Abstract
Shiga toxins produced by enterohemorrhagic Escherichia coli (EHEC) include Shiga toxin 1 (Stx1) as well as Shiga toxin 2 (Stx2). Stx1 is cell associated, whereas Stx2 is localized to the culture supernatant. We have analyzed the secretion of Stx2 by generating histidine-tagged StxB (StxB-H). Although neither StxB1-H nor StxB2-H was secreted in StxB-H-overexpressed EHEC, StxB2-H-overexpressed EHEC showed inhibited Stx2 secretion. On the other hand, StxB1-H-overexpressed EHEC showed no alteration of Stx2 secretion. B-subunit chimeras of Stx1 and Stx2 were used to identify the specific residue of StxB2 that the Stx2 secretory system recognizes. Alteration of the serine 31 residue to an asparagine residue (S31N) in StxB2-H enabled the recovery of Stx2 secretion. On the other hand, alteration of the asparagine 32 residue to a serine residue (N32S) in StxB1-H caused the partial secretion of a point-mutated histidine-tagged B subunit in EHEC. Based on the evidence, it appeared possible that this residue might contain secretion-related information for Stx2 secretion. To investigate this hypothesis, we constructed an isogenic mutant EHEC (Stx1B subunit, N32S) strain and an isogenic mutant EHEC (Stx2B subunit, S31N) strain. Although the mutant Stx2 was cell associated in isogenic mutant EHEC, mutant Stx1 was not extracellular. However, when we used plasmids for the expression of the mutant holotoxins, the overexpressed mutant Stx1 was found in the supernatant fraction, and the overexpressed mutant Stx2 was found in the cell-associated fraction in mutant holotoxin gene-transformed EHEC. These results indicate that the serine 31 residue of the B subunit of Stx2 contains secretion-related information.
Enterohemorrhagic Escherichia coli strains, which are associated with outbreaks of hemorrhagic colitis and hemolytic-uremic syndrome (HUS) (13, 15, 17), synthesize elevated levels of cytotoxins that are related to Shiga toxin, the potent cytotoxin produced by Shigella dysenteriae type 1 strains. These E. coli toxins are known as Stx1 and Stx2. Stx1 is identical to Shiga toxin (33), and Stx2 is genetically and functionally related to, but immunologically distinct from, Stx1 (22).
Stx1 and Stx2 consist of one molecule of the A subunit and five molecules of the B subunit and thus form an AB5 structure (22). The A subunit is enzymatically active and inhibits protein synthesis in eukaryotic cells (7), whereas the B subunits bind to receptor molecules of the target cells (2, 22). The structural genes of the two toxins share 54% amino acid sequence homology (14). Both toxins bind to the same glycolipid receptor, Gb3, and inhibit protein synthesis by the same mechanism as Shiga toxin. However, they are secreted in different ways. The cytotoxicity of Stx1 was almost completely cell associated, whereas most of the cytotoxicity of Stx2 was found in the extracellular fraction (30). The different distributions of the two toxins are due to the different translation across the outer membrane in EHEC because Stx1 is located in the periplasm fraction of the bacterial cells (38). Moreover, Weinstein et al. previously found that the B subunit dictated the cytotoxic localization of the toxin within bacterial cells (36). These results suggested that the B subunit of Stx2 was recognized by the Stx2 secretion system in EHEC.
A study of Vibrio cholerae demonstrated that the B pentamer of the cholera toxin (CT), which is very similar in structure to Shiga toxins, is formed in the periplasm before its secretion (11). This observation suggested that AB5 toxins, including CT and Shiga toxin, are translocated across the outer membrane in an assembled conformation. The principle upon which the AB5 toxin recognition process is based remains unclear, but this process may rely on the presence of a secretion motif within the B subunit of holotoxins.
After Stx1 was characterized, the molecular structure of Stx2 was determined by X-ray crystallography (9, 29), and four major differences between the structures of Stx1 and Stx2 were reported (9). Any of these structural differences might account for the differences in the properties of secretion of the two toxins, but this too remains unclear at present.
This study aimed to identify the specific residue of the B subunit of Stx2 that is essential for Stx2 secretion in EHEC.
MATERIALS AND METHODS
Bacterial strains, plasmids, and oligonucleotides.
The bacterial strains and plasmids used in this study are listed in Table 1. The oligonucleotides used for this study are listed in Table S1 in the supplemental material.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| E. coli K24 | EHEC O157, stx1 | This study |
| E. coli EDL933 | EHEC O157, stx1, stx2 | 24 |
| E. coli 86-24 | EHEC O157, stx2 | 10 |
| E. coli TS26 | EHEC O26H11, stx1 | This study |
| E. coli K47 | EHEC O157, stx2 but did not detect Stx2 | This study |
| E. coli BL21 | F−ompT hsdSB(rB− mB−) dcm gal | Promega |
| E. coli DH5αλpir | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoR (lacZYA-argF)U169 λpir+ | 6 |
| E. coli S17-1λpir | pro res mod+RP4-2 Tet::Mu-Kan::Tn7 | 27 |
| E. coli EDLN | Spontaneous variant of EDL933; Nalr | This study |
| E. coli N20-8 | Isogenic mutant of EDLN; stxB1 (N32S) | This study |
| E. coli N1-12 | Isogenic mutant of EDLN; stxB2 (S31N) | This study |
| Plasmids | ||
| pTrcHis2A | Ptrc laca; Codons for 6 histidine residues, Ampr | Invitrogen |
| pWM91 | Suicide vector; Ampr SacB+ | 18 |
| pi-1 | stxB1 in pTrcHis2A | This study |
| piii-1 | stxB2 in pTrcHis2A | This study |
| piS-3 | stxB1 His6 in pTrcHis2A | This study |
| piiiS-1 | stxB2 His6 in pTrcHis2A | This study |
| pAH-5 | (RBR-StxB1)a-stxB2 fusion His6 in pTrcHis2A | This study |
| pCH-6 | stxB110EstxB2 fusion His6 in pTrcHis2A | This study |
| pDH-22 | stxB123KstxB2 fusion His6 in pTrcHis2A | This study |
| pEH-7 | stxB135NstxB234NstxB1 fusion His6 in pTrcHis2A | This study |
| pOH-1 | stxB123KstxB2 fusion His6 in pTrcHis2A | This study |
| pFH-5 | stxB148MstxB2 fusion His6 in pTrcHis2A | This study |
| pHH-8 | (RBR-StxB1)a-stxB29EstxB1 fusion His6 in pTrcHis2A | This study |
| pNH-3 | stxB110EstxB222KstxB1 fusion His6 in pTrcHis2A | This study |
| pPH-2 | stxB135NstxB247MstxB1 fusion His6 in pTrcHis2A | This study |
| pXH-7 | stxB123KstxB226KstxB1 fusion His6 in pTrcHis2A | This study |
| pYH-2 | stxB127KstxB234NstxB1 fusion His6 in pTrcHis2A | This study |
| pL29Y-1 | stxB1(L29Y) His6 in pTrcHis2A | This study |
| pF30W-1 | stxB1(F30W) His6 in pTrcHis2A | This study |
| pN32S-1 | stxB1(N32S) His6 in pTrcHis2A | This study |
| pY28L-1 | (RBR-StxB1)a-stxB2(Y28L) His6 in pTrcHis2A | This study |
| pW29F-2 | (RBR-StxB1)a-stxB2(W29F) His6 in pTrcHis2A | This study |
| pS31N-6 | (RBR-StxB1)a-stxB2(S31N) His6 in pTrcHis2A | This study |
| pStx1-4 | stx1 in pTrcHis2A | This study |
| p2AKH-2 | stx2 in pTrcHis2A | This study |
| pStx1N32S-1 | stx1 mutant (B subunit, N32S) in pTrcHis2A | This study |
| pStx2S31N-22 | stx2 mutant (B subunit, N32S) in pTrcHis2A | This study |
| pWN32S-20 | stx1 mutant (B subunit, N32S) in pWM91 | This study |
| pWS31N-2 | stx2 mutant (B subunit, N32S) in pWM91 | This study |
RBR-StxB1 means ribosomal binding region of StxB1.
