Abstract
Enterococcus faecalis transposon insertion mutants were screened for attenuated killing of the nematode model host Caenorhabditis elegans. The genes disrupted in the attenuated mutants encode a variety of factors including transcriptional regulators, transporters, and damage control and repair systems. Five of nine mutants tested were attenuated in a mouse peritonitis model.
Enterococcus faecalis has emerged over the past few decades as one of the leading causes of hospital-acquired infection, causing diseases such as endocarditis, urinary tract infections, and bloodstream infections (14). E. faecalis's natural ruggedness, which causes intrinsic resistance to many antibiotics, and its versatility in swapping genetic information to gain additional resistance, have played large roles in its advance (12). Several E. faecalis virulence factors have been identified by their distinct biochemical properties, by their antigenic phenotypes, or by homology searches using known virulence factors from other bacteria (8). Surprisingly, given the importance of this pathogen, no in vitro or in vivo screens have been performed to isolate new virulence determinants in E. faecalis in an unbiased manner. We previously demonstrated that Caenorhabditis elegans could be used as a model host to identify potential mammalian virulence determinants (6). Additionally, we built an ordered library of transposon insertion mutants of E. faecalis strain OG1RF with approximately 25% of the nonessential genes disrupted (7). In this work, we identified 23 insertion mutants in the ordered library with attenuated killing of C. elegans. Five of nine mutants tested were also less virulent in a mouse peritonitis model.
To identify strains of E. faecalis mutants from our ordered library of 540 mutants that were deficient in C. elegans killing, the following strategy was used. Plates containing a bacterial lawn of each transposon mutant were generated by using growth conditions previously found optimal for killing of C. elegans by E. faecalis (6). Approximately 30 to 40 worms, strain N2 (the wild type), were placed on each mutant, and survival over time was assayed for 7 days at 24-h intervals. With GraphPad Prism 3.0 or STATA 6.0, survival was plotted by the Kaplan-Meier method and differences between the mutant and parent strain were compared by using the log-rank test. Seventy-two mutants with a difference resulting in a P value of 0.1 or less were tested in a second assay with a larger population of C. elegans (n = 60 to 80). P values of less than 0.05 were considered statistically significant. Mutants that caused significant attenuation by this criterion in both experiments are listed in Table 1, and an example of a typical killing assay is presented in Fig. 1A. Because mutants with a growth defect could cause a reduction in killing in a nonspecific manner, log-phase growth in liquid brain heart infusion (BHI) medium of all of the mutants was compared to that of the parent strain. All mutants displayed growth similar to that of the wild type, except for mutant 4H12 (Fig. 1B and Table 1). We also assayed growth in 50% serum from human volunteers as described previously (5), with and without heat inactivation. Growth in serum may more closely parallel conditions found in the mammalian host environment, and the comparison with heat-inactivated serum addresses whether or not there is increased sensitivity to complement. None of the mutants displayed a growth defect under either condition, including 4H12 (data not shown). Perhaps the deficiency of 4H12 in BHI is specific to conditions that result in a short doubling time.
TABLE 1.
