Abstract
Buruli ulcer, caused by Mycobacterium ulcerans, is a necrotizing skin disease emerging particularly in West Africa. M. bovis BCG vaccine offers only short-term protection against experimental footpad infection of C57BL/6 mice with M. ulcerans, and the duration of this protection cannot be prolonged by a booster vaccination.
Buruli ulcer, caused by Mycobacterium ulcerans, is an emerging infectious skin disease in tropical areas. In certain regions of West Africa, M. ulcerans is now the third most common mycobacterial disease in immunocompetent humans, after tuberculosis and leprosy. M. ulcerans produces a polyketide-like toxin, mycolactone, which is responsible for the extracellular phase of the infection (2) and for the deep necrotizing skin lesions typical for the advanced stages of the disease (4). To date, there is no specific vaccine against M. ulcerans, and surgery and skin grafting are often the only treatments administered to patients. Evidence in the literature has provided evidence for a cross-reactive but short-lived protective role of the attenuated Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine, used for the prophylaxis of tuberculosis (9, 15). One manifestation of Buruli ulcer against which BCG vaccination was reported to exert a sustained, immunoprophylactic effect is its most severe form, viz, osteomyelitis (12, 13). However, even these findings have to be considered with some caution, as they are based on data from BCG scars, which have been shown to be unreliable if the vaccine is administered at birth. We have previously reported that BCG vaccination of C57BL/6 (B6) mice can induce a substantial protective effect against experimental infection with M. ulcerans 5150 (16). Thus, BCG-vaccinated mice challenged with 3 × 104 acid-fast bacilli (AFB) and sacrificed 7 weeks after challenge demonstrated a 20-fold reduction in the number of AFB in the infected footpad compared to nonvaccinated mice (16).
BCG vaccination also displays cross-reactive and variable protection against leprosy, which is caused by another mycobacterial species, i.e., M. leprae (3). In 1996, the Karonga Prevention Trial Group in Malawi showed that a booster vaccination with BCG could offer additional protection against leprosy (but not against tuberculosis) compared to a single BCG immunization (8). In view of these findings, a booster BCG vaccination trial for the prevention of Buruli ulcer has been proposed by Paul Johnson (presented at the Sixth WHO Advisory Group on Buruli Ulcer, Geneva, Switzerland, 2003).
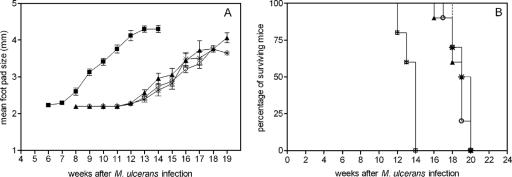
In order to provide an experimental basis for this trial, we have analyzed whether a booster immunization with BCG would increase the protective efficacy of the vaccine in our experimental mouse model. C57BL/6 mice (obtained from the animal breeding facilities of the WIV-Pasteur Institute) were vaccinated once or twice intravenously in a lateral tail vein with 0.2 mg (corresponding to 106 CFU) of M. bovis BCG (strain GL2) (grown for 2 weeks as a surface pellicle on synthetic Sauton medium and homogenized with a ball mill) as described before (16). In the first experiment, the interval between the two injections of BCG was 10 weeks. Mice were infected in the right footpad with 30 μl of M. ulcerans strain 04-855 (isolated in Benin and kindly provided by F. Portaels, Institute of Tropical Medicine, Antwerp, Belgium), corresponding to 5 × 104 AFB. Footpad swelling was measured with a calibrated Oditest (CH Kroeplin GmbH, Schlüchtern, Germany) apparatus with a resolution of 0.01 mm. The mean footpad size before infection was 2.0 ± 0.2 mm, and the cutoff value for positive swelling was set at 2.5 mm. Mice were sacrificed for ethical reasons by cervical dislocation when the footpad size was >4.00 mm. Mice were monitored weekly for footpad swelling until sacrifice. Four groups of 10 mice were compared, including naive mice, mice vaccinated once with BCG 16 weeks before infection, mice vaccinated once with BCG 6 weeks before infection, and finally, mice vaccinated twice with BCG, 16 weeks and 6 weeks before infection. As shown in Fig. 1A, prior BCG vaccination significantly retarded the apparition of footpad swelling. In naive mice, footpad size started to increase 8 weeks after M. ulcerans infection. In contrast, in mice vaccinated with BCG, the onset of footpad swelling was delayed for 5 to 6 weeks. There was no difference between mice vaccinated with BCG 6 or 16 weeks before infection, and likewise, there was no difference between mice vaccinated once or twice with BCG. Vaccination with BCG also delayed the moment when mice had to be euthanized. As shown in Fig. 1B, the median survival time of nonvaccinated mice was 14 weeks, whereas BCG-vaccinated mice demonstrated a median survival time of 19 weeks (P < 0.001 according to the log rank test). Like the case for footpad swelling, there was no difference in median survival time between mice vaccinated 6 or 16 weeks prior to M. ulcerans challenge or between mice vaccinated once or twice with BCG.
