Abstract
Colonization factor CS6 expressed by enterotoxigenic Escherichia coli (ETEC) is a nonfimbrial polymeric protein. A substantial proportion of ETEC strains isolated from patients in endemic settings and in people who travel to regions where ETEC is endemic are ETEC strains expressing CS6, either alone or in combination with fimbrial colonization factor CS5 or CS4. However, relatively little is known about the natural immune responses elicited against CS6 expressed by ETEC strains causing disease. We studied patients who were hospitalized with diarrhea (n = 46) caused by CS6-expressing ETEC (ETEC expressing CS6 or CS5 plus CS6) and had a disease spectrum ranging from severe dehydration (27%) to moderate or mild dehydration (73%). Using recombinant CS6 antigen, we found that more than 90% of the patients had mucosal immune responses to CS6 expressed as immunoglobulin (IgA) antibody-secreting cells (ASC) or antibody in lymphocyte supernatant (ALS) and that about 57% responded with CS6-specific IgA antibodies in feces. More than 80% of the patients showed IgA seroconversion to CS6. Significant increases in the levels of anti-CS6 antibodies of the IgG isotype were also observed in assays for ASC (75%), ALS (100%), and serum (70%). These studies demonstrated that patients hospitalized with the noninvasive enteric pathogen CS6-expressing ETEC responded with both mucosal and systemic antibodies against CS6. Studies are needed to determine if the anti-CS6 responses protect against reinfection and if protective levels of CS6 immunity are induced by vaccination.
Enterotoxigenic Escherichia coli (ETEC) strains are noninvasive enteropathogens which colonize the small intestine by means of colonization factors (CFs) and are the single most common cause of bacterial diarrhea in children in developing countries, as well as in adults, including travelers (24, 41). After colonization, the bacteria cause watery diarrhea due to production of heat-stable toxin (ST) (6) and/or heat-labile enterotoxin (LT) (30). CFs are antigens that are known to provide protection against infection with ETEC expressing homologous CFs (7, 15, 32, 34). Although ETEC can express more than 25 different CFs, 7 of these CFs are more prevalent than others (7). CS6 has been shown to promote binding of ETEC to rabbit and human enterocytes but not to cultured intestinal cells and other human-derived tissue (11). It is one of the most common CFs, and in some studies CS6 has been found to be present on more than 30% of the strains isolated from patients with ETEC diarrhea, as well as from military and civilian travelers to countries where ETEC is endemic (24, 28, 29, 41, 42). This nonfimbrial surface antigen is present either alone or in association with CS4 or CS5 on ETEC strains producing either ST or both enterotoxin types (7, 24, 42). Based on the high prevalence of CS6-positive ETEC, CS6 has been examined as a vaccine antigen and has been administered by different immunization routes, including the oral (5, 14, 32, 36), transcutaneous (9, 43), and intranasal routes (3, 4). These vaccines have been based on oral live attenuated strains expressing CS6 (36, 37) or recombinant CS6 antigen, and different routes and formulations have been used for immunization (3, 43). However, there is a need to better determine if disease caused by CS6-positive ETEC results in immune responses against CS6, to quantify the responses, and to evaluate if such responses protect against reinfection, since detailed studies have not been carried out with such patients (12). Such information is important for understanding the design and requirements for a vaccine against CS6-expressing ETEC. The aim of the present study was to determine the mucosal and systemic immune responses in patients hospitalized with ETEC expressing CS6 antigen alone or in combination with CS5 antigen in a setting in Bangladesh where ETEC is endemic.
MATERIALS AND METHODS
Study group.
Patients with acute watery diarrhea presenting to the hospital of the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B) between April 2003 and October 2005 were screened for ETEC and for Vibrio cholerae (19, 21). Forty-six of the patients with diarrhea caused by ETEC expressing CS6 or CS5 plus CS6 were recruited from the 324 patients who were ETEC positive. The degree of dehydration of the patients (mild to severe) was assessed by a physician using the Denver system (39). Ten adults (ages, 18 to 50 years) and 10 children (ages, 1 to 5 years) whose ages and socioeconomic backgrounds were similar to those of the patients and who had no history of diarrhea during the previous 3 months were included as Bangladeshi control subjects. Informed consent was obtained from the patients and controls; in the case of children, approval was obtained from the parents or guardians. The study was approved by the Institutional Review Board of ICDDR,B.
Bacteriological analysis of fecal samples.