Cell fractionation.
EHEC was cultured in LB broth at 37°C for 12 h. Cells were pelleted by centrifugation at 10,000 × g for 5 min, and the supernatant obtained was used as the supernatant fraction. The pellet was suspended in an equal volume of ice-cold phosphate-buffered saline (PBS) (pH 7.2) and sonicated for 30 s on ice. After sonication, the cell homogenate was centrifuged at 10,000 × g for 5 min, and the supernatant obtained was used as the cell-associated fraction. The protein concentration of the cell-associated fraction was determined according to the Bradford method (Bio-Rad, Richmond, CA). For low-level overexpression of the B subunit in transformed EHEC strain 86-24, ampicillin and IPTG (isopropyl-β-d-thiogalactopyranoside) were used at 50 μg/ml and 1 μM, respectively. The transformed EHEC strain K47 was cultured in LB broth containing 50 μg/ml of ampicillin.
SDS-PAGE and immunoblotting.
Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto polyvinylidene difluoride membranes. The membranes were incubated in anti-VT1 antiserum, anti-VT2 antiserum, and/or anti-His tag antibody, followed by horseradish peroxidase (HRP)-conjugated anti-rabbit immunoglobulin G (IgG) and/or HRP-conjugated anti-mouse IgG. Antibody-antigen complexes were detected using the ECL detection kit (Amersham Biosciences K.K., Tokyo, Japan) by the LAS-1000 luminescent image analyzer (Fujifilm, Tokyo, Japan). The bands corresponding to the A subunit of Stx2 and histidine-tagged StxB were quantified by Image Gauge software (Fujifilm, Tokyo, Japan).
Gb3 receptor ELISA.
Porcine erythrocyte Gb3 (Wako, Osaka, Japan) was coated onto microtiter plate wells (C96 Maxisorp, Nunc-immuno plate; Nalge Nunc International, Rochester, NY) by evaporation from an ethanolic solution. A 100-μl aliquot of ethanolic Gb3 (5 μg/ml) was added per microtiter plate well in triplicate, and the ethanol was allowed to evaporate at room temperature for 6 h. Each well was blocked with 200 μl of 0.2% (wt/vol) bovine serum albumin (BSA) in PBS (BSA-PBS) for 6 h and washed twice with 200 μl/well BSA-PBS. Dilutions of standard Shiga toxins were prepared in BSA-PBS. The solution was dispensed into the wells and incubated overnight at 4°C. The wells were emptied and washed three times (1 min each) with 200 μl of BSA-PBS. One hundred microliters of diluted rabbit antiserum against Stx1 or antiserum against Stx2 in BSA-PBS was then added to the wells for 1 h at room temperature. The wells were washed as described above, and diluted HRP-conjugated anti-rabbit IgG in BSA-PBS was added to the wells for 1 h at room temperature. The substrate 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic)diammonium salt (ABTS) was dissolved in 0.1 M citric acid (pH 4.35) at 0.3 mg/ml. A volume of 7 μl of 30% H2O2 was added per 8 ml of solution. The wells were emptied and washed three times with 200 μl of BSA-PBS for 3 min each. Finally, the wells were washed once with PBS, and 100 μl/well of ABTS solution was added to the plate, which was then shaken gently and placed in the dark. After sufficient color had developed (usually 20 to 40 min), the absorbance of each well at 415 nm was determined by using an enzyme-linked immunosorbent assay (ELISA) plate reader.
Plasmid construction of chimeric StxB-H.
To construct plasmids expressing histidine-tagged StxB1 (StxB1-H) and histidine-tagged StxB2 (StxB2-H), in which six histidine residues were added at the carboxyl termini, a two-step PCR-based strategy was adopted. The StxB1 gene and the 3′ noncoding region of the StxB1 gene were amplified by PCR from the genomic DNA of EHEC strain TS26 using the primer sets P301 and P309 and P310 and P304, respectively. The resulting fragment mixture was used as the template for reamplification using P301 and P304. The StxB2 gene and the 3′ noncoding region of the StxB2 gene were amplified by PCR from the genomic DNA of EHEC strain 86-24 using the primer sets P305 and P311 and P312 and P308, respectively. The resulting fragment mixture was used as the template for reamplification using P305 and P308. Each DNA fragment was cleaved with NcoI and EcoRI, and the cleaved fragments were inserted into these sites in pTrcHis2A to yield pi-1, expressing StxB1, and piii-1, expressing StxB2. These plasmids were cleaved with SalI and were then self-ligated to yield piS-3, expressing StxB1-H, and piiiS-1, expressing StxB2-H. Sequences derived by PCR were verified by dideoxy sequencing (ABI).
PCR fragments containing different portions of StxB1 or StxB2 were introduced into the appropriate site of StxB1-H or StxB2-H by two-step PCR in order to create in-frame translational fusions between StxB1 and StxB2 (see Table S2 in the supplemental material). Each DNA fragment was cleaved with NcoI and SalI, and each fragment was inserted into these sites in pTrcHis2A. Sequences derived by PCR were verified by dideoxy sequencing (ABI).
Purification of chimeric StxB2-H.
The plasmids were transformed into E. coli BL21. To purify toxins from these strains, a 1-liter culture was grown at 30°C to an approximate optical density (600 nm) of 0.5. One milliliter of 1 M IPTG solution was added, and the culture was incubated for 3 h at 30°C. Cells were pelleted by centrifugation, resuspended in 12 ml of PBS, and sonicated on ice. Cellular debris was removed by centrifugation at 12,000 × g for 30 min. The supernatant was separated by affinity chromatography using a Ni2+-loaded HiTrap Chelating HP column (1-ml column; Amersham Biosciences K.K., Tokyo, Japan) equilibrated with PBS, and chimeric StxB-H was eluted by a 0 to 0.5 M gradient of imidazole. The peak fractions of chimeric StxB-H were dialyzed against PBS, concentrated, and finally stored at −80°C. The protein concentration of each toxin was determined by the Bradford method using a protein assay reagent (Bio-Rad, Richmond, CA).
Gel filtration.
The purified chimeric StxB2-H was loaded onto a TSK-G3000SW column (Tosoh Corporation, Tokyo, Japan) at a flow rate of 0.8 ml/min in PBS, and elution was measured by the absorbance at 280 nm. The molecular mass standards were purchased from Sigma (St. Louis, MO).
Plasmid construction and purification of mutant holotoxins.
The operons encoding Stx1 and Stx2 were amplified by PCR from the genomic DNA of EHEC strains TS26 and 86-24 using the primer sets P320 and P304 and P319 and P375, and these fragments were cloned by the ligation of the NcoI-EcoRI fragment into pTrcHis2A to generate plasmids pStx1-4 and p2AKH-2, respectively.
To construct the operons encoding the mutant Stx1 (B subunit, N32S) and the mutant Stx2 (B subunit, S31N), a two-step PCR-based strategy was adopted (see Table S2 in the supplemental material). Each DNA fragment was cleaved with NcoI and EcoRI, and each cleaved fragment was inserted into the appropriate site in pTrcHis2A. Sequences derived by PCR were verified by dideoxy sequencing (ABI).
The plasmids were transformed on E. coli BL21 cells. To purify toxins from these strains, a 1-liter culture was grown at 30°C to an approximate optical density at 600 nm of 0.5. A 100-μl aliquot of 1 M IPTG solution was added to the cell culture, which was incubated for 3 h at 30°C. The cells were pelleted by centrifugation and resuspended in 12 ml of PBS in E. coli BL21(pStx1-4) and E. coli BL21(pStx1N32S-1) or 20 mM Tris-HCl (pH 8.6) in E. coli BL21(p2AKH-2) and E. coli BL21(pStx2S31N-22). The samples were then sonicated on ice to release the membrane-bound toxin. Cellular debris was removed by centrifugation at 12,000 × g for 30 min.