FIG. 1.
Evolution of footpad size (A) and survival curves (B) after M. ulcerans injection for unvaccinated B6 mice and B6 mice vaccinated with BCG once or twice 6 or 16 weeks prior to infection. ▪, no BCG; ▴, BCG at week 16; ○, BCG at week 6; *, BCG at weeks 6 and 16.
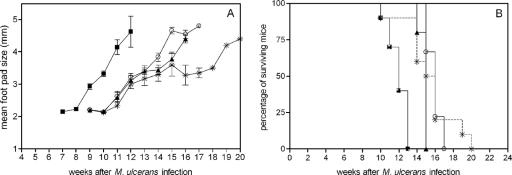
In a second experiment, the first BCG vaccination was given 25 weeks before challenge, and the time between the two vaccinations was prolonged to 18 weeks. As shown in Fig. 2A, BCG vaccination administered 25 or 7 weeks prior to M. ulcerans challenge protected mice to the same extent and delayed the apparition of footpad swelling by 4 weeks. As in the first experiment, BCG administered twice was not more effective than BCG administered once. Whereas 8 of 10 of these BCG-boosted mice had been sacrificed by week 16, two mice survived until week 19 or 20, explaining the slightly different mean footpad size curve (for surviving mice) for this group. In order to confirm our previous observation that footpad swelling correlates with bacterial replication in this experimental model, we also determined the number of AFB in infected footpads of all animals at the time of sacrifice, as described before (16). Because some animals died before we could euthanize them, the number of mice for which AFB counting could be performed was less than the initial 10 animals in each group. As shown in Table 1, the numbers of AFB recovered in experiment 1 were not significantly different between the three groups of BCG-vaccinated mice (P > 0.05 according to the Dunnett test), which were sacrificed between weeks 18 and 19, and the group of nonvaccinated mice, which were sacrificed between weeks 13 and 14 after challenge. Likewise, in experiment 2, the mean numbers of AFB recovered from BCG-vaccinated animals at weeks 16 to 17 after challenge and from nonvaccinated animals at weeks 12 to 13 were not significantly different, albeit that mean AFB counts recovered at the end of this experiment were higher than AFB counts recovered at the end of experiment 1, confirming the more rapid progression observed in footpad swelling in the second experiment.
FIG. 2.
Evolution of footpad size (A) and survival curves (B) after M. ulcerans injection for unvaccinated B6 mice and B6 mice vaccinated with BCG once or twice 7 or 25 weeks prior to infection. ▪, no BCG; ▴, BCG at week 25; ○, BCG at week 7; *, BCG at weeks 7 and 25.
TABLE 1.
Mycobacterial multiplication in B6 mice infected with 5 × 104 AFB of M. ulcerans strain 04-855
| Expt no. and vaccination group | Log10 AFB/ml (n)a |
|---|---|
| Expt 1 | |
| No BCG | 5.86 ± 0.17 (8) |
| BCG at wk 16 | 5.74 ± 0.28 (6) |
| BCG at wk6 | 6.02 ± 0.53 (6) |
| BCG at wk 6 and wk 16 | 5.76 ± 0.23 (6) |
| Expt 2 | |
| No BCG | 6.42 ± 0.17 (6) |
| BCG at wk 25 | 6.31 ± 0.19 (8) |
| BCG at wk 7 | 6.21 ± 0.44 (8) |
| BCG at wk 7 and wk 25 | 6.15 ± 0.43 (8) |
Data are means ± standard deviations for footpads homogenized in 2 ml of buffer.