Stool specimens from patients suffering from watery diarrhea were cultured on MacConkey agar and CFA agar with bile salts (21). Bacterial colonies on CFA agar were assayed for the presence of CFs by dot blot assays using monoclonal antibodies specific for CS5 and CS6 antigens (10). Production of LT by E. coli colonies isolated on MacConkey agar was determined by a previously described enzyme-linked immunosorbent assay (ELISA) procedure (21, 35). For detection of ST, an inhibition ELISA with an ST-cholera toxin B subunit conjugate was used (35) All stool samples were also cultured to detect other enteric pathogens, including V. cholerae, Salmonella, Shigella, and Campylobacter spp. (40), and were examined by direct microscopy to detect cyst and vegetative forms of parasites and ova of helminths. Stools of healthy controls were screened similarly.
Sample collection.
After rehydration, venous blood (10 ml from adults and 5 ml from children) was collected from the patients at the acute stage of the disease, i.e., on the second day of hospitalization, which was considered to be approximately 2 days after the onset of diarrhea (day 2), and then at different times after the onset (days 7 and 21). Blood and stool samples were collected once from the controls.
Peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation on Ficoll-Isopaque (Pharmacia, Uppsala, Sweden) from heparinized venous blood from adults on only study days 2 and 7 since specific antibody responses by antibody-secreting cells (ASC) and antibody in lymphocyte supernatant (ALS) can be detected only within 7 days of onset (1, 2, 13, 23). Serum separated from blood was stored in aliquots at −20°C until ELISA were performed.
CF-specific ASC.
PBMC from 12 adult patients were tested by the two-color enzyme-linked immunospot technique for ASC responses (13, 26, 38). Briefly, individual wells of 96-well plates with nitrocellulose bottoms (Millititer HA; Millipore Corp., Bedford, MA) were coated with 1 μg/well (100 μl) of recombinant CS6 (43) and incubated at 4°C overnight. The numbers of cells secreting antibodies of the immunoglobulin A (IgA), IgG, and IgM isotypes were determined. Affinity-purified goat anti-human immunoglobulins with IgA, IgM, or IgG specificity, conjugated to horseradish peroxidase, were used at a dilution of 1:250 in 1% fetal bovine serum in phosphate-buffered saline-Tween 20 (0.05%) as secondary antibodies (Southern Biotechnology Associates, Birmingham, AL). The numbers of ASC were determined manually by counting positive spots by low-power microscopy (magnification, ×40).
CS6-specific antibody in lymphocyte supernatant and serum.
PBMC (1 × 107 cells per ml) from a group of adult patients whose ASC responses were studied (n = 9) were cultured in 24-well tissue culture plates for 48 h in 5% CO2, and supernatants of the cultures were stored at −70°C and tested for antibody responses by ELISA (18, 23) using the ALS assay (16). Serum samples collected on study days 2, 7, and 21, and ALS specimens collected on days 2 and 7 were tested in microtiter plates (Nunc, Roskilde, Denmark) coated with 1 μg/ml of recombinant CS6 using previously described ELISA procedures (1, 18, 25, 33). For detection of CS5-specific antibody responses, ELISA plates were coated with purified CS5 antigen (17).
Titers were calculated using the computer-based program MULTI (DataTree Inc., Waltham, MA). Serum and ALS specimens collected from healthy controls were also tested by ELISA for immune responses against CS6.
Fecal extracts.
Fecal extracts were prepared, and aliquots were frozen at −70°C (19, 23). The total IgA content in fecal samples was determined by ELISA using pooled human Bangladeshi milk with a known IgA concentration (1 mg/ml) as the standard and using affinity-purified goat antibodies to the F(ab′)2 fragment of human IgG as the capture antibody which recognized IgA antibodies (Jackson ImmunoResearch Laboratories Inc, West Grove, PA), using methods described previously (1, 22, 38). The fecal antigen-specific IgA response was expressed as the interpolated IgA ELISA titer per μg total IgA; specimens with total IgA contents of <10 μg/ml, acute and convalescent specimens whose total IgA contents varied more than 10-fold, and acute and convalescent specimens with specific IgA titers of <1 were not included analyses (20). Anti-CS6 fecal IgA responses were analyzed by ELISA as described above, and based on the criteria described above, fecal extracts from 23 patients were analyzed.
Analyses.