The supernatant from E. coli BL21(pStx1-4) or E. coli BL21(pStx1N32S-1) was separated by affinity chromatography using a Gpro column (19) equilibrated with PBS, and the toxins were eluted with 4.5 M MgCl2 solution. Fractions that reacted against anti-Stx1 antiserum were dialyzed against PBS and concentrated. These fractions were separated by gel filtration chromatography using a Superose 12 column (Amersham Biosciences K.K., Tokyo, Japan) equilibrated with PBS. The peaks of Stx1 or mutant Stx1 (B subunit, N32S) were pooled and concentrated, and the samples were stored at −80°C.
The supernatants from E. coli BL21(p2AKH-2) or E. coli BL21(pStx2S31N-22) were separated by anion-exchange chromatography using a HiTrap Q HP column (two 5-ml columns connected in tandem; Amersham Biosciences K.K., Tokyo, Japan) equilibrated with 20 mM Tris-HCl (pH 8.6), and the toxins were eluted with a 0 to 1 M gradient of NaCl. Fractions that reacted against anti-Stx2 antiserum were dialyzed against 20 mM Tris-HCl (pH 8.6) and separated by anion-exchange chromatography using a RESOURCE Q column (1-ml column; Amersham Biosciences K.K., Tokyo, Japan) equilibrated with 20 mM Tris-HCl (pH 8.6), and the toxins were eluted with a 0 to 0.2 M gradient of NaCl. Fractions that reacted against anti-Stx2 antiserum were dialyzed against 20 mM Tris-HCl (pH 7.5) and separated by chromatofocusing chromatography using a Mono P column (Amersham Biosciences K.K., Tokyo, Japan) equilibrated with 25 mM histidine-HCl (pH 6.2), and the toxins were eluted with polybuffer 74-HCl (pH 4.0, 1:8; Amersham Biosciences K.K., Tokyo, Japan). Reacted fractions were separated once again under the same conditions. The chromatofocusing peaks of Stx2 or mutant Stx2 (B subunit, S31N) were pooled, and a final gel filtration step was carried out using a Superose 12 column. The peaks of Stx2 or mutant Stx2 (B subunit, S31N) were pooled and concentrated, and the samples were stored at −80°C. The protein concentration of each toxin was determined according to the Bradford method (Bio-Rad, Richmond, CA).
Construction of a single amino acid replacement mutant.
E. coli O157:H7 EDL933 (24) was the prototype for the construction of isogenic mutant strains. To obtain a selectable marker for conjugation, a spontaneously occurring nalidixic acid-resistant mutant of this organism (designated EDLN) was isolated from an LB agar plate containing nalidixic acid (20 mg/liter) onto which the centrifuged bacterial contents of 1 ml of an LB broth culture of E. coli O157:H7 EDL933 grown overnight were spread. The distribution of Stx1 and Stx2 in EHEC EDLN was identical to that in EHEC EDL933.
The plasmids pStx1(N32S)-1 and pStx2(S31N)-22 were digested with NcoI and EcoRI, and the DNA fragments were then blunted. The DNA fragments containing mutant Stx1 (B subunit, N32S) and Stx2 (B subunit, S31N) genes were inserted into the SmaI site in pWM91 (18) to generate plasmids pWN32S-20 and pWS31N-2, respectively, using E. coli DH5αλpir (6). The plasmids were electroporated into E. coli S17-1λpir (27). Matings were carried out by patch mating as described elsewhere previously (35). The entire bacterial lawn from each plate was suspended in PBS and diluted 10-fold to 10−4. A 100-μl aliquot of each dilution was spread onto an LB agar plate containing ampicillin (25 μg/ml) and nalidixic acid (20 μg/ml). Nalidixic acid-resistant and ampicillin-resistant colonies, representing potential EHEC EDLN exoconjugants, were picked and streaked onto an LB agar plate for single-colony isolation. Bacterial colonies were tested by PCR for the amplification of the Stx1 or Stx2 gene. An exoconjugant was grown in LB broth overnight at 37°C. Five 10-fold dilutions were spread out onto tryptic soy agar-20% sucrose agar plates and incubated at room temperature for 36 to 48 h. Colonies growing on the plates were picked and streaked onto LB agar plates for single-colony isolation. Colonies were tested for their sensitivity to ampicillin. The ampicillin-sensitive and sucrose-resistant colonies were further tested; one copy of the mutated toxin genes was left behind for PCR amplification of a DNA fragment of the mutant Stx1 (B subunit, N32S) or mutant Stx2 (B subunit, S31N) gene using primer sets P320 and P449 and P319 and P450, respectively. Finally, sequences of the holotoxin genes on the chromosomes of PCR-positive strains were verified by dideoxy sequencing (ABI).
Antibodies.
Polyclonal antisera for Stx1 and Stx2 were prepared as described previously (21, 39). The anti-Stx1 and anti-Stx2 antisera reacted primarily with the A subunits of Stx1 and Stx2 and hardly reacted with the B subunit of these proteins, respectively, as determined by immunoblotting. Anti-His tag antibody (27E8, a mouse monoclonal antibody) was obtained from Cell Signaling Technology, Inc. (Danvers, MA).
Mouse lethality studies.
Eight groups of 4-week-old ddY male mice (five mice per group) were injected intraperitoneally with various dilutions (5 μg to 300 pg) of purified holotoxins in sterile PBS (0.5 ml/mouse). The numbers of deaths were recorded twice daily, and the lethal dose corresponding to 50% mortality (LD50) was calculated by probit analysis (8).
RESULTS
Distribution of Shiga toxin family.
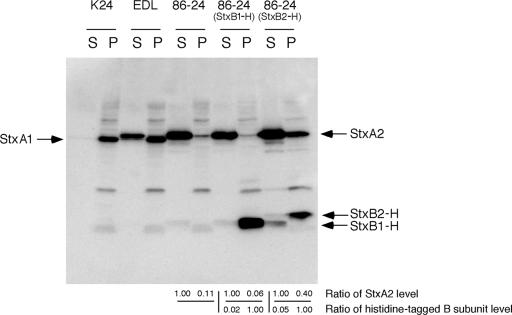
Measurements of the distribution of Stx1 and Stx2 in cultures of EHEC confirmed that Stx1 and Stx2 were secreted in different ways. EHEC strain K24, which produces Stx1, was cultured in LB broth and was found to produce Stx1 proteins that were exclusively cell associated, as determined by immunoblotting (Fig. 1). In contrast, measurement of the Stx2 distribution in cultures of EHEC strain 86-24 showed that Stx2 was produced extracellularly, as determined by immunoblotting (Fig. 1). EHEC strain EDL933, which produces both Stx1 and Stx2, was cultured in LB broth and was found to produce Stx1 proteins that were exclusively cell associated as well as Stx2 proteins that were extracellular, as determined by immunoblotting (Fig. 1). These results were consistent with those of a previous study (30).
FIG. 1.
Distribution of Stx1 and Stx2 and effect of overexpressed histidine-tagged StxB in EHEC. EHEC K24 (Stx1), EHEC EDL933 (Stx1 and Stx2), EHEC 86-24 (Stx2), and transformed EHEC 86-24 were fractionated into supernatant and cell-associated fractions. Each 2 μg of protein of the cell-associated fraction (P) and an equal volume of the supernatant fraction (S) were analyzed by SDS-PAGE and immunoblotting using a mixture of anti-Stx1 antiserum, anti-Stx2 antiserum, and anti-His tag antibody. Values represent the ratios of StxA2 protein levels to the cell-associated fraction in the supernatant fraction (upper) and the ratios of StxB-H protein levels to the supernatant fraction in the cell-associated fraction (lower).
To confirm the generality of Stx2 secretion in EHEC, we measured the distribution of Stx1 and Stx2 in 53 additional EHEC strains (21 Stx1- and Stx2-producing, 17 Stx1-producing, and 15 Stx2-producing strains) by Gb3 ELISA (Table 2). In Stx1- and Stx2-producing EHEC, approximately 88% of the total Stx2 production was located in the culture supernatant, and Stx1 was exclusively cell associated (average supernatant/total production of 8%). Similarly, in Stx2-producing EHEC strains, approximately 84% of the total Stx2 production was located in the culture supernatant. In 16 Stx1-producing EHEC strains, Stx1 was exclusively cell associated (average supernatant/total production of 4%). However, one Stx1-producing strain (EHEC K23) secreted Stx1 (supernatant/total production of 75%). Therefore, in general, Stx2 was predominantly extracellular, whereas Stx1 was mostly cell associated in EHEC strains.