In conclusion, the present results have confirmed our previous finding that BCG vaccination can exert a protective effect against experimental footpad infection with M. ulcerans in C57BL/6 mice (16). However, vaccine-induced protection was found to be only partial, and this did not seem to be caused by a waning of the efficacy, as similar protection levels were found when BCG was administered 1 or 6 months prior to challenge. Using the same experimental protocol, mice of the more Th2-prone BALB/c strain could not be protected by prior BCG vaccination, administered either as a single or as a double dose (data not shown). Our findings seem to indicate that the limited protection conferred by BCG against M. ulcerans is not caused by a waning of the immune protection, but rather, the quality of the immune response induced by the vaccine is not optimal.
Recently, a number of reports have discussed the use of improved BCG vaccines for the prevention of tuberculosis. An improved BCG vaccine overexpressing the protective antigens 85A and 85B (Ag85A and -B) (6) and a BCG vaccine improved for its induction of major histocompatibility complex class I-restricted responses are being tested in clinical phase 1 trials (5). Prime-boost combinations of BCG with recombinant pox- and adenoviruses are very powerful strategies to increase the immunogenicity of the vaccine (10, 17). Finally, priming with DNAs encoding immunodominant antigens prior to BCG vaccination can also dramatically increase the immunogenicity and protective efficacy induced by the vaccine (14). It remains to be determined whether a similar approach could be used for improving the efficacy of BCG against Buruli ulcer. In this respect, it is interesting that Ohara et al. have shown that a recombinant BCG strain overproducing the protective mycobacterial Ag85A reduced the multiplication of Mycobacterium leprae in the footpads of B6 mice (11). A DNA vaccine encoding the same Ag85A from M. bovis BCG (16), but not the heat shock protein Hsp65 from M. leprae (1), demonstrated a certain degree of protection against experimental M. ulcerans infection. On the other hand, it is possible that the cross-reactive immune response induced by the M. bovis BCG vaccine is not sufficient and that species-specific immune responses are required to effectively control the infection. For example, a comparative study of the protective efficacy of two DNA vaccines, encoding Ag85A from BCG and that from M. ulcerans, indicates that this might be the case (A. Tanghe et al., unpublished data). Buruli ulcer is an emerging infectious disease, and the number of reported cases increases annually. The disease imposes a serious economic burden for treating patients because of notably long hospital stays, often >3 months per patient. A safe and effective vaccine would undoubtedly be a better strategy to combat Buruli ulcer in the long run (7).
Acknowledgments
This work was partially supported by grants from the Damiaanaktie-Belgium and from FWO-Vlaanderen (Krediet aan Navorsers 1.5.144.04).
We are grateful to F. Portaels (Institute of Tropical Medicine, Antwerp, Belgium) for providing us the virulent African strain 04-855 of M. ulcerans.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 26 February 2007.
