, I. B., U. Vogel, S. Preger, B. Heymer, and J. Heesemann. 1993. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect. Immun. 61:2585-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrier, M., S. Lacroix-Lamande, R. Mancassola, G. Auray, N. Bernardet, A. M. Chausse, S. Uematsu, S. Akira, and F. Laurent. 2006. Oral and intraperitoneal administration of phosphorothioate oligodeoxynucleotides leads to control of Cryptosporidium parvum infection in neonatal mice. J. Infect. Dis. 193:1400-1407. [DOI] [PubMed] [Google Scholar]
- 8.Bjarnarson, S. P., H. Jakobsen, G. Del Giudice, E. Trannoy, C. A. Siegrist, and I. Jonsdottir. 2005. The advantage of mucosal immunization for polysaccharide-specific memory responses in early life. Eur. J. Immunol. 35:1037-1045. [DOI] [PubMed] [Google Scholar]
- 9.Black, R. E., R. J. Jackson, T. Tsai, M. Medvesky, M. Shayegani, J. C. Feeley, K. I. MacLeod, and A. M. Wakelee. 1978. Epidemic Yersinia enterocolitica infection due to contaminated chocolate milk. N. Engl. J. Med. 298:76-79. [DOI] [PubMed] [Google Scholar]
- 10.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandtzaeg, P. 2003. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine 21:3382-3388. [DOI] [PubMed] [Google Scholar]
- 12.Burns-Guydish, S. M., I. N. Olomu, H. Zhao, R. J. Wong, D. K. Stevenson, and C. H. Contag. 2005. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr. Res. 58:153-158. [DOI] [PubMed] [Google Scholar]
- 13.Capozzo, A. V., L. Cuberos, M. M. Levine, and M. F. Pasetti. 2004. Mucosally delivered Salmonella live vector vaccines elicit potent immune responses against a foreign antigen in neonatal mice born to naive and immune mothers. Infect. Immun. 72:4637-4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chensue, S. W. 2001. Molecular machinations: chemokine signals in host-pathogen interactions. Clin. Microbiol. Rev. 14:821-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlan, J. W. 1997. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect. Immun. 65:630-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cusumano, V., G. Mancuso, F. Genovese, M. Cuzzola, M. Carbone, J. A. Cook, J. B. Cochran, and G. Teti. 1997. Neonatal hypersusceptibility to endotoxin correlates with increased tumor necrosis factor production in mice. J. Infect. Dis. 176:168-176. [DOI] [PubMed] [Google Scholar]
- 17.Denecker, G., S. Totemeyer, L. J. Mota, P. Troisfontaines, I. Lambermont, C. Youta, I. Stainier, M. Ackermann, and G. R. Cornelis. 2002. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 70:3510-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fadel, S., and M. Sarzotti. 2000. Cellular immune responses in neonates. Int. Rev. Immunol. 19:173-193. [DOI] [PubMed] [Google Scholar]
- 19.Fotopoulos, S., A. Mouchtouri, G. Xanthou, N. Lipsou, E. Petrakou, and M. Xanthou. 2005. Inflammatory chemokine expression in the peripheral blood of neonates with perinatal asphyxia and perinatal or nosocomial infections. Acta Paediatr. 94:800-806. [DOI] [PubMed] [Google Scholar]
- 20.Garcia, A. M., S. A. Fadel, S. Cao, and M. Sarzotti. 2000. T cell immunity in neonates. Immunol. Res. 22:177-190. [DOI] [PubMed] [Google Scholar]
- 21.Garvy, B. A., and A. G. Harmsen. 1996. The importance of neutrophils in resistance to pneumococcal pneumonia in adult and neonatal mice. Inflammation 20:499-512. [DOI] [PubMed] [Google Scholar]
- 22.Genovese, F., G. Mancuso, M. Cuzzola, C. Biondo, C. Beninati, D. Delfino, and G. Teti. 1999. Role of IL-10 in a neonatal mouse listeriosis model. J. Immunol. 163:2777-2782. [PubMed] [Google Scholar]
- 23.Gonzalez, M. D., C. A. Lichtensteiger, and E. R. Vimr. 2001. Adaptation of signature-tagged mutagenesis to Escherichia coli K1 and the infant-rat model of invasive disease. FEMS Microbiol. Lett. 198:125-128. [DOI] [PubMed] [Google Scholar]
- 24.Grassl, G. A., E. Bohn, Y. Muller, O. T. Buhler, and I. B. Autenrieth. 2003. Interaction of Yersinia enterocolitica with epithelial cells: invasin beyond invasion. Int. J. Med. Microbiol. 293:41-54. [DOI] [PubMed] [Google Scholar]
- 25.Handley, S. A., P. H. Dube, P. A. Revell, and V. L. Miller. 2004. Characterization of oral Yersinia enterocolitica infection in three different strains of inbred mice. Infect. Immun. 72:1645-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Head, C. B., D. A. Whitty, and S. Ratnam. 1982. Comparative study of selective media for recovery of Yersinia enterocolitica. J. Clin. Microbiol. 16:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt, P. G., and C. A. Jones. 2000. The development of the immune system during pregnancy and early life. Allergy 55:688-697. [DOI] [PubMed] [Google Scholar]