TABLE 2.
Distribution of Shiga toxin familya
| Organism | Strain | Serotype | Stx1 or Shiga toxin
|
Stx2
|
||||
|---|---|---|---|---|---|---|---|---|
| S (ng/ml) | P (ng/ml) | S/(S + P) (%) | S (ng/ml) | P (ng/ml) | S/(S + P) (%) | |||
| EHEC | K1 | O157 | 4.5 | 15 | 23 | 12 | 2 | 86 |
| K2 | O157 | 5.5 | 20 | 22 | 200 | 8 | 96 | |
| K3 | O157 | 1.8 | 23 | 7 | 45 | 10 | 82 | |
| K4 | O157 | 1 | 19 | 5 | 160 | 8 | 95 | |
| K5 | O157 | 1.7 | 20 | 8 | 130 | 15 | 90 | |
| K6 | O157 | 1 | 28 | 3 | 310 | 8 | 97 | |
| K7 | O157 | 0 | 13 | 0 | 220 | 13 | 94 | |
| K8 | O157 | 1 | 14 | 7 | 330 | 15 | 96 | |
| K9 | O157 | 2.9 | 24 | 11 | 88 | 25 | 78 | |
| K10 | O157 | 1.3 | 16 | 8 | 350 | 32 | 92 | |
| K11 | O157 | 1.3 | 29 | 4 | 120 | 40 | 75 | |
| K12 | O157 | 1 | 16 | 6 | 4.6 | 1.4 | 77 | |
| K13 | O157 | 0 | 17 | 0 | 650 | 48 | 93 | |
| K14 | O157 | 1.2 | 13 | 8 | 670 | 28 | 97 | |
| K16 | O157 | 1.4 | 21 | 6 | 210 | 46 | 82 | |
| K17 | O157 | 1 | 13 | 7 | 1,000 | 28 | 97 | |
| K18 | O157 | 1.6 | 16 | 9 | 320 | 9 | 97 | |
| K19 | O157 | 1 | 12 | 8 | 420 | 34 | 93 | |
| K20 | O157 | 0 | 13 | 0 | 350 | 31 | 92 | |
| K21 | O157 | 3.5 | 22 | 14 | 30 | 37 | 45 | |
| K22 | O111 | 1 | 32 | 3 | 650 | 39 | 94 | |
| K23 | O157 | 38 | 13 | 75 | ||||
| K25 | O111 | 0.5 | 25 | 2 | ||||
| K26 | O111 | 0.6 | 11 | 5 | ||||
| K27 | O26 | 2.5 | 66 | 4 | ||||
| K28 | O26 | 1 | 58 | 2 | ||||
| K29 | O26 | 0.8 | 35 | 2 | ||||
| K30 | O26 | 1 | 61 | 2 | ||||
| K31 | O26 | 0.9 | 74 | 1 | ||||
| K32 | O26 | 1 | 66 | 1 | ||||
| K33 | O26 | 0.8 | 38 | 2 | ||||
| K34 | O26 | 1.7 | 43 | 4 | ||||
| K35 | O26 | 1 | 47 | 2 | ||||
| K36 | O26 | 1.4 | 52 | 3 | ||||
| K37 | O26 | 1.4 | 36 | 28 | ||||
| K38 | O26 | 1.2 | 68 | 2 | ||||
| K39 | O26 | 0.8 | 29 | 3 | ||||
| K40 | O26 | 1.9 | 52 | 4 | ||||
| K41 | O157 | 24 | 13 | 65 | ||||
| K42 | O157 | 560 | 15 | 97 | ||||
| K43 | O157 | 180 | 4 | 98 | ||||
| K44 | O157 | 4 | 1 | 80 | ||||
| K45 | O157 | 130 | 10 | 93 | ||||
| K46 | O157 | 360 | 4.5 | 99 | ||||
| K48 | O157 | 50 | 49 | 51 | ||||
| K49 | O157 | 310 | 90 | 78 | ||||
| K50 | O157 | 1,400 | 310 | 82 | ||||
| K51 | O157 | 430 | 8.6 | 98 | ||||
| K52 | O157 | 340 | 240 | 59 | ||||
| K53 | O157 | 140 | 13 | 92 | ||||
| K54 | O157 | 270 | 10 | 96 | ||||
| K55 | O157 | 180 | 20 | 90 | ||||
| K56 | O78 | 63 | 11 | 85 | ||||
| S. dysenteriae type 1 | S148 | 15 | 88 | 15 | ||||
| D1 | 3.7 | 51 | 7 | |||||
| D2 | 12 | 56 | 18 | |||||
| D3 | 31 | 110 | 22 | |||||
| D44 | 17 | 57 | 23 | |||||
| 6 | 14 | 100 | 12 | |||||
| 11 | 19 | 100 | 16 | |||||
| 16 | 29 | 340 | 8 | |||||
| 18 | 5.3 | 25 | 17 | |||||
| 19 | 0 | 28 | 0 | |||||
| 21 | 7.5 | 20 | 27 | |||||
| 22 | 0 | 28 | 0 | |||||
| 25 | 30 | 34 | 47 | |||||
| 28 | 0 | 39 | 0 | |||||
| 29 | 5.3 | 30 | 15 | |||||
S, supernatant; P, cell associated.
It is known that S. dysenteriae type 1 produces Shiga toxin, which is identical to Stx1 from EHEC (33). To determine the distribution of Shiga toxin in 15 Shigella dysenteriae type 1 strains, we assessed the distribution of Shiga toxin by Gb3 ELISA (Table 2). We detected approximately 15% of the total production of Shiga toxin in the culture supernatant. This result indicates that Shiga toxin, as well as Stx1, is secreted in only low amounts in S. dysenteriae type 1.
Effects of overexpressed histidine-tagged StxB in EHEC.
The localization of overexpressed Shiga toxins in E. coli K-12 was dictated by the type of B subunit (36). However, the mechanism by which Stx2 was specifically exported in EHEC has not yet been determined. In order to understand the mechanism of Stx2 secretion by EHEC, we have analyzed the secretion of Stx2 in EHEC strain 86-24, which produces Stx2, by generating histidine-tagged StxB1 (StxB1-H) or histidine-tagged StxB2 (StxB2-H) under low-expression conditions.
Unlike the expression of the StxB1 subunits, that of the StxB2 subunit has been more challenging (1). Since the 5′ noncoding regions of StxB2 differed from those of StxB1, we speculated that the difference in expression between the StxB1 and StxB2 subunits might take place at the translational step involved in mRNA ribosome binding. Following this line of reasoning, we discovered that interchanging the ribosomal binding regions of StxB1 and StxB2 resolved the StxB2 expression problem.
These strains were analyzed in terms of the distribution of exogenous StxB1-H or StxB2-H as well as in terms of endogenous Stx2. Although neither StxB1-H nor StxB2-H was secreted in StxB-H-overexpressed EHEC 86-24, StxB2-H-overexpressed EHEC 86-24 showed inhibited Stx2 secretion and an increase in the level of total Stx2 expression (Fig. 1). On the other hand, StxB1-H-overexpressed EHEC 86-24 did not show any alteration of Stx2 secretion and expression (Fig. 1). These results indicated that the overexpression of StxB2-H specifically affects the system of Stx2 secretion and expression in EHEC.
Identification of the StxB2 residue essential for the inhibition of Stx2 secretion.