A CF-specific ASC response of ≥10 ASC/106 PBMC on day 2 or 7 postinfection was considered positive. For ALS assays, patients with an ELISA titer against CS6 of ≥2 per 107 PBMC were considered responders. For analyses of serum and stool responses, the subjects with an antibody titer on day 7 or 21 after the onset of diarrhea that was ≥2-fold greater than the titer in the acute samples (day 2) were defined as positive responders. Responses were also compared to the responses observed for the healthy controls. The Wilcoxon signed-rank test and the Mann-Whitney U test were used where applicable for statistical analyses. The chi-square test was used for determining differences in proportions between groups. A P value of ≤0.05 was considered significant. Analyses were carried out using SigmaStat (SPSS, San Rafael, CA).
RESULTS
Study subjects.
About 1,800 diarrheal patients were screened during the study period, and 324 of these patients were positive for ETEC. Forty-six of the patients infected with ETEC expressing CS5 plus CS6 or ETEC expressing CS6 consented to participate in the study. Twenty-eight of these patients with ETEC diarrhea were adults (median age, 37 years), and 18 were children (median age, 1.9 years); both males (n = 41) and females (n = 5) were enrolled (Table 1). The patients had durations of diarrhea ranging from 2 to 72 h prior to arrival at the treatment center (median duration, 24 h), and the duration of hospitalization ranged from 14 h to 166 h (median duration, 53 h). A higher percentage of adults (46%) than of children (11%) suffered from severe dehydration (P = 0.03). It was observed that infection with ETEC strains expressing CS5 plus CS6 resulted in more severe disease than infection with ETEC strains expressing only CS6 (39 and 15%, respectively; P = 0.169).
TABLE 1.
Characteristics of patients with diarrhea caused by ETEC expressing CS6 and ETEC expressing CS5 plus CS6
| CF(s) on ETEC isolates | No. of (patients) | % Toxin types
|
% Adults/% children | Duration of diarrhea (median) (h)a | Duration of hospital stay (median) (h) | Dehydration status (%)b
|
|||
|---|---|---|---|---|---|---|---|---|---|
| ST | ST/LT | Mild | Moderate | Severe | |||||
| CS6 | 13 | 85 | 15 | 54/46 | 48 | 89 | 23 | 62 | 15 |
| CS5 + CS6 | 33 | 48 | 52 | 64/36 | 24 | 47 | 12 | 49 | 39 |
Duration of diarrhea prior to admission to the ICDDR,B hospital.
The dehydration status was graded as mild, moderate, or severe (39).
Most (85%) of the ETEC strains expressing only CS6 produced only ST, and 15% of these strains had the LT/ST phenotype; in contrast, almost equal proportions of the ETEC strains expressing CS5 plus CS6 had the ST (48%) and LT/ST (52%) phenotypes. No other bacterial enteropathogen was cocultured from the stools of the patients participating in the study. However, stool microscopy revealed the presence of Giardia lamblia in 7 of the 46 patients.
ASC and ALS responses.
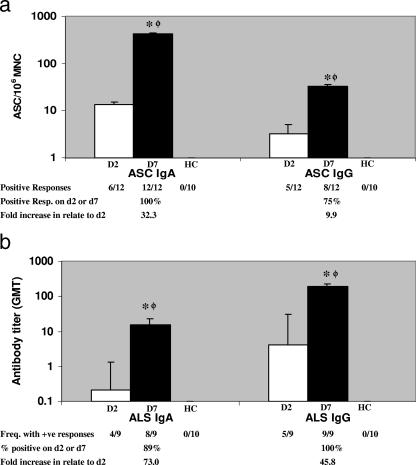
The ASC response to CS6 was studied in 12 adult patients infected with ETEC expressing CS6 (n = 9) or ETEC expressing CS5 plus CS6 (n = 3) at the acute stage and the early convalescent stage after diarrhea (Fig. 1a). The maximum ASC response was observed for the IgA isotype by day 7 postinfection (geometric mean, 430 ASC/106 PBMC); a lower response was observed for the IgG isotype (Fig. 1a), and a poor response was observed for the IgM isotype (not shown). On average, the CS6-specific IgA ASC response in patients infected with a CS6-expressing ETEC strain on day 7 was more than 30-fold higher than the response at the acute stage on day 2 (P = 0.005). A cumulative response rate of 100% was observed for the IgA isotype.
FIG. 1.