REFERENCES
- 1.Coutanceau, E., P. Legras, L. Marsollier, G. Reysset, S. T. Cole, and C. Demangel. 2006. Immunogenicity of Mycobacterium ulcerans Hsp65 and protective efficacy of a Mycobacterium leprae Hsp65-based DNA vaccine against Buruli ulcer. Microbes Infect. 8:2075-2081. [DOI] [PubMed] [Google Scholar]
- 2.Coutanceau, E., L. Marsollier, R. Brosch, E. Perret, P. Goossens, M. Tanguy, S. T. Cole, P. L. C. Small, and C. Demangel. 2005. Modulation of the host immune response by a transient intracellular stage of Mycobacterium ulcerans: the contribution of endogenous mycolactone toxin. Cell. Microbiol. 7:1187-1196. [DOI] [PubMed] [Google Scholar]
- 3.Fine, P. E., and P. G. Smith. 1996. Vaccination against leprosy—the view from 1996. Lepr. Rev. 67:249-252. [DOI] [PubMed] [Google Scholar]
- 4.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283:854-857. [DOI] [PubMed] [Google Scholar]
- 5.Grode, L., P. Seiler, S. Baumann, J. Hess, V. Brinkman, A. Nasser Eddine, P. Mann, C. Goosmann, S. Bandermann, D. Smith, G. J. Bancroft, J. M. Reyrat, D. Van Soolingen, B. Raupach, and S. Kaufmann. 2005. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 115:2472-2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic. 2000. Recombinant bacillus Calmette-Guérin (BCG) vaccines expressing the Mycobacterium tuberculosis 30 kD major secretory protein induce greater protective immunity against tuberculosis than conventional vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huygen, K. 2003. Prospects for vaccine development against Buruli disease. Expert Rev. Vaccines 2:561-569. [DOI] [PubMed] [Google Scholar]
- 8.Karonga Prevention Trial Group. 1996. Randomized controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet 348:17-24. [PubMed] [Google Scholar]
- 9.Lancet Publishing Group. 1969. BCG vaccination against Mycobacterium ulcerans infection (Buruli ulcer). Lancet i:111-115. [PubMed] [Google Scholar]
- 10.McShane, H., A. A. Pathan, C. Sander, S. M. Keating, S. C. Gilbert, K. Huygen, H. A. Fletcher, and A. V. S. Hill. 2004. Recombinant modified vaccinia Ankara expressing antigen 85A boosts BCG-primed and naturally acquired antimycobacterial immunity in humans. Nat. Med. 10:1240-1244. [DOI] [PubMed] [Google Scholar]
- 11.Ohara, N., M. Matsuoka, H. Nomaguchi, M. Naito, and T. Yamada. 2000. Inhibition of multiplication of Mycobacterium leprae in mouse foot pads by recombinant bacillus Calmette-Guérin (BCG). Vaccine 18:1294-1297. [DOI] [PubMed] [Google Scholar]
- 12.Portaels, F., J. Aguiar, M. Debacker, A. Guedenon, C. Steunou, O. Zinsou, and W. M. Meyers. 2004. Mycobacterium bovis BCG vaccination as prophylaxis against Mycobacterium ulcerans osteomyelitis in Buruli ulcer disease. Infect. Immun. 72:62-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portaels, F., J. Aguiar, M. Debacker, C. Steunou, C. Zinsou, A. Guedenon, and W. M. Meyers. 2002. Prophylactic effect of Mycobacterium bovis BCG vaccination against osteomyelitis in children with Mycobacterium ulcerans disease (Buruli ulcer). Clin. Diagn. Lab. Immunol. 9:1389-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romano, M., S. D'Souza, P.-Y. Adnet, R. Laali, F. Jurion, K. Palfliet, and K. Huygen. 2006. Priming but not boosting with plasmid DNA encoding mycolyl-transferase Ag85A from M. tuberculosis increases the survival time of M. bovis BCG vaccinated mice against low dose intravenous challenge with M. tuberculosis H37Rv. Vaccine 24:3353-3364. [DOI] [PubMed] [Google Scholar]
- 15.Smith, P. G., W. D. Revill, E. Lukwago, and Y. P. Rykushin. 1977. The protective effect of BCG against Mycobacterium ulcerans disease: a controlled trial in an endemic area of Uganda. Trans. R. Soc. Trop. Med. Hyg. 70:449-457. [DOI] [PubMed] [Google Scholar]
- 16.Tanghe, A., J. Content, J.-P. Van Vooren, F. Portaels, and K. Huygen. 2001. Protective efficacy of a DNA vaccine encoding Ag85A from M. bovis BCG against Buruli ulcer. Infect. Immun. 69:5403-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang, J., L. Thorson, R. W. Stokes, M. Santosuosso, K. Huygen, A. Zganiacz, M. Hitt, and Z. Xing. 2004. Single mucosal, but not parenteral immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 173:6357-6365. [DOI] [PubMed] [Google Scholar]