Abstract
Shigella flexneri uses its type III secretion apparatus (TTSA) to deliver invasins into human cells. This TTSA possesses an external needle with IpaD at its tip. We now show that deoxycholate promotes the stable recruitment of IpaB to the needle tip without inducing a rapid burst of type III secretion. The maintenance of IpaB at the needle tip requires a stable association of IpaD with the Shigella surface. This is the first demonstration of a translocator protein being stably associated with the TTSA needle.
Shigella flexneri uses its type III secretion system (TTSS) to invade colonic epithelial cells and trigger the onset of shigellosis (2, 4). A prominent feature of the TTS apparatus (TTSA) is an external needle composed of MxiH monomers (3). We recently demonstrated that IpaD localizes to the tip of the Shigella TTSA needle, where it controls the secretion of the translocators IpaB and IpaC (6). Neither IpaB nor IpaC was detected on the Shigella surface in this previous study (6). Because there have been reports of IpaB association with the Shigella surface (11, 14, 15), we explored different conditions that might favor controlled mobilization of IpaB to the surface without inducing the burst of type III secretion that is seen upon host cell contact. In particular, bile salts were examined based on observations that, when added to tryptic soy broth (TSB), they lead to increased Shigella adherence to and invasion of HeLa cells (12). The molecular basis for this effect is not known and has not been explored in detail.
IpaB and IpaD colocalize on the S. flexneri surface in the presence of DOC.
IpaD and IpaB were detected on the Shigella surface by using immunofluorescence microscopy as previously described (6). S. flexneri was grown to early log phase in TSB supplemented with 2.5 mM (0.1%, wt/vol) deoxycholate (DOC), a concentration well within the physiological range seen in the human intestine (Fig. 1) (5). As expected, IpaD continued to be detected on the bacterial surface by using rabbit anti-IpaD antiserum and Alexa Fluor 568 goat anti-rabbit immunoglobulin G (IgG) under these conditions (Fig. 1A). In the presence of DOC, however, IpaB was also detected on the Shigella surface by using rabbit anti-IpaB antiserum (Fig. 1B). Both proteins gave rise to similar punctate staining patterns. In contrast, IpaB was not surface exposed in either an mxiH-null (Fig. 1C) or an ipaD-null (Fig. 1D) mutant strain, indicating that IpaB surface localization relies upon a functional TTSS needle and IpaD. The maintenance of IpaD at the Shigella surface appears to be required for IpaB recruitment in the presence of DOC because a small C-terminal deletion that allows IpaD and IpaB secretion without concomitant retention of IpaD at the bacterial surface fails to permit IpaB labeling on the bacterial surface (Table 1).
FIG. 1.
IpaD and IpaB localize to the S. flexneri surface. IpaD and IpaB were detected on the surface of S. flexneri grown in TSB containing 2.5 mM DOC by confocal immunofluorescence microscopy using rabbit anti-IpaD antiserum (A) or rabbit anti-IpaB antiserum (B). IpaB was not detected on the surface of mxiH (C)- or ipaD (D)-null strains. Panels E to H are differential interference contrast (DIC) micrographs of panels A to D, respectively. IpaD and IpaB appeared to colocalize on the S. flexneri surface by double labeling. Panel I shows the DIC micrograph of panels J to L. Panel J is a green pseudocolored image indicating IpaD staining by using anti-IpaD monoclonal antibody. Panel K is a red pseudocolored image indicating IpaB staining by using rabbit anti-IpaB antiserum. Panel L is a merged image of panels J and K. The arrows point to regions where both IpaD and IpaB appear to be labeled.
TABLE 1.
IpaB recruitment to the TTSS needle tip of mutant S. flexneri strainsa
| S. flexneri strain | IpaD localization
|
IpaB localization | |
|---|---|---|---|
| Before DOC | After DOC | ||
| ipaD-null mutant (SF622) | NA | NA | − |
| ipaB-null mutant (SF621) | + | − | NA |
| IpaDΔ328-332 mutant | − | − | − |
Surface localization of IpaD and IpaB was determined by immunofluorescence microscopy. At least 100 bacteria in each of three randomly selected fields were counted. Strains with >80% of bacteria demonstrating surface staining for the given protein were considered positive. In most cases, surface localization was seen on either all bacteria or none. NA, not applicable.