(a) IgA and IgG ASC responses to CS6 antigen in adult patients infected with CS6-expressing ETEC. The responses to CS6 after the onset of diarrhea at the acute stage (day 2 [D2]) and at the early convalescence stage (day 7 [D7]) are shown, as are the responses in adult healthy controls (HC). The response frequency indicates the number of patients responding with ≥10 ASC/106 PBMC in all the patients studied. The bars indicate geometric means, and the error bars indicate standard errors of the means. Asterisks indicate statistically significant differences (one asterisk, P < 0.05 to 0.01; two asterisks, P < 0.01 to 0.001; three asterisks, P < 0.001) at day 7 compared to the responses at day 2. Phi indicates that there was a significant difference between patients at day 7 and healthy controls (P < 0.001). MNC, peripheral blood mononuclear cells. (b) IgA and IgG antibody responses to CS6 antigen in ALS specimens from adult patients with CS6-expressing ETEC. The responses to CS6 after the onset of acute-stage diarrhea (day 2 [D2]) and at the early convalescence stage (day 7 [D7]) are shown, as are the responses in adult healthy controls (HC). The response frequency indicates the number of patients responding with titers of ≥2/107 PBMC for all patients studied. The bars indicate geometric mean titers (GMT), and the error bars indicate standard errors of the means. Asterisks indicate statistically significant differences (one asterisk, P < 0.05 to 0.01; two asterisks, P < 0.01 to 0.001; three asterisks, P < 0.001) at day 7 compared to the responses at day 2. Phi indicates that there was a significant difference between patients at day 7 and healthy controls (P < 0.001). +ve, positive.
We also determined the ALS responses in 9 of 12 of the adult patients (9 patients with CS6-expressing ETEC and 3 patients with ETEC expressing CS5 plus CS6) whose ASC responses were studied (Fig. 1b). The CS6 IgA responses were low on day 2 (with 4/9 patients responding) but increased in most cases by day 7 of infection (89%). All the patients showed IgG responses to CS6 by study day 7 (P = 0.039). The three patients with diarrhea caused by ETEC expressing CS5 plus CS6 also responded with CS5-specific ASC and ALS IgA responses (geometric mean for IgA ASC, 330 ASC/106 PBMC; geometric mean titer for IgA ALS, 55/107 PBMC at day 7 after the onset of diarrhea). The ASC or ALS responses in the healthy controls were negligible for both the IgA and IgG isotypes compared to the responses observed for the patients at day 7 after the onset of diarrhea (P < 0.001).
Serological responses to CS6.
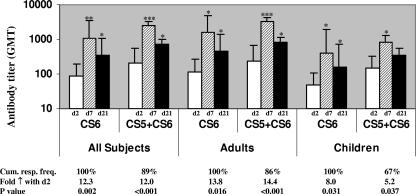
Patients infected with ETEC strains expressing CS6 either alone or in combination with CS5 showed comparable IgA responses to CS6 in serum (differences were not significant) (Fig. 2). By day 7 or 21 of infection, the majority of the adult ETEC patients showed increased IgA responses to CS6 compared to the responses seen at the acute stage (cumulative response, 100%). Similarly, about 90% of the children responded with IgA antibodies to CS6 in serum after infection. The IgA responses in both children and adults declined by day 21 compared to the responses seen at day 7 (P < 0.001). About 70% of the children and adults also responded with IgG antibodies to CS6 which peaked at the early convalescent stage on day 7 (geometric mean titer, 4,467; 4.6-fold increase) and remained elevated up to day 21 (geometric mean titer, 3,281; 3.4-fold increase) (data not shown). The differences in the IgA and IgG responses of adults and children with ETEC diarrhea to CS6 were not significant. The baseline IgA (geometric mean titer, 58) and IgG (geometric mean titer, 979) antibody levels to CS6, as well as the levels in the healthy controls, in both children and adults were lower than the levels seen in the patients at the convalescent phase (P = 0.047 to ≤0.001), probably reflecting the immune responses in the day 2 specimens collected soon after hospitalization.
FIG. 2.
IgA antibody responses to CS6 in sera from patients with diarrhea caused by ETEC expressing CS6 or ETEC expressing CS5 plus CS6. The responses to CS6 after the onset of diarrhea at the acute stage (day 2 [D2]) and at the early (day 7 [D7]) and late (day 21 [D2]1) convalescence stages in the patients are shown. The bars indicate geometric mean titers (GMT), and the error bars indicate standard errors of the means. The response frequency indicates the number of patients responding with ≥2-fold increases in antibody titers for all patients studied. Asterisks indicate that there were statistically significant differences between the responses in the acute stage of disease and the responses in the early or late convalescent stage of disease (one asterisk, P < 0.05; two asterisks, P = 0.001; three asterisks, P < 0.001).