Attenuated E. faecalis mutants identified by screening with C. elegans
| Type of mutation and mutant | V583 ORFa | Mouse model P valueb | Gene name | Gene product, function |
|---|---|---|---|---|
| Transcriptional regulator | ||||
| 4G3 | EF_0382 | NT | None | Helix-turn-helix, Fis type |
| 1C4 | EF_1302 | 0.13 | lysR | Helix-turn-helix, lysR type |
| 1D1 | EF_1569c | 0.14 | psr | CpsA/LytR/Psr family of transcriptional regulators |
| 5C11 | EF_1604 | 0.19 | scrR-1 | Negative regulator of sucrose operon |
| 4D4 | EF_1821 | 0.001e | fsrB | Peptide processor of Fsr two-component system |
| Transporter | ||||
| 2A1 | EF_0243 | NT | brnQ | Branched-chain amino acid transporter |
| 4C8 | EF_1513 | 0.31 | None | Pheromone binding protein |
| 4G6 | EF_2598 | NT | None | PEP-PTSf, beta-glucoside-specific IIABC component |
| Damage control and repair | ||||
| 6A3 | EF_1545 | 0.005 | recQ-1 | DNA helicase, SOS response |
| 3G8 | EF_1598 | 0.038 | phrB | Deoxyribodipyrimidine photolyase, DNA repair |
| 4G7 | EF_2591 | NT | None | glxI, glyoxalase, protective against electrophiles |
| Catabolism | ||||
| 4G8 | EF_0737 | NT | None | Putative amidase |
| 6C9 | EF_1603 | 0.01e | scrB-1 | Sucrose-6-phosphate hydrolase |
| 6E9 | EF_1623c | NT | pduJ | Carboxysome protein, metabolism of ethanolamine |
| Anabolism | ||||
| 1C9 | EF_1576 | NT | thyA | Thymidylate synthase, deoxyribonucleotide biosynthesis |
| 4H12 | EF_2200d | NT | map | Methionyl aminopeptidase, protein maturation |
| Other | ||||
| 4G10 | EF_0376 | NT | None | Hypothetical protein |
| 6C10 | EF_0861c | NT | None | GNAT family acetyltransferase |
| 6B3 | EF_1542 | NT | None | Conserved hypothetical membrane protein |
| 4F8 | EF_1590c | 0.0003 | paiA | SSAT acetyltransferase, negative regulator of sporulation |
| 6D5 | EF_1792 | NT | None | Conserved hypothetical |
| 4G9 | EF_2675 | NT | None | Putative competence protein, CoiA-like family |
| 6D6 | EF_2957 | NT | None | Hexapeptide repeat acetyltransferase |
ORF, open reading frame.
Regulated by Fsr system.
Growth defect.
PEP-PTS, phosphoenolpyruvate-dependent phosphotransferase system.
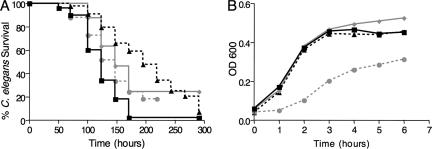
FIG. 1.
Example killing assay and growth curve of E. faecalis mutants with attenuated C. elegans killing. (A) Survival of C. elegans on OG1RF (squares) compared to mutants with disruptions in 4D4 (triangles) (P < 0.0001), 4G6 (diamonds) (P < 0.0001), and 4H12 (circles) (P < 0.0194). (B) Growth curves of E. faecalis mutants listed in panel A grown in BHI medium. OD 600, optical density at 600 nm.
The mutants found in the screen were classified by the probable function of the protein encoded by the disrupted gene according to the annotation provided by the V583 genome sequence (19). We subjected the sequences to BLAST analysis and researched the literature for additional information on possible functions. Several putative transcriptional regulators were identified. EF_1302, for example, resembles a lysR helix-turn-helix transcriptional regulator involved in virulence and stress response in other bacteria such as Pseudomonas aeruginosa (21). EF_1569 encodes Psr, which belongs to a family of negative transcriptional regulators that control cell surface properties such as cell wall and exopolysaccharide composition and synthesis (15). Such a mutant could be affected in adhesion or biofilm formation, properties that affect the infectivity of E. faecalis. We also identified EF_1604, which encodes the transcriptional repressor ScrR. In previous work (6) (as well as this study), we found that a mutation in scrB, a gene likely regulated by ScrR and encoding sucrose-6-hydrolase, caused attenuation in C. elegans killing. Sucrose utilization plays an important role in biofilm formation and pathogenicity in S. mutans during caries formation (16) and endocarditis (17), as it is a substrate for the synthesis of the extracellular polymers glucan and fructan (9). We also identified FsrB in this screen; the Fsr two-component regulatory system is a major regulator of virulence in E. faecalis, and mutation of FsrB attenuates virulence in a variety of infection models, including C. elegans (6, 11, 18, 20).
Transporters were found, including a pheromone binding protein possibly involved in quorum sensing and a phosphoenolpyruvate-dependent phosphotransferase system beta-glucoside-specific IIABC component. Genes involved in beta-glucoside metabolism were found to be upregulated in Streptococcus gordonii on infected heart valves and contributed to biofilm formation (13).