We previously reported that IpaB is not detected on the surface of early-log-phase Shigella in the absence of DOC (6); however, immunoblot analysis suggested that IpaB is associated with the TTSS needle (6). When bacteria were grown to early log phase in TSB and then DOC was added, IpaB mobilized to the bacterial surface within 15 min of DOC exposure. IpaB and IpaD were also surface localized when S. flexneri was exposed to chenodeoxycholate and taurodeoxycholate (data not shown). IpaC was never seen at the Shigella surface during these experiments, and the levels of IpaB in the bacterial cytoplasm did not appear to change (data not shown), presumably due to the minimal effect that DOC addition had on the short-term induction of type III secretion (see below).
To determine whether IpaD and IpaB actually colocalize on the Shigella surface, bacteria were double labeled with anti-IpaD monoclonal antibody and rabbit anti-IpaB antiserum. Primary antibodies were detected using Alexa Fluor 488 goat anti-mouse IgG (Fig. 1J) and Alexa Fluor 568 goat anti-rabbit IgG (Fig. 1K). When the images were merged (Fig. 1L), IpaD and IpaB appeared to colocalize. While IpaD could be readily observed without IpaB costaining, IpaB was not typically observed on the surface without colocalizing with at least some population of IpaD (Fig. 1, panels J to L). The fact that a short C-terminal truncation of IpaD eliminated its association with the Shigella surface (Table 1) but allowed uncontrolled secretion of IpaD and IpaB indicated that none of the observed results are due to the readsorption of secreted IpaD, IpaB, or IpaD/IpaB complexes to the bacterial surface. These findings prompted a closer look at the localization of IpaB and IpaD with respect to the Shigella TTSA.
IpaB and IpaD colocalize at the S. flexneri TTSA needle tip.
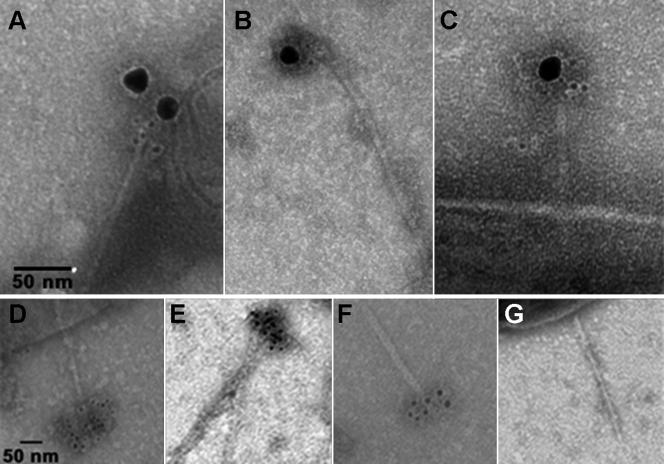
Bacteria and sheared MxiH needles were examined by transmission electron microscopy with negative staining and immunogold labeling. Shigella was grown to early log phase in TSB containing 2.5 mM DOC. The bacteria or needles sheared from the bacteria were applied to carbon-Formvar grids and treated with monoclonal anti-IpaD IgG, followed by 5-nm gold particle-labeled goat anti-mouse F(ab′)2 and rabbit anti-IpaB antiserum and 20-nm gold particle-labeled goat anti-rabbit IgG (Fig. 2). The grids were then stained with 2% uranyl acetate and imaged using a Jeol 1200 EX II transmission electron microscope at an electron acceleration voltage of 120 keV as described previously (6). In Fig. 2A and B, needles sheared from the bacterial surface show IpaD labeling (5-nm gold particles) near the needle tip with IpaB labeling (20-nm gold particles) associated with the same needle. Similar micrographs showing bacterium-associated needles indicated that the observed staining was at the needle tip and not at the base of sheared needles (Fig. 2C).
FIG. 2.