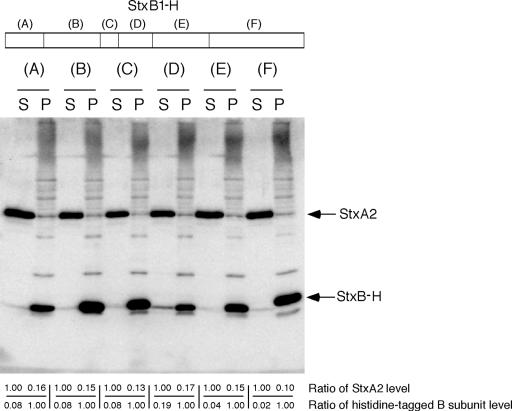
To identify the specific region of StxB2 that is essential for the inhibition of Stx2 secretion, we constructed a B-subunit chimera gene using Stx1 and Stx2. The B-subunit monomers of Stx1 and Stx2 contain 69 and 70 residues, respectively, and they have typical oligomer-binding folds that consist of a six-stranded antiparallel β-barrel capped by an α-helix (9, 29). Regions selected for the replacements corresponded to the secondary structure units of StxB1 and StxB2. The various histidine-tagged chimeras of the StxB1 subunits were generated by a two-step PCR-based strategy consisting of StxB2 replacements within residues 1 to 9 (region A), 10 to 22 (region B), 23 to 26 (region C), 27 to 34 (region D), 35 to 47 (region E), or 48 to 69 (region F) of StxB1 (Fig. 2). We analyzed the distribution of exogenous chimeric StxB1-H and endogenous Stx2 in EHEC strain 86-24 by generating histidine-tagged chimeras of StxB under low-expression conditions. The generation of chimeric StxB1-H in EHEC strain 86-24 did not alter the distribution of Stx2 (Fig. 2). However, expressed StxB1-H (region D) was partially secreted in EHEC strain 86-24 (Fig. 2). This suggested that region D of StxB2 includes the specific residue(s) related to Stx2 secretion in EHEC.
FIG. 2.
Effect of chimeric StxB1-H on Stx2 secretion in EHEC 86-24. Each 2 μg of protein of the cell-associated fraction (P) and an equal volume of the supernatant fraction (S) were analyzed by SDS-PAGE and immunoblotting using a mixture of anti-Stx2 antiserum and anti-His tag antibody. Values represent the ratios of StxA2 protein levels to the cell-associated fraction in the supernatant fraction (upper) and the ratios of StxB-H protein levels to the supernatant fraction in the cell-associated fraction (lower).
StxB1 and StxB2 shared three different residues in region D. To identify the StxB2 residues in region D that are related to Stx2 secretion in EHEC 86-24, we generated point-mutated StxB1-H to investigate the effects of the replacement of the tyrosine 28 residue, the tryptophan 29 residue, or the serine 32 residue in region D of StxB2. Single mutations of the leucine 29 residue to a tyrosine residue (L29Y) or of the phenylalanine 30 residue to a tryptophan residue (F30W) of StxB1-H did not exhibit any changes in the distribution of the histidine-tagged B subunit in any of the point-mutated StxB1-H-overexpressed EHEC 86-24 isolates (Fig. 3). However, single-mutant StxB1-H (asparagine 32 residue to a serine residue [N32S])-overexpressed EHEC strain 86-24 showed partially secreted StxB1-H(N32S) (Fig. 3).
FIG. 3.
Effect of point-mutated StxB-H on Stx2 secretion in EHEC 86-24. Each 2 μg of protein of the cell-associated fraction (P) and an equal volume of the supernatant fraction (S) were analyzed by SDS-PAGE and immunoblotting using a mixture of anti-Stx2 antiserum and anti-His tag antibody. Values represent the ratios of StxA2 protein levels to the cell-associated fraction in the supernatant fraction (upper) and the ratios of StxB-H protein levels to the supernatant fraction in the cell-associated fraction (lower).
On the other hand, when the serine 31 residue of StxB2-H was replaced with an asparagine residue, StxB2-H(S31N) showed a loss of Stx2 secretion inhibition (Fig. 3). As a control, in StxB2-H, the tyrosine 28 residue was exchanged for a leucine (Y28L) or the tryptophan 29 residue was exchanged for a phenylalanine (W29F), and the inhibitory activity against Stx2 secretion was retained even with low expression levels (Fig. 3). These results indicate that the serine 31 residue of StxB2 is an essential component for Stx2 secretion inhibition in EHEC.
The B-subunit structure of Stx2 is a pentamer in which each monomer comprises two three-stranded antiparallel β-sheets and an α-helix (Fig. 4). The fold is very similar to that of Stx1 (9). The serine 31 residues of the B-subunit monomer of Stx2 are exposed on the Gb3-binding surface of the B pentamer (Fig. 4). The serine 31 residue of the B subunit is located on the carboxyl-terminal end of the first three-stranded β-sheets. A side chain of the serine 31 residues of the B-subunit monomer of Stx2 as well as a side chain of the asparagine 32 residues of the B-subunit monomer of Stx1 are both toward the Gb3-binding surface of the B pentamer. In the Stx2 structure, the A subunit is located on the face opposite the Gb3-binding site shown in Fig. 4B.
FIG. 4.
Two orthogonal views of the Stx2 B pentamer. (A) View along the five-fold axis. The surface toward the viewer is the sugar-binding surface, which corresponds to the bottom surface in the view shown in B. Each serine 31 residue of the B subunit is located in the shaded circle. Opened arrows indicate the carboxyl-terminal end of each B-subunit monomer. In the Stx2 structure, the A subunit is located on the face opposite the binding sites in A (side of the carboxyl-terminal end in B). Stx1 is also very similar structurally to Stx2. The figures were made with Cn3D software (http://www.ncbi.nlm.nih.gov/Structure/CN3D/cn3d.shtml).
We found that expressed StxB2-H (regions A and B), consisting of StxB2 replacements in combination with regions A and B of StxB1, which contained the serine 31 residue of the B subunit, did not show inhibited Stx2 secretion in EHEC 86-24 (Fig. 5A). Gel filtration analysis revealed that StxB2-H (regions A and B) had not formed a B-subunit pentamer (Fig. 5B). This result suggested that the B-subunit pentamer is necessary for the inhibition of Stx2 secretion in EHEC.
FIG. 5.
Effect of StxB2-H (regions A and B) on Stx2 secretion in EHEC 86-24 (A) and size determination of StxB2-H (regions A and B) by gel filtration (B). (A) Two micrograms of protein of the cell-associated fraction (P) and an equal volume of the supernatant fraction (S) were analyzed by SDS-PAGE and immunoblotting using a mixture of anti-Stx2 antiserum and anti-His tag antibody. Values represent the ratios of StxA2 protein levels to the cell-associated fraction in the supernatant fraction (upper) and the ratios of StxB-H protein levels to the supernatant fraction in the cell-associated fraction (lower). (B) Purified StxB2-H (regions A and B) was loaded onto a TSK-G3000SW column and measured by reading the absorbance at 280 nm. Closed arrows indicate the elution of molecular mass standards. The fraction (shaded arrow) of StxB2-H contained the binding activity towards Vero cells, but the fraction (opened arrow) of StxB2-H (regions A and B) showed no binding activity.
Change in the distribution of mutant holotoxins.
Hence, it appears likely that this serine residue (i.e., serine 31) contains secretion-related information for Stx2 secretion in EHEC. To further investigate this hypothesis, we constructed isogenic mutant strains, EHEC N20-8 (StxB1 subunit, N32S) and EHEC N1-12 (StxB2 subunit, S31N), from EHEC EDL933, which produced both Stx1 and Stx2. EHEC strains EDLN, N20-8, and N1-12 were cultured in LB broth, and samples at various growth phases were analyzed by immunoblotting (Fig. 6). EHEC EDLN was found to produce the StxA1 proteins that were exclusively cell associated and the StxA2 proteins that were extracellular at the stationary phase (10 h). However, time course analysis revealed that the expression patterns differed between Stx1 and Stx2 in EHEC EDLN (Fig. 6). Although the StxA1 protein was detected at the mid-log phase in EHEC EDLN by immunoblotting, the StxA2 protein was detected from the late log phase (Fig. 6). Moreover, the system for Stx2 secretion may be expressed in EHEC EDLN at the stationary phase, because the StxA2 protein in the supernatant fraction was found at the stationary phase in EHEC EDLN (Fig. 6).
FIG. 6.