Intestinal IgA antibody responses.
Both children and adults (n = 23) infected with ETEC expressing CS6 and ETEC expressing CS5 plus CS6 also responded with CS6-specific IgA antibodies in their stools by day 7 of infection, and the response declined by day 21 (cumulative responder frequency, 57%) (Table 2). The day 7 antibody levels in the patients were significantly higher than the antibody levels in stool specimens from healthy controls (P < 0.001).
TABLE 2.
IgA antibody responses to CS6 in fecal extracts obtained from study subjects
| Patients | Day | Mean IgA titer/total IgAa | Responder frequency (%)b | Fold increasec |
|---|---|---|---|---|
| Diarrheal | 2 | 3.3 (1.3) | ||
| 7 | 8.5 (1.7) | 50 | 2.6 (5.0)d | |
| 21 | 2.0 (1.6) | 22 | 1.0 (1.2) | |
| Healthy controls | 0 | 1.7 (0.7) |
Geometric mean titer (standard error of the mean) for IgA titer per μg total IgA.
Percentage of responders at day 7 or 21 after the onset of the study compared to the response at day 2. The cumulative frequency of responses on day 7 and/or day 21 was 57%.
Fold increase in the geometric mean titer compared to the titer at the acute stage or in relation to healthy controls (in parentheses).
There was a significant difference between the acute-stage healthy control and the early convalescent stage (P < 0.05).
DISCUSSION
Diarrhea due to CS6-expressing ETEC strains is now recognized as one of the most common ETEC infections, and recent data show that between 25 and 30% of cases may be due to strains having this phenotype (27, 29, 31, 41). The CS6 subtype of the multivalent group of ETEC not only is isolated from children and adults in areas where ETEC is endemic but also is responsible for a majority of ETEC diarrhea in civilian and military travelers (41).
In this report we describe the first study in which mucosal and systemic immune responses were studied quantitatively and in detail for patients hospitalized with diarrhea due to CS6-expressing ETEC strains, although in a previous study using an immunoblot assay and CS6 antigen poorly purified from a wild-type strain, patients infected with ETEC expressing CS5 plus CS6 were shown to respond to CS6 antigen (12). However, with the availability of purified recombinant CS6 antigen it has been possible to analyze anti-CS6 immune responses using more quantitative assays. In addition, we were able to compare responses in patients infected with ETEC strains expressing CS6 and responses in patients infected with ETEC strains expressing CS5 plus CS6.
The results of this investigation also show that by early convalescence (study day 7), most patients had responded with IgA and IgG antibodies to CS6 in lymphocytes isolated from the circulation. These early responses suggest that patients had been infected for at least 1 or 2 days before admission to the hospital. Thus, we showed previously that mucosal immune responses might be induced by 3 days after immunization in immunologically primed subjects (33). There was a poor response of the IgM isotype to CS6, which also suggests that the antibodies generated in sera reflect a primed and booster response. As a surrogate marker of mucosal immunity, assessment of IgA responses against CS6 using ASC or ALS specimens was found to be very useful. Previous studies with cholera patients (2, 23) and with ETEC vaccine recipients (16) have shown that the ALS and ASC assays are comparable for estimating mucosal immune responses and for detecting a recent infection. The results obtained in this study demonstrate that ALS specimens can be used for evaluating responses to CS6 antigen and may be a good alternative to the ASC testing procedure. Of the many advantages of ALS specimens, the most important is that samples can be stored and tested at an appropriate time, whereas the ASC assay must be performed as soon as blood samples are obtained. Due to this, as well as the ease and convenience of the assay, the ALS assay is a preferable technique for studying recent mucosal immune responses to ETEC infections.
We also examined the intestinal IgA antibody responses to CS6 in feces, which increased in 57% of the patients, mostly by day 7 after the onset of diarrhea. The lower rate of responses seen in analyses of the feces than in tests for ASC or ALS responses is similar to the rate seen in other studies of cholera and ETEC diarrhea (23, 25, 38). This may be due to lower levels of specific IgA antibodies in the intestinal secretions, but it may also be due to rapid degradation of antibodies in fecal samples by proteases. Although precautions were taken to store feces at low temperatures and to add protease inhibitors to extracts, the responder rates in most studies have been found to be lower than the rates observed for other secretions (23).