Transmission electron micrographs of immunogold-labeled IpaD and IpaB at the S. flexneri TTSA needle tip. Free TTSA needles (A, B, E, and F) or S. flexneri-associated needles (C, D, and G) were immunolabeled with secondary gold-labeled IgG or F(ab′)2 fragments and negatively stained as described in the text. Typical dissociated needles are shown in panels A and B after being double labeled for IpaD (5-nm gold particles) and IpaB (20-nm gold particles), respectively. In panel C, a bacterium-associated needle is labeled for IpaD with 5-nm gold particles and for IpaB with 20-nm gold particles. A Shigella-associated needle (D) and a needle dissociated from Shigella (E) (another needle with neither end exposed crosses the field) are shown after being stained with anti-IpaB IgG only following exposure to DOC. A needle dissociated from Shigella (F) is shown after being stained with anti-IpaD only following exposure to DOC. In panel G, a Shigella-associated needle stained for IpaB after the bacteria were grown without any exposure to DOC is shown. Although needle breakage could account for the lack of IpaB detection, no IpaB staining was found to be associated with needles from multiple samples.
When IpaB labeling alone was examined for Shigella grown in the presence of DOC, labeling at the needle tip was readily apparent for both bacterium-associated (Fig. 2D) and sheared (Fig. 2E) needles by using secondary F(ab′)2 labeled with 5-nm gold particles. Likewise, IpaD staining alone (using rabbit antiserum in this case) continued to be seen for needles prepared from Shigella grown in the presence of DOC (Fig. 2F), as was previously seen for needles prepared from bacteria grown without DOC (6). In the absence of DOC, no IpaB was seen at the TTSA needle tip (Fig. 2G). Thus, the postulated IpaB-IpaD secretion plug appears to be located at the Shigella TTSA needle tip. From the presented data, one might also speculate that IpaB assumes a position that is external to IpaD relative to the needle tip and bacterial surface, but this will require further work to confirm. It is therefore possible that IpaD localizes initially to the needle tip, where it detects environmental signals that trigger the recruitment of IpaB to a distal position at the needle tip to form an MxiH-IpaD-IpaB ternary complex.
DOC promotes maturation to a secretion-competent state.
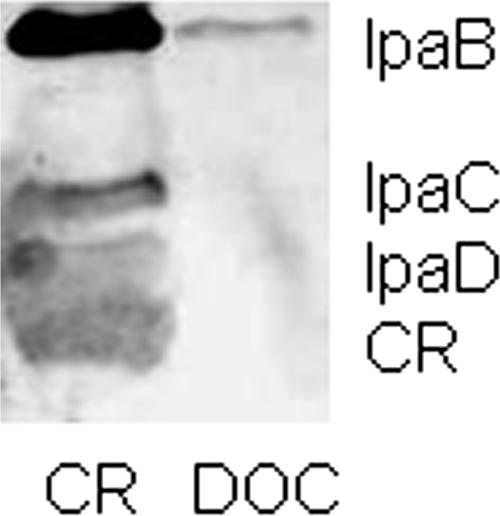
The small amphipathic dye Congo red (CR) is known to induce a burst of Shigella type III secretion which mimics that seen upon host cell contact (1). DOC causes a minor, low-level increase in type III secretion during sustained growth (12), but whether it induces a rapid burst of secretion has not been explored. Thus, early-log-phase bacteria were resuspended in phosphate-buffered saline containing 35 μg/ml CR or 2.5 mM DOC for 30 min. The supernatants were then collected, and the levels of IpaB, IpaC, and IpaD were determined by immunoblot analysis using monoclonal anti-IpaB, anti-IpaC, and anti-IpaD antibodies followed by IRDye 800 goat anti-mouse secondary antibodies. Immunoblots were visualized using an Odyssey infrared imager (LI-COR, Lincoln, NE). Unlike CR, DOC does not induce the burst of secretion associated with TTSS activation (Fig. 3). CR and DOC therefore seem to influence the TTSS differently. Because DOC does not actually induce a rapid burst of type III secretion, it is unlikely that IpaB localization at the needle tip is an artifact of the secretion process. The molecular basis for DOC-mediated recruitment of IpaB to the Shigella TTSA needle tip remains to be determined. We speculate that DOC association with IpaD results in a conformational change that allows IpaB to exit the TTSA and remain bound to IpaD at the needle tip in the absence of further secretion stimuli.