Growth curve (A) and distribution of Stx1 and Stx2 (B) in EHEC EDLN, isogenic mutant EHEC strain N20-8 (StxB1 subunit, N32S), and EHEC N1-12 (StxB2 subunit, S31N). EHEC strains were fractionated into a supernatant fraction and a cell-associated fraction at mid-log phase (4 h), late log phase (6 h), early stationary phase (8 h), and stationary phase (10 h). Each 2 μg of protein of the cell-associated fraction (P) and an equal volume of the supernatant fraction (S) were analyzed by SDS-PAGE and immunoblotting using a mixture of anti-Stx1 and anti-Stx2 antisera. Closed rectangles, EHEC EDLN; closed circles, EHEC N20-8 (StxB1 subunit, N32S); open circles, EHEC N1-12 (StxB2 subunit, S31N).
Isogenic mutant EHEC strain N1-12 was found to produce both the StxA1 and StxA2 proteins that were exclusively cell associated at the stationary phase (10 h). This result indicated that the serine 31 residue of the B subunit of Stx2 is essential for Stx2 secretion in EHEC. However, isogenic mutant EHEC strain N20-8 was not found to produce the StxA1 proteins that were extracellular in EHEC. This result indicated that the alteration of the asparagine 32 residue to a serine residue in the B subunit of Stx1 did not suffice for the secretion of mutant Stx1 in both Stx1- and Stx2-producing EHEC strains.
Therefore, to confirm whether or not overexpressed mutant Stx1 protein using the expression vector was secreted in EHEC, we constructed plasmids for the expression of mutant Stx1 (B subunit, N32S) and mutant Stx2 (B subunit, S31N). These plasmids were transformed into EHEC K47, which retained the stx2 gene, but no Stx2 protein was detected by immunoblotting (Fig. 7). Expressed Stx1 was found in the cell-associated fraction, and expressed Stx2 was found in the supernatant in each gene-transformed EHEC K47 strain, when maintained under low-expression conditions (Fig. 7). On the other hand, using E. coli BL21 as a host strain, both Stx1 and Stx2 were distributed in the cell-associated fraction (data not shown). This result indicates that E. coli BL21 did not possess a system for Stx2 secretion. However, mutant Stx1 (B subunit, N32S) was found in the supernatant, and mutant Stx2 (B subunit, S31N) was found in the cell-associated fraction in transformed EHEC K47 under low-expression conditions (Fig. 7). These results indicate that the serine 31 residue of the B subunit of Stx2 is also necessary for the secretion of Stx2 in holotoxin gene-transformed EHEC; moreover, the alteration of the asparagine 32 residue to a serine residue in the B subunit of Stx1 induced the secretion of mutant Stx1 in holotoxin gene-transformed EHEC. These results suggested that Stx1 potentially possesses secretable properties, as it lacks one piece of secretion-related information in holotoxin gene-transformed EHEC.
FIG. 7.
Distribution of point-mutated holotoxins in EHEC K47. EHEC strain K47 strain retained the stx2 gene, but no Stx2 protein was detected by immunoblotting. EHEC K47 harboring the stx gene was fractionated into supernatant (S) and cell-associated (P) fractions. The samples were analyzed by SDS-PAGE and immunoblotting using anti-Stx1 or anti-Stx2 antiserum.
Analysis of binding affinity and toxicity of mutant holotoxins.
The binding affinity of Stx2 for immobilized Gb3 was approximately 10-fold lower than that of Stx1 (32). To confirm the relationship between secretion property and binding affinity of Shiga toxins, the binding of mutant holotoxins to immobilized Gb3 was determined by Gb3 ELISA using anti-Stx1 or anti-Stx2 antiserum. The affinity of mutant Stx1 (B subunit, N32S) was almost identical to that of Stx1 (Fig. 8). In the same way, the affinity of mutant Stx2 (B subunit, S31N) was almost identical to that of Stx2 (Fig. 8). These results indicated that there were no differences in the affinity for Gb3 between Stx1 and mutant Stx1 (B subunit, N32S) or between Stx2 and mutant Stx2 (B subunit, S31N).
FIG. 8.
Comparison of binding between Stx1 and mutant Stx1 (B subunit, N32S) (A) or between Stx2 and mutant Stx2 (B subunit, S31N) (B) to Gb3 immobilized on ELISA plates coated with Gb3. Bound holotoxin was detected with anti-Stx1 or anti-Stx2 antiserum, followed by HRP conjugated to anti-rabbit IgG. Closed rectangles, Stx1; open rectangles, mutant Stx1 (B subunit, N32S); closed circles, Stx2; open circles, mutant Stx2 (B subunit, S31N). The data are presented as the percentages of the values for the maximum binding of Stx1 or Stx2 (means ± standard errors; n = 3).
Tesh et al. previously demonstrated that Stx2 had an approximately 400-fold-lower LD50 for mice than did Stx1 (32). Therefore, to clarify the relationship between secretion property and toxicity of Shiga toxins, we examined the LD50 of mutant holotoxins in mice. Groups of five mice were injected intraperitoneally with various dilutions of purified holotoxins. The LD50 of Stx1 and that of mutant Stx1 (B subunit, N32S) were 450 ng and 240 ng, respectively (Table 3). On the other hand, the LD50 of Stx2 and that of mutant Stx2 (B subunit, S31N) were 3.8 ng and 1.4 ng, respectively (Table 3). These results indicated that there were no differences in mouse lethality between Stx1 and mutant Stx1 (B subunit, N32S) or between Stx2 and mutant Stx2 (B subunit, S31N). Therefore, the secretion property of Shiga toxins was not related to affinity and toxicity.
TABLE 3.
Toxicity of mutant holotoxins in micea
| Toxin | LD50 (ng) (95% confidence limit) |
|---|---|
| Stx1 | 450 (99 < LD50 < 1,200) |
| Stx1 (B subunit, N32S) | 240 (110 < LD50 < 540) |
| Stx2 | 3.8 (0.5 < LD50 < 10) |
| Stx2 (B subunit, S31N) | 1.4 (0.08 < LD50 < 5.3) |
LD50 value was estimated by Probit analysis (8), and values in parentheses are 95% confidence limits.
DISCUSSION
Although its structure is highly homologous with that of Stx2, Stx1 tended not to be secreted in EHEC when the host strain was grown in LB broth. All 15 S. dysenteriae type 1 strains examined also rarely secreted Shiga toxin under LB broth conditions. Secreted proteins present in the supernatant fluid of EHEC have been shown to depend on the growth medium used (5). However, when Stx1-producing EHEC strain K24 was grown in serum-free tissue culture medium (minimal essential medium) and M9 medium, Stx1 was not detected in the supernatant fraction (data not shown). These results indicated that the system for Stx1 secretion was not present in EHEC strain K24 under LB medium, minimal essential medium, or M9 medium growth conditions.
The three-dimensional structure of Stx2 was similar to that of CT (9, 28, 29, 40). It was previously reported that CT was secreted by a type II secretion system in V. cholerae (26). Although the failure to identify a protein secretory pathway in enterotoxigenic E. coli (ETEC) for heat-labile enterotoxin (LT), which is immunologically, structurally, and functionally similar to CT, fostered the view that ETEC strains release LT only when the bacteria undergo lysis (34), Tauschek et al. recently reported the identification of a type II secretion pathway for LT secretion in ETEC (31). Therefore, in contrast to Stx1 and Shiga toxin, there is a system for LT secretion in ETEC.