More than 70% of patients seroconverted with CS6-specific IgA antibodies in serum, and the peak responses occurred at early convalescence and decreased at late convalescence. However, this reflected the nature of the IgA isotype, which lasts for a shorter time in the systemic circulation. Responses were also seen in the IgG isotype; however, these responses persisted up to late convalescence. The response for the two antibody isotypes is similar to the responses of patients to other enteric pathogens, such as V. cholerae (22). Whether the memory response to these antigens can protect individuals after natural infection or vaccination must be validated in longitudinal studies.
The antibody response seen after natural infection in Bangladeshi patients is similar to the response seen after transcutaneous immunization with CS6 together with LT in humans (43). Intranasal vaccination of mice with CS6 incorporated in microspheres also induced both IgA and IgG antibody titer responses and IgA responses in feces (3). Thus, natural oral infection with CS6-expressing ETEC induced both a mucosal response and a systemic response, as did infection by the transcutaneous and intranasal routes. Since natural infection induces responses in the mucosa as well as in the circulation, vaccination strategies using the different routes of immunization must stimulate these two compartments, may be needed for induction of an appropriate immune response, and should be targeted for better vaccine efficacy.
CS6 is among the most predominant CF types associated with ETEC disease in humans (41). Most CS6-expressing ETEC strains express ST (LT/ST or only ST), and immunity to this toxin type has been difficult to stimulate (8). Thus, if CS6 proves to be a protective antigen, then using it to overcome this problem seems to be a reasonable way to target a large proportion of disease-producing ETEC strains.
The results of this study demonstrate that natural infection with CS6-expressing ETEC in children and adults induces a robust immune response in patients hospitalized with diarrhea. Since CS6 is a highly prevalent colonization factor in ETEC strains, efforts to include it in vaccine formulations need to be intensified. Studies also need to be carried out to determine if natural infection with CS6 protects against further infections with CS6-positive ETEC, as well as to identify correlates of protection against such infections.
Acknowledgments
This research was supported by the Swedish Agency for Research and Economic Cooperation (Sida-SAREC grant 2004-0578), the Swedish Medical Research Council, and the ICDDR,B. The ICDDR,B is supported by countries and agencies which share its concern for the health problems of developing countries.
We acknowledge the advice and help of Stephen Savarino with this work.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Ahren, C., M. Jertborn, and A. M. Svennerholm. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asaduzzaman, M., E. T. Ryan, M. John, L. Hang, A. I. Khan, A. S. Faruque, R. K. Taylor, S. B. Calderwood, and F. Qadri. 2004. The major subunit of the toxin-coregulated pilus TcpA induces mucosal and systemic immunoglobulin A immune responses in patients with cholera caused by Vibrio cholerae O1 and O139. Infect. Immun. 72:4448-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd, W., and F. J. Cassels. 2006. Intranasal immunization of BALB/c mice with enterotoxigenic Escherichia coli colonization factor CS6 encapsulated in biodegradable poly(dl-lactide-co-glycolide) microspheres. Vaccine 24:1359-1366. [DOI] [PubMed] [Google Scholar]
- 4.de Lorimier, A. J., W. Byrd, E. R. Hall, W. M. Vaughan, Tang, D., Z. J. Roberts, C. E. McQueen, and F. J. Cassels. 2003. Murine antibody response to intranasally administered enterotoxigenic Escherichia coli colonization factor CS6. Vaccine 21:2548-2555. [DOI] [PubMed] [Google Scholar]
- 5.Favre, D., S. Ludi, M. Stoffel, J. Frey, M. P. Horn, G. Dietrich, S. Spreng, and J. F. Viret. 2006. Expression of enterotoxigenic Escherichia coli colonization factors in Vibrio cholerae. Vaccine 24:4354-4368. [DOI] [PubMed] [Google Scholar]
- 6.Field, M., L. H. Graf, Jr., W. J. Laird, and P. L. Smith. 1978. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc. Natl. Acad. Sci. USA 75:2800-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 8.Girard, M. P., D. Steele, C. L. Chaignat, and M. P. Kieny. 2006. A review of vaccine research and development: human enteric infections. Vaccine 24:2732-2750. [DOI] [PubMed] [Google Scholar]