- 28.Ichinohe, H., M. Yoshioka, H. Fukushima, S. Kaneko, and T. Maruyama. 1991. First isolation of Yersinia enterocolitica serotype O:8 in Japan. J. Clin. Microbiol. 29:846-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito, S., K. J. Ishii, M. Gursel, H. Shirotra, A. Ihata, and D. M. Klinman. 2005. CpG oligodeoxynucleotides enhance neonatal resistance to Listeria infection. J. Immunol. 174:777-782. [DOI] [PubMed] [Google Scholar]
- 30.Jones, R. G., X. Li, P. D. Gray, J. Kuang, F. Clayton, W. S. Samowitz, B. B. Madison, D. L. Gumucio, and S. K. Kuwada. 2006. Conditional deletion of β-1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J. Cell Biol. 175:505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby, A. C., U. Yrlid, and M. J. Wick. 2002. The innate immune response differs in primary and secondary Salmonella infection. J. Immunol. 169:4450-4459. [DOI] [PubMed] [Google Scholar]
- 32.Koenig, J. M., and M. C. Yoder. 2004. Neonatal neutrophils: the good, the bad, and the ugly. Clin. Perinatol. 31:39-51. [DOI] [PubMed] [Google Scholar]
- 33.Lee, L. A., J. Taylor, G. P. Carter, B. Quinn, J. J. Farmer III, and R. V. Tauxe. 1991. Yersinia enterocolitica O:3: an emerging cause of pediatric gastroenteritis in the United States. J. Infect. Dis. 163:660-663. [DOI] [PubMed] [Google Scholar]
- 34.Levy, O., E. E. Suter, R. L. Miller, and M. R. Wessels. 2006. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 108:1284-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levy, O., K. A. Zarember, R. M. Roy, C. Cywes, P. J. Godowski, and M. R. Wessels. 2004. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 173:4627-4634. [DOI] [PubMed] [Google Scholar]
- 36.Lotz, M., D. Gutle, S. Walther, S. Menard, C. Bogdan, and M. W. Hornef. 2006. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J. Exp. Med. 203:973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mamroud, E., Y. Flashner, A. Tidhar, R. Ber, D. Gur, M. Aftalion, S. Lazar, B. Velan, A. Shafferman, and S. Cohen. 2003. Evaluation of protective immunity induced by Yersinia enterocolitica type-III secretion system mutants. Adv. Exp. Med. Biol. 529:425-430. [DOI] [PubMed] [Google Scholar]
- 38.Marchant, A., T. Goetghebuer, M. O. Ota, I. Wolfe, S. J. Ceesay, D. De Groote, T. Corrah, S. Bennett, J. Wheeler, K. Huygen, P. Aaby, K. P. McAdam, and M. J. Newport. 1999. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J. Immunol. 163:2249-2255. [PubMed] [Google Scholar]
- 39.Marodi, L. 2006. Neonatal innate immunity to infectious agents. Infect. Immun. 74:1999-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metchock, B., D. R. Lonsway, G. P. Carter, L. A. Lee, and J. E. McGowan, Jr. 1991. Yersinia enterocolitica: a frequent seasonal stool isolate from children at an urban hospital in the southeast United States. J. Clin. Microbiol. 29:2868-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mordue, D. G., and L. D. Sibley. 2003. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. J. Leukoc. Biol. 74:1015-1025. [DOI] [PubMed] [Google Scholar]
- 42.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naqvi, S. H., E. M. Swierkosz, J. Gerard, and J. R. Mills. 1993. Presentation of Yersinia enterocolitica enteritis in children. Pediatr. Infect. Dis. J. 12:386-389. [DOI] [PubMed] [Google Scholar]
- 44.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 45.Rhee, S. J., W. A. Walker, and B. J. Cherayil. 2005. Developmentally regulated intestinal expression of IFN-gamma and its target genes and the age-specific response to enteric Salmonella infection. J. Immunol. 175:1127-1136. [DOI] [PubMed] [Google Scholar]
- 46.Schuit, K. E., and R. DeBiasio. 1980. Kinetics of phagocyte response to group B streptococcal infections in newborn rats. Infect. Immun. 28:319-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siegrist, C. A. 2001. Neonatal and early life vaccinology. Vaccine 19:3331-3346. [DOI] [PubMed] [Google Scholar]
- 48.Sun, C.-M., L. Fiette, M. Tanguy, C. Leclerc, and R. Lo-Man. 2003. Ontogeny and innate properties of neonatal dendritic cells. Blood 102:585-591. [DOI] [PubMed] [Google Scholar]
- 49.Yasui, H., J. Kiyoshima, and T. Hori. 2004. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin. Diagn. Lab. Immunol. 11:675-679. [DOI] [PMC free article] [PubMed] [Google Scholar]