Although EHEC 86-24 was shown to secrete Stx2, EHEC 86-24 did not secrete StxB2-H, in which six histidine residues were added at the carboxyl termini (Fig. 1). Moreover, EHEC K47, which expressed no Stx2 protein, also did not secrete StxB2-H (data not shown). The carboxyl termini of the B-subunit monomer were located near a central pore along the five-fold axis close to an adjacent A subunit (Fig. 4). These results suggest that the conformation of the holotoxin might be important for the secretion of Stx2. However, instead of the inhibition of Stx2 secretion, the overexpressed StxB1-H(N32S) was partially secreted in EHEC 86-24 and EHEC K47 (Fig. 3 and data not shown). These results suggested that the pentamer of StxB1-H(N32S), without the A subunit, was secreted in EHEC. The immunoreactivity of native StxB1-H against anti-His tag antibody (27E8) was largely different from that of native StxB2-H by ELISA (data not shown). This suggests that StxB1-H and StxB2-H differ in the local structure and/or orientation of carboxyl termini. These results are consistent with the previous finding that the orientation of the A subunit with respect to the B pentamer differs in both holotoxins (9). Therefore, the different effects of expressed histidine-tagged B subunits in EHEC might be caused by the difference in the local structure and/or orientation of carboxyl termini of StxB1-H and StxB2-H.
The overexpression of StxB2-H in EHEC 86-24 led to the inhibition of Stx2 secretion. Moreover, the overexpression of StxB1-H affected the secretion in EHEC K23, which secreted Stx1 (data not shown). These results suggest that the hypothetical regulatory molecule(s) of the secretion system for Stx2 secretion in EHEC 86-24 specifically interacts with the B subunit of Stx2 and that, in EHEC K23, this molecule recognizes the B subunit of Stx1. The hypothetical regulatory molecule gene(s) of the secretion system in EHEC K23 may be mutated in response to changes in specificity for secreted proteins. However, it remains unclear how StxB2-H specifically inhibits Stx2 secretion, and the regulatory molecules required for the secretion of Stx2 in EHEC also remain unknown.
The overexpression of StxB1-H in EHEC had no effect on the distribution of endogenous Stx2. We confirmed by gel filtration that StxB1-H and StxB2-H each form a pentamer and show binding activity against immobilized Gb3, as determined by Gb3 ELISA (data not shown). Therefore, differences in the secretion system between StxB1-H and StxB2-H were not found to be related to the assembly and binding activity of the B subunit.
StxB2-H (regions A and B), which contained the serine 31 residue of the B subunit, did not show any changes in the distribution of Stx2 in EHEC and did not form a B-subunit pentamer (Fig. 5). These results suggest that interaction between the B subunit and the hypothetical regulatory molecule(s) of the secretion system is necessary for the assembly of the B subunit. Information for the secretion of CT has been shown to reside in the B-subunit pentamer, as the expression of B subunits in the absence of the A subunit results in their assembly into pentamers in the periplasm followed by secretion across the outer membrane of V. cholerae (12). In this case, however, there were unsuccessful attempts to identify the targeting signal in the B pentamer by the construction of fusion proteins between a portion of the B subunit and either beta-lactamase or alkaline phosphatase (25). This lack of success was due apparently to the inability of the fusion proteins to form pentamers; this supports the hypothesis that the assembly of the B subunit into a pentameric structure is a prerequisite for the interaction between the B subunit and the secretion machinery in AB5 toxins.
In this study, it became clear that the alteration of the serine 31 residue to an asparagine residue in the B subunit of Stx2 leads to the inhibition of mutant Stx2 secretion in EHEC (Fig. 6 and 7). Four major structural differences between Stx1 and Stx2 were found by X-ray crystallography (9, 29). However, the serine 31 residue of the B subunit of Stx2 was not present in four regions that were found to differ structurally between Stx1 and Stx2. Therefore, the difference in a side chain between the serine and asparagine residues may be important for the secretion of Shiga toxins. One piece of secretion-related information for Stx2 secretion in EHEC was the serine 31 residue of the B subunit, which was exposed on the Gb3-binding surface (9). Therefore, the putative hypothetical regulatory molecule(s) of the system for Stx2 secretion in EHEC may specifically interact with the Gb3-binding surface of the assembled B subunits of Stx2. However, the mechanism by which Shiga toxins undergo recognition remains unclear.
Although the overexpressed mutant Stx1 (B subunit, N32S) was secreted in holotoxin gene-transformed EHEC K47, the isogenic mutant EHEC strain N20-8, which produced mutant Stx1 (B subunit, N32S) and Stx2, did not secrete mutant Stx1 (B subunit, N32S) at any growth phase (Fig. 6). One possible reason for this is that the expression patterns between mutant Stx1 and Stx2 are different in EHEC. The machinery for Stx2 secretion might not be expressed in EHEC at the log phase. Another possibility is that mutant Stx1 (B subunit, N32S) interacts with the machinery for Stx2 secretion in EHEC but that the affinity of mutant Stx1 for the secretion machinery in EHEC is lower than that of Stx2. It seems that the efficiency of Stx2 secretion in EHEC N20-8 is lower than that in EHEC EDLN (Fig. 6).
It was reported previously that the phenylalanine 30 residue of the B subunit of Stx1 (i.e., the counterpart of the tryptophan 29 residue of the Stx2 B subunit) on the Gb3-binding surface also plays an important role in receptor binding (4, 20). Asparagine 32 and serine 31 of the B subunit of Stx1 and Stx2 were very close to phenylalanine 30 and tryptophan 29, respectively, in the B subunit on the same Gb3-binding surface. Since an alteration of the serine 31 residue to an asparagine residue in the B subunits of Stx2 or an alteration of the asparagine 32 residue to a serine residue in the B subunits of Stx1 did not dramatically alter the affinity of the holotoxins for Gb3, the Gb3 binding sites might not be directly involved with the asparagine 32 and serine 31 residues of the B subunits of Stx1 and Stx2, respectively.
It was previously determined that a single tryptophan residue is important for aerolysine secretion by Aeromonas salmonicida as well as for the secretion of Erwinia chrysanthemi Cel5 cellulase (3, 37). Those two studies suggested that tryptophan residues might be part of the secretion motif of secretion proteins. However, in the present study, the secretion motif of Stx2 did not include tryptophan residues, and the serine residue appeared to contain secretion-related information for both Stx1 and Stx2. However, there is a tryptophan residue at position 33, near the serine 31 residue, in the B subunit of Stx2. Since this tryptophan 33 residue was also exposed on the Gb3-binding surface, this residue of StxB2 may also participate in the secretion motif of Stx2.
Epidemiological studies have indicated that EHEC strains producing Stx2 are more commonly associated with serious human disease, such as HUS, than are strains producing Stx1 (16, 23). The link between Stx2 production and HUS may be a direct consequence of the increased in vivo toxicity of Stx2, one possible explanation for which could be that the levels of production and secretion of Stx2 in vivo are also higher than those of Stx1. This pathway for Stx2 secretion likely plays a significant role in EHEC pathogenicity.
Supplementary Material
Acknowledgments
We are grateful to Ayako Kiuti and Reiko Komine for their technical assistance and Bradley G. Stiles for critical reading of the manuscript.
This research was supported by grants-in-aid for scientific research (17590382) from the Ministry of Education, Culture, Sports, Science, and Technology and the Japan Society for the Promotion of Science (JSPS).
Editor: A. D. O'Brien
Footnotes
Published ahead of print on 26 February 2007.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Acheson, D. W., S. A. De Breucker, M. Jacewicz, L. L. Lincicome, A. Donohue-Rolfe, A. V. Kane, and G. T. Keusch. 1995. Expression and purification of Shiga-like toxin II B subunits. Infect. Immun. 63:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acheson, D. W. K., A. Donohue-Rolfe, and G. T. Keusch. 1991. The family of Shiga and Shiga-like toxins, p. 415-433. In J. E. Alouf and J. H. Freer (ed.), Sourcebook of bacterial protein toxins. Academic Press Ltd., London, United Kingdom.