- 9.Guerena-Burgueno, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helander, A., H. M. Grewal, W. Gaastra, and A. M. Svennerholm. 1997. Detection and characterization of the coli surface antigen 6 of enterotoxigenic Escherichia coli strains by using monoclonal antibodies. J. Clin. Microbiol. 35:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helander, A., G. C. Hansson, and A. M. Svennerholm. 1997. Binding of enterotoxigenic Escherichia coli to isolated enterocytes and intestinal mucus. Microb. Pathog. 23:335-346. [DOI] [PubMed] [Google Scholar]
- 12.Helander, A., C. Wenneras, F. Qadri, and A. M. Svennerholm. 1998. Antibody responses in humans against coli surface antigen 6 of enterotoxigenic Escherichia coli. Infect. Immun. 66:4507-4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jertborn, M., C. Ahren, and A. M. Svennerholm. 2001. Dose-dependent circulating immunoglobulin A antibody-secreting cell and serum antibody responses in Swedish volunteers to an oral inactivated enterotoxigenic Escherichia coli vaccine. Clin. Diagn. Lab. Immunol. 8:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz, D. E., A. J. DeLorimier, M. K. Wolf, E. R. Hall, F. J. Cassels, J. E. van Hamont, R. L. Newcomer, M. A. Davachi, D. N. Taylor, and C. E. McQueen. 2003. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 21:341-346. [DOI] [PubMed] [Google Scholar]
- 15.Levine, M. M. 1990. Modern vaccines. Enteric infections. Lancet 335:958-961. [DOI] [PubMed] [Google Scholar]
- 16.McKenzie, R., A. L. Bourgeois, F. Engstrom, E. Hall, H. S. Chang, J. G. Gomes, J. L. Kyle, F. Cassels, A. K. Turner, R. Randall, M. Darsley, C. Lee, P. Bedford, J. Shimko, and D. A. Sack. 2006. Comparative safety and immunogenicity of two attenuated enterotoxigenic Escherichia coli vaccine strains in healthy adults. Infect. Immun. 74:994-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qadri, F., F. Ahmed, T. Ahmed, and A. M. Svennerholm. 2006. Homologous and cross-reactive immune responses to enterotoxigenic Escherichia coli colonization factors in Bangladeshi children. Infect. Immun. 74:4512-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qadri, F., T. Ahmed, F. Ahmed, Y. A. Begum, D. A. Sack, and A. M. Svennerholm. 2006. Reduced dose of oral killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine is safe and immunogenic in Bangladeshi infants 6-17 months of age: dosing studies in different age groups. Vaccine 24:1726-1733. [DOI] [PubMed] [Google Scholar]
- 19.Qadri, F., T. Azim, A. Chowdhury, J. Hossain, R. B. Sack, and M. J. Albert. 1994. Production, characterization, and application of monoclonal antibodies to Vibrio cholerae O139 synonym Bengal. Clin. Diagn. Lab. Immunol. 1:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qadri, F., M. I. Chowdhury, S. M. Faruque, M. A. Salam, T. Ahmed, Y. A. Begum, A. Saha, M. S. Alam, K. Zaman, L. V. Seidlein, E. Park, K. P. Killeen, J. J. Mekalanos, J. D. Clemens, and D. A. Sack. 2005. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J. Infect. Dis. 192:573-579. [DOI] [PubMed] [Google Scholar]
- 21.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuchs, M. J. Albert, R. B. Sack, and A. M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qadri, F., G. Jonson, Y. A. Begum, C. Wenneras, M. J. Albert, M. A. Salam, and A. M. Svennerholm. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O0139. Clin. Diagn. Lab. Immunol. 4:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri, F., E. T. Ryan, A. S. Faruque, F. Ahmed, A. I. Khan, M. M. Islam, S. M. Akramuzzaman, D. A. Sack, and S. B. Calderwood. 2003. Antigen-specific immunoglobulin A antibodies secreted from circulating B cells are an effective marker for recent local immune responses in patients with cholera: comparison to antibody-secreting cell responses and other immunological markers. Infect. Immun. 71:4808-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadri, F., C. Wenneras, F. Ahmed, M. Asaduzzaman, D. Saha, M. J. Albert, R. B. Sack, and A. Svennerholm. 2000. Safety and immunogenicity of an oral, inactivated enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Bangladeshi adults and children. Vaccine 18:2704-2712. [DOI] [PubMed] [Google Scholar]