FIG. 3.
Analysis of protein secretion by S. flexneri. IpaB, IpaC, and IpaD from the supernatants of equal numbers of S. flexneri following 30 min of incubation in phosphate-buffered saline containing 35 μg/ml CR or 2.5 mM DOC were analyzed by immunoblotting using monoclonal anti-IpaB, anti-IpaC, and anti-IpaD antibodies and IRDye 800 goat anti-mouse IgG.
This is the first report of a TTSS translocator protein being specifically recruited to the tip of the TTSA needle in a controlled fashion. IpaB localization to the needle tip requires the presence of IpaD and MxiH. This event can be triggered by bile salts, leading to speculation that IpaD could serve as an environmental sensor that gives rise to a secretion-competent or secretion-primed TTSA. These findings fit well with observations that both IpaB and IpaD are needed to control type III secretion (10). In the same respect, this important observation suggests that a needle tip complex composed of IpaB and IpaD (with IpaB perhaps taking up a more distal position relative to the bacterial surface) may represent the primary sensor of host cell contact. This is consistent with the fact that IpaB is a cholesterol binding protein (7, 8) and that invasion occurs at lipid rafts on host cells (9, 13). It will now be important to determine the fate of this needle complex following host cell contact.
Acknowledgments
We thank M. Olive, E. V. Oaks, and K. R. Turbyfill for critical reading of the manuscript.
Funding was provided by grants AI034428 and AI057927 to W.D.P. and by grant NIH39334 and the KU Center for Research, Inc., to the Microscopy and Analytical Imaging Laboratory.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 12 February 2007.
REFERENCES
- 1.Bahrani, F. K., P. J. Sansonetti, and C. Parsot. 1997. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 65:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri ‘needle complex’, a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 4.Blocker, A., K. Komoriya, and S. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 100:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral, D. J., and D. M. Small. 1989. Physical chemistry of bile, p. 621-662. In S. G. Schultz, J. G. Forte, and B. R. Bauner (ed.), Handbook of physiology, vol. 3. American Physiology Society, Bethesda, MD. [Google Scholar]
- 6.Espina, M., A. J. Olive, R. Kenjale, D. S. Moore, S. F. Ausar, R. W. Kaminski, E. V. Oaks, C. R. Middaugh, W. D. Picking, and W. L. Picking. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74:4391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington, A., N. Darboe, R. Kenjale, W. L. Picking, C. R. Middaugh, S. Birket, and W. D. Picking. 2006. Characterization of the interaction of single tryptophan containing mutants of IpaC from Shigella flexneri with phospholipid membranes. Biochemistry 45:626-636. [DOI] [PubMed] [Google Scholar]
- 8.Hayward, R. D., R. J. Cain, E. J. McGhie, N. Phillips, M. J. Garner, and V. Koronakis. 2005. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol. Microbiol. 56:590-603. [DOI] [PubMed] [Google Scholar]
- 9.Lafont, F., G. Tran Van Nhieu, K. Hanada, P. Sansonetti, and F. G. van der Goot. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ménard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills, J. A., J. M. Buysse, and E. V. Oaks. 1988. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect. Immun. 56:2933-2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pope, L. M., K. E. Reed, and S. M. Payne. 1995. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect. Immun. 63:3642-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Goot, F. G., G. Tran van Nhieu, A. Allaoui, P. Sansonetti, and F. Lafont. 2004. Rafts can trigger contact-mediated secretion of bacterial effectors via a lipid-based mechanism. J. Biol. Chem. 279:47792-47798. [DOI] [PubMed] [Google Scholar]
- 14.Watarai, M., T. Tobe, M. Yoshikawa, and C. Sasakawa. 1995. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 14:2461-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West, N. P., P. Sansonetti, J. Mounier, R. M. Exley, C. Parsot, S. Guadagnini, M. C. Prevost, A. Prochnicka-Chalufour, M. Delepierre, M. Tanguy, and C. M. Tang. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313-1317. [DOI] [PubMed] [Google Scholar]