- 3.Chapon, V., H. D. Simpson, X. Morelli, E. Brun, and F. Barras. 2000. Alteration of a single tryptophan residue of the cellulose-binding domain blocks secretion of the Erwinia chrysanthemi Cel5 cellulase (ex-EGZ) via the type II system. J. Mol. Biol. 303:117-123. [DOI] [PubMed] [Google Scholar]
- 4.Clark, C., D. Bast, A. M. Sharp, P. M. St. Hilaire, R. Agha, P. E. Stein, E. J. Toone, R. J. Read, and J. L. Brunton. 1996. Phenylalanine 30 plays an important role in receptor binding of verotoxin-1. Mol. Microbiol. 19:891-899. [DOI] [PubMed] [Google Scholar]
- 5.Ebel, F., C. Deibel, A. U. Kresse, C. A. Guzman, and T. Chakraborty. 1996. Temperature- and medium-dependent secretion of proteins by Shiga toxin-producing Escherichia coli. Infect. Immun. 64:4472-4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott, S. J., and J. B. Kaper. 1997. Role of type 1 fimbriae in EPEC infections. Microb. Pathog. 23:113-118. [DOI] [PubMed] [Google Scholar]
- 7.Endo, Y., K. Tsurugi, T. Yutsudo, Y. Takeda, T. Ogasawara, and K. Igarashi. 1988. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171:45-50. [DOI] [PubMed] [Google Scholar]
- 8.Finney, D. J. 1971. Probit analysis, 3rd ed. Cambridge University Press, London, United Kingdom.
- 9.Fraser, M. E., M. Fujinaga, M. M. Cherney, A. R. Melton-Celsa, E. M. Twiddy, A. D. O'Brien, and M. N. James. 2004. Structure of Shiga toxin type 2 (Stx2) from Escherichia coli O157:H7. J. Biol. Chem. 279:27511-27517. [DOI] [PubMed] [Google Scholar]
- 10.Griffin, P. M., S. M. Ostroff, R. V. Tauxe, K. D. Greene, J. G. Wells, J. H. Lewis, and P. A. Blake. 1988. Illnesses associated with Escherichia coli O157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109:705-712. [DOI] [PubMed] [Google Scholar]
- 11.Hirst, T. R., and J. Holmgren. 1987. Conformation of protein secreted across bacterial outer membranes: a study of enterotoxin translocation from Vibrio cholerae. Proc. Natl. Acad. Sci. USA 84:7418-7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirst, T. R., J. Sanchez, J. B. Kaper, S. J. Hardy, and J. Holmgren. 1984. Mechanism of toxin secretion by Vibrio cholerae investigated in strains harboring plasmids that encode heat-labile enterotoxins of Escherichia coli. Proc. Natl. Acad. Sci. USA 81:7752-7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann, S. L. 1993. Southwestern Internal Medicine Conference: Shiga-like toxins in hemolytic-uremic syndrome and thrombotic thrombocytopenic purpura. Am. J. Med. Sci. 306:398-406. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, M. P., J. W. Newland, R. K. Holmes, and A. D. O'Brien. 1987. Nucleotide sequence analysis of the structural genes for Shiga-like toxin I encoded by bacteriophage 933J from Escherichia coli. Microb. Pathog. 2:147-153. [DOI] [PubMed] [Google Scholar]
- 15.Keusch, G. T., and D. W. Acheson. 1997. Thrombotic thrombocytopenic purpura associated with Shiga toxins. Semin. Hematol. 34:106-116. [PubMed] [Google Scholar]
- 16.Kleanthous, H., H. R. Smith, S. M. Scotland, R. J. Gross, B. Rowe, C. M. Taylor, and D. V. Milford. 1990. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with verocytotoxin producing Escherichia coli. Part 2: microbiological aspects. Arch. Dis. Child. 65:722-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansbury, L. E., and H. Ludlam. 1997. Escherichia coli O157: lessons from the past 15 years. J. Infect. 34:189-193. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf, W. W., W. Jiang, L. L. Daniels, S. K. Kim, A. Haldimann, and B. L. Wanner. 1996. Conditionally replicative and conjugative plasmids carrying lacZ alpha for cloning, mutagenesis, and allele replacement in bacteria. Plasmid 35:1-13. [DOI] [PubMed] [Google Scholar]
- 19.Miyake, M., E. Utsuno, and M. Noda. 2000. Binding of avian ovomucoid to Shiga-like toxin type 1 and its utilization for receptor analog affinity chromatography. Anal. Biochem. 281:202-208. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa, K., K. Matsuoka, M. Watanabe, K. Igai, K. Hino, K. Hatano, A. Yamada, N. Abe, D. Terunuma, H. Kuzuhara, and Y. Natori. 2005. Identification of the optimal structure required for a Shiga toxin neutralizer with oriented carbohydrates to function in the circulation. J. Infect. Dis. 191:2097-2105. [DOI] [PubMed] [Google Scholar]
- 21.Noda, M., T. Yutsudo, N. Nakabayashi, T. Hirayama, and Y. Takeda. 1987. Purification and some properties of Shiga-like toxin from Escherichia coli O157:H7 that is immunologically identical to Shiga toxin. Microb. Pathog. 2:339-349. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien, A. D., and R. K. Holmes. 1987. Shiga and Shiga-like toxins. Microbiol. Rev. 51:206-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostroff, S. M., P. I. Tarr, M. A. Neill, J. H. Lewis, N. Hargrett-Bean, and J. M. Kobayashi. 1989. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. J. Infect. Dis. 160:994-998. [DOI] [PubMed] [Google Scholar]
- 24.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 25.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 26.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, and V. J. DiRita. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon, R., U. Priefer, and A. Pühler. 1982. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 28.Sixma, T. K., S. E. Pronk, K. H. Kalk, E. S. Wartna, B. A. van Zanten, B. Witholt, and W. G. Hol. 1991. Crystal structure of a cholera toxin-related heat-labile enterotoxin from E. coli. Nature 351:371-377. [DOI] [PubMed] [Google Scholar]
- 29.Stein, P. E., A. Boodhoo, G. J. Tyrrell, J. L. Brunton, and R. J. Read. 1992. Crystal structure of the cell-binding B oligomer of verotoxin-1 from E. coli. Nature 355:748-750. [DOI] [PubMed] [Google Scholar]
- 30.Strockbine, N. A., L. R. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauschek, M., R. J. Gorrell, R. A. Strugnell, and R. M. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tesh, V. L., J. A. Burris, J. W. Owens, V. M. Gordon, E. A. Wadolkowski, A. D. O'Brien, and J. E. Samuel. 1993. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infect. Immun. 61:3392-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesh, V. L., and A. D. O'Brien. 1991. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 5:1817-1822. [DOI] [PubMed] [Google Scholar]
- 34.Wai, S. N., A. Takade, and K. Amako. 1995. The release of outer membrane vesicles from the strains of enterotoxigenic Escherichia coli. Microbiol. Immunol. 39:451-456. [DOI] [PubMed] [Google Scholar]
- 35.Wanner, B. L. 1994. Gene expression in bacteria using TnphoA and TnphoA′ elements to make and switch phoA gene, lacZ(op), and lacZ(pr) fusions. Methods Mol. Genet. 3:291-310. [Google Scholar]
- 36.Weinstein, D. L., M. P. Jackson, L. P. Perera, R. K. Holmes, and A. D. O'Brien. 1989. In vivo formation of hybrid toxins comprising Shiga toxin and the Shiga-like toxins and role of the B subunit in localization and cytotoxic activity. Infect. Immun. 57:3743-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong, K. R., and J. T. Buckley. 1991. Site-directed mutagenesis of a single tryptophan near the middle of the channel-forming toxin aerolysin inhibits its transfer across the outer membrane of Aeromonas salmonicida. J. Biol. Chem. 266:14451-14456. [PubMed] [Google Scholar]
- 38.Yoh, M., E. K. Frimpong, and T. Honda. 1997. Effect of antimicrobial agents, especially fosfomycin, on the production and release of Vero toxin by enterohaemorrhagic Escherichia coli O157:H7. FEMS. Immunol. Med. Microbiol. 19:57-64. [DOI] [PubMed] [Google Scholar]
- 39.Yutsudo, T., N. Nakabayashi, T. Hirayama, and Y. Takeda. 1987. Purification and some properties of a Vero toxin from Escherichia coli O157:H7 that is immunologically unrelated to Shiga toxin. Microb. Pathog. 3:21-30. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, R. G., D. L. Scott, M. L. Westbrook, S. Nance, B. D. Spangler, G. G. Shipley, and E. M. Westbrook. 1995. The three-dimensional crystal structure of cholera toxin. J. Mol. Biol. 251:563-573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.