- 26.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao, M. R., R. Abu-Elyazeed, S. J. Savarino, A. B. Naficy, T. F. Wierzba, I. Abdel-Messih, H. Shaheen, R. W. Frenck, Jr., A. M. Svennerholm, and J. D. Clemens. 2003. High disease burden of diarrhea due to enterotoxigenic Escherichia coli among rural Egyptian infants and young children. J. Clin. Microbiol. 41:4862-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao, M. R., T. F. Wierzba, S. J. Savarino, R. Abu-Elyazeed, N. El-Ghoreb, E. R. Hall, A. Naficy, I. Abdel-Messih, R. W. Frenck, Jr., A. M. Svennerholm, and J. D. Clemens. 2005. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J. Infect. Dis. 191:562-570. [DOI] [PubMed] [Google Scholar]
- 29.Rockabrand, D. M., H. I. Shaheen, S. B. Khalil, L. F. Peruski, Jr., P. J. Rozmajzl, S. J. Savarino, M. R. Monteville, R. W. Frenck, A. M. Svennerholm, S. D. Putnam, and J. W. Sanders. 2006. Enterotoxigenic Escherichia coli colonization factor types collected from 1997 to 2001 in US military personnel during operation Bright Star in northern Egypt. Diagn. Microbiol. Infect. Dis. 55:9-12. [DOI] [PubMed] [Google Scholar]
- 30.Sixma, T. K., K. H. Kalk, B. A. van Zanten, Z. Dauter, J. Kingma, B. Witholt, and W. G. Hol. 1993. Refined structure of Escherichia coli heat-labile enterotoxin, a close relative of cholera toxin. J. Mol. Biol. 230:890-918. [DOI] [PubMed] [Google Scholar]
- 31.Steinsland, H., P. Valentiner-Branth, H. K. Gjessing, P. Aaby, K. Molbak, and H. Sommerfelt. 2003. Protection from natural infections with enterotoxigenic Escherichia coli: longitudinal study. Lancet 362:286-291. [DOI] [PubMed] [Google Scholar]
- 32.Svennerholm, A. M., and J. Holmgren. 1995. Oral vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. Adv. Exp. Med. Biol. 371B:1623-1628. [PubMed] [Google Scholar]
- 33.Svennerholm, A. M., M. Jertborn, L. Gothefors, A. M. Karim, D. A. Sack, and J. Holmgren. 1984. Mucosal antitoxic and antibacterial immunity after cholera disease and after immunization with a combined B subunit-whole cell vaccine. J. Infect. Dis. 149:884-893. [DOI] [PubMed] [Google Scholar]
- 34.Svennerholm, A. M., Y. L. Vidal, J. Holmgren, M. M. McConnell, and B. Rowe. 1988. Role of PCF8775 antigen and its coli surface subcomponents for colonization, disease, and protective immunogenicity of enterotoxigenic Escherichia coli in rabbits. Infect. Immun. 56:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svennerholm, A. M., M. Wikstrom, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner, A. K., J. C. Beavis, J. C. Stephens, J. Greenwood, C. Gewert, N. Thomas, A. Deary, G. Casula, A. Daley, P. Kelly, R. Randall, and M. J. Darsley. 2006. Construction and phase I clinical evaluation of the safety and immunogenicity of a candidate enterotoxigenic Escherichia coli vaccine strain expressing colonization factor antigen CFA/I. Infect. Immun. 74:1062-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turner, A. K., T. D. Terry, D. A. Sack, P. Londono-Arcila, and M. J. Darsley. 2001. Construction and characterization of genetically defined aro omp mutants of enterotoxigenic Escherichia coli and preliminary studies of safety and immunogenicity in humans. Infect. Immun. 69:4969-4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenneras, C., F. Qadri, P. K. Bardhan, R. B. Sack, and A. M. Svennerholm. 1999. Intestinal immune responses in patients infected with enterotoxigenic Escherichia coli and in vaccinees. Infect. Immun. 67:6234-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. 1990. Manual for the treatment of diarrhoea: for use by physicians and other senior healthworkers. WOH/CDD/SER/80-2, revision 2. World Health Organization, Geneva, Switzerland.
- 40.WHO. 1987. Manual for laboratory investigations of acute enteric infections, p. 9-20. World Health Organization, Geneva, Switzerland.
- 41.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf, M. K., L. A. de Haan, F. J. Cassels, G. A. Willshaw, R. Warren, E. C. Boedeker, and W. Gaastra. 1997. The CS6 colonization factor of human enterotoxigenic Escherichia coli contains two heterologous major subunits. FEMS Microbiol. Lett. 148:35-42. [DOI] [PubMed] [Google Scholar]
- 43.Yu, J., F. Cassels, T. Scharton-Kersten, S. A. Hammond, A. Hartman, E. Angov, B. Corthesy, C. Alving, and G. Glenn. 2002. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 70:1056-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]