Some of the mutants with attenuated C. elegans killing have insertions in genes encoding enzymes that could be involved in damage control and repair (Table 1). For example, recQ (EF_1545) and phrB (EF_1598) homologs encode DNA repair enzymes. Loss of such enzymes in other pathogens such as Salmonella results in attenuated killing of mice (2) and sensitivity to the oxidative burst in macrophages (3). glxI (EF_2591) has been found to be upregulated in macrophage-engulfed Salmonella and may have roles in dealing with oxidative stress (4).
EF_1623 is one of a cluster of genes that are orthologs of the eut-pdu operons in Salmonella. The E. faecalis operon appears to be involved in the use of ethanolamine, a readily available lipid component, as a carbon and nitrogen source. This gene cluster was previously identified as being strongly regulated by the Fsr system (1), and some of the components have been found to be upregulated in Salmonella engulfed by macrophages (10).
To determine if any of the newly identified mutants caused loss of infectivity in a mammalian model, seven were tested in a mouse peritonitis model. Mice were inoculated intraperitoneally with E. faecalis as previously described (6, 22). The significance of differences in survival time compared to that of the wild type was evaluated in the same manner as described for C. elegans. The experiment was repeated twice and carried out under approved protocols. Three mutants, 6A3, 3G8, and 4F8, were attenuated (P < 0.05) (Fig. 2 and Table 1). Additionally, two of the mutants identified, 6C9 and 4D4, had insertions in genes characterized as causing attenuation in the mouse model in previous work (6, 20). It is conceivable that use of a different animal model, such as a model of endocarditis, would identify a different subset of attenuated mutants.
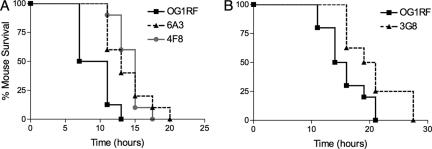
FIG. 2.
Survival of mice infected intraperitoneally with E. faecalis mutants. Shown are representative experiments with the mutants characterized as attenuated in this model but not previously published (6A3, 4F8, and 3G8). (A) Survival of mice infected with 7.35 × 108 CFU of OG1RF, 7.37 × 108 CFU of 6A3, and 7.41 × 108 CFU of 4F8. (B) Survival of mice infected with 5.55 × 108 CFU of OG1RF and 5.65 × 108 CFU of 3G8.
In summary, we identified 23 insertion mutants with attenuated C. elegans killing, 2 of which were previously known to affect pathogenesis in the worm and the mouse (6, 20). Several orthologs of the genes disrupted by the transposon insertions are known to affect mammalian pathogenesis in other bacterial species, and more than half of those tested were attenuated in a mouse peritonitis model. In conclusion, C. elegans is an efficient way to identify potential virulence determinants and screening a complete ordered library would likely uncover additional factors.
Acknowledgments
We thank Frederick M. Ausubel and Stephen B. Calderwood for early support of this project and Michael C. Lorenz for critical reading of the manuscript.
This work was supported by Public Health Service grants AI603084 (to E.M.) and AI064470 (to D.A.G.) and New Scholar Awards in Global Infectious Diseases from the Ellison Medical Foundation to E.M. and D.A.G. Initial stages were supported by Public Health Service grant AI064332 to Frederick M. Ausubel.
Editor: A. Camilli
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Bourgogne, A., S. G. Hilsenbeck, G. M. Dunny, and B. E. Murray. 2006. Comparison of OG1RF and an isogenic fsrB deletion mutant by transcriptional analysis: the Fsr system of Enterococcus faecalis is more than the activator of gelatinase and serine protease. J. Bacteriol. 188:2875-2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchmeier, N. A., S. J. Libby, Y. Xu, P. C. Loewen, J. Switala, D. G. Guiney, and F. C. Fang. 1995. DNA repair is more important than catalase for Salmonella virulence in mice. J. Clin. Investig. 95:1047-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchmeier, N. A., C. J. Lipps, M. Y. So, and F. Heffron. 1993. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol. Microbiol. 7:933-936. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47:103-118. [DOI] [PubMed] [Google Scholar]
- 5.Figdor, D., J. K. Davies, and G. Sundqvist. 2003. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol. Immunol. 18:234-239. [DOI] [PubMed] [Google Scholar]
- 6.Garsin, D. A., C. D. Sifri, E. Mylonakis, X. Qin, K. V. Singh, B. E. Murray, S. B. Calderwood, and F. M. Ausubel. 2001. A simple model host for identifying gram-positive virulence factors. Proc. Natl. Acad. Sci. USA 98:10892-10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garsin, D. A., J. Urbach, J. C. Huguet-Tapia, J. E. Peters, and F. M. Ausubel. 2004. Construction of an Enterococcus faecalis Tn917-mediated gene disruption library offers insight into Tn917 insertion patterns. J. Bacteriol. 186:7280-7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore, M. S., P. S. Coburn, S. R. Nallapareddy, and B. E. Murray. 2002. Enterococcal virulence, p. 301-354. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 9.Hamada, S., and H. D. Slade. 1980. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heithoff, D. M., C. P. Conner, U. Hentschel, F. Govantes, P. C. Hanna, and M. J. Mahan. 1999. Coordinate intracellular expression of Salmonella genes induced during infection. J. Bacteriol. 181:799-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jha, A. K., H. P. Bais, and J. M. Vivanco. 2005. Enterococcus faecalis mammalian virulence-related factors exhibit potent pathogenicity in the Arabidopsis thaliana plant model. Infect. Immun. 73:464-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kak, V., and J. W. Chow. 2002. Acquired antibiotic resistances in enterococci, p. 355-383. In D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 13.Kiliç, A. O., L. Tao, Y. Zhang, Y. Lei, A. Khammanivong, and M. C. Herzberg. 2004. Involvement of Streptococcus gordonii beta-glucoside metabolism systems in adhesion, biofilm formation, and in vivo gene expression. J. Bacteriol. 186:4246-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malani, P. N., C. A. Kauffman, and M. J. Zervos. 2002. Enterococcal disease, epidemiology, and treatment, p. 385-408. In M. S. Gilmore, D. B. Clewell, P. Courvalin, G. M. Dunny, B. E. Murray, and L. B. Rice (ed.), The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM Press, Washington, DC.
- 15.Massidda, O., O. Dardenne, M. B. Whalen, W. Zorzi, J. Coyette, G. D. Shockman, and L. Daneo-Moore. 1998. The PBP 5 synthesis repressor (psr) gene of Enterococcus hirae ATCC 9790 is substantially longer than previously reported. FEMS Microbiol. Lett. 166:355-360. [DOI] [PubMed] [Google Scholar]
- 16.Munro, C., S. M. Michalek, and F. L. Macrina. 1991. Cariogenicity of Streptococcus mutans V403 glucosyltransferase and fructosyltransferase mutants constructed by allelic exchange. Infect. Immun. 59:2316-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munro, C. L., and F. L. Macrina. 1993. Sucrose-derived exopolysaccharides of Streptococcus mutans V403 contribute to infectivity in endocarditis. Mol. Microbiol. 8:133-142. [DOI] [PubMed] [Google Scholar]
- 18.Mylonakis, E., M. Engelbert, X. Qin, C. D. Sifri, B. E. Murray, F. M. Ausubel, M. S. Gilmore, and S. B. Calderwood. 2002. The Enterococcus faecalis fsrB gene, a key component of the fsr quorum-sensing system, is associated with virulence in the rabbit endophthalmitis model. Infect. Immun. 70:4678-4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071-2074. [DOI] [PubMed] [Google Scholar]
- 20.Qin, X., K. V. Singh, G. M. Weinstock, and B. E. Murray. 2000. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect. Immun. 68:2579-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 22.Singh, K. V., X. Qin, G. M. Weinstock, and B. E. Murray. 1998. Generation and testing of mutants of Enterococcus faecalis in a mouse peritonitis model. J. Infect. Dis. 178:1416-1420. [DOI] [PubMed] [Google Scholar]